Abstract
Environmental stress often leads to an increased production of reactive oxygen species that are involved in plastid-to-nucleus retrograde signaling. Soon after the release of singlet oxygen (1O2) in chloroplasts of the flu mutant of Arabidopsis, reprogramming of nuclear gene expression reveals a rapid transfer of signals from the plastid to the nucleus. We have identified extraplastidic signaling constituents involved in 1O2-initiated plastid-to-nucleus signaling and nuclear gene activation after mutagenizing a flu line expressing the luciferase reporter gene under the control of the promoter of a 1O2-responsive AAA-ATPase gene (At3g28580) and isolating second-site mutations that lead to a constitutive up-regulation of the reporter gene or abrogate its 1O2-dependent up-regulation. One of these mutants, caa39, turned out to be a weak mutant allele of the Topoisomerase VI (Topo VI) A-subunit gene with a single amino acid substitution. Transcript profile analysis of flu and flu caa39 mutants revealed that Topo VI is necessary for the full activation of AAA-ATPase and a set of 1O2-responsive transcripts in response to 1O2. Topo VI binds to the promoter of the AAA-ATPase and other 1O2-responsive genes, and hence could directly regulate their expression. Under photoinhibitory stress conditions, which enhance the production of 1O2 and H2O2, Topo VI regulates 1O2-responsive and H2O2-responsive genes in a distinct manner. These results suggest that Topo VI acts as an integrator of multiple signals generated by reactive oxygen species formed in plants under adverse environmental conditions.
Keywords: oxidative stress, light stress, cell death
Plants are often exposed to environmental changes that adversely affect their growth and development and may ultimately result in the death of the plant. Most of these stress conditions disrupt the metabolic balance of cells and increase the production of reactive oxygen species (ROS) (1). ROS may be toxic and cause oxidative damage, or they may act as signaling molecules and activate the plant’s defenses against environmental stress (2, 3). The specificity of these responses is largely determined by the chemical identity of the ROS (4, 5). Research in the past has primarily been concerned with studying the biological activities of superoxide anion (O2•–) and hydrogen peroxide (H2O2) (6, 7), whereas the analysis of singlet oxygen (1O2) and hydroxyl radical (•OH) has been impeded by the lack of suitable experimental systems and detection techniques. Only recently, by using the conditional flu mutant of Arabidopsis thaliana, has 1O2 been shown to be involved in plastid-to-nucleus retrograde signaling. Immediately after the onset of 1O2 generation in plastids of Arabidopsis, changes in nuclear gene expression reveal a rapid transfer of 1O2-derived signals from the plastid to the nucleus (8–10). Several 1O2-responsive genes are different from those activated by O2•− or H2O2, suggesting that O2•−/H2O2- and 1O2-dependent signaling occurs via distinct pathways (8, 10, 11). Other consequences of increased 1O2 generation inside plastids include a drastic reduction in the growth rate of mature plants and the bleaching and death of seedlings (10). All these 1O2-mediated responses are genetically regulated by the two plastid proteins, EXECUTER1 and EXEXUTER2, required for the translocation of 1O2-derived signals from the plastid to the nucleus (12, 13).
1O2 signaling does not seem to operate via an isolated signaling pathway but rather as part of a complex signaling network that integrates various extra- and intracellular cues (14). We previously used a genetic approach to penetrate this complexity. A transgenic flu line expressing an 1O2-responsive reporter gene was mutagenized, and we isolated second-site mutations that either led to a constitutive up-regulation of the reporter gene or abrogated its 1O2-dependent up-regulation (14). The reporter gene consisted of the luciferase ORF and the promoter of the 1O2-responsive AAA-ATPase nuclear gene of Arabidopsis (8, 10). Here we report on the identification and characterization of mutant caa39, whose response to 1O2 is impaired. We found that caa39 is a weak mutant allele of the Topoisomerase VI (Topo VI) A-subunit (AtTOP6A) gene with a single amino acid substitution. Under photoinhibitory stress conditions, AtTOP6A is indispensable for the selective activation of several 1O2-responsive nuclear genes and at the same time may act as a repressor of H2O2-responsive genes. This dual activity assigns a key role to Topo VI as an integrator of multiple ROS signals that are released by plants in response to adverse environmental conditions.
