Abstract
Vast expanses of oxygen-deficient and nitrite-rich water define the major oxygen minimum zones (OMZs) of the global ocean. They support diverse microbial communities that influence the nitrogen economy of the oceans, contributing to major losses of fixed nitrogen as dinitrogen (N2) and nitrous oxide (N2O) gases. Anaerobic microbial processes, including the two pathways of N2 production, denitrification and anaerobic ammonium oxidation, are oxygen-sensitive, with some occurring only under strictly anoxic conditions. The detection limit of the usual method (Winkler titrations) for measuring dissolved oxygen in seawater, however, is much too high to distinguish low oxygen conditions from true anoxia. However, new analytical technologies are revealing vanishingly low oxygen concentrations in nitrite-rich OMZs, indicating that these OMZs are essentially anoxic marine zones (AMZs). Autonomous monitoring platforms also reveal previously unrecognized episodic intrusions of oxygen into the AMZ core, which could periodically support aerobic metabolisms in a typically anoxic environment. Although nitrogen cycling is considered to dominate the microbial ecology and biogeochemistry of AMZs, recent environmental genomics and geochemical studies show the presence of other relevant processes, particularly those associated with the sulfur and carbon cycles. AMZs correspond to an intermediate state between two “end points” represented by fully oxic systems and fully sulfidic systems. Modern and ancient AMZs and sulfidic basins are chemically and functionally related. Global change is affecting the magnitude of biogeochemical fluxes and ocean chemical inventories, leading to shifts in AMZ chemistry and biology that are likely to continue well into the future.
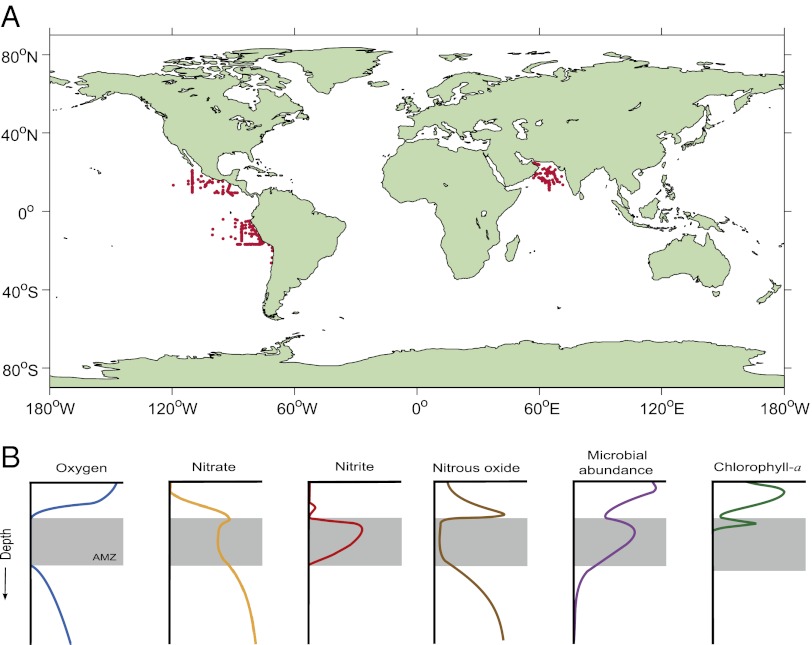
Midwater depths in the ocean typically contain reduced oxygen concentrations. This is due to the decomposition of surface-derived sinking organic material by aerobic respiration, combined with the introduction of high-latitude, oxygen-rich surface water to deeper zones (1). In some areas of the open ocean, high rates of phytoplankton productivity, coupled with poor ventilation and sluggish circulation, lead to an extensive, oxygen-deficient intermediate layer a few hundred meters in depth, where nitrite accumulates (2) and oxygen concentrations drop to low levels undetectable by the most sensitive modern techniques (3). Also, in this layer, nitrous oxide (N2O) does not accumulate but covaries inversely with nitrite concentration (4–6). Henceforth, we will designate these essentially anoxic marine zones as AMZs to differentiate them from oxygen-containing oxygen minimum zones (OMZs) as commonly found in the global ocean. The nitrite-rich AMZs are prominent in the eastern tropical North Pacific (ETNP), the eastern tropical South Pacific (ETSP), and in the Arabian Sea (Fig. 1).
Fig. 1.
Location and characteristic biogeochemical profiles of AMZs. (A) Global map of major AMZs derived from the distribution of waters with ≤2 μM oxygen and ≥0.5 μM nitrite based on data from the US National Oceanographic Database (NODC) and from Thamdrup et al. (3) and Canfield et al. (17) (observations from inland seas, such as the Baltic Sea, have not been included). Note that oxygen measurements reported in the NODC database are based on traditional standard techniques, with detection limits in the low micromolar range, and not the new highly sensitive STOX sensors. (B) Cartoon of characteristic profiles in AMZs illustrate the accumulation of nitrite within the AMZ, due to the anaerobic microbial process of nitrate reduction, and the high N2O concentrations at the boundaries (oxyclines). The figure also shows the presence of high microbial cell abundance and of a secondary chlorophyll-a maximum due to picocyanobacteria within the AMZ waters.
Due to the vanishingly low oxygen concentrations in these AMZ waters, higher eukaryotic taxa, such as fish, are normally excluded or persist due to special adaptations (7) and anaerobic microbial processes (i.e., those that do not depend on free oxygen) dominate (8). Indeed, significant amounts of nitrate are converted to dinitrogen (N2) gas in AMZs, generating large “fixed nitrogen” (nitrate plus nitrite and ammonium) deficits, thereby contributing to an estimated 30–50% of all the nitrogen lost to N2 in the oceans (9). The extent of these AMZs, however, is uncertain due to the limitations of standard techniques for measuring very low oxygen concentrations. These limitations hinder our ability to delineate the spatial distribution and intensity of aerobic and anaerobic processes in OMZ settings. Climate-induced warming of the upper ocean appears to have already contributed to deoxygenation of the global ocean (10, 11) and to an expansion of OMZ waters (12). This trend is expected to increase into the future (13), expanding the AMZ area and influencing rates of fixed nitrogen loss from the oceans.
Here, we highlight how recent advances in oxygen sensing and observational technologies (applied initially to the study of the AMZ of the ETSP) are redefining our understanding of oxygen and its dynamics in OMZs. These results, combined with novel molecular and experimental approaches, reveal unexpected complexity to elemental cycling in AMZs and show how modern AMZs represent an intermediate state of a geochemical and microbiological continuum to sulfidic basins like the modern Black Sea and ancient equivalents.
How Much Oxygen Is in OMZs?
