Abstract
Platelets express a variety of membrane and secreted glycoproteins, but the importance of glycosylation to platelet functions is poorly understood. To explore the importance of O-glycosylation, we generated mice with a targeted deletion of Cosmc in murine endothelial/hematopoietic cells (EHC) (EHC Cosmc−/y). X-linked Cosmc encodes an essential chaperone that regulates protein O-glycosylation. This targeted mutation resulted in lethal perinatal hemorrhage in the majority of mice, and the surviving mice displayed severely prolonged tail-bleeding times and macrothrombocytopenia. EHC Cosmc−/y platelets exhibited a marked decrease in GPIb-IX-V function and agonist-mediated integrin αIIbβ3 activation, associated with loss of interactions with von Willebrand factor and fibrinogen, respectively. Significantly, three O-glycosylated glycoproteins, GPIbα, αIIb, and GPVI normally on platelet surfaces that play essential roles in platelet functions, were partially proteolyzed in EHC Cosmc−/y platelets. These results demonstrate that extended O-glycans are required for normal biogenesis of the platelets as well as the expression and functions of their essential glycoproteins, and that variations in O-glycosylation may contribute to altered hemostasis.
Keywords: platelet glycoproteins, T-synthase, Tn antigen
Platelets play a pivotal role in hemostasis by promoting clot formation at sites of vascular injury. Platelets rapidly adhere to exposed subendothelial matrices (primary platelet adhesion) and to one another (platelet aggregation), forming the primary hemostatic plug. Glycoproteins on the platelet surface, especially glycoprotein GPIb-IX-V complex and integrin αIIbβ3 (GPIIbIIIa), mediate these adhesive events by their interactions with extracellular glycoproteins, including von Willebrand factor (VWF) and fibrinogen, respectively. Although the functions of platelet surface glycoproteins are well known, the roles of posttranslational modifications of platelet glycoproteins, such as O-glycosylation, are poorly understood (1). Most studies focus on recombinant glycoproteins expressed in heterologous cell lines (2–4), and few studies have explored the roles of platelet glycoprotein O-glycosylation in vivo. In addition, altered glycoprotein expression or glycosylation may contribute to some types of platelet disorders associated with thrombocytopenia and giant platelets (5), thus highlighting the importance of a molecular understanding of the roles of platelet glycans in physiological processes.
Glycoprotein O-glycans in all cells are extended by the key Golgi glycosyltransferase T-synthase (core 1 β3galactosyltransferase) that adds galactose to GalNAcα1-Ser/Thr (Tn antigen) to generate the core 1 O-glycan, Galβ1-3GalNAcα1-Ser/Thr, also known as the T antigen (6). Formation of active T-synthase dimers is unique in that it requires Cosmc (7, 8), an essential and specific molecular chaperone in the endoplasmic reticulum (6, 9) encoded by X-linked Cosmc. In cells lacking Cosmc, the active T-synthase is not expressed, the Tn antigen cannot be extended, and cells express the uncommon Tn antigen (10). Individuals with Tn syndrome, who have an acquired hematopoietic mutation in the X-linked Cosmc, demonstrate mosaicism in expression of the Tn antigen in a subpopulation of blood cells of all lineages (6, 7, 11) and can develop thrombocytopenia and anemia in association with altered platelet glycosylation (12). We recently reported that deletion of Cosmc in mice causes embryonic death at ∼embryonic day (E) 12.5 that is associated with hemorrhaging, similar to that observed for deletion of the T-synthase (10), a phenotype that could be a defect in either endothelial or hematopoietic lineages.
To explore the roles of O-glycans in platelet function, we generated mice lacking Cosmc in endothelial/hematopoietic cells (EHC) through Tie2-Cre recombinase-targeted deletion. The remarkable changes in platelet formation and function revealed by altering O-glycosylation pathways provide fresh insights into the roles of O-glycans in platelet glycoprotein stability and function (Fig. S1).
Results
Disruption of Cosmc Causes Tn Antigen Expression.
Mice with specific deletion of Cosmc in murine EHCs (EHC Cosmc−/y) were generated by breeding floxed Cosmc (Cosmcflox/flox) female and Tie2-Cre transgenic male mice, where Cre recombinase is primarily expressed in EHCs (13). A high incidence (∼90%) of perinatal or postnatal lethality (<3 wk) was observed in EHC Cosmc−/y mice. Autopsy revealed gross hemorrhage within the body cavities as the predominant cause of death, and hemorrhage was clearly observed in EHC Cosmc−/y mouse embryos as early as E15.5 (Fig. 1A). The few surviving mice demonstrated growth retardation and hepatosplenomegaly. Even among these few survivors, the primary cause of death after 3–5 mo was pulmonary or gastrointestinal hemorrhage. We examined whether survival within these few mice might be because of expression of trace amounts of the Cosmc transcript. Consistent with this possibility, a trace amount of Cosmc transcript was observed in platelets from EHC Cosmc−/y mice (Fig. 1B). Levels of Cosmc transcript were unaffected in other tissues, indicating efficient and specific deletion of Cosmc in EHCs. However, the trace amount of transcript from surviving EHC Cosmc−/y mice is likely to be responsible for their brief survival, a fortuitous outcome allowing us to explore specific functions of O-glycans in platelets.
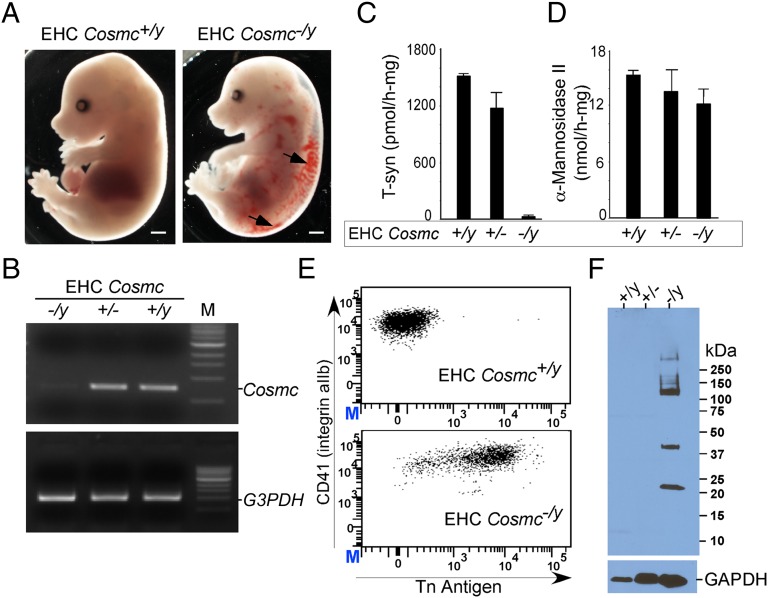
Fig. 1.
