Abstract
Most chemiluminescence (CL) reactions usually generate only one-step CL, which is rarely dependent on the highly reactive and biologically/environmentally important hydroxyl radicals (•OH). Here, we show that an unprecedented two-step CL can be produced by the carcinogenic tetrachloro-1,4-benzoquinone (also known as p-chloranil) and H2O2, which was found to be well-correlated to and directly dependent on its two-step metal-independent production of •OH. We proposed that •OH-dependent formation of quinone-dioxetane and electronically excited carbonyl species might be responsible for this unusual two-step CL production by tetrachloro-1,4-benzoquinone/H2O2. This is a unique report of a previously undefined two-step CL-producing system that is dependent on intrinsically formed •OH. These findings may have potential applications in detecting and quantifying •OH and the ubiquitous polyhalogenated aromatic carcinogens, which may have broad biological and environmental implications for future research on these types of important species.
Keywords: halogenated quinones; trichlorohydroxy-1,4-benzoquinone; dichloromaleic acid; halogenated phenols; light emission
Polyhalogenated quinones are a class of toxicological intermediates that can cause acute hepatoxicity, nephrotoxicity, and carcinogenesis (1, 2). They have also been observed as reactive oxidation intermediates or products in processes used to oxidize or destroy polychlorinated persistent organic pollutants in various chemical and enzymatic systems (3–7). More recently, several polyhalogenated quinones, which are suspected bladder carcinogens, were identified as new chlorination disinfection byproducts in drinking water (7). Tetrachloro-1,4-benzoquinone (TCBQ) is one of the major genotoxic and carcinogenic quinoid metabolites of the widely used wood preservative pentachlorophenol (PCP), which has been found in over one-fifth of the National Priorities List sites identified by the US Environmental Protection Agency and classified as a group 2B environmental carcinogen (3). TCBQ itself has been widely used as a fungicide for treatment of seeds, and as an oxidizing or dehydrating agent in organic synthesis (often called p-chloranil).
The hydroxyl radical (•OH) is an extremely reactive oxidant, important in chemistry, biology, medicine, and atmospheric and environmental science (3, 8–13). In biology, •OH is recognized as the most reactive and harmful of the so-called reactive oxygen species (ROS), which can cause DNA, protein, and lipid oxidation (8–10). Cancer, arthritis, and Parkinson’s disease are but a few of the ailments that are linked to •OH (8). In environmental and atmospheric chemistry, •OH is also important because of its ability to oxidize and destroy organic pollutants efficiently and has been referred to as the atmosphere’s “detergent” (3–6, 11–13). One of the most widely accepted mechanisms for •OH production is through the transition metal-catalyzed Fenton reaction (3, 8–10). Recently, however, we found that •OH could be produced independent of transition metal ions by hydrogen peroxide (H2O2) with TCBQ and other halogenated quinones (14, 15). A unique nucleophilic substitution coupled with homolytic decomposition reaction mechanism was proposed (3, 15). We also found that alkoxyl and carbon-centered quinone ketoxy radicals could be produced by halogenated quinone-mediated decomposition of organic hydroperoxides through a similar mechanism (16–18). Interestingly, it was recently observed by others that, in the presence of fluorescent agent riboflavin, chemiluminescence (CL) could be produced by H2O2 and brominated quinoid compounds isolated from acorn worm, a luminous marine organism (19). However, neither the underlying molecular mechanism for CL production nor its possible correlation with •OH production is clear.
Therefore, in the present study we addressed the following questions: (i) Can CL be produced by halogenated quinones and H2O2 in the absence of a fluorescent agent; (ii) If so, are there any correlations between •OH and CL production; (iii) Is CL dependent on •OH production; (iv) What are the molecular mechanisms underlying CL and •OH production; and (v) What are the potential biological and environmental implications?
Results and Discussion
TCBQ and H2O2 Can Produce Two-Step CL, Even in the Absence of Fluorescent Agents.