Results
Isolation and Characterization of the caa39 Mutant.
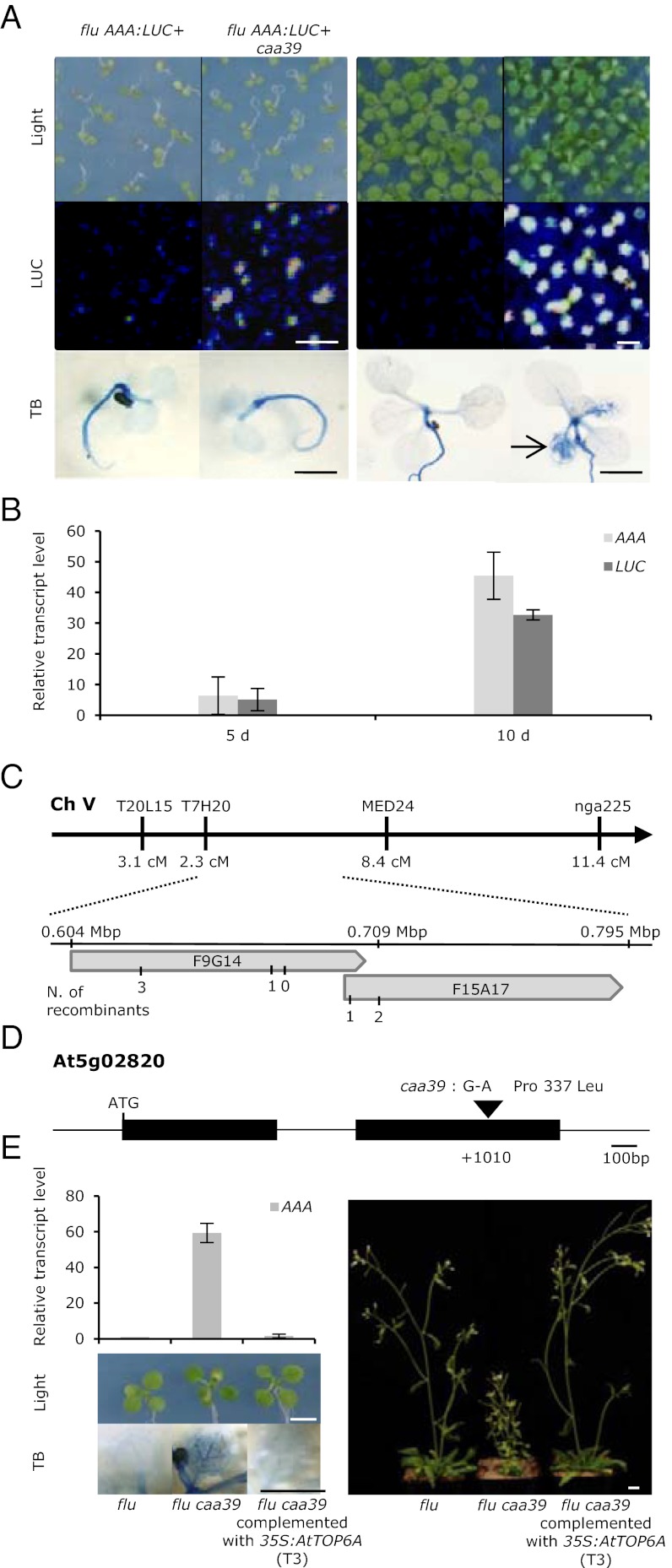
The Arabidopsis flu AAA:LUC+ line, which expresses the luciferase (LUC) reporter gene under the control of the 1O2-responsive AAA-ATPase promoter (14), was mutagenized and screened for second-site mutants that either constitutively up-regulate the 1O2-responsive reporter gene or have lost the ability to respond to 1O2. Six flu AAA:LUC+ caa mutants were isolated that in 10-d-old seedlings constitutively activate both the reporter gene and the endogenous AAA-ATPase gene, and hence carry trans-acting mutations (14). In caa mutants with mutations in genes that genetically form part of the 1O2-signaling pathway and that also contain the flu mutation, generation of 1O2 by a dark-to-light (D/L) shift should not further enhance the constitutive expression of 1O2-responsive genes. Based on this criterion, only one of the six caa mutants, caa39, could be directly linked to 1O2 signaling (14–16). Until 5 d old, caa39 mutant seedlings grown under continuous light were phenotypically similar to wild-type seedlings (Fig. 1A). Afterward they developed chlorotic spots and spontaneous cell death, and their growth slowed relative to wild-type (Fig. 1 A and E).
Fig. 1.
Identification of the CAA39 gene. (A) Constitutive luciferase activity and phenotype of the flu AAA:LUC+ caa39 and the flu AAA:LUC+ parental line. Seedlings were grown in continuous light on Murashige and Skoog agar plates for 5 d and 10 d. Bioluminescence (LUC), corresponding visible images (Light), and Trypan blue (TB) staining of cell death are presented. (Scale bars, 0.5 cm.) (B) Relative transcript levels of the AAA-ATPase gene (AAA) and the Luciferase reporter gene (LUC) in cotyledons of 5-d-old and 10-d-old seedlings of the flu AAA:LUC+ caa39 mutant and the flu AAA:LUC+ parental line. Transcript levels were determined by quantitative RT-PCR. Results represent mean values of two biological replicates ± SE. (C) Map-based cloning of CAA39. Initial mapping analysis revealed genetic linkage of CAA39 to markers located on top of chromosome V. Fine mapping localized the caa39 mutation in a 100-kb region covered by BAC clones F9G14 and F15A17. (D) A single G-to-A nucleotide substitution was found in the second exon of the At5g28020 locus in the caa39 mutant, resulting in a Pro-337 to Leu amino acid exchange. Filled boxes indicate exons, lines indicate transcribed regions. (E) Complementation of caa39. AAA-ATPase expression, morphological phenotype and Trypan blue staining of cell death in 10-d-old seedlings, (Left) and morphological phenotype of 35-d-old mature plants (Right) of flu, flu caa39, and flu caa39 complemented with the wild-type copy of the At5g28020 gene. (Scale bars, 0.5 cm.)
CAA39 Encodes the A-Subunit of Arabidopsis Topo VI.
The visible morphological alterations and constitutive expression of the AAA:LUC+ reporter gene cosegregated as a single recessive Mendelian trait when crossed with the parental flu AAA:LUC+ line (14). By map-based cloning, the caa39 mutation could be assigned to a fragment of ∼90 kb covered by the two BAC clones, F9G14 and F15A17 (Fig. 1C and Fig. S1). This region is predicted to contain 22 ORFs (http://www.tigr.org). By sequencing DNA in this region we identified a single G-to-A nucleotide substitution in locus At5g02820 of flu AAA:LUC+ caa39 relative to the parental and wild-type lines (Fig. 1D). At5g02820 encodes the Topo VI A-subunit AtTOP6A/AtSPO11-3/RHL2/BIN5, which is homologous to the eukaryotic SPO11 meiotic recombination endonuclease. The caa39 mutation results in the conversion of proline to leucine at position 337 within the topoisomerase-primase domain (Fig. 1D). Proline 337 is highly conserved in TOP6A/SPO11-3 homologs (Fig. S2). The identification of caa39 was confirmed by complementing the mutant with the complete ORF of AtTOP6A under the control of the cauliflower mosaic virus 35S promoter and by allelism tests. Complemented flu caa39 35S:AtTOP6A plants were phenotypically indistinguishable from the parental flu plants, and AAA-ATPase transcript levels returned to the low basal level found in light-grown flu (Fig. 1E). For allelism tests, caa39 was crossed with the allelic mutant rhl2-1 (17). F1 seedlings from this cross retained high LUC activity and displayed a phenotype intermediate between the parental lines (Fig. S3). Significantly, in rhl2-1 seedlings expression of the endogenous AAA-ATPase was also constitutively up-regulated to a similar high level as in caa39, despite a more severe morphological phenotype (Fig. 2).