Cosmc disruption (EHC Cosmc−/y) results in hemorrhage and loss of T-synthase activity. (A) E15.5 embryos (EHC Cosmc−/y) reveal hemorrhaging, denoted by arrows. (Scale bars, 1 mm.) (B) RT-PCR analysis of Cosmc transcription level in different platelet genotypes. (C and D) T-synthase activity in platelets isolated from EHC Cosmc+/y, EHC Cosmc+/−, and EHC Cosmc−/y. α-Mannosidase II activity shown as a control. Error bars indicate ±1 SEM of two independent experiments. (E) Flow cytometry analysis of EHC Cosmc+/y and EHC Cosmc−/y platelets, stained with anti-CD41 and anti-Tn antigen antibodies. (F) Western blot of platelet lysates from wild-type mice (Cosmc+/y), EHC Cosmc+/− mice, and EHC Cosmc−/y mice, using biotin labeled anti-Tn antigen antibody. GAPDH was used as a loading control.
Platelets from EHC Cosmc−/y mice lacked T-synthase activity compared with wild-type (Cosmc+/y) and EHC Cosmc+/− (Fig. 1C), whereas activity of control enzymes in the Golgi apparatus, such as α-mannosidase-II, was not affected (Fig. 1D). Loss of T-synthase activity correlated with expression of the Tn antigen on the surface of the majority of platelets from EHC Cosmc−/y mice (Fig. 1E). When platelets were analyzed by Western blot with biotin labeled anti-Tn mAb, only extracts of platelets from the EHC Cosmc−/y mice were stained and several major Tn(+) O-glycoproteins were observed (Fig. 1F).
Lethal Perinatal Hemorrhage and Macrothrombocytopenia.
An unexpected observation was that surviving EHC Cosmc−/y mice exhibited excessive bleeding when tails were snipped for genotyping and blood sampling. Bleeding times in EHC Cosmc−/y mice were prolonged compared with wild-type (Fig. 2A) and required tail cauterization to stop hemorrhaging. Platelet counts were significantly lower in EHC Cosmc−/y mice (165.57 ± 57 × 103/mm3) compared with wild-type (787.43 ± 124 × 103/mm3) (Fig. 2B and Table S1); unexpectedly, the size of the Cosmc−/y platelets was markedly increased [Fig. 2C (C-1 and C-2) and Table S1]. Electron microscopy further confirmed these profound differences in morphology, showing that Cosmc−/y platelets lacked a normal discoid shape and were at least twice the diameter of wild-type platelets [Fig. 2C (C-3 and C-4)].
Fig. 2.
EHC Cosmc-deficient animals have prolonged bleeding times and macrothrombocytopenia. (A) Tail-bleeding time (s) in three different mouse genotypes (n = 6–7). Cautery of mice with continued bleeding was performed at 600 s (red arrow). (B) Peripheral platelet counts in EHC Cosmc+/y, EHC Cosmc+/−, and EHC Cosmc−/y mice. *P = 0.00023, **P = 0.0000002 (equal variance). Error bars indicate ± 1 SD (n = 6). (C) Panels C-1 and C-3 (EHC Cosmc+/y), and C-2 and C-4 (EHC Cosmc−/y) show images of platelets (arrows) from blood smears stained by Wright-Giemsa (C-1 and C-2), or viewed by transmission electron microscopy (C-3 and C-4).
Decreased Expression of GPIbα
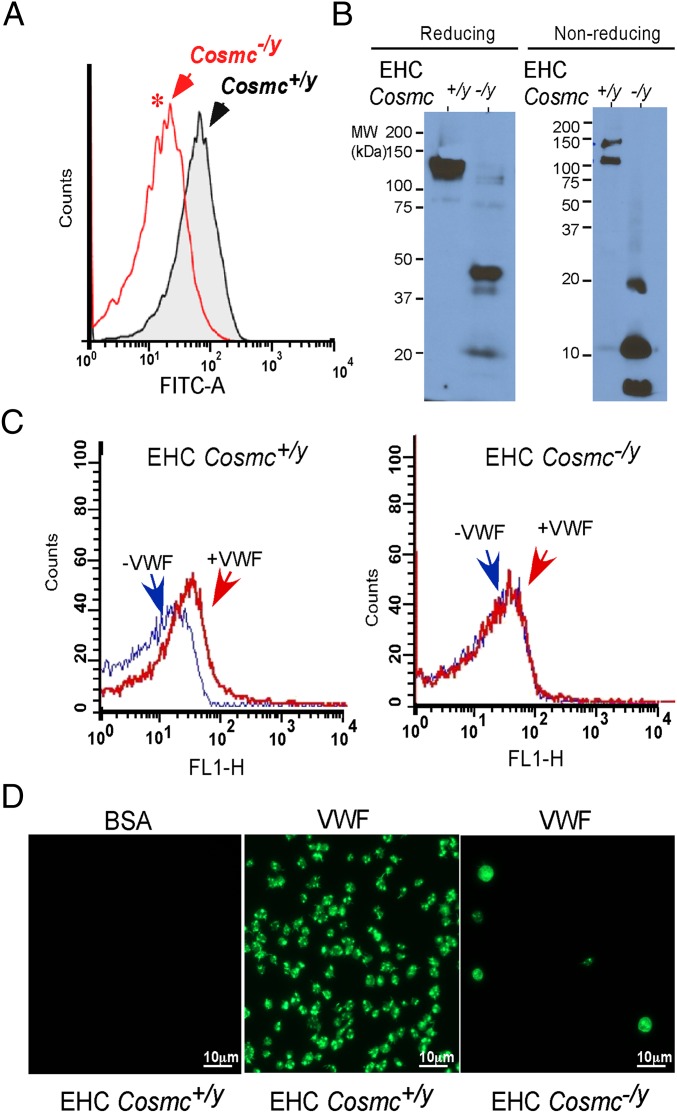
GPIb-IX-V is the primary platelet receptor for VWF, and deficient expression of this receptor complex is the cause of the major bleeding diathesis Bernard-Soulier syndrome (BSS) (14). Unexpectedly, EHC Cosmc−/y platelets showed significantly lower expression of surface GPIbα compared with platelets from wild-type mice (Fig. 3A). Examination of GPIbα in EHC Cosmc−/y platelet extracts revealed a major loss of full-length GPIbα, and residual GPIbα fragments in both reduced and nonreduced conditions, with prominent fragments of ∼45 and ∼21 kDa, and a minor fragment of 40 kDa being present in EHC Cosmc−/y extracts (Fig. 3B). The size of the peptide fragments in comparison with the intact GPIbα polypeptide (∼100 kDa) is consistent with proteolysis as the cause of this mobility change, as opposed to altered glycosylation of an intact GPIbα. The residual amount of full-length GPIbα migrated at ∼120 kDa, corresponding to one of the Tn(+) bands with ∼120 kDa (Fig. 1F), and the ∼21-kDa and ∼40-kDa fragments of GPIbα also corresponded the Tn(+) bands, respectively seen in Fig. 1F. Interestingly, the major ∼45-kDa GPIbα fragment from EHC Cosmc−/y platelet extracts was not stained by anti-Tn antibody (Figs. 1F and 3B), suggesting that this fragment comprising a portion of GPIbα containing a large portion of the extracellular domain of GPIbα with Tn antigens might be shed into the plasma. Because of the loss of expression of intact GPIbα, and considering its role as the primary platelet VWF receptor, we directly assessed the interaction of EHC Cosmc−/y platelets with VWF. We observed reduced binding of VWF to EHC Cosmc−/y platelets in both flow cytometry (Fig. 3C) and in plate-based assays (Fig. 3D), in the presence of botrocetin, a snake venom protein that enhances GPIbα affinity for VWF. Thus, loss of expression of intact GPIbα is associated with a loss of functional binding of platelets to VWF.