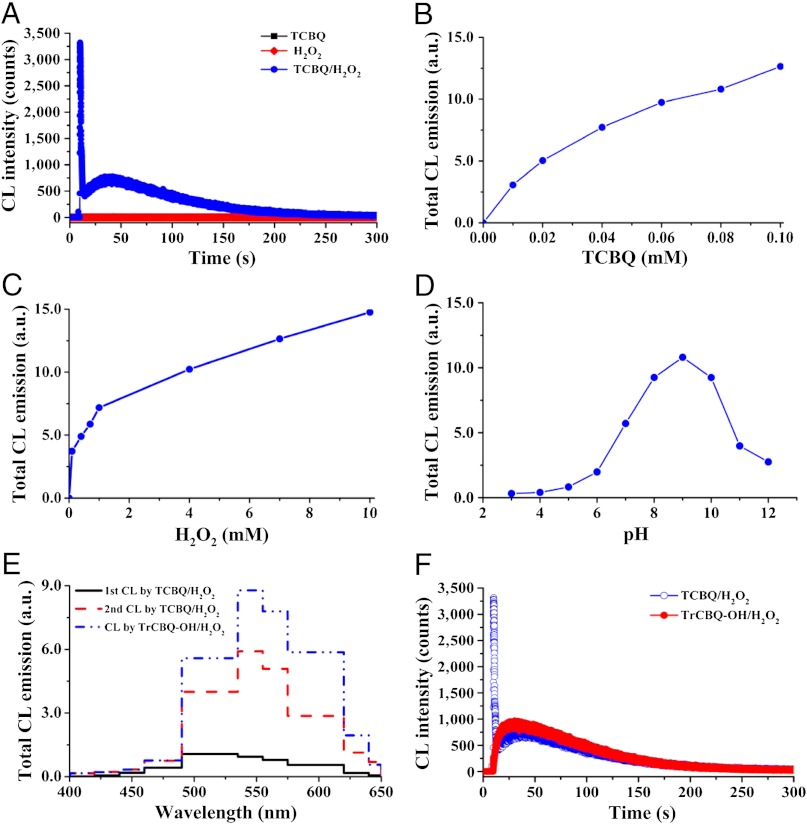
We found, surprisingly, that even in the absence of fluorescent agents, CL could be produced by TCBQ and H2O2 (Fig. 1A). In contrast, neither TCBQ nor H2O2 alone could produce any CL (Fig. 1A). The intensity of the CL was found to be dependent on both TCBQ and H2O2: The higher the concentrations of TCBQ and H2O2, the stronger the CL (Fig. 1B and C). The CL was also found to depend on pH of the buffer: No CL was observed at pH ≤ 3, but as the pH was increased the intensity of CL increased progressively, then reached maximum at pH 9; further increase of pH, however, led to a decline of CL intensity (Fig. 1D). The approximate CL quantum yield for TCBQ/H2O2 system was found to be about  , which was determined by using luminol as the relative standard (20). This suggests that the CL emission from TCBQ/H2O2 system is a low-level CL.
, which was determined by using luminol as the relative standard (20). This suggests that the CL emission from TCBQ/H2O2 system is a low-level CL.
Fig. 1.
TCBQ and H2O2 can produce two-step CL, even in the absence of fluorescent agents. (A) Two-step CL could be produced by TCBQ and H2O2. TCBQ, 0.1 mM; H2O2, 100 mM. (B–D) The effects of TCBQ, H2O2, and pH on CL production by TCBQ/H2O2. TCBQ, 0.2 mM; H2O2, 5 mM. (E and F) The second-step CL produced by TCBQ/H2O2 is the same as the CL by TrCBQ–OH/H2O2: Emission spectra (E) and time course (F). TCBQ, 0.1 mM; TrCBQ–OH, 0.1 mM; H2O2, 100 mM. All reactions were carried out in Chelex-pretreated phosphate buffer (0.1 M, pH 7.4).
More interestingly, an unusual two-step CL was found to be produced by TCBQ and H2O2: The first one was strong but short-lived (2–6 sec) with maximum CL emission at band 490–535 nm, and the second one was weak but lasted longer (≥120 sec) with maximum at band 535–555 nm (Fig. 1A and E).
Analogous two-step CL was observed when TCBQ was substituted with other tetrahalogenated quinones, including tetrabromo-1,4-benzoquinones and their corresponding hydroquinone forms, as well as tetrabromo- and tetrachloro-1,2-benzoquinones (Figs. S1 and S2). These findings demonstrated that the two-step CL production is a general phenomenon between H2O2 and all tetrahalogenated quinoid compounds.
The Two-Step CL Production by TCBQ/H2O2 Was Found to Be Well Correlated to Its Two-Step Hydroxyl Radical Production.
We showed recently that •OH could be produced by TCBQ with H2O2 through a previously undefined mechanism of nucleophilic substitution followed by homolytical decomposition (15). Based on this mechanism, we hypothesized that the initial transient intermediate trichlorohydroxy-1,4-benzoquinone (TrCBQ–O-) may further react with excess H2O2 to produce another molecule of •OH and 2,5-dichloro-3,6-dihydroxy-1,4-benzoquinone (DDBQ, also called chloranilic acid) (3). In other words, the production of •OH by TCBQ/H2O2 might be also a two-step process.
To test whether this is the case and whether there is any correlation between •OH and CL production by TCBQ/H2O2, the reaction between TCBQ and H2O2 was further studied by complementary application of various analytical methods.
