Abstract
The only evidence-based behavioral treatment for anxiety and stress-related disorders involves desensitization techniques that rely on principles of extinction learning. However, 40% of patients do not respond to this treatment. Efforts have focused on individual differences in treatment response, but have not examined when, during development, such treatments may be most effective. We examined fear-extinction learning across development in mice and humans. Parallel behavioral studies revealed attenuated extinction learning during adolescence. Probing neural circuitry in mice revealed altered synaptic plasticity of prefrontal cortical regions implicated in suppression of fear responses across development. The results suggest a lack of synaptic plasticity in the prefrontal regions, during adolescence, is associated with blunted regulation of fear extinction. These findings provide insight into optimizing treatment outcomes for when, during development, exposure therapies may be most effective.
Fear learning is a highly adaptive, evolutionarily conserved process that allows one to respond appropriately to cues associated with danger. In the case of psychiatric disorders, however, fear may persist long after an environmental threat has passed. This unremitting and often debilitating form of fear is a core component of many anxiety disorders, including posttraumatic stress disorder (PTSD), and involves exaggerated and inappropriate fear responses. Existing treatments, such as exposure therapy, are based on principles of fear extinction, during which cues previously associated with threat are presented in the absence of the initial aversive event until cues are considered safe and fear responses are reduced. Extinction-based exposure therapies have the strongest empirical evidence for benefitting adult patients suffering from PTSD (1), yet a comparative lack of knowledge about the development of fear neural circuitry prohibits similarly successful treatment outcomes in children and adolescents (2). Adolescence, in particular, is a developmental stage when the incidence of anxiety disorders peaks in humans (3–6), and it is estimated that over 75% of adults with fear-related disorders met diagnostic criteria as children and adolescents (7, 8). Because of insufficient or inaccurate diagnoses and a dearth of pediatric and adolescent specialized treatments, fewer than one in five children or adolescents are expected to receive treatment for their anxiety disorders (9), leaving a vast number with inadequate or no treatment (2, 10). The increased frequency of anxiety disorders in pediatric and adolescent populations highlights the importance of understanding neural mechanisms of fear regulation from a developmental perspective, as existing therapies directly rely upon principles of fear-extinction learning. Converging evidence from human and rodent studies suggests that insufficient top-down regulation of subcortical structures (11–14), such as the amygdala, may coincide with diminished prototypical extinction learning (15), as well as ongoing fine-tuning of excitatory–inhibitory balance in the prefrontal cortex that may coincide with diminished prototypical extinction learning (16). Because top-down prefrontal regulation has been postulated to mediate extinction learning and may determine the efficacy of exposure therapy often used as part of cognitive behavioral therapy, it is important to discern how changes in the development of prefrontal circuitry influences fear extinction. Studying the development of fear learning and memory in humans, while examining, in parallel, the underlying neural mechanisms in rodent models, may offer insights into optimizing treatment strategies for developing populations by clarifying when, during development, a particular intervention or treatment may be more or less effective.
Results
Behavioral Results.
Human fear-extinction learning.