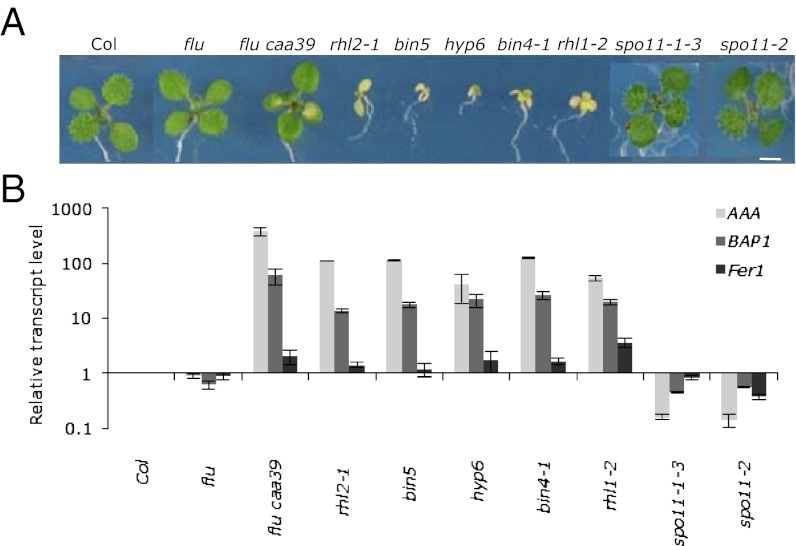
Fig. 2.
Phenotype of mutants with impaired Arabidopsis Topo VI subunits. (A) Visible phenotypes of 10-d-old seedlings grown under continuous light. (B) Relative transcript levels of endogenous AAA-ATPase (AAA), BAP1 and FER1 marker genes were analyzed in cotyledons of 10-d-old seedlings by quantitative RT-PCR and compared with Col. spo11-1–3 and spo11-2 were included as controls. Results represent mean values of two biological replicates ± SE. (Scale bars, 2 mm.)
Topo VI is an ATP-dependent type II topoisomerase (type IIB) enzyme found in archaea, plants, red algae, diatoms, and a few protists (18). Archaea Topo VI forms an A2B2 heterotetramer (19). In Arabidopsis the B subunit, which is closely related to the ATPase region of type IIA topoisomerases (20, 21), is encoded by a single gene, At3g20780 AtTOP6B/RHL3/BIN3/HYP6. In addition to the homologs of the archaeal Topo VI A and B subunits, Arabidopsis Topo VI also contains two small subunits, ROOT HAIRLESS 1 (RHL1) (22)/HYPOCOTYL 7 (HYP7) (23) and BRASSINOSTEROID-INSENSITIVE 4 (BIN4) (24)/MIDGET (MID) (25). If the regulatory role of AtTOP6A is linked to its function as a subunit of the Arabidopsis Topo VI complex, deletion of either subunit should have the same effect as in the caa39 mutant. Therefore, we analyzed mutants for each protein in the plant Topo VI complex and for two AtTOP6A homologs that are not associated with Topo VI but are required for meiotic recombination [AtSPO11-1 (spo11-1-3) and AtSPO11-2 (spo11-2)] (26). These mutants were grown for 10 d under the same continuous light conditions together with flu caa39, flu, and wild-type Col. spo11-1-3 and spo11-2 mutant plants were morphologically indistinguishable from wild-type, whereas mutants in subunits of the Topo VI complex were severely affected in their growth and showed chlorotic cotyledons (Fig. 2). Seedlings of caa39 displayed a much less severe phenotype. Inactivation of different subunits of the Topo VI complex resulted in very similar expression signatures (constitutive up-regulation of the 1O2-responsive genes AAA-ATPase and BAP1, whereas the H2O2-responsive marker gene FER1 was not or barely affected) despite the fact that caa39 growth phenotype is much less severe than in the AtTOP6A knock-out mutants or the other Topo VI mutants (Fig. 2). In contrast, impairment of the SPO11 meiotic recombination endonuclease function in spo11-1-3 and spo11-2 mutants resulted in very different expression profiles (Fig. 2). These results support the notion that AtTOP6A modulates 1O2-responsive gene expression while being part of the Topo VI complex.
AtTOP6A Regulates 1O2-Triggered Stress Responses.
To confirm that AtTOP6A represents a genuine 1O2 signaling component, we further analyzed the flu caa39 mutant under 1O2-producing conditions. As cotyledons of 10-d-old flu seedlings grown under continuous light are no longer able to accumulate significant amounts of the photosensitizer protochlorophyllide (Pchlide) in the dark and, hence, do not release 1O2 during reillumination, this analysis was performed in 5-d-old seedlings. The expression of LUC and the endogenous AAA-ATPase was only moderately enhanced in 5-d-old flu caa39 seedlings under steady-state conditions relative to flu (Fig. 1 A and B). Following a D/L shift, 1O2-induced accumulation of AAA-ATPase and BAP1 transcripts was strongly suppressed in flu caa39 seedlings (Fig. 3A), even though they accumulated similar excess amounts of Pchlide as the parental flu line (14); a full induction was restored in complemented flu caa39 35S:AtTOP6A plants (Fig. 3A). The impaired 1O2-induced accumulation of AAA-ATPase transcripts in 5-d-old flu caa39 seedlings after a D/L shift could not be attributed to saturated expression of AAA-ATPase before 1O2 production because: (i) 5-d-old flu caa39 seedlings only moderately accumulated AAA-ATPase transcripts compared with 10-d-old seedlings (Fig. 1B), and (ii) other flu caa mutants that constitutively accumulated similar or higher amounts of AAA-ATPase transcripts than flu caa39 were still able to further accumulate AAA-ATPase transcripts in response to 1O2 in 5-d-old seedlings (14–16).
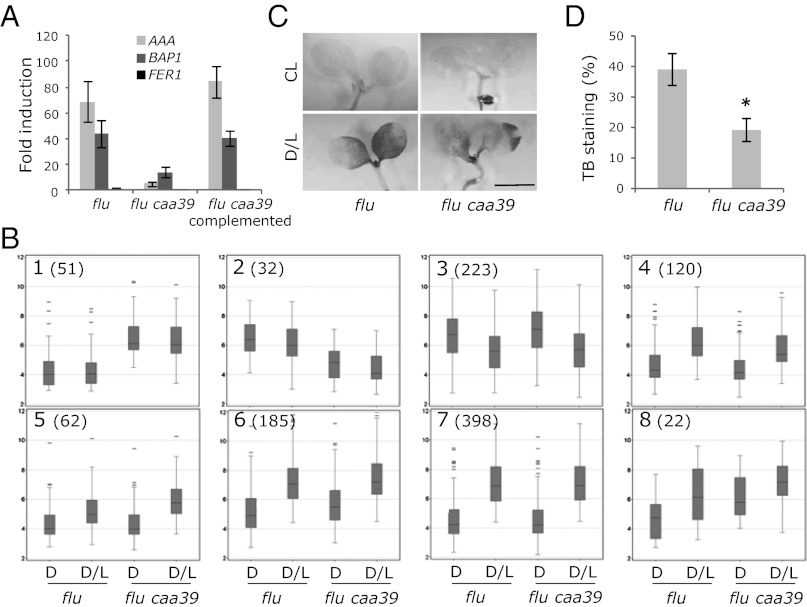
Fig. 3.