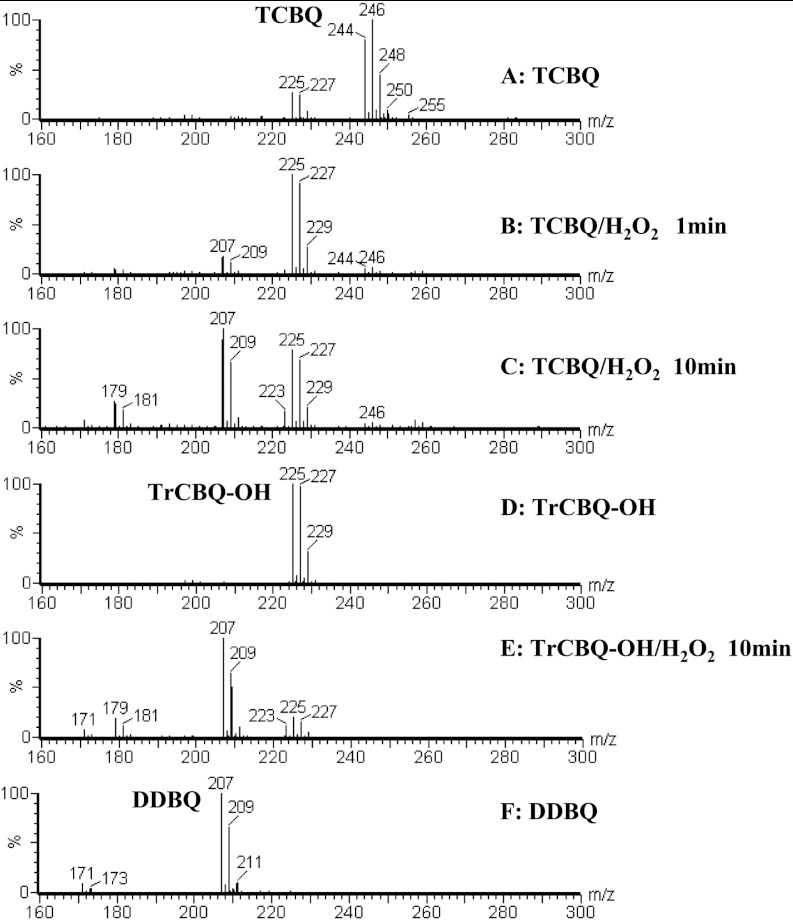
The initial transient intermediate and final products between TCBQ and H2O2 were identified by electrospray ionization quadrupole time-of-flight mass spectrometry (ESI-Q-TOF-MS). As expected, we found that, in the presence of excess H2O2, TCBQ was first converted to the initial transient intermediate TrCBQ–OH, and then further to the final product DDBQ (Fig. 2 A–C and SI Materials and Methods). To test whether TrCBQ–OH could also be directly converted to DDBQ in the presence of H2O2, TrCBQ–OH was synthesized according to a published method (15); we found that it was indeed the case (Fig. 2 D–F). However, no further reaction was observed between DDBQ and H2O2. These results suggest that the reaction between TCBQ and H2O2 is a two-step process.
Fig. 2.
Identification of reaction intermediate and product for TCBQ and H2O2 by ESI-Q-TOF-MS. (A) ESI-negative-Q-TOF-MS spectra of TCBQ; (B) TCBQ/H2O2, 1 min; (C) TCBQ/H2O2, 10 min; (D) TrCBQ–OH; (E) TrCBQ–OH/H2O2, 10 min; (F) DDBQ. TCBQ, 0.5 mM; TrCBQ–OH, 0.5 mM; DDBQ, 0.5 mM; H2O2, 100 mM. All reactions were carried out in Chelex-pretreated ammonia acetate buffer (0.1 M, pH 7.4).
The time course for •OH production by TCBQ/H2O2 was followed by both ESR with DMPO (5,5-dimethyl-1-pyrroline-N-oxide) as the spin-trapping agent, and fluorescent method with coumarin-3-carboxylic acid (3-CCA) as an •OH probe. Two clear-cut phases of •OH production were observed: The first phase is fast, whereas the second is much slower (Fig. 3 A and B). This demonstrated that two-step •OH could be produced by two-step reactions between TCBQ and H2O2.
Fig. 3.
The two-step CL production by TCBQ/H2O2 was well-correlated to and directly dependent on its two-step hydroxyl radical production. (A and B) The ESR and fluorescent kinetic study on •OH production by TCBQ/H2O2. TCBQ, 0.1 mM; TrCBQ–OH, 0.1 mM; H2O2, 1 mM; DMPO, 100 mM; 3-CCA, 4 mM. (C) ESR detection of •OH production by TCBQ/H2O2 and TrCBQ–OH/H2O2. TCBQ, 0.5 mM; TrCBQ–OH, 0.5 mM; H2O2, 5 mM; DMPO, 100 mM. (D) The pH-dependent •OH production by TCBQ/H2O2 by fluorescent method. TCBQ, 0.1 mM; H2O2, 1 mM; 3-CCA, 1 mM. (E) The CL production by TCBQ/H2O2 was quenched by the classic •OH scavengers. TCBQ, 0.1 mM; H2O2, 100 mM. (F) CL production by TCBQ/H2O2 was further enhanced by addition of Fe(II)–EDTA. TCBQ, 0.1 mM; H2O2, 100 mM. All reactions were carried out in Chelex-pretreated phosphate buffer (0.1 M, pH 7.4).