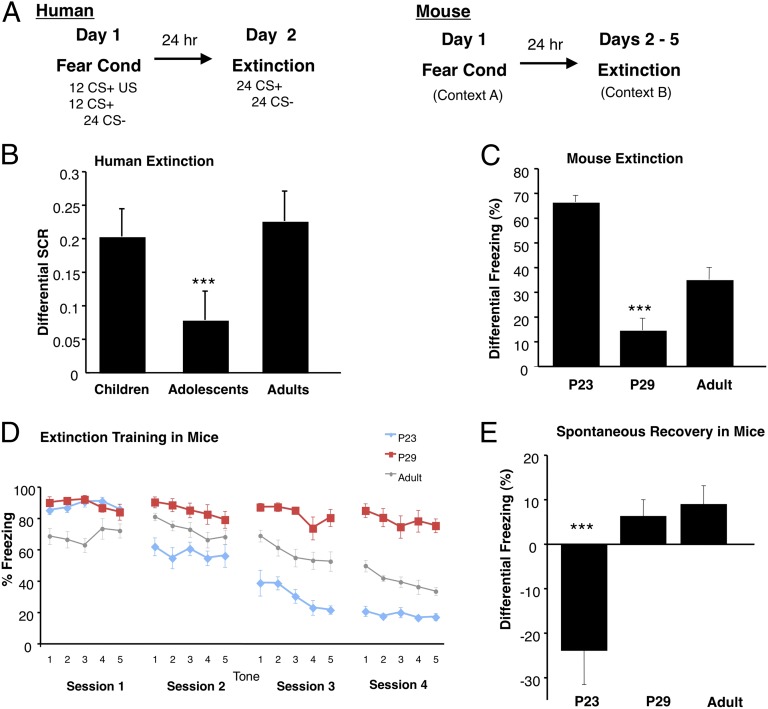
Because immature functional connectivity between the ventromedial prefrontal cortex (vmPFC) and amygdala in adolescents has previously been shown in tasks of emotion regulation (13), we initially sought to investigate age-dependent differences in fear-extinction learning in humans. Using age delineations for children, adolescents, and adults, we assessed skin conductance response across development in humans to measure prototypical physiological fear responses during conditioned fear acquisition and fear memory extinction (17–21) (Fig. 1A). A two-way ANOVA on skin conductance response during late acquisition (the last of three acquisition runs) with main factors of age group (children, adolescents, adults) and stimulus type [paired conditioned stimulus (CS+) or unpaired (CS−) with an aversive noise] showed a main effect of stimulus type (CS+ > CS−) [F(1, 79) = 10.786, P = 0.002] and no Group × Stimulus type interaction [F(2, 79) = 0.032, P = 0.968] (Fig. S1A), demonstrating that all subjects learned to discriminate between the threat cue and the safety cue. Furthermore, there was no main effect of age group on responses to either stimulus type [CS+: F(2,79) = 0.581, P = 0.562; CS−: F(2, 79) = 0.655, P = 0.522] or the differential acquisition measure [CS+ − CS−: F(2, 79) = 0.021, P = 0.979] during late acquisition. Thus, any subsequent group effects in extinction learning are not related to differences in fear acquisition. In contrast, analysis of extinction indices revealed a main effect of age group for humans [F(2, 80) = 3.228, P = 0.038], such that adolescents showed attenuated fear-extinction learning compared with children [t(56) = 2.34, P = 0.023] and adults [t(51) = 1.802, P = 0.078] (Fig. 1B). This effect of age group on fear extinction was present when sex and trait anxiety are entered as covariates [F(2, 73) = 3.086, P = 0.052] (see SI Materials and Methods). There was no significant difference in extinction learning between children and adults (P = 0.701).
Fig. 1.
Cued extinction learning and spontaneous recovery across development in mice and humans. (A) Behavioral paradigms for parallel fear conditioning experiments in humans and mice. (B) Analysis of extinction indices [(averaged first two extinction trials) − (averaged last two extinction trials)] reveals a main effect of age group for humans, such that adolescents display attenuated fear extinction learning compared with children and adults, (adolescent 0.05916 ± 0.06904; children 0.25435 ± 0.04839; adults 0. 22510 ± 0.05931). (C) A lack of extinction learning and retention of extinction memory in is observed in adolescent mice, as displayed by a significantly decreased differential extinction indices [(Day 1, Tone 1) − (Day 4, Tone 5)] compared with older and younger ages, (P23 66.5% ± 2.75; P29 14.72% ± 4.79; P70 35.17% ± 4.89). (D) Adolescent mice display attenuated extinction learning over the course of four days compared with preadolescent and older adult mice. (E) Preadolescent mice demonstrate a lack of spontaneous recovery [(Day 2, Tone 1) − (Day 1, Tone 5)] compared with older ages. All results are presented as a mean ± SEM ***P < 0.001. See also Table S1 and Fig. S1.