It is well known that oxygen concentrations can reach very low values in some OMZ waters. Using the Winkler titration method to determine oxygen concentrations, Johs Schmidt (14) observed in 1921 that the water off the western coast of Panama “contains practically no oxygen at all” at depths between 400 and 500 m. Introduced in 1888 by the Hungarian chemist Lajos W. Winkler (15), Winkler titrations remain a standard method today, and if carefully applied, they can reach a detection limit of about 1 μM. These concentrations are quite low compared with air-saturation values of about 250 μM in equatorial waters and with values in most of the open ocean, including abyssal depths. However, is 1 μM sufficiently low in concentration to delineate where anaerobic metabolism dominates and where aerobic metabolism becomes less relevant? Based on recent results enabled by newly developed advances in sensor technology (see below), we argue that it likely is not and that the role of oxygen in structuring OMZ microbial metabolism is actually much more complex.
The switchable trace oxygen (STOX) amperometric microsensor (16) has been developed recently for measuring ultralow oxygen concentrations. Its construction is based on the presence of a front guard secondary cathode that, when polarized, prevents oxygen from reaching the primary internal sensing cathode. With this design, a zero calibration is obtained in each measuring cycle, bringing the detection limit of the sensor to the nanomolar range (∼1–10 nM), depending on the instrument’s configuration and electronics (16).
STOX sensors provide strong clues about the oxygen levels supporting anaerobic metabolism in AMZs. The first STOX measurements in the OMZ off the Peruvian coast demonstrated oxygen concentrations of less than the 3-nM detection limit of the sensor (16). These values are about three orders of magnitude lower than those revealed by the Winkler method and underscore Schmidt’s sentiment of “practically no oxygen at all” in the core of AMZs. Oxygen concentrations below the detection limit of the STOX sensor were also measured off the coast of Iquique, Chile (17). Moreover, in a survey of nearly 1,600 km of the ETSP, numerous STOX measurements through OMZ waters reveal that the observations made off Peru and Iquique generally apply and that nitrite accumulates (>0.5 μM) only when oxygen falls below 50 nM (3). This observation, when coupled with known oceanographic nitrite distributions, suggests that essentially oxygen-free waters occupy a volume of at least 2.4 × 1014 km3 in the ETSP (3). Essentially oxygen-free waters have also been found using STOX sensors in the nitrite-rich regions of the Arabian Sea (18). It is clear, therefore, that vast volumes of nitrite-rich essentially anoxic waters (AMZs) occur in the ETSP and the Arabian Sea. STOX sensor measurements have not yet been reported for the ETNP, but the combined distribution of oxygen, nitrite, and N2O (4) suggests that anoxic waters are also present in this system. In contrast, other oceanic OMZs, such as those in the eastern tropical South Atlantic or the eastern subarctic North Pacific, do not show any signs of anoxia; here, anoxic waters, when present, are restricted to coastal regions or fjords.
Anaerobic microbes are known to be oxygen-sensitive, but they differ in their oxygen sensitivities depending on the specific metabolic pathways involved. Notably, sulfate reduction and methanogenesis are believed to require strictly anoxic conditions in most environments (19). In contrast, based on experiments with natural populations from the Black Sea and the Namibian and Peruvian OMZs, anaerobic ammonium oxidation (anammox) may occur at oxygen concentrations up to about 10–20 μM (20, 21). Experiments with cultures of OMZ bacteria have shown a switch between oxygen and nitrate respiration at about 2–4 μM oxygen (22), whereas those with natural populations from the Namibian and Peruvian OMZs show that dissimilative nitrate reduction to nitrite is still active at 25 μM oxygen (21).
It seems apparent, however, that although some microbes may tolerate low micromolar levels of oxygen during their anaerobic metabolism, they may not establish naturally abundant populations at these oxygen levels. The Bay of Bengal offers an interesting test case in which oxygen concentrations drop to about 3 μM (or possibly lower, given uncertainties of the Winkler method used to establish these levels); however, there is no evidence for anaerobic metabolism or N2 production through denitrification or anammox (23, 24). Therefore, despite the ability of anaerobic bacteria to metabolize in up to 20–25 μM oxygen (21), they may require significantly lower oxygen levels to grow and to persist at high abundance in the environment.
The STOX sensor (16) also has been used to explore the low oxygen metabolism of Escherichia coli when grown as a strict aerobe (25). E. coli possesses a low-oxygen affinity dioxygen reductase of the cytochrome bd superfamily, and it can grow at oxygen concentrations below the 3-nM oxygen detection limit of the sensor, with a half-saturation constant with respect to growth value of 121 nM (25). If other low-oxygen–adapted aerobes behave similar to E. coli, the 1-μM detection limit of the Winkler method is clearly insufficient to mark the limits of aerobic metabolism.
How Does Aerobic Metabolism Vary with Time and Space in AMZs?
Although recent STOX sensor measurements have demonstrated essentially anoxic conditions in the core of the ETSP and Arabian Sea AMZs, even the current resolution of 3 nM oxygen cannot rule out the possibility of aerobic metabolism in some of these waters. Indeed, aerobic metabolism likely persists in the dimly lit zones of the upper OMZ off the coast of Chile, where cyanobacteria, particularly of the genus Prochlorococcus, form a deep chlorophyll-a maximum (26) (Fig. 1B). This same feature is also observed in the AMZs of the Arabian Sea and the ETNP (27). Oxygen produced by oxygenic photosynthesis by cyanobacteria would provide oxygen to feed aerobic processes at these depths.
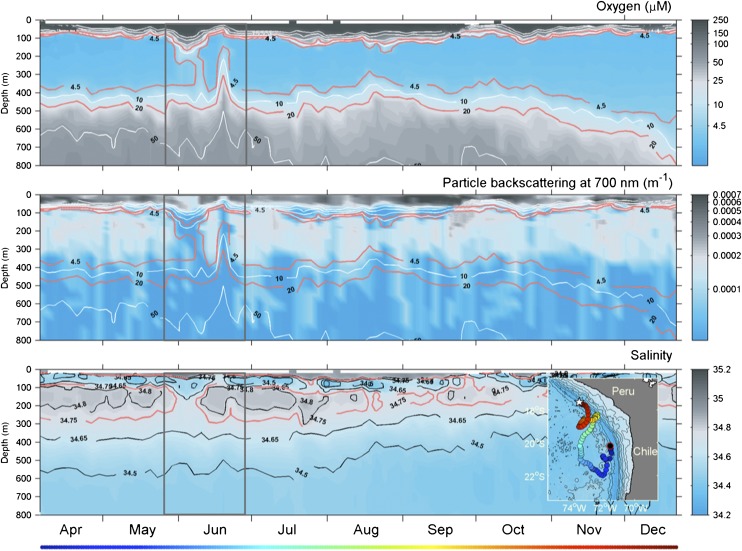
Deeper in the AMZ, however, and outside of the photic zone, any aerobic metabolism must be fueled by oxygen supplied from turbulent diffusion or by the episodic injection and mixing of oxygen-rich water into the AMZ. Turbulent diffusion seems an unlikely oxygen source, because the oxygen gradients and mixing rates necessary to drive it have not been reported. In contrast, autonomous continuously profiling floats deployed off the coasts of Chile and Peru (28) have revealed episodic intrusions of oxygen into the AMZ core (Fig. 2). The available data do not reveal the frequency or spatial distribution of such intrusions, but such oxygen dynamics may play an important role in establishing and maintaining aerobic microbial communities in essentially anoxic waters and in regulating biogeochemical cycling in AMZs. More work with autonomous sensing platforms, such as floats and gliders, coupled with highly sensitive oxygen sensors, would help characterize these dynamics in detail. More importantly, the impacts of oxygen intrusions on AMZ biogeochemical cycling remain to be assessed.