The above results suggested that each step of reaction between TCBQ and H2O2 might produce •OH and CL, and the second-step •OH formation and CL emission should be produced by the reaction of H2O2 with TrCBQ–OH, the transient intermediate produced during the first-step reaction between TCBQ and H2O2. We found that this was exactly the case: The time course and the emission spectrum of the second-step CL produced by TCBQ and H2O2 were almost the same as compared with that produced by TrCBQ–OH and H2O2 (Fig. 1F), and •OH could also be produced by TrCBQ–OH and H2O2, which is much weaker and slower as compared with the first-step •OH production by TCBQ/H2O2 (Fig. 3C). Similar pH-dependent effects were also observed for both •OH and CL production by TCBQ/H2O2 (Fig. 3D).
Taken together, the above findings showed that the production of •OH by TCBQ/H2O2 is a two-step process, and the two-step •OH production was correlated well with the two-step CL production by TCBQ/H2O2. These results indicate that •OH might be involved in the two-step CL production.
The CL Production by TCBQ/H2O2 Was Directly Dependent on •OH Formation.
We found that the two-step CL production by TCBQ/H2O2 was indeed directly dependent on •OH formation. This was based on the following lines of evidence: (i) The CL produced by TCBQ/H2O2 was markedly inhibited by several well-known •OH scavengers, such as DMSO, formate, and ethanol (Fig. 3E), and the concentrations for each •OH scavenger to inhibit 50% of CL (IC50) were found to be 9.3, 8.5, and 45 mM, respectively, which are inversely correlated with their rate constants with •OH (8) (Fig. 3E); (ii) CL production by TCBQ/H2O2 could be further enhanced significantly (up to four times) by extra •OH produced by addition of the iron complex Fe(II)–EDTA, a classic Fenton reagent that can react with H2O2 to produce •OH (Fig. 3F); (iii) CL could be produced by TCBQ only with H2O2, but not with organic hydroperoxides such as tert-butyl hydroperoxide (t-BuOOH) and cumene hydroperoxide (CuOOH) under the same experimental conditions (Fig. S3), which is consistent with the fact that •OH could be produced by TCBQ only with H2O2, but not with organic hydroperoxides (only alkoxyl radicals were produced in these cases); and (iv) CL could be produced by H2O2 not only with TCBQ, but also with all other halogenated quinones, but not with non- or methyl-substituted quinones (Fig. S4). This is also in agreement with our previous finding that •OH could be produced only by halogenated quinones, but not with non- or methyl-substituted quinones (see ref. 14). To our knowledge, this is a unique report showing that the CL production is well-correlated and directly dependent on •OH formation.
The above findings may have some potential applications. For example, the method could be used as a rapid and specific chemiluminescent method for detecting and measuring metal-independent •OH production by halogenated quinones and H2O2; estimating the rate constant with •OH of an unknown compound; and evaluating the •OH-scavenging abilities of various antioxidants, food extracts, and herbal preparations. The unique advantage of this method is that the source of •OH can be produced independent of transition metal ions by halogenated quinones and H2O2.
The Major Ring-Opening Products Between TCBQ and H2O2 Were Identified as 2,3-Dichloromaleic Acid and CO2.
As shown above, the two hydroxylated quinones TrCBQ–OH and DDBQ were identified as transient and final product between TCBQ and H2O2. To our surprise, the quantitative analysis by HPLC showed that the yield of the final product DDBQ after TCBQ reaction with excessive H2O2 was only 9.8% (Table S1). This indicates that other products (other than DDBQ) should be formed, too. However, no other products could be readily identified by MS or HPLC using previously published methods (15, 18). To solve this problem, qualitative and quantitative analysis of all reaction products from TCBQ/H2O2 were conducted systematically by complementary applications of multiple analytical methods: GC-MS, Q-TOF-MS-HPLC, and ion chromatography (IC). We found that, unexpectedly, beside DDBQ, three quinone ring-opening products were also formed. The major ring-opening product was identified as dichloromaleic acid (DCMA), and the other two minor ring-opening products were chloromalonic acid (CMA) and oxalic acid (Figs. S5 and S6). The IC quantitative results showed that the yields of DCMA, CMA, and oxalic acid from TCBQ/H2O2 were 32.3%, 13.4%, and 12.2%, respectively (Table S1). The remaining 32.3% TCBQ was found to be converted to CO2 (and/or CO) through total organic carbon (TOC) analysis.
DDBQ, CMA, oxalic acid, and CO2 (and/or CO), but not DCMA, were identified as the final products for the reaction between TrCBQ–OH and H2O2, and their yields were 33.1%, 17.8%, 21.3%, and 27.8%, respectively (Fig. S6 and Table S1).