Mouse extinction learning.
To examine the mechanistic basis of this altered fear-extinction learning, we performed parallel studies in mice across comparable postnatal ages. Using classic Pavlovian-based fear conditioning, fear-learning studies were conducted in preadolescent postnatal day (P)23, adolescent (P29), and adult (P70) mice to assess developmental differences in conditioned fear acquisition and extinction learning at ages comparable to human children, adolescents, and adults (22, 23). Freezing behavior was assessed in mice as the prototypical species-specific fear response. First, consistent with previous reports delineating erasure of conditioned fear memories in very young mice because of immature perineuronal net framework in the lateral amygdala (24), we found that preadolescent mice exhibit rapid reductions in freezing behavior and a lack of spontaneous recovery of fear responses between sessions, demonstrating persistent attenuation of fear memories with multiple extinction trials [F(2, 21) = 11.563, P < 0.001] (Fig. 1 D and E). However, preadolescent fear memory does not degrade solely with the passage of time but requires extinction sessions to weaken the memory trace (Fig. S1C). Second, adolescent mice, like the human subjects, display significantly attenuated fear-extinction learning compared with their preadolescent and adult counterparts (Fig. 1 C and D). Analysis of extinction indices revealed a main effect of age for mice [F(2, 22) = 25.426, P < 0.001], demonstrating attenuated extinction learning in adolescent mice. These findings are consistent with previous rodent studies that show adolescent rats require twice as many extinction trials as adults, a pharmacological manipulation such as D-cycloserine, or CS presentations of prolonged duration to achieve comparable reductions in conditioned fear behavior (23, 25, 26). This lack of extinction in adolescent mice was not a result of higher baseline activity levels or heightened auditory sensitivity (Fig. S1B).
Physiological Correlates.
Having demonstrated attenuated adolescent extinction learning in both humans and rodents, we sought to perform detailed immunohistochemical and electrophysiological experiments across development in mice to examine potential underlying synaptic changes in the neural circuitry implicated in fear learning. Strong cross-species preservation of the neural circuitry implicated in fear-extinction learning is supported by human and nonhuman animal extinction studies, further bolstering the translational credibility of rodent experiments to explore mechanistic underpinnings that are otherwise precluded from experiments with human subjects (18, 27).
Immunohistochemical results.
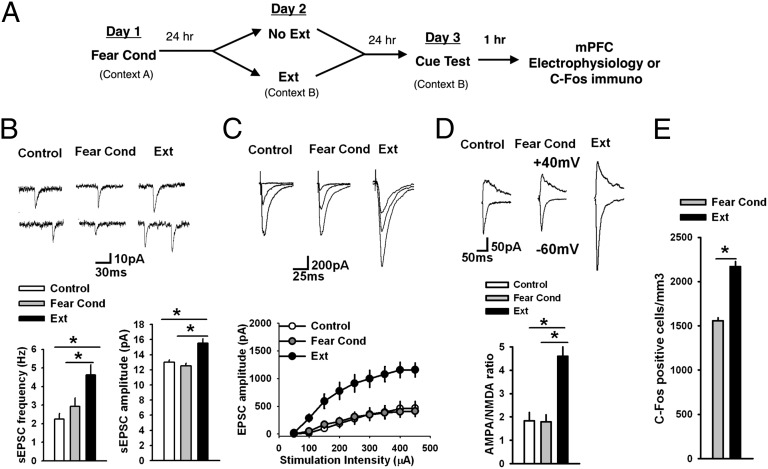
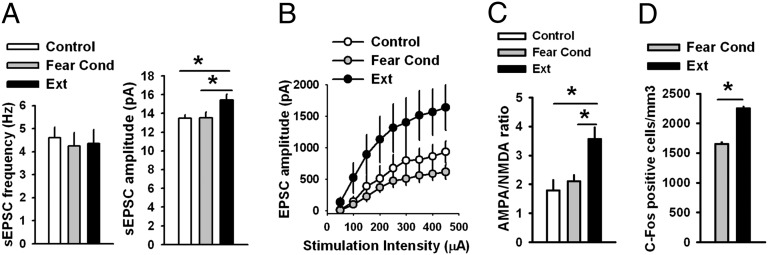
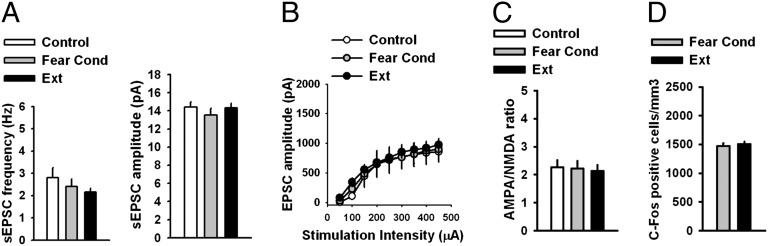