Response of the flu caa39 mutant to the release of singlet oxygen. (A) Fold-inductions of AAA-ATPase, BAP1, and FER1 in flu, flu caa39, and flu caa39 complemented with the wild-type copy of the AtTOP6A gene. The 5-d-old seedlings were subjected to 8 h dark and 30 min reillumination (D/L). Transcript levels were determined by quantitative RT-PCR. Results represent mean values of two biological replicates ± SE (B) Genome-wide identification of 1,093 genes differently regulated in flu or flu caa39 prior or after the release of 1O2. K-means analysis was used to define eight clusters identifying different expression profiles. (C) Trypan blue staining showing cell death in continuous light (CL) and after D/L shift in 5-d-old seedlings. Plants were subjected to 8-h dark. Trypan blue staining was done after 12 h of light exposure. (Scale bar, 2 mm.) (D) Quantification of cell death. Values are expressed as percentage of positive Trypan blue staining area relative to total area (n = 8; mean ± SE). *t test P < 0.01.
It is conceivable that Topo VI may also regulate the expression of other 1O2-responsive genes and affect phenotypic changes, such as cell death, that are triggered by the release of 1O2 in the flu mutant (10). Therefore, we tried to identify genes that were affected by the caa39 mutation before and after the release of 1O2. Genome-scale gene expression profiling was performed using Affymetrix Arabidopsis AGRONOMICS1 microarrays (http://www.agron-omics.eu/); 5-d-old flu and flu caa39 seedlings were grown under continuous light, transferred to darkness for 8 h, and reilluminated for 30 min to generate 1O2. Based on the expression data of biological triplicates and a selection criterion of 1.5-fold change, 1,093 genes were identified that exhibited reproducible activation or repression in caa39 or after a D/L shift in flu. The AAA-ATPase, the very low basal expression level of which exceeded the background level on the microarrays only in flu after reillumination, was not retained in this analysis. K-means clustering was performed to identify genes that may form coregulated clusters. Eight different gene clusters could be clearly distinguished (Fig. 3B). Clusters 1 and 2 principally comprised genes that are constitutively up-regulated and down-regulated, respectively, in caa39, but not affected by a D/L shift in flu. Cluster 1 is highly enriched in genes related to DNA repair/response to DNA damage stimulus, such as ATMND1, RAD51, BRCA1, XRI1, PARP2, ATRAD17, and TSO2 [cluster frequency 15.2%, background TAIR (The Arabidopsis Information Resource database, www.arabidopsis.org) frequency 0.6%, P value 2.31 e−06] (Dataset S1), indicating that although no cell death could be detected in 5-d-old flu caa39 seedlings under continuous light (Fig. 3C), the Topo VI mutation caa39 causes DNA damages, the accumulation of which will eventually lead to the appearance of cell death at later stages. Conversely, such a DNA damage response is not activated in the flu mutant following the release of 1O2. Cluster 4 comprised genes that are up-regulated after a D/L shift in flu and less induced in the flu caa39 mutant, but not affected by the caa39 mutation before the D/L shift (i.e., genes that are induced by 1O2 in a Topo VI-dependent manner) (Fig. 3B and Dataset S1). Conversely, genes in cluster 5 (Fig. 3B and Dataset S1) are further activated in flu caa39 following a D/L shift; interestingly, this cluster contains four two-component response regulator genes (cluster frequency 7.3%, background TAIR frequency 0.1%, P value 1.99 e−04). Clusters 6 and 8 comprised genes that are up-regulated in caa39 both before and after a D/L shift in flu. Finally, the main cluster (cluster 7) consists of 398 genes that are activated after a D/L shift and do not seem to be regulated by AtTOP6A (Fig. 3B and Dataset S1). Similarly, genes that are down-regulated after a D/L shift are not or barely affected by the caa39 mutation (cluster 3). Collectively, these expression data identify subfractions of 1O2-responsive genes that are controlled by Topo VI, either in a positive or negative manner. When we examined the phenotypic responses of flu AAA:LUC+ caa39 to 1O2 generated by a D/L shift, we found that the cell-death response was significantly reduced compared with the parental line (Fig. 3 C and D).
Contrasting Roles of AtTOP6A During Expression Changes of 1O2- and H2O2-Responsive Genes Under High Light Stress.