In summary, the product analysis showed that H2O2 not only causes the dechlorination and hydroxylation of TCBQ to form TrCBQ–OH and DDBQ, but also causes quinone ring-opening to form DCMA, CMA, oxalic acid, and CO2 (and/or CO). To our knowledge, this is also a previously uncharacterized report showing TCBQ could decompose to form small-molecule organic acids by H2O2. It has been suggested that H2O2-dependent decomposition pathway for halogenated quinones may be important in systems where H2O2 is either used or produced. However, the exact molecular mechanisms underlying such further transformations are not clear. Our findings may provide a unique perspective to understand better such transformation mechanisms during wastewater treatment or remediation processes in which halogenated quinones are formed.
Possible Light-Emitting Intermediates.
Chemiluminescence is a phenomenon in which chemically generated molecules in excited states liberate energy with light emission, which is generally accompanied by decomposition of organic peroxides (21–26). Based on the luminescent properties of electronically excited product, CL could be divided as direct CL, in which excited product directly exhibits CL, and indirect CL, such as chemically initiated electron-exchange luminescence (CIEEL), in which excited product first transfers the excitation energy to suitable luminescer, and then the electronically excited luminescer emits CL (21–23). If the CL production by TCBQ/H2O2 were through the CIEEL mechanism, the addition of fluorescent agents such as riboflavin or 9,10-diphenylanthracene (DPA) should have marked impact on CL production (21–23). However, no such effect was observed. These results indicate that CIEEL mechanism may not be responsible for the CL production by TCBQ/H2O2; in other words, the CL produced by TCBQ/H2O2 must be a direct or intrinsic one: Some light-emitting intermediates must be generated during the reaction of TCBQ with H2O2. The fact that several ring-opening products were formed during TCBQ/H2O2 reaction also suggests that the process of quinone ring opening might be responsible for generating excited light-emitting species and the corresponding energy required for CL emission.
A class of high-energy four membered-ring peroxides, 1,2-dioxetanes, has been proposed as the critical intermediate in many chemiluminescent reactions (21–24); 1,2-dioxetanes have received a great deal of attention because of their unique ability to decompose thermally into electronically excited carbonyl products. We propose that an unusual quinone 1,2-dioxetane intermediate might be produced during the reaction between TCBQ and H2O2. The following thermal decomposition of quinone 1,2-dioxetane might generate electronically excited carbonyl-containing compound as light emitter, which emits CL directly. If the above hypothesis were correct, the energy released during the decomposition of quinone 1,2-dioxetane should meet the energy requirement for light-emitter transiting from ground state to excited state. As anticipated, our preliminary thermochemical calculations supported the above hypothesis—the energy released from the decomposition of the proposed quinone 1,2-dioxetane could reach up to 80.2 kcal/mol (1 kcal = 4.18 kJ), which is sufficient to meet the demand of CL emission of TCBQ/H2O2.
To differentiate between singlet- and triplet-excited carbonyl species as the potential light-emitting intermediates, two energy acceptors (DPA and 9,10-dibromoanthracene sulfonate) and three different dyes (fluoresin, eosin, and rose bengal) were used. We found that the CL spectra were not changed by these energy acceptors and dyes, and the CL intensity was not enhanced, but rather inhibited, at high concentrations of them. These results suggest that singlet and triplet species could not be readily differentiated by this approach. Further investigations on this issue are needed for our future studies.
Molecular Mechanism of •OH-Dependent Two-Step CL Production by TCBQ and H2O2.
On the basis of all the above results and our previous findings (3, 14–18), a unique molecular mechanism was proposed for •OH-dependent two-step CL production by TCBQ/H2O2 (Scheme 1): A nucleophilic reaction may take place between TCBQ and H2O2, forming a quinone–hydroperoxide reaction intermediate TrCBQ–OOH, which could decompose homolytically to produce •OH and the oxygen-centered enoxy radical TrCBQ–O•. TrCBQ–O• then could either disproportionate to form the initial transient intermediate TrCBQ–OH or isomerize to form the carbon-centered ketoxy radical •TrCBQ = O, which could be attacked by •OH to form multicarbonyl compound I. The unstable multicarbonyl compound I may further react with H2O2 to form a quinone 1,2-dioxetane, which would further decompose to form the ring-opening excited light emitter [A]*. The first CL was emitted concurrently when electronically excited state of [A]* returned to ground state of [A]; the subsequent decomposition of [A] leads to the formation of final products DCMA and CO2 (and/or CO).
Scheme 1.
Proposed molecular mechanism for the first-step •OH-dependent CL production by TCBQ and H2O2.
The second-step CL could be produced by the transient intermediate TrCBQ–OH with excessive H2O2 through similar pathways, during which DDBQ and other ring-opening products (CMA, oxalic acid, CO2 and/or CO) would be formed (Scheme S1). However, the second CL exhibits the maximum emission at band 535–555 nm as compared to band 490–535 nm for the first CL. This might be because the light emitter [B]* has an electron-donating hydroxyl group, which caused the second CL to be red shifted to longer wavelength.