Given the critical role of the vmPFC in fear-extinction learning and retention of extinction memory, we hypothesized that there would be alterations in vmPFC synaptic plasticity across development. We focused on two subregions of the vmPFC, the dorsally located prelimbic cortex (PL), which is associated with production of conditioned-fear responses and expression of conditioned-fear behaviors (28), and the more ventrally located infralimbic cortex (IL), which is associated with suppression of conditioned-fear responses typically seen during successful extinction learning and upon recall of extinction memory (29–32). To investigate neuronal activity levels in each of these regions, we used immunohistochemical techniques to measure c-Fos protein levels in vmPFC of P23, P29, and adult mice after fear-extinction learning. Downstream of the immediate-early gene c-fos, c-Fos activity has been shown to be associated with successful fear-extinction learning in the IL of adult rodents (33). Consistent with previous studies, density of c-Fos–labeled cells in the IL of fear-extinguished mice was significantly higher than nonextinguished, fear-conditioned controls in both P23 and adult mice (Fig. 2E; see also Fig. 4D), whereas there was no change in density of c-Fos labeling in adolescent mice (Fig. 3D), suggesting that neural activity in the vmPFC of adolescent mice differs from the prototypical adult neural activity observed during fear extinction. These immunohistochemical results suggesting enhanced activity in IL of P23 and adult mice, compared with adolescents, parallel the lack of extinction learning in adolescent mice (as shown in Fig. 1 C and D). In addition to increased c-Fos in the IL of P23 and adult mice, decreased c-Fos expression was observed in the PL of P23 (Fig. S8D) and adult (Fig. S10D) fear-extinguished mice compared with age-matched fear-conditioned mice, with no change in c-Fos expression pattern for either group during adolescence (Fig. S9D) (see Fig. S3 for corresponding behavioral data and Figs. S4–S6 for representative images).
Fig. 2.
Fear extinction modifies glutamatergic synaptic transmission in IL L5 pyramidal neurons of P23 mice. (A) Paradigm design. (B) Frequency and amplitude of sEPSCs in IL L5 pyramidal neurons from control (n = 17 neurons, 6 mice), fear-conditioned (Fear Cond, n = 17 neurons, 6 mice), and fear-extinguished P23 mice (Ext, n = 16 neurons, 6 mice). Both the frequency and amplitude of sEPSCs were significantly increased in fear extinguished mice. (Upper) Examples of sEPSCs. (C) EPSC amplitude in IL L5 pyramidal neurons from control (n = 10 neurons, 5 mice), fear-conditioned (n = 9 neurons, 5 mice), and fear-extinguished P23 mice (n = 11 neurons, 5 mice). EPSC amplitude was significantly increased in fear extinguished mice compared with control group. (Upper) Examples of EPSCs evoked by 100-, 200-, and 300-μA stimulation of L2/3. (D) AMPA/NMDA ratio in IL L5 pyramidal neurons from control (n = 13 neurons, 5 mice), fear-conditioned (n = 8 neurons, 5 mice), and fear-extinguished P23 mice (n = 11 neurons, 5 mice). AMPA/NMDA ratio was significantly increased in fear extinguished mice. (Upper) Examples of EPSCs evoked at −60 and +40 mV by 150-μA stimulation of L2/3. (E) Histograms showing the average density of c-Fos–labeled nuclei in the IL across behavioral treatment group reveal increased density of c-Fos expression in the IL of P23 fear-extinguished mice compared with fear-conditioned mice (P23 Fear Cond 1,557 ± 34.15; P23 Ext 2,169 ± 56.06), Student t test, *P < 0.01. See also Figs. S2–S10.