Fig. 2.
Profiling float observations of oxygen, particle backscattering at 700 nm (an index of particle abundance), and salinity in the upper 800 m of the AMZ of the ETSP. Oxygen-deficient conditions associated with a high particle load persist over several months within the AMZ but are occasionally interrupted by instructions of waters with higher oxygen concentrations and lower salinities. (Inset) Trajectory of the float, which profiled each 3 d during the 9 mo in 2007. Data are from Whitmire et al. (28).
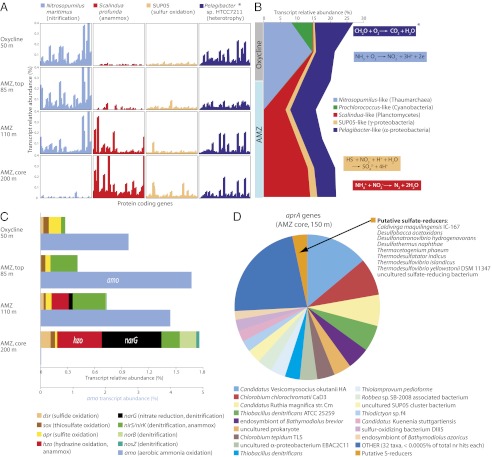
The emerging picture suggests that AMZs have extremely low oxygen levels of <3 nM. In their upper sunlit portions, aerobic respiration may be maintained by local oxygenic photosynthesis or turbulent diffusion, but below this layer, the waters are essentially anoxic, save for periodic injections of oxygen during mixing events. This picture is supported by recent microbial community gene content (metagenomic) and gene expression (metatranscriptomic) surveys of the permanent AMZ waters off the Chilean coast (29). Regular patterns emerge when the genes or transcripts are sorted by key taxa of specific metabolic pathways (Fig. 3). In particular, sequences matching “Candidatus Nitrosopumilus maritimus,” a member of the archaeal phylum Thaumarchaeota known to oxidize ammonium to nitrite aerobically during the autotrophic nitrification process (Fig. 4), dominate the gene expression libraries in the upper oxic waters (50 m), in the lower oxic zone (85 m), and in the transition zone (110 m). Transcripts of these aerobic organisms, however, are essentially absent in the core of the open-ocean AMZ at 200 m, where sequences matching the anammox bacterium “Candidatus Scalindua profunda” (30) are prominent (Fig. 3). These patterns are consistent with the general vertical distribution of archaeal groups in the ETSP AMZ (31) and with the segregation of aerobic ammonia-oxidizing archaea and anaerobic anammox bacteria in the Arabian Sea (32).
Fig. 3.
Key patterns in metabolic protein-coding transcripts and gene sequences in the AMZ off Iquique, Chile. (A) Metatranscriptome sequencing reveals diverse transcripts matching the genomes or metagenomes of functionally diagnostic taxa. (*Pelagibacter-like transcripts encoding enzymes of heterotrophic aerobic respiration are mostly restricted to oxycline depths. Pelagibacter-like transcripts at the AMZ core primarily encode transport-related proteins.) (B) Relative transcriptional activity of genera representing distinct functional clades varies with depth. Reactions (boxed) are based on the characterized metabolisms of closely related taxa. Colors match those in A. (C) Genes for dissimilatory sulfur and nitrogen metabolism are transcribed in depth-specific patterns. amo, ammonia monooxygenase (amoABC subunits combined); apr, APS reductase (subunits aprAB and membrane anchor aprM combined); dsr, dissimilatory sulfite reductase pathway (all dsr genes combined); hzo, hydrazine oxidoreductase; narG, nitrate reductase; nirS/nirK, NO-forming nitrite reductase; norB, NO reductase; nosZ, N2O reductase; sox, sulfur oxidation pathway (all sox genes combined). The scale differs for the amo gene. (D) Metagenome (DNA) sequences matching the aprA gene suggest a diverse AMZ community of both sulfur oxidizers and sulfate reducers. Abundances are shown as percentages of the total number of protein-coding sequences in shotgun-sequenced community RNA (A–C) and DNA (D) datasets. Protein-coding genes are arrayed along the x axis in A, with per-gene transcript abundance normalized to kilobases of gene length. Figures are based on samples collected from the ETSP AMZ in June 2008 (29) (A–C) and January 2010 (17) (D).
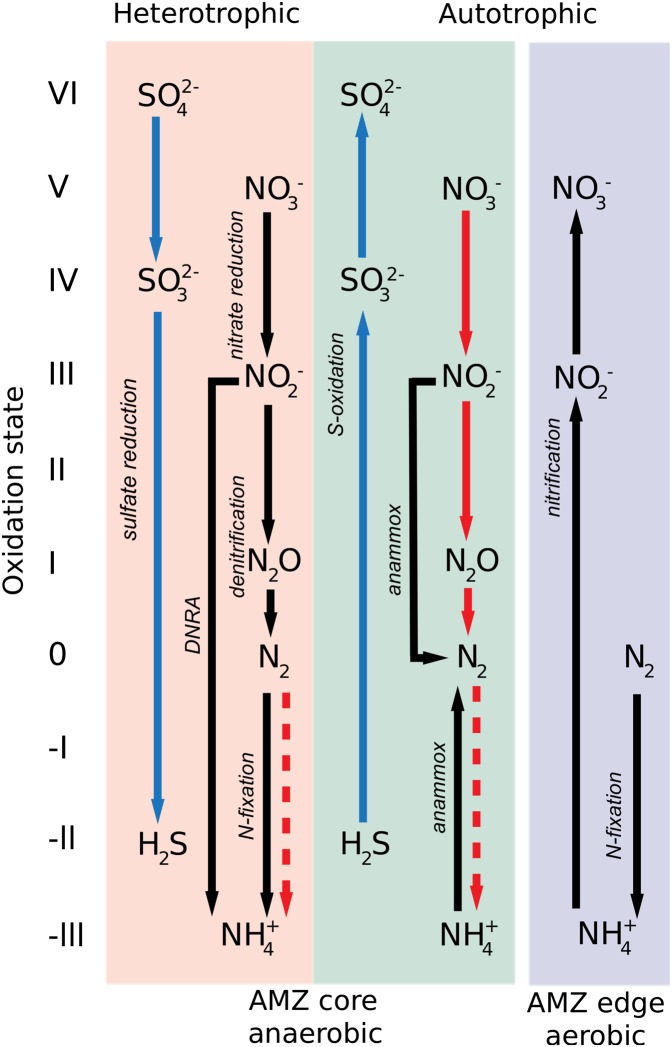
Fig. 4.