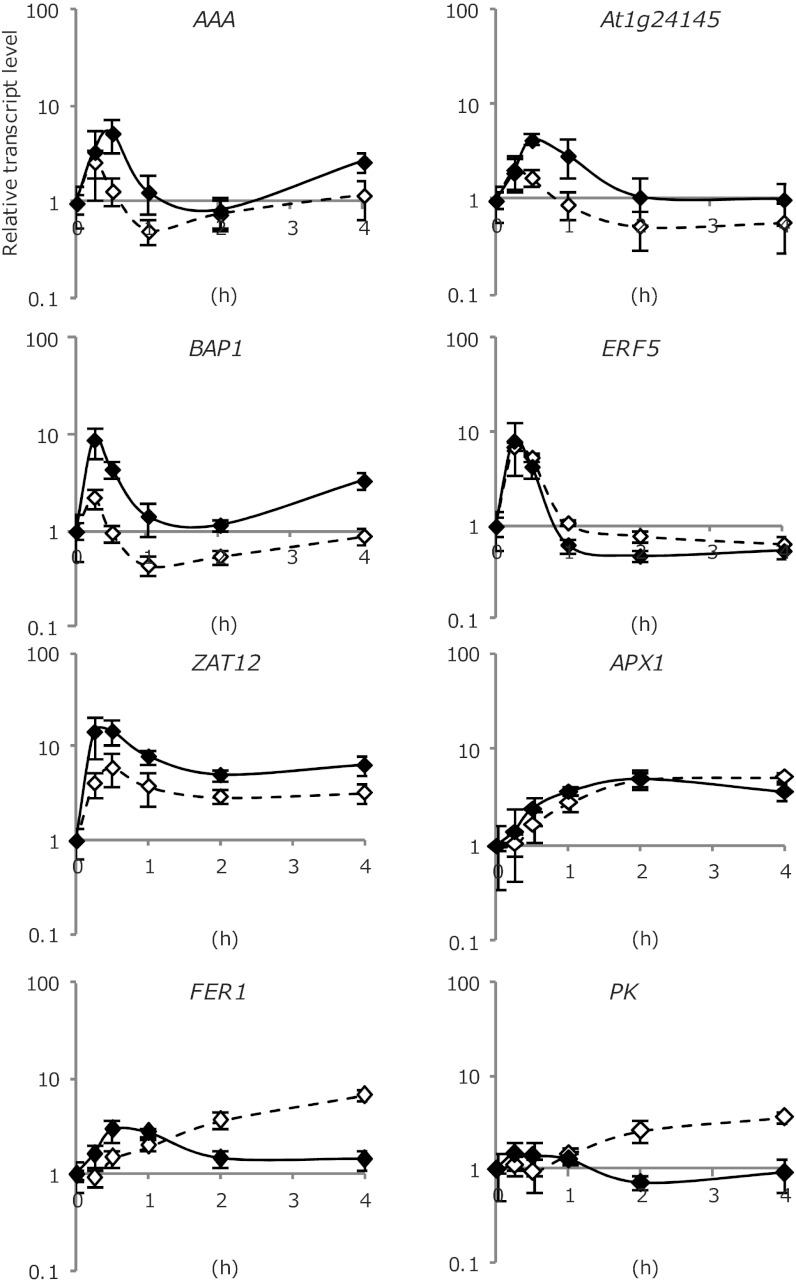
The physiological role of AtTOP6A was further assessed in wild-type and caa39 plants exposed to a high light treatment that causes photoinhibition and increases the production of 1O2 and H2O2 (27). We analyzed the expression of the AAA-ATPase and other 1O2-induced genes that are selectively activated by 1O2 from the three main clusters [i.e., At1g24145 from cluster 4, BAP1 from cluster 6 (10) and ERF5 from cluster 7 (28, 29)], as well as three H2O2-responsive genes [i.e., FER1, APX1, and At3g49160 (PK, pyruvate kinase-like)], and finally ZAT12, which was shown to be induced in several ROS-producing conditions (29). Seedlings were initially grown for 5 d under 80-μmol photons m−2⋅s−1 before transferring them to high light (2,000 μmol⋅m−2⋅s−1). In wild-type plants, transcripts of the 1O2-responsive genes rapidly and transiently accumulated first within 15–30 min after the start of high light treatment (Fig. 4), but the induction of H2O2-responsive genes was weaker and delayed. Expression of the general oxidative stress-response gene ZAT12 displayed an intermediate kinetic relative to that of the 1O2 and H2O2-responsive genes (Fig. 4). In caa39, induction of the 1O2-responsive genes was reduced (Fig. 4), except ERF5 that was also unaffected by the caa39 mutation in the flu mutant (Fig. 3B, cluster 7). Conversely, the caa39 mutation augmented the stress-induced activation of the H2O2-responsive genes FER1 and PK, but hardly affected APX1. Very similar induction patterns and effects of the caa39 mutation were observed in an experiment where the original flu AAA:LUC+ and flu AAA:LUC+ caa39 lines were grown for 6 d under continuous low light (when they reach a developmental stage similar to seedlings grown for 5 d under continuous normal light) before transferring them to continuous moderate high light (1,050 μmol⋅m−2⋅s−1) (Fig. S4). These results suggest that AtTOP6A can act as a positive regulator of 1O2-responsive genes and simultaneously suppress the expression of some H2O2-responsive genes under photoinhibitory stress conditions that generate both ROS.
Fig. 4.
Response of the wild-type Col and caa39 mutant to high-light stress conditions. Relative transcript levels of the 1O2-responsive AAA-ATPase, At1g24145, BAP1, ERF5, the H2O2-responsive FER1, PK, and APX1 genes, and the general ROS-responsive marker gene ZAT12 were analyzed in wild-type Col (closed symbols) and caa39 (open symbols) seedlings at the onset and 15 min, 30 min, 1 h, 2 h, and 4 h after the initiation of the high light (HL, 2,000 ± 50 μmol⋅m−2 s−1) treatment. Before HL stress, seedlings were grown for 5 d in normal light (NL, 80 ± 5 μmol⋅m−2⋅s−1) 16-h light/8-h dark photoperiod conditions. Relative transcript levels were determined by quantitative RT-PCR and expressed relative to the levels in NL. Results represent mean quantification cycle values of four biological replicates ± SE.
Topo VI Directly Binds to the Proximal Promoter Region of 1O2-Responsive Genes.