To our knowledge, this is a unique report that two-step CL could be produced by tetrahalogenated quinones with H2O2 and that the CL production is directly and specifically dependent on two-step •OH formation. These findings, together with the identification and quantitative analysis of the transient and final products (especially the new ring-opening products), have further confirmed and expanded our previously proposed molecular mechanism for metal-independent production of •OH by TCBQ/H2O2.
Why This CL-Producing System Is Unique.
Compared with the previously reported classic CL-producing systems (21–26), the TCBQ/H2O2 CL-producing system in this study showed the following unique characteristics: The two-step CL produced by TCBQ/H2O2 was directly and specifically dependent on the two-step intrinsic production of •OH, but not on other reactive oxygen species such as superoxide anion radical and singlet oxygen. It should also be noted that the CL produced by TCBQ/H2O2 could be observed under normal physiological pH, or even under weak acidic conditions, whereas alkaline or strong alkaline conditions are required for the classic luminol and lucigen systems. Also, no fluorescent agents are needed for CL production by TCBQ/H2O2, whereas fluorescent agents are frequently required for some classic CL-producing systems (such as peroxalates).
Potential Biological and Environmental Implications.
We found that when TCBQ was substituted with other polyhalogenated quinones, analogous •OH-dependent CL could also be observed (Figs. S2 and S4). Therefore, our findings may have interesting biological and environmental implications: Many widely used polyhalogenated aromatic compounds, which are considered persistent organic pollutants and probable human carcinogens—including polyhalogenated phenols (such as the ubiquitous wood preservative PCP and the brominated flame-retardant 3,3′,5,5′-tetrabromobisphenol A), Agent Orange, hexachlorobenzene, and polychlorinated biphenyls—can be metabolized in vivo (1–3, 27–29) or dehalogenated chemically and enzymatically (4–6) to their corresponding quinones. Recently, polychlorinated quinoid compounds were also identified as new chlorination disinfection byproducts in drinking water (7) and found in discharges from pulp and paper mills (3). These polyhalogenated quinones not only cause oxidative damage to DNA and other macromolecules, but also form protein and DNA adducts both in vitro and in vivo (1–7, 27–29), and therefore are potentially carcinogenic toward mammalian organisms. Therefore, it is important to detect and measure these halogenated quinones and/or their parent compounds both in the environment and in biological systems. It is well-known that CL-based methods are inherently sensitive because of the relative ease with which low light emission can be quantified by photon-counting techniques. The fact that CL could be produced by halogenated quinones/H2O2 may imply that we can use this unique CL-producing property to detect and measure halogenated quinones. Indeed, we found that as little as 10 nM TCBQ could be quantitatively detected by this new CL method, and the linear range for TCBQ is 0.01 to approximately 100 μM (SI Materials and Methods and Table S2).
Recently, H2O2 has been increasingly favored in the advanced oxidation processes (AOPs) as an environmentally safe oxidant for remediation of environmental pollutants, such as chlorinated phenols (3–6, 30, 31). In these “environmentally green” systems, H2O2 is often used at high concentrations. The most reactive radical intermediate formed during AOPs is •OH (29). Several previous studies showed that PCP could be oxidized and degraded by H2O2 in the presence of certain catalysts through the formation of tetrachloroquinoid intermediates (3–6, 31). This suggests that CL could also be produced during PCP degradation by AOPs such as the classic Fenton system, Fe(II)–EDTA/H2O2. We found that this is indeed the case. We took advantage of this finding and employed this highly sensitive CL method to measure quantitatively trace amounts of halogenated phenols such as PCP, triclosan, and 3,3′,5,5′-tetrabromobisphenol A (SI Materials and Methods, Fig. S7, and Table S3). We found that the detection limit of PCP in the PCP/Fe(II)–EDTA/H2O2 system is as low as 2.6 parts per billion (ppb), and the linear range for PCP is 8 to approximately 8,000 ppb. This is lower than the PCP concentration level of 40 ppb found in the body fluids of people who are not occupationally exposed to PCP, and much lower than the level of 19,580 ppb in occupationally exposed individuals (3). Because of the low PCP detection limit, this CL method might be used to measure PCP both in our environment and in biological systems.
Materials and Methods
CL Analysis.
The CL produced by halogenated quinones and H2O2 was measured by an ultraweak CL analyzer (Institute of Biophysics, Chinese Academy of Science) with a CR-120 red-sensitive photomultiplier tube (operated at the range of -900 to approximately  ; Hamamatsu); the CL analyzer was operated in pulse mode. The CL determination was carried out in a 3-mL glass cuvette and started by the injection of halogenated quinones. The CL signal was recorded by a computer equipped with a data-acquisition interface. Data acquisition and treatment were performed with BPCL software. The total intensity of CL was integrated during the whole process for CL measurements. The CL emission spectra were obtained using a set of interference filters with wavelengths from 400 to 640 nm, which were placed between the sample cuvette and the photomultiplier tube. During the determination of CL wavelength, appropriate corrections were applied for both spectral response of the photomultiplier tube and transmissivity of filters.