Major microbial biogeochemical processes in the AMZ core and in the adjacent oxic waters. The heterotrophic processes within the core are anaerobic and include sulfate reduction, nitrate reduction to nitrite, nitrate reduction to ammonium (DRNA), and denitrification to N2 gas. These processes oxidize organic matter and liberate ammonia for use by anammox bacteria. The sulfide produced by sulfate reducers is oxidized again to sulfate through autotrophic microbial metabolisms with nitrate and nitrite as electron acceptors. Sulfur metabolisms are given with blue arrows, and nitrogen metabolisms are given with black arrows, except those coupled to sulfur (sulfide plus S-intermediate compounds), which are given by red arrows. Anammox is also an autotrophic microbial process. Ammonium oxidation (nitrification) is a significant aerobic microbial process in the oxic waters surrounding the AMZ core and most probably occurring during oxygen intrusions into the core. Nitrogen fixation is the energy-intensive fixation of N2 gas to organic nitrogen (the oxidation state of ammonium) and is accomplished by a wide range of microorganisms, including sulfate reducers, sulfur oxidizers, and cyanobacteria. It occurs in both the oxygenated upper layers of AMZ settings and in the AMZ core.
These observations, however, seem to contradict other studies reporting nitrification activity in the core of AMZ waters off the coast of Peru (33, 34). In these prior studies, nitrification was measured in 2–3 μM oxygen, which was believed to be the ambient oxygen level. These oxygen levels are clearly higher than ambient levels as revealed by recent STOX sensor measurements, and elevated oxygen may have stimulated nitrification activity not otherwise found in the AMZ core. Nevertheless, these earlier studies, combined with more recent studies also showing nitrification activity (21, 35) as well as the expression of ammonia oxidation genes (36) at very low oxygen concentrations, demonstrate the significant potential for aerobic metabolism in the heart of AMZs. As described above, this aerobic process may lay dormant until activated by recurrent oxygen injection events.
How Are Microbial Communities and Biogeochemical Processes Coupled in AMZs?
In the classic view, anaerobic microbial metabolisms found in AMZs were thought to be dominated by heterotrophic denitrification to N2 gas (Fig. 4). This view has changed substantially, in part, because the anaerobic oxidation of ammonium with nitrite, (anammox) has now been identified as a significant or even dominant pathway of N2 formation in many oxygen-deficient marine systems (e.g., 8, 37, 38). However, denitrification has recently been shown to be important for the removal of fixed nitrogen in the AMZs of the Arabian Sea and ETSP (39, 40). Modern measurements of anammox and denitrification have not yet been reported for the ETNP AMZ. In addition, the dissimilatory reduction of nitrate to ammonia (DNRA; Fig. 4) has been shown to be a significant nitrogen cycling process in the Peruvian AMZ (36), as well as in waters over the Oman Shelf, where DNRA represents an important ammonium source for anammox (18).
These insights are largely based on biogeochemical studies using 15N tracer experiments and on gene expression assays with specific gene markers to explore the nitrogen cycle (8). However, recent environmental genomic approaches have also advanced our understanding of both the nitrogen cycle in AMZ waters as well as the cycling of other elements previously unrecognized. Regarding anammox, gene surveys of AMZ waters have documented an anammox community of relatively low diversity, characterized by members of the marine genus “Candidatus Scalindua” of the bacterial Planctomycetes phylum (41). A metatranscriptome depth profile from the Chilean open-ocean AMZ (Fig. 3) revealed few anammox bacterial transcripts in surface waters and through the oxycline, whereas anammox gene transcripts were a prominent feature in the AMZ core. This is consistent with predicted distributions of N2 production by anammox bacteria in AMZ settings (42). Anammox rates, however, have also been shown to be highest at the top of the AMZ and not in the core (36, 38, 40, 43, 44), suggesting that other factors, such as water circulation (e.g., upwelling intensity) and ammonium availability, are also important in determining the vertical partitioning of anammox rates at any given time.
Despite the apparent dominance of anammox in N2 production in many AMZ settings, gene surveys reveal a complex assemblage of diverse taxonomic groups potentially capable of denitrification (39, 45, 46). Sequencing of community RNA (29) from an AMZ site off Iquique, Chile, has uncovered all the genes required for a complete denitrification pathway, and these are transcriptionally most active in the core of the AMZ (∼200 m) (Fig. 3C). Although the capacity for classic heterotrophic denitrification appears large in AMZs, it is unclear why denitrification appears less significant than other processes in some cases, although appearing relatively more important in other settings (39, 40). One possibility is that heterotrophic denitrification is limited by the availability of organic matter (38–40, 47), and hence much more variable in space and time than anammox.
The first step of the denitrification pathway, the reduction of nitrate to nitrite, accounts for the nitrite accumulation in AMZ waters (Fig. 1B). Microbial community transcriptomes sampled off the Chilean coast (Fig. 3) revealed a diverse and abundant pool of nitrate reductase gene (narG) transcripts used in dissimilatory nitrate reduction to nitrite in the AMZ core (29). Heterotrophic nitrate reducers might supply significant amounts of both ammonia (via organic matter decomposition) and nitrite (via nitrate reduction) for the anammox process (36). Transcripts from the anammox gene hydrazine oxidoreductase (hzo), found at relatively high abundance in the AMZ core (Fig. 3), support a possible link between anammox and dissimilatory nitrate reduction to nitrite.
Perhaps the most significant recent redirection of the classic view of AMZ biogeochemistry and microbiology has been the recognition of a cryptic pelagic sulfur cycle in these regions. In traditional thinking, the anaerobic organic matter mineralization process of sulfate reduction, the first step in the sulfur cycle, should not begin until both oxygen and nitrate/nitrite are fully used (48). Therefore, a sulfur cycle was never envisioned for nitrate- and nitrite-rich AMZs (Fig. 1), although transient accumulations of H2S have been observed in anoxic, nitrate- and nitrite-depleted, continental shelf waters (49, 50). Surprisingly, recent taxonomic, metagenomic, and metatranscriptomic surveys have uncovered an abundant and diverse sulfur-oxidizing microbial community in the AMZ water column. This community is particularly enriched in γ-proteobacteria related to sulfur-oxidizing symbionts of deep-sea bivalves (17, 29, 51–54) (Fig. 3). Metagenomic analysis of this clade, referred to as “SUP05,” reveals a versatile metabolism with genes mediating carbon fixation, dissimilatory reduction of nitrate to N2O, and oxidation pathways for diverse reduced sulfur species (sulfide, sulfite, elemental sulfur, and thiosulfate) (55). SUP05 bacteria thus have the genetic repertoire for carrying out autotrophic denitrification coupled with sulfur oxidation (Fig. 4).