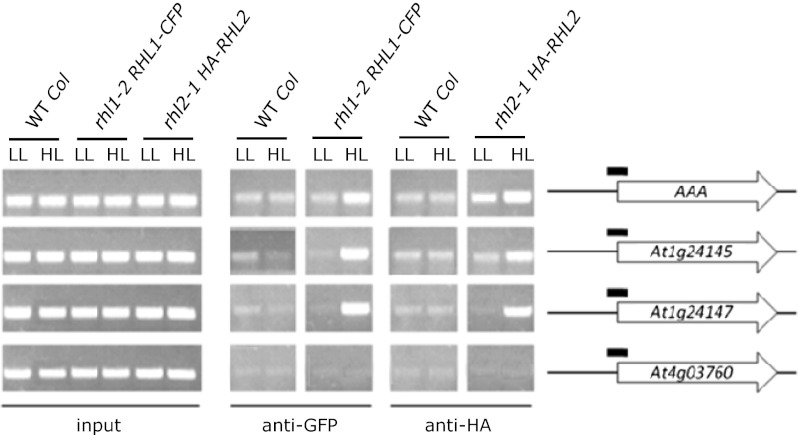
Topo VI could regulate gene expression either directly by binding to the promoter of a particular target gene or indirectly. Therefore, we used ChIP assays to examine Topo VI complex association with the promoter of the AAA-ATPase gene and two new loci identified in cluster 4, At1g24145 and At1g24147, the induction of which in the flu mutant following a D/L shift is very high and dramatically reduced in flu caa39 (Dataset S1), making them very suitable for such analysis. Arabidopsis transgenic lines that express tagged versions of the AtTOP6A/AtSPO11-3/RHL2/BIN5 (HA-RHL2) and RHL1/HYP7 (RHL1-CFP) Topo VI subunits (25) were crossed with mutant plants deficient for the corresponding genes (i.e., rhl2-1 and rhl1-2) to control functionality of the fusion proteins by phenotypic and molecular complementation. HA-RHL2 and RHL1-CFP restored a wild-type phenotype in rhl2-1 and rhl1-2, respectively, as well as a near wild-type level of transcripts (Fig. S5). ChIP-PCR experiments with the double homozygous lines rhl2-1 HA-RHL2 and rhl1-2 RHL1-CFP revealed that AAA-ATPase sequences were enriched in precipitated chromatin from rhl1-2 RHL1-CFP and, to a lesser extent, rhl2-1 HA-RHL2 (Fig. 5). Enrichment was even more pronounced for At1g24145 and At1g24147 sequences under high-light stress conditions that activate the genes (Fig. 5 and Fig. S6). In contrast, no enrichment was observed in the pseudogene At4g03760 that was used as a control. These results suggest that the plant Topo VI complex directly binds to the promoter/transcription initiation site of AAA-ATPase, At1g24145, and At1g24147 genes and, hence, appears to be directly involved in initiation and elongation of 1O2-responsive gene transcription.
Fig. 5.
Identification of Topo VI binding sites in vivo. ChIP assays were carried out using anti-GFP and anti-HA antibodies with wild-type (Col), rhl1-2 RHL1-CFP, and rhl2-1 HA-RHL2 plants that were grown for 6 d in low light (LL; 12 μmol⋅m−2⋅s−1) and then transferred to moderate high light (HL; 1,050 μmol⋅m−2⋅s−1) for 30 min. PCR analysis was carried out to probe RHL1 and RHL2 binding to the promoter region of the 1O2-responsive AAA-ATPase, At1g24145, and At1g24147 genes and the control locus At4g07360. Filled boxes indicate position and size of PCR-amplified fragments. The experiment was repeated three times with comparable results.
Discussion
Topo VI, A Genuine Component of 1O2 Retrograde Signaling.
The caa39 mutant was isolated in a genetic screen aimed at identifying factors involved in 1O2-specific retrograde signaling from the plastid to the nucleus and was shown in the present work to be impaired in the Topo VI A-subunit. Initially, six caa mutants were isolated, which constitutively activate the AAA-ATPase gene in the absence of enhanced 1O2 production (14). The characterization of these mutants suggested that 1O2-signaling does not operate as an isolated linear pathway but rather forms an integral part of a signaling network that is modified by other signaling routes and impacts not only on stress responses of plants, but also on their development. The work suggested further that most of the factors mutated in caa mutants repress the basal expression of the AAA-ATPase gene but are unlikely directly linked to 1O2-signaling. caa39 was the only caa mutant that showed an impaired 1O2-induced accumulation of AAA-ATPase transcripts and other 1O2-responsive genes, and hence AtTOP6A seems to form a genuine part of 1O2 signaling. The regulatory role of AtTOP6A depends on its function as a subunit of the plant Topo VI complex. As shown by our ChIP analysis, the Topo VI directly interacts with the upstream region of the AAA-ATPase, At1g24145, and At1g24147 genes under high-light stress conditions that activate the genes; therefore, we propose that Topo VI is directly involved in initiation and elongation of the transcription of these and other 1O2-responsive genes. Nevertheless, a fairly large proportion of nuclear genes activated in the flu mutant following a D/L shift are unaffected by the caa39 mutation. Because plastid-generated 1O2 is unlikely to leave the chloroplast in Arabidopsis (13), this observation supports that several 1O2-derived signals might be transferred from the plastid to the nucleus and modify the activity of Topo VI—or Topo VI-dependent nuclear factors—as well as of other still unknown nuclear factors that control the expression of Topo VI-independent 1O2-responsive genes. A candidate 1O2-derived signal could be β-cyclocitral, a carotenoid oxidation product that was recently found to accumulate under high light conditions that generate 1O2 (30). However, because β-cyclocitral–mediated signaling does not appear to require the EXECUTER1 function, it seems to be different from the EXECUTER1-dependent 1O2-signaling pathway operating in the flu mutant subjected to a D/L shift and wild-type plants exposed to mild light stress (31).
Topo VI, An Integrator of Different ROS Signals.
Our findings show that the plant Topo VI complex represses the expression of AAA-ATPase under nonstress conditions but can act as an activator of the AAA-ATPase and other genes in response to 1O2. At the same time, the identification of genes that were further activated in flu caa39 following a D/L shift supports a role of Topo VI in both activation and repression of 1O2-regulated genes. Furthermore, under ROS-producing high-light stress conditions, Topo VI can work simultaneously as an activator and a repressor of different ROS-responsive genes (i.e., 1O2-responsive vs. H2O2-responsive genes), suggesting that it might act as a molecular switch that relays the known antagonistic effect of H2O2 to 1O2-signaling (11). This dual activity assigns a key role to Topo VI as an integrator of different ROS signals that are released by plants in response to adverse environmental conditions. Other type II topoisomerases have also been shown to act as transcriptional repressors (32). The human topoisomerase II (hTopo II) represses RNA polymerase II transcription in vitro by binding to the promoter and blocking the formation of stable preinitiation complexes. Significantly, hTopo II-mediated repression can be relieved by the addition of sequence-specific transcriptional activators (32, 33). In addition, a dual role for human DNA topoisomerase I (hTopo I) as transcriptional repressor and activator has been reported (34). It is proposed that hTopoI is loaded onto the transcription complex to repress transcription by interacting with the transcription factor II D (TFIID) complex. In the presence of an activator, hTopo I is assumed to then be translocated from the TFIID complex to the elongation complex, thereby removing the superhelical tension caused by the elongation process and enhancing the efficiency of elongation (34). The dual activity of Arabidopsis Topo VI as a repressor and activator of 1O2-responsive gene expression could be explained by an analogous mechanism. This hypothesis is further supported by the recent finding that the Arabidopsis BIN4/MID subunit of Topo VI can interact in a yeast two-hybrid assay with the TFIIB, which is involved in RNA polymerase II recruitment and transcription initiation in eukaryotes (35, 36). Quite recently, Ju et al. provided in vivo molecular evidence that human Topo IIβ can generate a transient dsDNA strand break that permits a nucleosome-specific histone H1–HMGB protein exchange event, which promotes local changes of chromatin architecture and leads to the transcriptional activation of target genes (37). Topoisomerase-mediated chromatin factor exchange represents an attractive mechanism for the transcriptional activation and repression of different sets of genes. The mechanism of Arabidopsis Topo VI activation following the release of different ROS remains to be elucidated, as does the identification of other determinants that may control the selectivity of response to different ROS.