Fig. 3.
Cosmc-deficient platelets have impaired platelet GPIbα expression and function. (A) Platelet surface GPIbα expression as measured by flow cytometry (n = 4, *P = 0.0122, equal variance). (B) Western blot of GPIbα in platelet lysates in reducing and nonreducing conditions. (C) Flow cytometry of botrocetin-treated EHC Cosmc+/y and EHC Cosmc−/y platelets in the presence of VWF and incubated with FITC-labeled anti-VWF antibody. (D) Botrocetin-activated EHC Cosmc+/y and EHC Cosmc−/y platelets were allowed to adhere to BSA or VWF. Adherent platelets were stained with fluorescein-labeled phalloidin.
EHC Cosmc−/y Platelets Are Defective in Activation.
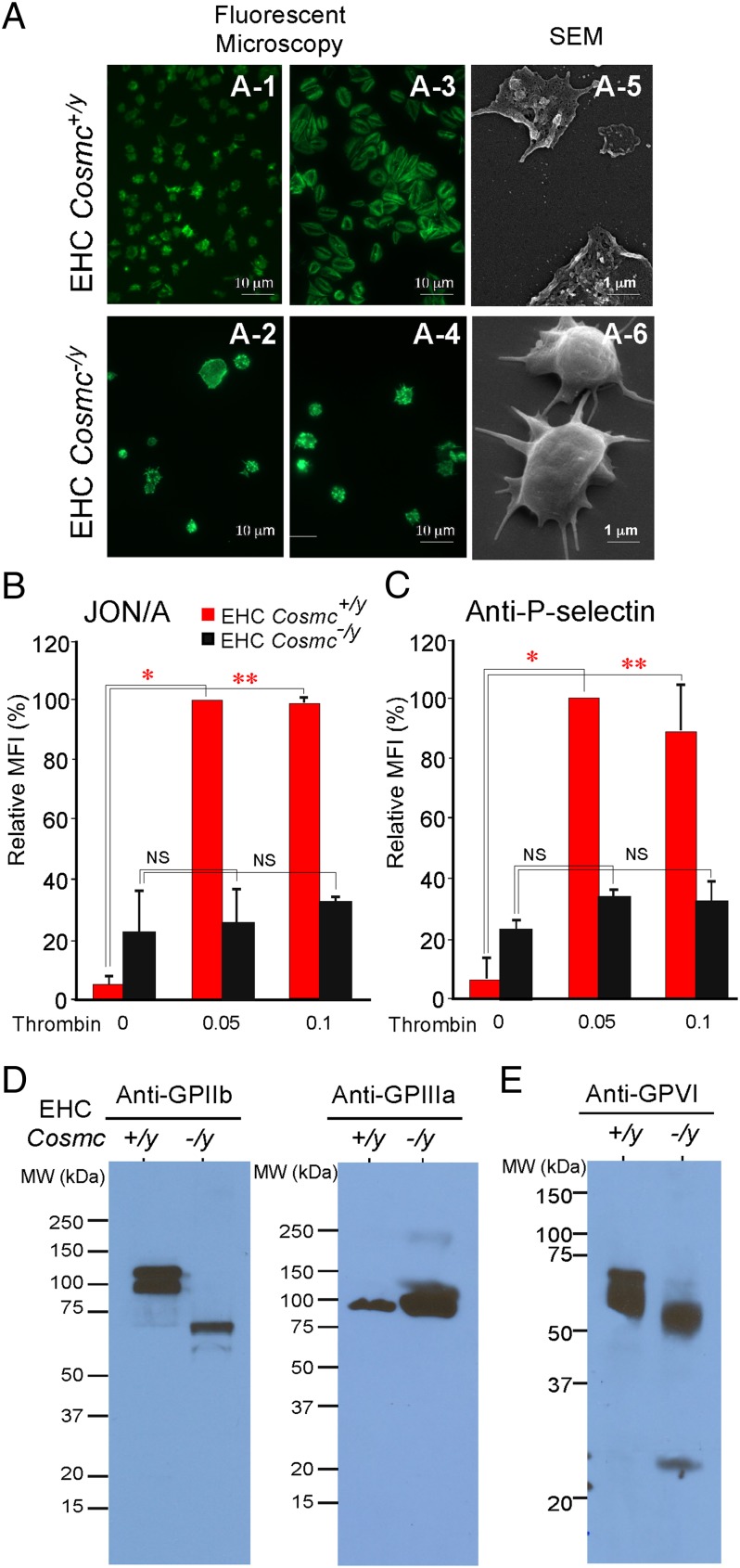
We also examined whether the platelets from EHC Cosmc−/y mice could be activated by the agonist thrombin. After stimulation through the protease activated receptor (PAR-1) pathway, activated platelets normally spread and release P-selectin on the cell surface, and convert integrin αIIbβ3 (GPIIb/IIIa) into its active form (conformation) (15). Scanning electron microscopy (SEM) revealed that wild-type platelets display peripheral flattening, lamellipodia, and filopodia extensions on fibrinogen, whereas EHC Cosmc−/y platelets were rounded with few filopodia and greatly reduced spreading (Fig. 4A). As platelet adhesion and spreading on fibrinogen is primarily dependent on the platelet integrin αIIbβ3, we examined expression of αIIbβ3 activation and P-selectin expression on platelets from wild-type and EHC Cosmc−/y mice. Consistent with the absence of platelet spreading in EHC Cosmc−/y platelets, integrin αIIbβ3 activation, as detected by the activation-dependent JON/A antibody, was impaired in thrombin-stimulated EHC Cosmc−/y platelets (Fig. 4B), whereas surface expression of integrin αIIb was unchanged (Fig. 1E). There was also no significant increase in surface expression of P-selectin in EHC Cosmc−/y platelets compared with wild-type at either thrombin concentration (0.05 or 0.1 U/mL) (Fig. 4C). These results demonstrate that EHC Cosmc−/y platelets have a defect in thrombin-induced activation of key platelet glycoproteins. Unexpectedly, we also observed that GPIIb from EHC Cosmc−/y platelets exhibited a marked reduction in apparent molecular weight compared with GPIIb from wild-type platelets, although there was no apparent size difference in GPIIIa from either platelet source (Fig. 4D). These results indicate that expression of full-length GPIIb is defective in EHC Cosmc−/y platelets, and associated with apparent proteolysis of GPIIb. We also analyzed whether a major collagen receptor glycoprotein VI (GPVI) (16) is altered in expression. Western blot analysis showed GPVI from EHC Cosmc−/y platelets also exhibited a marked reduction in apparent molecular weight compared with GPVI (Mr ∼62 kDa) in wild-type platelets (Fig. 4E). In control studies, we performed RT-PCR to explore expression of these and other platelet proteins and glycoproteins in wild-type versus EHC Cosmc−/y platelets. The transcript levels of GPIbα, GPIIb, GPIIIa, P-selectin, and other platelet markers were similar to wild-type platelets (Fig. S2 and Table S2). These results demonstrate that although loss of Cosmc in platelets results in loss-of-function, but not change in expression, of GPIbα, GPIIb, GPIIIa, and P-selectin, either because of proteolysis or loss of surface expression arising from loss of normal O-glycan extensions.
Fig. 4.
Altered expression and function of integrin αIIb and other platelet glycoproteins in Cosmc-deficient platelets. (A) Fluorescent microscopy: EHC Cosmc+/y and EHC Cosmc−/y platelet binding to fibrinogen detected by fluorescein-labeled phalloidin; EHC Cosmc+/y platelets without (A-1) or with (A-3) thrombin stimulation. EHC Cosmc−/y platelets without (A-2) or with (A-4) thrombin stimulation. SEM: thrombin-stimulated EHC Cosmc+/y and EHC Cosmc−/y platelets (A-5 and A-6). (B and C) In the absence of Cosmc, thrombin fails to stimulate activated αIIbβ3 measured by JON/A antibody (B) and P-selectin expression on the surface of platelets (C). Comparing resting to thrombin-stimulated platelets, *P = 0.0388, **P = 0.0219 (B), *P = 0.0102, **P = 0.00037 (C), NS, not significant as noted in graph (*, unequal variance; NS and **, equal variance). (D) Western blots of EHC Cosmc+/y and EHC Cosmc−/y platelets with anti-GPIIb and anti-GPIIIa antibodies. (E) Western blot of GPVI in platelet lysates in reducing conditions.