The fact that sulfide-oxidizing microorganisms are abundant in AMZ waters immediately raises the question, “Where does the sulfide come from?” Radiolabeled sulfate tracer experiments from the AMZ off the Chilean coast revealed significant sulfate reduction in the upper reaches of the AMZ water column at rates estimated to contribute up to 20% of the AMZ carbon mineralization in this region (17). Consistent with these results, metagenomic and metatranscriptomic datasets from the same AMZ revealed the presence of dissimilatory sulfur respiratory genes (aprA and dsrB) similar to those found in the genomes of known δ-proteobacterial sulfate reducers (17, 29) (Fig. 3D). Further experiments revealed that the rapid removal of sulfide was coupled to the reduction of nitrate to nitrite, or N2O reduction to N2 (17). The rapidity of sulfide oxidation in high backgrounds of seawater sulfate, as well as its coupling with denitrification processes typically ascribed to heterotrophic carbon decomposition, partially explains why this pelagic sulfur cycle went previously unrecognized. Overall, these recent findings suggest the presence of an active pelagic sulfur cycle in AMZ waters. This sulfur cycle fuels nitrate reduction, thereby supplying additional substrates (nitrite and ammonia) for anammox bacteria (Fig. 4). Until now, an active planktonic sulfur cycle has only been measured in the ETSP AMZ, but we expect it to be present also in the other AMZs. Taxonomic molecular surveys in the AMZs of the Arabian Sea and ETNP (51, 56) have suggested the presence of sulfate reducers and sulfur oxidizers in the water column.
What Processes Control AMZ Chemistry?
Despite complex interactions between the sulfur and nitrogen cycles in modern AMZs, nitrate and nitrite are normally present in excess. Why should AMZ water chemistry be poised in this chemical state? Modeling has revealed that natural feedbacks operate to maintain a nitrate/nitrite-rich AMZ chemistry if nitrogen fixation is limited (57). With limited nitrogen fixation, the nitrate reduced to N2 in the AMZ will not be replenished, ensuring nitrogen-limited primary production in the overlying waters (57). The feedback control is quite simple: If denitrification becomes too intense, nitrogen will become further limiting, decreasing primary production. This will diminish the organic carbon flux into the OMZ, reducing the rate of denitrification and allowing nitrate (plus nitrite) to persist. Rather paradoxically, nitrate and nitrite-rich OMZs persist in this chemical state due to nitrogen limitation in the overlying waters.
This scenario changes, however, if nitrogen fixation balances the N2 loss in the AMZ. In this case, sulfidic conditions would develop (57) when upwelling rates become high enough to generate sufficient primary production and a downward carbon flux to remove all nitrate (plus nitrite) by denitrification and anammox. This does not normally happen because nitrogen fixation in AMZ regions appears to be insufficient to replace the N2 lost in the AMZ core. Nitrogen fixation, however, does occur, and has been documented in the surface waters of the Arabian Sea (58) and in the upwelling waters off the coasts of Peru and Chile (59). Also, blooms of the nitrogen-fixing cyanobacterium Trichodesmium, derived from ocean color satellite data, have been reported for surface waters of the Arabian Sea and the ETNP off Central America (60). In the case of the ETSP AMZ (59), nitrogen fixation appears to be accomplished by a mixed population of prokaryotes, including sulfate reducers and sulfur oxidizers but apparently not cyanobacteria, which are believed to be responsible for most of the nitrogen fixation in the oceans (61). Nitrogen fixation is distributed in both the oxic upper layers of the water column and well into the AMZ at rates varying considerably from year to year (59). Still, even at the maximum observed rate, nitrogen fixation would only replenish about 5% of the nitrate lost to N2 in the ETSP AMZ.
Although the factors regulating nitrogen fixation would be paramount in establishing AMZ chemistry, there is no consensus as to why nitrogen fixation is so limited in AMZ areas. One possibility is that the turbid highly productive waters typically overlying AMZs are not well suited to the growth of Trichodesmium-like and unicellular nitrogen-fixing cyanobacteria, which prefer open-ocean, oligotrophic, and calm conditions (62). It is not clear, however, why AMZ settings do not support other nitrogen fixers whose physiological traits are more compatible with the ambient physicochemical conditions. As another possibility, iron limitation demonstrably hinders primary production in the ETSP AMZ region (63). Severe iron limitation could exclude nitrogen-fixing cyanobacteria whose iron requirements are particularly high (64).
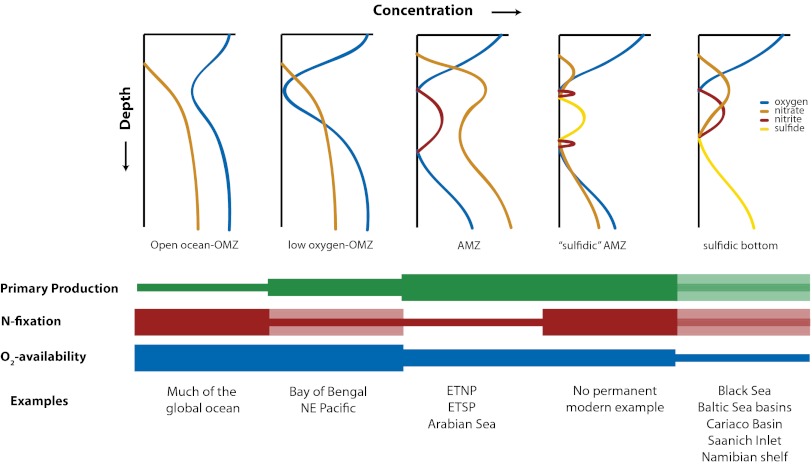
Nevertheless, there is no inherent reason why AMZs must maintain a nitrate/nitrite-rich chemistry or why such chemistry should have always defined AMZs in the past. Indeed, there is evidence for sulfidic AMZs in the North Atlantic during the Cretaceous ocean anoxic event 2 at the Cenomanian/Turonian boundary (65), at the Permian/Triassic boundary (66), and for time periods in the Precambrian (67). Because modern AMZs support the same biogeochemical cycles as sulfidic water bodies today, and presumably also sulfidic AMZs of the past, one can view them as a particular chemical intermediate state between two “end points” represented by fully oxic systems and fully sulfidic systems (Fig. 5). Thus, one can picture periods in Earth history in which the ocean resembled modern AMZs with a nitrate/nitrite-rich chemistry but without the accumulation of H2S. As nitrogen fixation increases, sulfidic AMZs become possible, and with basin restriction or contact with underlying sediments, sulfidic conditions can extend to the sediment–water interface (a conceptual model is provided in Fig. 5). Other factors, such as atmospheric oxygen concentrations (controlling oxygen availability to deep ocean waters), iron availability, and large-scale ocean ventilation, may also influence AMZ chemistry, and these factors should inform the further development of AMZ biogeochemical models (67, 68).
Fig. 5.