Topo VI, An Integrator of Environmental Cues.
Until now, Topo VI was primarily implicated in DNA endoreduplication, and the physiological significance of plant topoisomerases was largely unknown (17, 23–25). Interestingly, recent studies have shown that the constitutive expression of rice OsTOP6A or OsTOP6B increases the expression of stress-responsive genes and confers abiotic stress tolerance to transgenic Arabidopsis plants (38, 39). Our study shows that the plant Topo VI complex is a key regulatory factor during the activation of ROS-responsive genes that can eventually modulate the intensity of the 1O2-induced cell-death response. The Arabidopsis genome contains a second type II topoisomerase that is, however, differentially regulated (23, 40) and, based on our preliminary data, doesn’t seem to participate in ROS-responsive gene activation. Taken together, these findings suggest that Topo VI may have a specific function in regulating plant responses to adverse environmental conditions by reprogramming the expression of specific sets of ROS-responsive genes.
Materials and Methods
Plant Material and Growth Conditions.
All experiments were performed with A. thaliana ecotype Columbia (Col-0). Details of lines and growth conditions are given in SI Materials and Methods.
Luciferase Imaging, Trypan Blue Staining, and Quantification of Cell Death.
Luciferase imaging in plants was performed as previously described (14). Dead cells were identified by staining with lacto-phenol Trypan blue (41). Seedlings were photographed and the area of positive Trypan blue staining was quantified using ImageJ software (http://rsb.info.nih.gov/ij/).
ChIP.
ChIP was performed as previously described (42). Anti-GFP antibodies (11 814 460 001; Roche), anti-HA antibodies (11 867 423 001; Roche), antidimethyl-histone H3 Lys-9 antiserum (07-690; Upstate), and anti-IgG antibody (I5381; Sigma-Aldrich) were used for immunoprecipitation. Immunoprecipitated DNA was analyzed by PCR using AAA-ATPase, At1g24145, and At1g24147 gene-specific primers and primers against the control locus At4g03760 (Tables S1–S5).
Identification and complementation of the caa39 mutation, RNA isolation, quantitative RT-PCR, and microarray analysis are described in SI Materials and Methods. See Fig. S7 for validation of reference genes.
Supplementary Material
Acknowledgments
We thank André Imboden, Mena Nater, and Geoffrey Foucher for technical assistance, and Dr. Ben Field for critical reading of the manuscript. This study was supported by the Eidgenössiche Technische Hochschule Zurich; the Swiss National Science Foundation; the Functional Genomic Center Zürich; National Institutes of Health Grant R01-GM085036 (to K.A.); Swiss National Science Foundation Grant 31003A_13027 (to L.H.); and French National Research Agency Grant ANR 2010 JCJC 1205 01 (to C.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. F.V.B. is a guest editor invited by the Editorial Board.
Data deposition: The microarray data have been deposited in ArrayExpress, www.ebi.ac.uk/arrayexpress, (ID no. E-TABM-1076).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202041109/-/DCSupplemental.
References
- 1.Miller G, Shulaev V, Mittler R. Reactive oxygen signaling and abiotic stress. Physiol Plant. 2008;133:481–489. doi: 10.1111/j.1399-3054.2008.01090.x. [DOI] [PubMed] [Google Scholar]
- 2.Apel K, Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 3.Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays. 2006;28:1091–1101. doi: 10.1002/bies.20493. [DOI] [PubMed] [Google Scholar]
- 4.Laloi C, Apel K, Danon A. Reactive oxygen signalling: The latest news. Curr Opin Plant Biol. 2004;7:323–328. doi: 10.1016/j.pbi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Møller IM, Jensen PE, Hansson A. Oxidative modifications to cellular components in plants. Annu Rev Plant Biol. 2007;58:459–481. doi: 10.1146/annurev.arplant.58.032806.103946. [DOI] [PubMed] [Google Scholar]
- 6.Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant. 1997;11:1187–1194. [Google Scholar]
- 7.Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 8.Laloi C, Przybyla D, Apel K. A genetic approach towards elucidating the biological activity of different reactive oxygen species in Arabidopsis thaliana. J Exp Bot. 2006;57:1719–1724. doi: 10.1093/jxb/erj183. [DOI] [PubMed] [Google Scholar]
- 9.Meskauskiene R, et al. FLU: A negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2001;98:12826–12831. doi: 10.1073/pnas.221252798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.op den Camp RG, et al. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laloi C, et al. Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2007;104:672–677. doi: 10.1073/pnas.0609063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KP, Kim C, Landgraf F, Apel K. EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc Natl Acad Sci USA. 2007;104:10270–10275. doi: 10.1073/pnas.0702061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner D, et al. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science. 2004;306:1183–1185. doi: 10.1126/science.1103178. [DOI] [PubMed] [Google Scholar]
- 14.Baruah A, Simková K, Apel K, Laloi C. Arabidopsis mutants reveal multiple singlet oxygen signaling pathways involved in stress response and development. Plant Mol Biol. 2009;70:547–563. doi: 10.1007/s11103-009-9491-0. [DOI] [PubMed] [Google Scholar]
- 15.Baruah A, Simková K, Hincha DK, Apel K, Laloi C. Modulation of O-mediated retrograde signaling by the PLEIOTROPIC RESPONSE LOCUS 1 (PRL1) protein, a central integrator of stress and energy signaling. Plant J. 2009;60:22–32. doi: 10.1111/j.1365-313X.2009.03935.x. [DOI] [PubMed] [Google Scholar]