GPIIb and GPVI Are O-Glycoproteins.
The proteolysis observed for glycoproteins GPIIb and GPVI in EHC Cosmc−/y platelets suggested that these glycoproteins might contain O-glycosylated glycoproteins. To further explore this possibility, we immunoprecipitated GPIIbα (complex) in wild-type platelet lysates using a specific anti-GPIIb antibody; the immunoprecipitated material was blotted with peanut agglutinin (PNA) from Arachis hypogaea before and after neuraminidase. PNA specifically recognizes and binds to terminal Gal-GalNAc disaccharide within either core 1 or core 2 O-glycans on glycoproteins (10). PNA stained a major O-glycoprotein migrating at ∼120 kDa in the anti-GPIIb pull-down material only after desialylation, indicating that this glycoprotein carried sialylated core 1 or core 2 O-glycans (Fig. S3A). Upon stripping and reblotting with anti-GPIIb mAb, we observed that GPIIb shifted to a slightly lower molecular weight because of desialylation; the migration of desialylated GPIIb was similar to the glycoprotein band recognized by PNA, confirming that GPIIbα contains O-glycans (Fig. S3A).
For GPVI, both EHC Cosmc+/y and EHC Cosmc−/y platelets were immunoprecipitated with anti-GPVI antibody and then blotted with Helix pomatia agglutinin (HPA), a lectin that specifically recognizes terminal α-GalNAc, such as Tn antigen, on glycoproteins (10). HPA(+) protein bands were seen in EHC Cosmc−/y samples, but not in EHC Cosmc+/y, indicating GPVI carried the truncated O-glycan Tn antigen (Fig. S3B, Upper). The Western blot of this material with anti-GPVI antibody confirmed the ∼50 kDa HPA(+) band was GPVI (Fig. S3B, Lower), demonstrating that GPVI contains O-glycans. Taken together, these results demonstrate that both GPIIb and GPVI are O-glycosylated glycoproteins.
Discussion
Our results indicate an essential role for Cosmc, and thus the extensions of O-glycans, in the expression and function of the primary platelet adhesive proteins GPIb-IX-V, GPVI, and integrin αIIbβ3. The platelet GPIb-IX-V complex is the key platelet receptor for VWF (17–19). Human platelet GPIbα has abundant O-glycans, including sialylated versions of core 1 and core 2 O-glycans (20, 21), and a majority of sialic acid on the platelet surface appears to be associated with GPIbα (22). Although the O-glycan structures in murine GPIbα are unknown, there are 73 potential glycosylation sites in murine GPIbα in comparison with the 42 potential O-glycosylation sites in human GPIbα. Interestingly, although human GPIbα has four potential N-glycosylation sites, murine GPIbα has no predicted N-glycosylation sites. Previous studies that have evaluated the importance of O-glycosylation in GPIbα expression and function have been contradictory. A study that used exoglycosidases and endoglycosidases to partly remove O-glycans (23) suggested that O-glycans on platelet GPIbα were not important for interaction with VWF. On the other hand, it has been reported that sialylation of GPIbα and the presence of O-glycans on VWF promotes their interactions (4). Our results are unique in showing in vivo that loss of galactose on the core 1 O-glycans, and resultant loss of core 2 O-glycans of platelet GPIbα, lead to its instability and decreased expression. It is unlikely that deficiency of sialic acid on the O-glycans alone is the contributing factor to these phenotypes, because mice deficient in ST3Gal-I, the principal enzyme for sialylating O-glycans in hematopoietic cells (24), do not exhibit platelet deficiencies or bleeding disorders (see phenotype analysis of ST3Gal-I–null mice by the Consortium for Functional Glycomics at www.functionalglycomics.org/glycomics/publicdata/phenotyping.jsp).