Cartoon shows the development of different chemistries depending on the relative mix of different driving parameters, including rates of primary production, rates of nitrogen fixation, and the availability of oxygen. Oxygen availability could imply oxygen limitation as observed in modern anoxic basins, such as the Black Sea, or the limitation of oxygen availability as occurred during times in the geological past when atmospheric oxygen concentrations were lower. If oxygen availability becomes limiting enough, rates of primary production and nitrogen fixation may take a secondary role in determining the development of water column chemistry. Based on information provided in the text, OMZs include those regions of the global ocean where oxygen is decreased as a result of respiration but where nitrite does not accumulate (except a nitrite maximum that typically occurs in well-oxygenated, near-surface waters as a result of nitrification). Modern measurements suggest that nitrite accumulation occurs at oxygen levels of less than 50 nM. AMZs occur as oxygen falls below about 50 nM and nitrite begins to accumulate.
AMZs in the Anthropocene
OMZs are microbial reactors of global significance. Models have predicted their expansion in response to global warming, both as a result of reduced oxygen solubility at higher temperatures and from an increased water-column stratification resulting from stronger temperature gradients in the upper waters (69, 70). Examination of a 50-y dissolved oxygen database suggests such an expansion (12), which should continue well into the future (13). The increasing deposition of atmospheric anthropogenically fixed nitrogen on the open ocean (71) is also expected to contribute to AMZ expansion and intensification, and eventually to the development of sulfidic conditions. Expanded areas of reduced oxygen will alter marine ecosystems, including the distributions and ecology of marine animals. Expanding AMZs should also increase rates of fixed nitrogen loss as N2 (57, 72), as well as production rates of N2O, an important greenhouse gas, at their boundaries (73). It is unclear how these changes will affect human welfare. One might assume, however, that a contraction of animal habitat and alterations in the elemental cycles would produce negative effects, particularly if AMZs expand further onto the continental shelf and compound the expansion of shelf anoxia and euxinia from increasing nutrient inputs from land (74).
For these reasons, recent scientific progress in our understanding of the microbiological function, dynamics, and distribution of oceanic AMZ waters is most fortunate. However, we view this progress as only a beginning. New oxygen-sensing technologies have barely been applied to outlining the spatial distribution and dynamics of oxygen accurately in AMZ waters. We still do not know the nature of the episodic oxygen intrusions, as revealed by autonomous platforms, or their role in structuring AMZ microbial communities and biogeochemical cycling. Accurate budgets of fixed nitrogen loss in AMZs are still under debate, as are the processes controlling this loss, and we have just begun to understand the role of sulfur cycling in AMZ waters. We are also uncertain as to the rates of nitrogen fixation in AMZ waters and as to what processes regulate these rates. Despite high rates of nitrogen loss through denitrification and anammox, with the establishment of severe nitrogen limitation, high concentrations of unused nitrogen still persist in surface waters overlying many AMZ settings. Iron limitation may be partly responsible for this, but is this the only reason? Will these nutrients become more available to fuel primary production under future global climate change or anthropogenic emissions?
New tools and methods have revealed novel complexity in AMZ ecosystems, but we anticipate that AMZs may support an even more diverse assemblage of indigenous microbial species that remain completely uncharacterized genetically, physiologically, and ecologically. Although our knowledge of AMZs has been growing at a breathtaking pace, the potential impacts of AMZs on marine ecosystem structure and global geochemical cycling highlight a need to accelerate this rapid pace of exploration and discovery further.
Acknowledgments
We thank all our colleagues who have contributed ideas and data making this review possible and the Agouron Institute for encouragement. We also thank Winston Rojas for help with the preparation of some of the figures. Funding was provided by the Agouron Institute. Additional financial support was provided by the Chilean National Commission for Scientific and Technological Research through the Fondap Program (O.U.), the Gordon and Betty Moore Foundation (O.U., E.F.D., and R.M.L.), the Danish National Research Foundation (D.E.C.), and the European Research Council (D.E.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Wyrtki K. The oxygen minima in relation to ocean circulation. Deep-Sea Res. 1962;9:11–23. [Google Scholar]
- 2.Kamykowski D, Zentara S-J. Spatio-temporal and process-oriented views of nitrite in the world oceans as recorded in the historical data set. Deep-Sea Res. 1991;38:445–464. [Google Scholar]
- 3.Thamdrup B, Dalsgaard T, Revsbech NP. Widespread functional anoxia in the oxygen minimum zone of the eastern South Pacific. Deep Sea Res Part I Oceanogr Res Pap. 2012;65:36–45. [Google Scholar]
- 4.Cohen Y, Gordon LI. Nitrous oxide in the oxygen minimum of the eastern tropical North Pacific: Evidence for its consumption during denitrification and possible mechanisms for its production. Deep-Sea Res. 1978;25:509–524. [Google Scholar]
- 5.Nicholls JC, Davies CA, Trimmer M. High-resolution profiles and nitrogen isotope tracing reveal a dominant source of nitrous oxide and multiple pathways of nitrogen gas formation in the central Arabian Sea. Limnol Oceanogr. 2007;52:156–168. [Google Scholar]
- 6.Farías L, Paulmier A, Gallegos M. Nitrous oxide and N-nutrient cycling in the oxygen minimum zone off northern Chile. Deep Sea Res Part I Oceanogr Res Pap. 2007;54:113–128. [Google Scholar]
- 7.Childress JJ, Seibel BA. Life at stable low oxygen levels: Adaptations of animals to oceanic oxygen minimum layers. J Exp Biol. 1998;201:1223–1232. doi: 10.1242/jeb.201.8.1223. [DOI] [PubMed] [Google Scholar]
- 8.Lam P, Kuypers MMM. Microbial nitrogen cycling processes in oxygen minimum zones. Annu Rev Mar Sci. 2011;3:317–345. doi: 10.1146/annurev-marine-120709-142814. [DOI] [PubMed] [Google Scholar]