- 16.Šimková K, et al. The chloroplast division mutant caa33 of Arabidopsis thaliana reveals the crucial impact of chloroplast homeostasis on stress acclimation and retrograde plastid-to-nucleus signaling. Plant J. 2012;69:701–712. doi: 10.1111/j.1365-313X.2011.04825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugimoto-Shirasu K, Stacey NJ, Corsar J, Roberts K, McCann MC. DNA topoisomerase VI is essential for endoreduplication in Arabidopsis. Curr Biol. 2002;12:1782–1786. doi: 10.1016/s0960-9822(02)01198-3. [DOI] [PubMed] [Google Scholar]
- 18.Malik SB, Ramesh MA, Hulstrand AM, Logsdon JM., Jr Protist homologs of the meiotic Spo11 gene and topoisomerase VI reveal an evolutionary history of gene duplication and lineage-specific loss. Mol Biol Evol. 2007;24:2827–2841. doi: 10.1093/molbev/msm217. [DOI] [PubMed] [Google Scholar]
- 19.Bergerat A, Gadelle D, Forterre P. Purification of a DNA topoisomerase II from the hyperthermophilic archaeon Sulfolobus shibatae. A thermostable enzyme with both bacterial and eucaryal features. J Biol Chem. 1994;269:27663–27669. [PubMed] [Google Scholar]
- 20.Corbett KD, Benedetti P, Berger JM. Holoenzyme assembly and ATP-mediated conformational dynamics of topoisomerase VI. Nat Struct Mol Biol. 2007;14:611–619. doi: 10.1038/nsmb1264. [DOI] [PubMed] [Google Scholar]
- 21.Forterre P, Gribaldo S, Gadelle D, Serre MC. Origin and evolution of DNA topoisomerases. Biochimie. 2007;89:427–446. doi: 10.1016/j.biochi.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Schneider K, et al. The ROOT HAIRLESS 1 gene encodes a nuclear protein required for root hair initiation in Arabidopsis. Genes Dev. 1998;12:2013–2021. doi: 10.1101/gad.12.13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugimoto-Shirasu K, et al. RHL1 is an essential component of the plant DNA topoisomerase VI complex and is required for ploidy-dependent cell growth. Proc Natl Acad Sci USA. 2005;102:18736–18741. doi: 10.1073/pnas.0505883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breuer C, et al. BIN4, a novel component of the plant DNA topoisomerase VI complex, is required for endoreduplication in Arabidopsis. Plant Cell. 2007;19:3655–3668. doi: 10.1105/tpc.107.054833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirik V, Schrader A, Uhrig JF, Hulskamp M. MIDGET unravels functions of the Arabidopsis topoisomerase VI complex in DNA endoreduplication, chromatin condensation, and transcriptional silencing. Plant Cell. 2007;19:3100–3110. doi: 10.1105/tpc.107.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stacey NJ, et al. Arabidopsis SPO11-2 functions with SPO11-1 in meiotic recombination. Plant J. 2006;48:206–216. doi: 10.1111/j.1365-313X.2006.02867.x. [DOI] [PubMed] [Google Scholar]
- 27.Fryer MJ, Oxborough K, Mullineaux PM, Baker NR. Imaging of photo-oxidative stress responses in leaves. J Exp Bot. 2002;53:1249–1254. [PubMed] [Google Scholar]
- 28.Danon A, Miersch O, Felix G, Camp RG, Apel K. Concurrent activation of cell death-regulating signaling pathways by singlet oxygen in Arabidopsis thaliana. Plant J. 2005;41:68–80. doi: 10.1111/j.1365-313X.2004.02276.x. [DOI] [PubMed] [Google Scholar]
- 29.Gadjev I, et al. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 2006;141:436–445. doi: 10.1104/pp.106.078717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramel F, et al. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc Natl Acad Sci USA. 2012;109:5535–5540. doi: 10.1073/pnas.1115982109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim C, et al. Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell. 2012;24:3026–3039. doi: 10.1105/tpc.112.100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brou C, et al. Sequence-specific transactivators counteract topoisomerase II-mediated inhibition of in vitro transcription by RNA polymerases I and II. Nucleic Acids Res. 1993;21:4011–4018. doi: 10.1093/nar/21.17.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNamara S, Wang H, Hanna N, Miller WH., Jr Topoisomerase IIbeta negatively modulates retinoic acid receptor alpha function: A novel mechanism of retinoic acid resistance. Mol Cell Biol. 2008;28:2066–2077. doi: 10.1128/MCB.01576-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merino A, Madden KR, Lane WS, Champoux JJ, Reinberg D. DNA topoisomerase I is involved in both repression and activation of transcription. Nature. 1993;365:227–232. doi: 10.1038/365227a0. [DOI] [PubMed] [Google Scholar]
- 35.Evans-Roberts KM, Breuer C, Wall MK, Sugimoto-Shirasu K, Maxwell A. Arabidopsis thaliana GYRB3 does not encode a DNA gyrase subunit. PLoS ONE. 2010;5:e9899. doi: 10.1371/journal.pone.0009899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szklarczyk D, et al. The STRING database in 2011: Functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011;39(Database issue):D561–D568. doi: 10.1093/nar/gkq973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ju BG, et al. A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science. 2006;312:1798–1802. doi: 10.1126/science.1127196. [DOI] [PubMed] [Google Scholar]
- 38.Jain M, Tyagi AK, Khurana JP. Overexpression of putative topoisomerase 6 genes from rice confers stress tolerance in transgenic Arabidopsis plants. FEBS J. 2006;273:5245–5260. doi: 10.1111/j.1742-4658.2006.05518.x. [DOI] [PubMed] [Google Scholar]
- 39.Jain M, Tyagi AK, Khurana JP. Constitutive expression of a meiotic recombination protein gene homolog, OsTOP6A1, from rice confers abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Rep. 2008;27:767–778. doi: 10.1007/s00299-007-0491-8. [DOI] [PubMed] [Google Scholar]
- 40.Hruz T, et al. Genevestigator V3: A reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics. 2008 doi: 10.1155/2008/420747. 2008:Article ID 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keogh RC, Deverall BJ, Mcleod S. Comparison of histological and physiological responses to Phakopsora pachyrhizi in resistant and susceptible soybean. Trans Br Mycol Soc. 1980;74:329–333. [Google Scholar]
- 42.Bowler C, et al. Chromatin techniques for plant cells. Plant J. 2004;39:776–789. doi: 10.1111/j.1365-313X.2004.02169.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.