Our findings relate to those of Alexander et al. (25), who generated a mouse line with a point mutation T961A in T-synthase (C1GALT1) cDNA using N-ethyl-N-nitrosourea mutagenesis. The mutation resulted in Y321N change in the protein leading to severe loss of enzymatic activity. Although the mutated mice were viable, they exhibited thrombocytopenia, but had no bleeding phenotype. The phenotypic differences between the T-synthase mutant mice and EHC Cosmc−/y mice are most likely because of the near quantitative loss of T-synthase activity in EHC Cosmc−/y platelets in comparison with the 5–10% residual T-synthase activity in T-synthase mutant mice. In contrast to EHC Cosmc−/y mouse platelets, the majority of GPIbα from the platelets in T-synthase mutant mice were intact, with only limited Tn antigen expression, and thus demonstrate that 5∼10% of T-synthase activity is sufficient for platelet glycoprotein function. This possibility is also consistent with the observation that disruption of T-synthase in mouse EHCs (EHC T-syn−/−) caused a similar phenotype of perinatal death and hemorrhage as for EHC Cosmc−/y mice (10, 26).
A particularly unique aspect of our findings is that EHC Cosmc−/y platelets do not have significantly altered expression of integrin αIIbβ3 (GPIIb/IIIa) on the surface (Fig. 1E), but exhibit proteolytic fragments of αIIb (Fig. 4D). GPIIb/IIIa, the key platelet glycoproteins in EHC Cosmc−/y platelets are not activated by thrombin, with the resulting consequence of reduced expression of surface P-selectin and reduced binding to fibrinogen. Moreover, the collagen receptor GPVI is also partly degraded. Such results suggest that O-glycan elongation is critical to the function of αIIbβ3 and GPVI. Indeed, our results (Fig. S3) demonstrate that both murine αIIb and GPVI are modified with O-glycans. Interestingly, the whole blood of EHC Cosmc-/y platelet extracts with anti-Tn mAb did not detect the protein bands corresponding to either αIIb or GPVI, which is probably because of the fact that the anti-Tn mAb used in this study has a restricted specificity for two adjacent Tn antigens in glycoproteins (27), as is often seen in mucins and GPIbα. The low abundance of GPVI in the EHC Cosmc−/y platelet extracts could also be a problem for detection by the anti-Tn antibody. At present there are no glycosylation studies on either murine αIIbβ3 or GPVI. Although the detailed N- and O-glycan structures of human αIIbβ3 are unknown, prior studies on biosynthesis of αIIbβ3 in human HEL cells explored modifications such as N-glycans, but did not report the presence of O-glycans (28). However, human αIIb appears to contain at least one O-glycan important for expression of the Baka (HPA-3a) alloantigen (29, 30). The functions of O-glycans on αIIbβ3 are unknown, but our results suggest that complex O-glycan structures beyond the Tn antigen are required for conformation/proteolytic stability of αIIb and its movement to the cell surface and activation. Further studies are needed to define potential sites of O-glycosylation on αIIb and the effect of decreased O-glycan expression on association with β3 and subsequent activation at the plasma membrane. Potentially, changes in thrombin signaling could also account for some of the defects observed in EHC Cosmc−/y platelets. In this regard, it should be noted that GPIbα has a high affinity binding site for α-thrombin and accounts for most of the total α-thrombin that can bind to platelets (31), resulting in induced platelet adhesion and spreading, secretion, and aggregation (32). Thus, a deficiency in thrombin signaling could occur as a result of defective GPIbα expression. Alternatively, there could be changes in the thrombin receptor activities of multiple signaling pathways (33). The changes in thrombin-mediated effects in EHC Cosmc−/y platelets are subjects of future studies.
Interestingly, although several important glycoproteins, including GPIbα, GPVI, and integrin αIIbβ3 from EHC Cosmc−/y platelets are partly proteolyzed into smaller fragments because of aberrant O-glycosylation, other glycoproteins carrying Tn antigen are still high molecular weight migrating >100 kDa (Fig. 1F). Although the identity of these O-glycosylated glycoproteins in murine platelets awaits identification in future studies, it is possible that the ∼120-kDa Tn(+) band is intact GPIbα from EHC Cosmc−/y platelets (Fig. 3B, Reducing), but the >250-kDa Tn(+) band could be VWF. This finding further indicates that the under O-glycosylated glycoproteins are highly susceptible to some unknown proteases, and the role of O-glycans on glycoproteins does not merely play a role in the stability of the glycoproteins. The identification of all O-glycosylated glycoproteins and the O-glycosylation sites on those O-glycoproteins in platelets will aid us in understanding the roles of glycosylation in platelet biology, and will be investigated using proteomics approaches in future studies.
Our results using Cosmc-disrupted mice, which express truncated O-glycans, resemble individuals with either of two major platelet disorders, BSS and Glanzmann thrombasthenia (GT). BSS is a rare, severe thrombopathy caused by mutations resulting in loss of GPIb-IX-V complex on platelet surfaces, and typically arises from heritable mutations in genes encoding either GPIbα or GPIbβ (34) manifesting as thrombocytopenia, loss of platelet adhesion, and megathrombocytes (24). Early studies using radiolabeling of surface glycans in platelets suggested that platelets from BSS patients had reduced expression of surface GPIbα and potentially altered glycosylation (35). GT arises from a lack of platelet aggregation and spreading after activation because of defective expression of the integrin αIIbβ3, arising from heritable mutations in genes encoding either αIIb or β3 (33). Although a number of studies have examined the roles of N-linked glycans in platelet glycoprotein function, our study is unique in providing fresh insights into the physiological roles of O-glycosylation in platelet biogenesis and function. The Cosmc-null mice produce platelets that resemble a combination of both BSS and GT and will help to define the roles of O-glycans in the formation and function of platelet GPIb-IX-V complex and αIIbβ3, and may contribute insights into rare disorders of platelet function associated with thrombocytopenia and giant platelets.