; Hamamatsu); the CL analyzer was operated in pulse mode. The CL determination was carried out in a 3-mL glass cuvette and started by the injection of halogenated quinones. The CL signal was recorded by a computer equipped with a data-acquisition interface. Data acquisition and treatment were performed with BPCL software. The total intensity of CL was integrated during the whole process for CL measurements. The CL emission spectra were obtained using a set of interference filters with wavelengths from 400 to 640 nm, which were placed between the sample cuvette and the photomultiplier tube. During the determination of CL wavelength, appropriate corrections were applied for both spectral response of the photomultiplier tube and transmissivity of filters.
The Kinetic Study of •OH Formation by Both ESR and Fluorescent Methods.
The time courses for •OH formation by TCBQ/H2O2 and TrCBQ–OH/H2O2 were monitored by both ESR with DMPO as the spin-trapping agent, and by fluorescence with 3-CCA as an •OH probe. The basic system consisted of 0.1 mM TCBQ (or TrCBQ–OH), 1 mM H2O2, 100 mM DMPO for ESR study, or 4 mM 3-CCA for fluorescent study, in 0.1 M Chelex-treated phosphate buffer (pH 7.4) at room temperature. ESR spectra were recorded either immediately after the interaction of TCBQ (or TrCBQ–OH) with H2O2, or at indicated time intervals on a Bruker ER 200 D-SRC spectrometer operating at 9.8 GHz. Fluorescence detection was preformed on a Cary Eclipse (Varian) spectrofluorometer. Samples with 3-CCA as the •OH probe were excited at 388 nm, and the resulting fluorescence was measured at 446 nm.
Supplementary Material
ACKNOWLEDGMENTS.
The authors acknowledge the technical help provided by Drs. Jin-Ming Lin, Li-Xia Zhao, and Frederick Villamena. This work was supported by Project 973 (2008CB418106); Hundred-Talent Project, Chinese Academy of Sciences; National Science Foundation China Outstanding Youth Award (20925724); National Science Foundation China Grants 20877081, 20890112, 20921063, 21207139, and 21237005; and National Institutes of Health Grants ES11497, RR01008, and ES00210.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204479109/-/DCSupplemental.
References
- 1.Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 2.Song Y, Wagner BA, Witmer JR, Lehmler HJ, Buettner GR. Nonenzymatic displacement of chlorine and formation of free radicals upon the reaction of glutathione with PCB quinones. Proc Natl Acad Sci USA. 2009;106:9725–9730. doi: 10.1073/pnas.0810352106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu BZ, Shan GQ. Potential mechanism for pentachlorophenol-induced carcinogenicity: A novel mechanism for metal-independent production of hydroxyl radicals. Chem Res Toxicol. 2009;22:969–977. doi: 10.1021/tx900030v. [DOI] [PubMed] [Google Scholar]
- 4.Meunier B. Catalytic degradation of chlorinated phenols. Science. 2002;296:270–271. doi: 10.1126/science.1070976. [DOI] [PubMed] [Google Scholar]
- 5.Gupta SS, et al. Rapid total destruction of chlorophenols by activated hydrogen peroxide. Science. 2002;296:326–328. doi: 10.1126/science.1069297. [DOI] [PubMed] [Google Scholar]
- 6.Sorokin A, Seris JL, Meunier B. Efficient oxidative dechlorination and aromatic ring-cleavage of chlorinated phenols catalyzed by iron sulfophthalocyanine. Science. 1995;268:1163–1166. doi: 10.1126/science.268.5214.1163. [DOI] [PubMed] [Google Scholar]
- 7.Zhao YL, Qin F, Boyd JM, Anichina J, Li XF. Characterization and determination of chloro- and bromo-benzoquinones as new chlorination disinfection byproducts in drinking water. Anal Chem. 2010;82:4599–4605. doi: 10.1021/ac100708u. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Oxford University Press; 2007. p. 45. [Google Scholar]
- 9.Wagner JR, Cadet J. Oxidation reactions of cytosine DNA components by hydroxyl radical and one-electron oxidants in aerated aqueous solutions. Acc Chem Res. 2010;43:564–571. doi: 10.1021/ar9002637. [DOI] [PubMed] [Google Scholar]
- 10.Xu G, Chance MR. Hydroxyl radical-mediated modification of proteins as probes for structural proteomics. Chem Rev. 2007;107:3514–3543. doi: 10.1021/cr0682047. [DOI] [PubMed] [Google Scholar]
- 11.Voinov MA, Pagan JOS, Morrison E, Smirnova TI, Smirnov AI. Surface-mediated production of hydroxyl radicals as a mechanism of iron oxide nanoparticle biotoxicity. J Am Chem Soc. 2011;133:35–41. doi: 10.1021/ja104683w. [DOI] [PubMed] [Google Scholar]