- 9.Codispoti LA, et al. The oceanic fixed nitrogen and nitrous oxide budgets: Moving targets as we enter the Anthropocene? Scientia Marina. 2001;65:85–105. [Google Scholar]
- 10.Keeling RE, Körtzinger A, Gruber N. Ocean deoxygenation in a warming world. Annu Rev Mar Sci. 2010;2:199–229. doi: 10.1146/annurev.marine.010908.163855. [DOI] [PubMed] [Google Scholar]
- 11.Helm KP, Bindoff NL, Church JA. Observed decreases in oxygen content of the global ocean. Geophys Res Lett. 2011;38:L23602. [Google Scholar]
- 12.Stramma L, Johnson GC, Sprintall J, Mohrholz V. Expanding oxygen-minimum zones in the tropical oceans. Science. 2008;320:655–658. doi: 10.1126/science.1153847. [DOI] [PubMed] [Google Scholar]
- 13.Shaffer G, Olsen SM, Olaf J, Pedersen P. Long-term ocean oxygen depletion in response to carbon dioxide emissions from fossil fuels. Nat Geosci. 2009;2:105–109. [Google Scholar]
- 14.Schmidt J. On the contents of oxygen in the ocean on both sides of Panama. Science. 1925;61:592–593. doi: 10.1126/science.61.1588.592. [DOI] [PubMed] [Google Scholar]
- 15.Winkler LW. Die Bestimmung des im Wasser Gelösten Sauerstoffes. Berichte der Deutschen Chemischen Gesellschaft. 1888;21:2843–2855. [Google Scholar]
- 16.Revsbech NP, et al. Determination of ultra-low oxygen concentrations in oxygen minimum zones by the STOX sensor. Limnol Oceanogr Methods. 2009;7:371–381. [Google Scholar]
- 17.Canfield DE, et al. A cryptic sulfur cycle in oxygen-minimum-zone waters off the Chilean coast. Science. 2010;330:1375–1378. doi: 10.1126/science.1196889. [DOI] [PubMed] [Google Scholar]
- 18.Jensen MM, et al. Intensive nitrogen loss over the Omani Shelf due to anammox coupled with dissimilatory nitrite reduction to ammonium. ISME J. 2011;5:1660–1670. doi: 10.1038/ismej.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canfield DE, Kristensen E, Tamdrup B. Aquatic Geomicrobiology. Academic Press, San Diego; 2005. [DOI] [PubMed] [Google Scholar]
- 20.Jensen MM, Kuypers MMM, Lavik G. Rates and regulation of anaerobic ammonium oxidation and denitrification in the Black Sea. Limnol Oceanogr. 2008;53:23–36. [Google Scholar]
- 21.Kalvelage T, et al. Oxygen sensitivity of anammox and coupled N-cycle processes in oxygen minimum zones. PLoS ONE. 2011;6:e29299. doi: 10.1371/journal.pone.0029299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devol AH. Bacterial oxygen uptake kinetics as related to biological processes in oxygen deficient zones of the oceans. Deep-Sea Res. 1978;25:137–146. [Google Scholar]
- 23.Rao CK, et al. Hydrochemistry of the Bay of Bengal: Possible reasons for a different water-column cycling of carbon and nitrogen from the Arabian Sea. Mar Chem. 1994;47:279–290. [Google Scholar]
- 24.Howell EA, Doney SC, Fine RA, Olson DB. Geochemical estimates of denitrification in the Arabian Sea and Bay of Bengal during WOCE. Geophys Res Lett. 1997;24:2549–2552. [Google Scholar]
- 25.Stolper DA, Revsbech NP, Canfield DE. Aerobic growth at nanomolar oxygen concentrations. Proc Natl Acad Sci USA. 2010;107:18755–18760. doi: 10.1073/pnas.1013435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavin P, González B, Santibáñez JF, Scanlan DJ, Ulloa O. Novel lineages of Prochlorococcus thrive within the oxygen minimum of the eastern tropical South Pacific. Environ Microbiol Rep. 2010;2:728–738. doi: 10.1111/j.1758-2229.2010.00167.x. [DOI] [PubMed] [Google Scholar]
- 27.Goericke R, Olson R, Shalapyonok A. A novel niche for Prochlorococcus sp. in low-light suboxic environments in the Arabian Sea and the Eastern Tropical North Pacific. Deep Sea Res Part I Oceanogr Res Pap. 2000;47:1183–1205. [Google Scholar]
- 28.Whitmire AL, Letelier RM, Villagrán V, Ulloa O. Autonomous observations of in vivo fluorescence and particle backscattering in an oceanic oxygen minimum zone. Opt Express. 2009;17:21992–22004. doi: 10.1364/OE.17.021992. [DOI] [PubMed] [Google Scholar]
- 29.Stewart FJ, Ulloa O, DeLong EF. Microbial metatranscriptomics in a permanent marine oxygen minimum zone. Environ Microbiol. 2012;14:23–40. doi: 10.1111/j.1462-2920.2010.02400.x. [DOI] [PubMed] [Google Scholar]
- 30.van de Vossenberg J, et al. The metagenome of the marine anammox bacterium ‘Candidatus Scalindua profunda’ illustrates the versatility of this globally important nitrogen cycle bacterium. Environ Microbiol. 2012 doi: 10.1111/j.1462-2920.2012.02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belmar L, Molina V, Ulloa O. Abundance and phylogenetic identity of archaeoplankton in the permanent oxygen minimum zone of the eastern tropical South Pacific. FEMS Microbiol Ecol. 2011;78:314–326. doi: 10.1111/j.1574-6941.2011.01159.x. [DOI] [PubMed] [Google Scholar]
- 32.Pitcher A, et al. Niche segregation of ammonia-oxidizing archaea and anammox bacteria in the Arabian Sea oxygen minimum zone. ISME J. 2011;5:1896–1904. doi: 10.1038/ismej.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward BB, Glover HE, Lipschultz F. Chemoautotrophic activity and nitrification in the oxygen minimum zone off Peru. Deep-Sea Res. 1989;36:1031–1051. [Google Scholar]
- 34.Lipschultz F, et al. Bacterial transformations of inorganic nitrogen in the oxygen-deficient waters of the Eastern Tropical South Pacific Ocean. Deep-Sea Res. 1990;37:1513–1541. [Google Scholar]
- 35.Füssel J, et al. Nitrite oxidation in the Namibian oxygen minimum zone. ISME J. 2012;6:1200–1209. doi: 10.1038/ismej.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lam P, et al. Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc Natl Acad Sci USA. 2009;106:4752–4757. doi: 10.1073/pnas.0812444106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuypers MMM, et al. Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proc Natl Acad Sci USA. 2005;102:6478–6483. doi: 10.1073/pnas.0502088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thamdrup B, et al. Anaerobic ammonium oxidation in the oxygen-deficient waters off northern Chile. Limnol Oceanogr. 2006;51:2145–2156. [Google Scholar]
- 39.Ward BB, et al. Denitrification as the dominant nitrogen loss process in the Arabian Sea. Nature. 2009;461:78–81. doi: 10.1038/nature08276. [DOI] [PubMed] [Google Scholar]
- 40.Dalsgaard T, Thamdrup B, Farias L, Revsbech NP. Anammox and denitrification in the oxygen minimum zone of the eastern South Pacific. Limnol Oceanogr. 2012;57:1331–1346. [Google Scholar]