It is noteworthy that in complementary experiments, we attempted to delete Cosmc specifically in megakaryocytes, taking advantage of Pf4-cre transgenic male mice, in which the expression of Cre recombinase is under control of Pf4 (platelet factor 4). Pf4-cre transgenic mice express a codon-improved Cre recombinase (iCre) controlled by mouse Pf4, or Cxcl4, promoter. Cre recombinase is expressed in a majority of terminally differentiated megakaryocytes (36). This strategy was successful for studying roles of several proteins in megakaryocyte differentiation and platelet function (37). Although we successfully generated mice with the genotype Cosmcflox/y-Crepf4/+ (Fig. S4A) (37, 38), the Cosmc in these mice were unaffected. Examination of T-synthase levels showed similar levels of T-synthase in platelets from Cosmcflox/y-Crepf4/+ mice compared with wild-type and only a moderate decrease in Cosmc transcript in the platelets isolated from Cosmcflox/y-Crepf4/+ mice (Fig. S4 B and C). Thus, Cosmc transcripts and consequently T-synthase levels remain relatively normal because of the late expression of Pf4 controlled Cre recombinase in megakaryocytes and the long half-life of Cosmc transcript or Cosmc/T-synthase proteins in platelets and megakaryocytes.
Tn syndrome, characterized by the expression of Tn antigen on a subpopulation of blood cells of all lineages (6), is caused by somatic mutation of Cosmc in hematopoietic stem cells and in some patients is accompanied by a mild bleeding diathesis. In some ways, the EHC Cosmc−/y mice partly represent Tn syndrome, although no evidence shows that endothelial cells are involved in Tn syndrome, and fewer hematopoietic cells, ranging from 20∼80%, are affected in different patients (11). Thrombocytopenia and bleeding disorder in some patients with Tn syndrome may arise from dysfunction of key platelet glycoproteins, and the megathrombocytopenia in EHC Cosmc−/y mice greatly resembles the clinical symptoms of Tn syndrome. Our results also suggest the potential of pharmacologic intervention to inhibit O-glycosylation as a potential antiplatelet and antithrombotic target. Advantages over other modalities may include potential long-lasting effect due to effects on structural moieties and mild affects on multiple critical adhesion pathways.
Materials and Methods
Generation of EHC Cosmc−/y Mice.
Cosmcflox/flox female mice were crossed with Tie2Cre Tg mice [Tg (Tek-cre) 1Ywa] male mice (13, 39). Because Cosmc is X-linked, male mice carrying Tie2+Cre will have complete deletion in the affected tissues. EHC Cosmc−/y mice were of mixed genetic background (129SvEv/TAC and C57BL/6J). Animal studies were performed according to the approved Institutional Animal Care and Use Committee protocol of Emory University. Wild-type and floxed Cosmc Tie2Cre alleles were identified by PCR. See Table S3 for PCR primers for mouse genotyping.
Preparation of Murine Washed Platelets.
Mice were killed with 100% CO2. Whole blood was obtained by cardiac puncture and washed platelets were isolated as previously described (40, 41).
Hematology.
Blood was collected by retroorbital bleeding of isoflurane-anesthetized mice into EDTA-containing polypropylene microtubes (Becton Dickinson). Complete blood count was carried out with HESKA CBC-Diff Veterinary Hematology System and platelet counts were confirmed by microscopy and hemacytometry. Blood smears were analyzed by microscopy (1 × 51S8F-3; Olympus) using SPOT software (Olympus) with Wright-Giemsa stain (Electron Microscopy Sciences).
Bleeding Times.
Mice were intraperitoneally injected with avertin (0.4 mg/g) to induce general anesthesia. Next, 0.5 cm of the distal tail was briskly cut and the tail was immediately transferred to 37 °C saline. The time point that the bleeding stopped was recorded as bleeding time; tails were cauterized when bleeding times >10 min.
Flow Cytometry.
Binding of VWF to EHC Cosmc+/y and EHC Cosmc−/y platelets was analyzed as previously described (42, 43). To analyze platelet activation under stimulation by different concentrations of bovine thrombin, murine platelets were washed as above. Washed platelets were resuspended at 107/mL in Tyrode’s buffer containing 2 mM CaCl2. Platelets were unstimulated or stimulated with thrombin (0.05 U/mL, 0.1 U/mL; Haematological Technologies). Platelets were stimulated for 5 min with the indicated agonist followed by addition of the indicated antibody for 2 min: FITC-labeled anti–mouse CD62P (P-selectin), PE-labeled JON/A (Emfret Analytics), FITC-labeled anti-Tn, and APC-labeled anti-CD41 antibody (eBioscience). Following incubation, platelets were immediately fixed in 1% PFA, diluted, and analyzed on a Becton Dickinson FACscan as previously described (44). Platelets were gated by light scatter and expression of CD41.
Platelet Spreading on VWF or Fibrinogen.
Platelet spreading on immobilized VWF or fibrinogen after stimulation with botrocetin and bovine thrombin was performed as previously described (45) using fluorescein-labeled phalloidin (Invitrogen) (46). Additional information on flow cytometry and platelet spreading is presented in SI Materials and Methods.
Immunoprecipitation and Immunoblotting.
Immunoprecipitation was performed as previously described (47, 48). Briefly, 1 × 108 washed platelets were surface-labeled with EZ-Link sulfo-NHS-LC-biotin (Pierce; 100 μg/mL in PBS) and subsequently solubilized in 1 mL of lysis buffer (25 mM Tris⋅HCl buffer pH 7.5) containing 150 mM NaCl and proteinase inhibitor mixture (Roche Molecular Biochemicals). Cell debris was removed by centrifugation (15,000 × g, 10 min). Two micrograms of mAb MWReg 30 (BD Pharmingen) to GpIIb or 2 μg of mAb to GpVI (rat anti-mouse mAb; Emfret Analytics), was added together with 50 μL of Dynabeads protein G-Sepharose (Invitrogen), and precipitated overnight at 4 °C. Samples were separated by 4–20% gradient SDS/PAGE along with molecular weight markers and transferred to nitrocellulose membrane. The membrane was incubated with streptavidin/horseradish peroxidase (1 μg/mL) for 1 h after blocking. After extensive washing, biotinylated proteins were visualized by SuperSignal WestPico Chemiluminescent substrate (Thermo Scientific). For immunoblotting, platelets were not surface-labeled. Washed platelets (1 × 107/mL) were resuspended in lysis buffer and extracts were obtained by adding 0.5% Triton X-100 to platelet supernatant and solubilizing on ice for 30 min. After lysis, whole-platelet extract was analyzed by SDS/PAGE and transferred to nitrocellulose membranes. Western blotting with biotin labeled anti-Tn, and anti-GPIbα anti-GPIIIa antibodies (Emfret Analytics), was performed as previously described (8).