- 12.Montzka SA, et al. Small interannual variability of global atmospheric hydroxyl. Science. 2011;331:67–69. doi: 10.1126/science.1197640. [DOI] [PubMed] [Google Scholar]
- 13.Rohrer F, Berresheim H. Strong correlation between levels of tropospheric hydroxyl radicals and solar ultraviolet radiation. Nature. 2006;442:184–187. doi: 10.1038/nature04924. [DOI] [PubMed] [Google Scholar]
- 14.Zhu BZ, Zhao HT, Kalyanaraman B, Frei B. Metal-independent production of hydroxyl radicals by halogenated quinones and hydrogen peroxide: An ESR spin trapping study. Free Radical Biol Med. 2002;32:465–473. doi: 10.1016/s0891-5849(01)00824-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhu BZ, Kalyanaraman B, Jiang GB. Molecular mechanism for metal-independent production of hydroxyl radicals by hydrogen peroxide and halogenated quinones. Proc Natl Acad Sci USA. 2007;104:17575–17578. doi: 10.1073/pnas.0704030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu BZ, et al. Mechanism of metal-independent decomposition of organic hydroperoxides and formation of alkoxyl radicals by halogenated quinones. Proc Natl Acad Sci USA. 2007;104:3698–3702. doi: 10.1073/pnas.0605527104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu BZ, Shan GQ, Huang CH, Kalyanaraman B, Mao L, Du YG. Metal-independent decomposition of hydroperoxides by halogenated quinones: Detection and identification of a quinone ketoxy radical. Proc Natl Acad Sci USA. 2009;106:11466–11471. doi: 10.1073/pnas.0900065106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu BZ, Zhu JG, Mao L, Kalyanaraman B, Shan GQ. Detoxifying carcinogenic polyhalogenated quinones by hydroxamic acids via an unusual double Lossen rearrangement mechanism. Proc Natl Acad Sci USA. 2010;107:20686–20690. doi: 10.1073/pnas.1010950107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanakubo A, Isobe M. Isolation of brominated quinones showing chemiluminescence activity from luminous acorn worm, Ptychodera flava. Bioorg Med Chem. 2005;13:2741–2747. doi: 10.1016/j.bmc.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 20.Stevani CV, Silva SM, Badder WJ. Studies on the mechanism of the excitation step in peroxyoxalate chemiluminescence. Eur J Org Chem. 2000;24:4037–4046. [Google Scholar]
- 21.Schuster GB. Chemiluminescence of organic peroxides. Conversion of ground-state reactants to excited-state products by the chemically initiated electron-exchange luminescence mechanism. Acc Chem Res. 1979;12:366–373. [Google Scholar]
- 22.Matsumoto M. Advanced chemistry of dioxetane-based chemiluminescent substrates originating from bioluminescence. J Photochem Photobiol C. 2004;5:27–53. [Google Scholar]
- 23.Adam W, Kazakov DV, Kazakov VP. Singlet-oxygen chemiluminescence in peroxide reactions. Chem Rev. 2005;105:3371–3387. doi: 10.1021/cr0300035. [DOI] [PubMed] [Google Scholar]
- 24.Bos R, et al. In search of a chemiluminescence 1,4-dioxy biradical. J Am Chem Soc. 2009;131:2770–2771. doi: 10.1021/ja808401p. [DOI] [PubMed] [Google Scholar]
- 25.Saleh L, Plieth C. Total low-molecular-weight antioxidants as a summary parameter, quantified in biological samples by a chemiluminescence inhibition assay. Nat Protoc. 2010;5:1627–1634. doi: 10.1038/nprot.2010.120. [DOI] [PubMed] [Google Scholar]
- 26.Widder EA. Bioluminescence in the ocean: Origins of biological, chemical, and ecological diversity. Science. 2010;328:704–708. doi: 10.1126/science.1174269. [DOI] [PubMed] [Google Scholar]
- 27.Chignell CF, et al. EPR studies of in vivo radical production by 3,3′,5,5′-tetrabromobisphenol A (TBBPA) in the Sprague-Dawley rat. Toxicol Appl Pharmacol. 2008;230:17–22. doi: 10.1016/j.taap.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 28.Teuten EL, Us L, Reddy CM. Two abundant bioaccumulated halogenated compounds are natural products. Science. 2005;307:917–920. doi: 10.1126/science.1106882. [DOI] [PubMed] [Google Scholar]
- 29.Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FAPC. Food web-specific biomagnification of persistent organic pollutants. Science. 2007;317:236–239. doi: 10.1126/science.1138275. [DOI] [PubMed] [Google Scholar]
- 30.Pera-Titus M, García-Molina V, Baños MA, Giménez J, Esplugas S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl Catal B. 2004;47:219–256. [Google Scholar]
- 31.Zimbron JA, Reardon KF. Fenton’s oxidation of pentachlorophenol. Water Res. 2009;43:1831–1840. doi: 10.1016/j.watres.2009.01.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.