- 41.Schmid MC, et al. Anaerobic ammonium-oxidizing bacteria in marine environments: Widespread occurrence but low diversity. Environ Microbiol. 2007;9:1476–1484. doi: 10.1111/j.1462-2920.2007.01266.x. [DOI] [PubMed] [Google Scholar]
- 42.Chang BX, Devol AH, Emerson SR. Denitrification and the nitrogen gas excess in the eastern tropical South Pacific oxygen deficient zone. Deep Sea Res Part I Oceanogr Res Pap. 2010;57:1092–1101. [Google Scholar]
- 43.Galán A, et al. Anammox bacteria and the anaerobic oxidation of ammonium in the oxygen minimum zone off northern Chile. Deep Sea Res Part II Top Stud Oceanogr. 2009;56:1021–1031. [Google Scholar]
- 44.Lam P, et al. Origin and fate of the secondary nitrite maximum in the Arabian Sea. Biogeosciences. 2011;8:1565–1577. [Google Scholar]
- 45.Jayakumar DA, Francis CA, Naqvi SWA, Ward BB. Diversity of nitrite reductase genes (nirS) in the denitrifying water column of the coastal Arabian Sea. Aquat Microb Ecol. 2004;34:69–78. [Google Scholar]
- 46.Castro-González M, Braker G, Farías L, Ulloa O. Communities of nirS-type denitrifiers in the water column of the oxygen minimum zone in the eastern South Pacific. Environ Microbiol. 2005;7:1298–1306. doi: 10.1111/j.1462-2920.2005.00809.x. [DOI] [PubMed] [Google Scholar]
- 47.Ward BB, et al. Organic carbon, and not copper, controls denitrification in oxygen minimum zones of the ocean. Deep Sea Res Part I Oceanogr Res Pap. 2008;55:1672–1683. [Google Scholar]
- 48.Froelich PN, et al. Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: Suboxic diagenesis. Geochim Cosmochim Acta. 1979;43:1075–1090. [Google Scholar]
- 49.Dugdale R, Goering J, Barber R, Smith R, Packard T. Denitrification and hydrogen sulfide in the Peru upwelling region during 1976. Deep-Sea Res. 1977;24:601–608. [Google Scholar]
- 50.Naqvi SWA, et al. Increased marine production of N2O due to intensifying anoxia on the Indian continental shelf. Nature. 2000;408:346–349. doi: 10.1038/35042551. [DOI] [PubMed] [Google Scholar]
- 51.Fuchs B, Woebken D, Zubkov M, Burkill P, Amann R. Molecular identification of picoplankton populations in contrasting waters of the Arabian Sea. Aquat Microb Ecol. 2005;39:145–157. [Google Scholar]
- 52.Stevens H, Ulloa O. Bacterial diversity in the oxygen minimum zone of the eastern tropical South Pacific. Environ Microbiol. 2008;10:1244–1259. doi: 10.1111/j.1462-2920.2007.01539.x. [DOI] [PubMed] [Google Scholar]
- 53.Lavik G, et al. Detoxification of sulphidic African shelf waters by blooming chemolithotrophs. Nature. 2009;457:581–584. doi: 10.1038/nature07588. [DOI] [PubMed] [Google Scholar]
- 54.Wright JJ, Konwar KM, Hallam SJ. Microbial ecology of expanding oxygen minimum zones. Nat Rev Microbiol. 2012;10:381–394. doi: 10.1038/nrmicro2778. [DOI] [PubMed] [Google Scholar]
- 55. Walsh DA, et al. (2009) Metagenome of a versatile chemolithoautotroph from expanding oceanic dead zones. Science 326:578–582. [DOI] [PubMed]
- 56.Podlaska A, Wakeham SG, Fanning KA, Taylor GT. Microbial community structure and productivity in the oxygen minimum zone of the eastern tropical North Pacific. Deep Sea Res Part I Oceanogr Res Pap. 2012;66:77–89. [Google Scholar]
- 57.Canfield DE. Models of oxic respiration, denitrification and sulfate reduction in zones of coastal upwelling. Geochim Cosmochim Acta. 2006;70:5753–5765. [Google Scholar]
- 58.Capone DG, et al. An extensive bloom of the N2-fixing cyanobacterium Trichodesmium erythraeum in the central Arabian Sea. Mar Ecol Prog Ser. 1998;172:281–292. [Google Scholar]
- 59.Fernandez C, Farías L, Ulloa O. Nitrogen fixation in denitrified marine waters. PLoS ONE. 2011;6:e20539. doi: 10.1371/journal.pone.0020539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Westberry TK, Siegel DA. Spatial and temporal distribution of Trichodesmium blooms in the world’s oceans. Global Biogeochem Cycles. 2006;20:GB4016. doi: 10.1029/2005GB002673. [DOI] [Google Scholar]
- 61.Zehr JP, Kudela RM. Nitrogen cycle of the open ocean: From genes to ecosystems. Annu Rev Mar Sci. 2011;3:197–225. doi: 10.1146/annurev-marine-120709-142819. [DOI] [PubMed] [Google Scholar]
- 62.Hood RR. Modeling the distribution of Trichodesmium and nitrogen fixation in the Atlantic Ocean. J Geophys Res. 2004;109:C06006. doi: 10.1029/2002JC001753. [DOI] [Google Scholar]
- 63.Hutchins DA, et al. Phytoplankton iron limitation in the Humboldt Current and Peru Upwelling. Limnol Oceanogr. 2002;47:997–1011. [Google Scholar]
- 64.Falkowski PG. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature. 1997;387:272–275. [Google Scholar]
- 65.Hetzel A, Böttcher ME, Wortmann UG, Brumsack H-J. Paleo-redox conditions during OAE 2 reflected in Demerara Rise sediment geochemistry (ODP Leg 207) Palaeogeogr Palaeoclimatol Palaeoecol. 2009;273:302–328. [Google Scholar]
- 66.Algeo TJ, et al. Spatial variation in sediment fluxes, redox conditions, and productivity in the Permian–Triassic Panthalassic Ocean. Palaeogeogr Palaeoclimatol Palaeoecol. 2011;308:65–83. [Google Scholar]
- 67.Lyons TW, Anbar AD, Severmann S, Scott C, Gill BC. Tracking euxinia in the ancient ocean: A multiproxy perspective and Proterozoic case study. Annu Rev Earth Planet Sci. 2009;37:507–534. [Google Scholar]
- 68.Poulton SW, Canfield DE. Ferruginous conditions: A dominant feature of the ocean through Earth’s history. Elements. 2011;7:107–112. [Google Scholar]
- 69.Bopp L, Le Quéré C, Heimann M, Manning AC, Monfray P. Climate-induced oceanic oxygen fluxes: Implications for the contemporary carbon budget. Global Biogeochem Cycles. 2002;16:1022. doi: 10.1029/2001GB001445. [DOI] [Google Scholar]
- 70.Matear RJ. Long-term changes in dissolved oxygen concentrations in the ocean caused by protracted global warming. Global Biogeochem Cycles. 2003;17:1125. doi: 10.1029/2002GB001997. [DOI] [Google Scholar]
- 71. Duce RA, et al. (2008) Impacts of atmospheric anthropogenic nitrogen on the open ocean. Science 320:893–897. [DOI] [PubMed]
- 72.Schmittner A, Oschlies A, Matthews HD, Galbraith ED. Future changes in climate, ocean circulation, ecosystems, and biogeochemical cycling simulated for a business-as-usual CO2 emission scenario until year 4000 AD. Global Biogeochem Cycles. 2008;22:GB1013. doi: 10.1029/2007GB002953. [DOI] [Google Scholar]
- 73.Codispoti LA. Oceans. Interesting times for marine N2O. Science. 2010;327:1339–1340. doi: 10.1126/science.1184945. [DOI] [PubMed] [Google Scholar]
- 74.Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321:926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]