Enzyme Assays.
T-synthase and α-mannosidase activity from murine washed platelet extracts were measured using the acceptor GalNAc1α-(4-MU) and mannoside1α-(4-MU), respectively, as previously described (49).
Statistical Analysis.
For P value determination, a two-sample independent t test was used (95% confidence interval). To determine equal or unequal variance, F statistics were applied and variance is noted for each P value.
Supplementary Material
Acknowledgments
We thank the late Dr. Georg F. Springer for mouse anti-Tn monoclonal antibody; Dr. Changgeng Ruan (Jiangsu Institute of Hematology) for the SZ-29 antibody; Drs. Lijun Xia (Oklahoma Medical Research Foundation) and Masashi Yanagisawa (University of Texas Southwestern Medical Center) for providing Tie2-Cre transgenic mice; Drs. Jamie Heimburg-Molinaro, David F. Smith, Rajindra Aryal, and Connie Arthur for their suggestions and editing of the manuscript; and the Robert P. Apkarian integrated electron microscopy core facility at Emory University for technical support. This work was supported by National Institutes of Health Grants R01GM068559 (to R.D.C.), R01DK80876 (to T.J.), and R01HL095858 (to S.M.J.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208253109/-/DCSupplemental.
References
- 1.McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost. 2001;86:746–756. [PubMed] [Google Scholar]
- 2.Schulte am Esch J, 2nd, et al. Impact of O-linked glycosylation of the VWF-A1-domain flanking regions on platelet interaction. Br J Haematol. 2005;128:82–90. doi: 10.1111/j.1365-2141.2004.05253.x. [DOI] [PubMed] [Google Scholar]
- 3.Miura S, et al. Structural elements influencing von Willebrand factor (vWF) binding affinity for platelet glycoprotein Ib within a dispase-digested vWF fragment. Blood. 1994;84:1553–1558. [PubMed] [Google Scholar]
- 4.Carew JA, Quinn SM, Stoddart JH, Lynch DC. O-linked carbohydrate of recombinant von Willebrand factor influences ristocetin-induced binding to platelet glycoprotein 1b. J Clin Invest. 1992;90:2258–2267. doi: 10.1172/JCI116112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mhawech P, Saleem A. Inherited giant platelet disorders. Classification and literature review. Am J Clin Pathol. 2000;113:176–190. doi: 10.1309/FC4H-LM5V-VCW8-DNJU. [DOI] [PubMed] [Google Scholar]
- 6.Ju T, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angew Chem Int Ed Engl. 2011;50:1770–1791. doi: 10.1002/anie.201002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ju T, Cummings RD. Protein glycosylation: Chaperone mutation in Tn syndrome. Nature. 2005;437:1252. doi: 10.1038/4371252a. [DOI] [PubMed] [Google Scholar]
- 8.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci USA. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ju T, Aryal RP, Stowell CJ, Cummings RD. Regulation of protein O-glycosylation by the endoplasmic reticulum-localized molecular chaperone Cosmc. J Cell Biol. 2008;182:531–542. doi: 10.1083/jcb.200711151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, et al. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc Natl Acad Sci USA. 2010;107:9228–9233. doi: 10.1073/pnas.0914004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger EG. Tn-syndrome. Biochim Biophys Acta. 1999;1455:255–268. doi: 10.1016/s0925-4439(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 12.Judson PA, Spring FA, Taylor MA, Anstee DJ. Evidence for carbohydrate-deficient forms of the major sialoglycoproteins of human platelets, granulocytes and T lymphocytes in individuals with Tn syndrome. Immunology. 1983;50:415–422. [PMC free article] [PubMed] [Google Scholar]
- 13.Kisanuki YY, et al. Tie2-Cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 14.Nurden A, Nurden P. Advances in our understanding of the molecular basis of disorders of platelet function. J Thromb Haemost. 2011;9(Suppl 1):76–91. doi: 10.1111/j.1538-7836.2011.04274.x. [DOI] [PubMed] [Google Scholar]
- 15.Martorell L, et al. Thrombin and protease-activated receptors (PARs) in atherothrombosis. Thromb Haemost. 2008;99:305–315. doi: 10.1160/TH07-08-0481. [DOI] [PubMed] [Google Scholar]
- 16.Nurden AT. Sustaining platelet counts in chronic ITP. Lancet. 2011;377:358–360. doi: 10.1016/S0140-6736(10)61090-2. [DOI] [PubMed] [Google Scholar]
- 17.López JA. The platelet glycoprotein Ib-IX complex. Blood Coagul Fibrinolysis. 1994;5:97–119. [PubMed] [Google Scholar]
- 18.Ruggeri ZM. Mechanisms initiating platelet thrombus formation. Thromb Haemost. 1997;78:611–616. [PubMed] [Google Scholar]
- 19.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 20.Korrel SA, et al. Structural studies on the O-linked carbohydrate chains of human platelet glycocalicin. Eur J Biochem. 1984;140:571–576. doi: 10.1111/j.1432-1033.1984.tb08140.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsuji T, et al. The carbohydrate moiety of human platelet glycocalicin. J Biol Chem. 1983;258:6335–6339. [PubMed] [Google Scholar]
- 22.Solum NO, Hagen I, Filion-Myklebust C, Stabaek T. Platelet glycocalicin. Its membrane association and solubilization in aqueous media. Biochim Biophys Acta. 1980;597:235–246. doi: 10.1016/0005-2736(80)90102-9. [DOI] [PubMed] [Google Scholar]
- 23.Moshfegh K, et al. Fine structural and functional consequences of deglycosylation of the platelet adhesion receptor GPIb-IX (CD 42b) Biochem Biophys Res Commun. 1998;249:903–909. doi: 10.1006/bbrc.1998.9125. [DOI] [PubMed] [Google Scholar]
- 24.Priatel JJ, et al. The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity. 2000;12:273–283. doi: 10.1016/s1074-7613(00)80180-6. [DOI] [PubMed] [Google Scholar]
- 25.Alexander WS, et al. Thrombocytopenia and kidney disease in mice with a mutation in the C1galt1 gene. Proc Natl Acad Sci USA. 2006;103:16442–16447. doi: 10.1073/pnas.0607872103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu J, et al. Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J Clin Invest. 2008;118:3725–3737. doi: 10.1172/JCI36077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borgert A, et al. Deciphering structural elements of mucin glycoprotein recognition. ACS Chem Biol. 2012;7:1031–1039. doi: 10.1021/cb300076s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosa JP, McEver RP. Processing and assembly of the integrin, glycoprotein IIb-IIIa, in HEL cells. J Biol Chem. 1989;264:12596–12603. [PubMed] [Google Scholar]
- 29.Calvete JJ, Muñiz-Diaz E. Localization of an O-glycosylation site in the alpha-subunit of the human platelet integrin GPIIb/IIIa involved in Baka (HPA-3a) alloantigen expression. FEBS Lett. 1993;328:30–34. doi: 10.1016/0014-5793(93)80959-x. [DOI] [PubMed] [Google Scholar]
- 30.Djaffar I, Vilette D, Pidard D, Wautier JL, Rosa JP. Human platelet antigen 3 (HPA-3): Localization of the determinant of the alloantibody Lek(a) (HPA-3a) to the C-terminus of platelet glycoprotein IIb heavy chain and contribution of O-linked carbohydrates. Thromb Haemost. 1993;69:485–489. [PubMed] [Google Scholar]
- 31.Mazzucato M, et al. Characterization of the initial alpha-thrombin interaction with glycoprotein Ib alpha in relation to platelet activation. J Biol Chem. 1998;273:1880–1887. doi: 10.1074/jbc.273.4.1880. [DOI] [PubMed] [Google Scholar]
- 32.Adam F, Guillin MC, Jandrot-Perrus M. Glycoprotein Ib-mediated platelet activation. A signalling pathway triggered by thrombin. Eur J Biochem. 2003;270:2959–2970. doi: 10.1046/j.1432-1033.2003.03670.x. [DOI] [PubMed] [Google Scholar]
- 33.Gandhi PS, Chen Z, Appelbaum E, Zapata F, Di Cera E. Structural basis of thrombin-protease-activated receptor interactions. IUBMB Life. 2011;63:375–382. doi: 10.1002/iub.461. [DOI] [PubMed] [Google Scholar]
- 34.Nurden P, Nurden AT. Congenital disorders associated with platelet dysfunctions. Thromb Haemost. 2008;99:253–263. doi: 10.1160/TH07-09-0568. [DOI] [PubMed] [Google Scholar]
- 35.McGregor JL, Clemetson KJ, James E, Luscher EF, Dechavanne M. A comparison of the major platelet membrane glycoproteins from Bernard-Soulier syndrome with normals after radiolabelling of sialic acid or terminal galactose/N-acetylgalactosamine residues. Thromb Res. 1980;17:713–718. doi: 10.1016/0049-3848(80)90374-6. [DOI] [PubMed] [Google Scholar]
- 36.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 37.Hitchcock IS, et al. Roles of focal adhesion kinase (FAK) in megakaryopoiesis and platelet function: Studies using a megakaryocyte lineage specific FAK knockout. Blood. 2008;111:596–604. doi: 10.1182/blood-2007-05-089680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen Q, et al. Survivin is not required for the endomitotic cell cycle of megakaryocytes. Blood. 2009;114:153–156. doi: 10.1182/blood-2008-11-190801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams SA, Xia L, Cummings RD, McEver RP, Stanley P. Fertilization in mouse does not require terminal galactose or N-acetylglucosamine on the zona pellucida glycans. J Cell Sci. 2007;120:1341–1349. doi: 10.1242/jcs.004291. [DOI] [PubMed] [Google Scholar]
- 40.Judd BA, et al. Hematopoietic reconstitution of SLP-76 corrects hemostasis and platelet signaling through alpha IIb beta 3 and collagen receptors. Proc Natl Acad Sci USA. 2000;97:12056–12061. doi: 10.1073/pnas.97.22.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jobe SM, et al. Role of FcRgamma and factor XIIIA in coated platelet formation. Blood. 2005;106:4146–4151. doi: 10.1182/blood-2005-03-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Englund GD, Bodnar RJ, Li Z, Ruggeri ZM, Du X. Regulation of von Willebrand factor binding to the platelet glycoprotein Ib-IX by a membrane skeleton-dependent inside-out signal. J Biol Chem. 2001;276:16952–16959. doi: 10.1074/jbc.M008048200. [DOI] [PubMed] [Google Scholar]
- 43.Bodnar RJ, Xi X, Li Z, Berndt MC, Du X. Regulation of glycoprotein Ib-IX-von Willebrand factor interaction by cAMP-dependent protein kinase-mediated phosphorylation at Ser 166 of glycoprotein Ib(beta) J Biol Chem. 2002;277:47080–47087. doi: 10.1074/jbc.M208329200. [DOI] [PubMed] [Google Scholar]
- 44.Leo L, Di Paola J, Judd BA, Koretzky GA, Lentz SR. Role of the adapter protein SLP-76 in GPVI-dependent platelet procoagulant responses to collagen. Blood. 2002;100:2839–2844. doi: 10.1182/blood-2002-04-1234. [DOI] [PubMed] [Google Scholar]
- 45.Yin H, et al. Src family tyrosine kinase Lyn mediates VWF/GPIb-IX-induced platelet activation via the cGMP signaling pathway. Blood. 2008;112:1139–1146. doi: 10.1182/blood-2008-02-140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu M, Xi X, Englund GD, Berndt MC, Du X. Analysis of the roles of 14-3-3 in the platelet glycoprotein Ib-IX-mediated activation of integrin alpha(IIb)beta(3) using a reconstituted mammalian cell expression model. J Cell Biol. 1999;147:1085–1096. doi: 10.1083/jcb.147.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nieswandt B, et al. Expression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcRgamma chain. J Biol Chem. 2000;275:23998–24002. doi: 10.1074/jbc.M003803200. [DOI] [PubMed] [Google Scholar]
- 48.Rehli M, Krause SW, Kreutz M, Andreesen R. Carboxypeptidase M is identical to the MAX.1 antigen and its expression is associated with monocyte to macrophage differentiation. J Biol Chem. 1995;270:15644–15649. doi: 10.1074/jbc.270.26.15644. [DOI] [PubMed] [Google Scholar]
- 49.Ju T, et al. A novel fluorescent assay for T-synthase activity. Glycobiology. 2011;21:352–362. doi: 10.1093/glycob/cwq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.