Fig. 4.
Fear extinction modifies glutamatergic synaptic transmission in IL L5 pyramidal neurons of adult mice. (A) Frequency and amplitude of sEPSCs in IL L5 pyramidal neurons from control (n = 14 neurons, 7 mice), fear-conditioned (n = 17 neurons, 8 mice), and fear-extinguished adult mice (n = 15 neurons, 8 mice). The amplitude of sEPSCs was significantly increased in fear-extinguished mice. (B) EPSC amplitude in IL L5 pyramidal neurons from control (n = 8 neurons, 5 mice), fear-conditioned (n = 10 neurons, 5 mice), and fear-extinguished adult mice (n = 9 neurons, 5 mice). EPSC amplitude was significantly increased in fear-extinguished mice. (C) AMPA/NMDA ratio in IL L5 pyramidal neurons from control (n = 9 neurons, 5 mice), fear-conditioned (n = 12 neurons, 5 mice), and fear-extinguished adult mice (n = 13 neurons, 5 mice). AMPA/NMDA ratio was significantly increased in fear-extinguished mice. (D) Histograms showing the average density of c-Fos–labeled nuclei in the IL across behavioral treatment group reveal increased density of c-Fos expression in the IL of adult fear-extinguished mice compared with fear-conditioned mice (Adult Fear Cond 1,650 ± 29.08; Adult Ext 2,257 ± 26.13), Student t test, *P < 0.01. See also Fig. S2–S10.
Fig. 3.
Absence of modification of glutamatergic synaptic transmission in IL L5 pyramidal neurons of P29 mice after fear acquisition and extinction trials. (A) Frequency and amplitude of sEPSCs in IL L5 pyramidal neurons from control (n = 13 neurons, 8 mice), fear-condition (n = 15 neurons, 8 mice), and fear-extinction groups (n = 15 neurons, 8 mice). (B) EPSC amplitude in IL L5 pyramidal neurons from control (n = 9 neurons, 5 mice), fear-condition (n = 14 neurons, 7 mice), and fear-extinction groups (n = 13 neurons, 7 mice). (C) AMPA/NMDA ratio in IL L5 pyramidal neurons from control (n = 11 neurons, 4 mice), fear-condition (n = 9 neurons, 3 mice), and fear-extinction groups (n = 10 neurons, 3 mice). Fear acquisition and extinction trials did not affect sEPSCs, EPSCs, and AMPA/NMDA ratio in IL L5 pyramidal neurons of P29 mice. (D) Histograms showing the average density of c-Fos–labeled nuclei in the IL across the behavioral treatment group reveal no change in density of c-Fos expression in the IL of P29 fear-extinguished mice compared with fear-conditioned mice (P29 Fear Cond 1,468 ± 51.25; P29 Ext 1,506 ± 40.49). See also Figs. S2–S10.
Electrophysiological results.
To probe developmental influences on fear-associated synaptic plasticity within the PL and IL, we performed electrophysiological recordings in vmPFC brain slices of mice after both fear acquisition and fear extinction. An earlier study showed that fear conditioning involved a decrease in intrinsic excitability of IL neurons, whereas fear extinction reversed this decrease in excitability (34). However, the specific synaptic mechanisms in the vmPFC that are involved in fear conditioning or extinction have not been explored. Therefore, we measured spontaneous excitatory postsynaptic currents (sEPSCs), evoked excitatory postsynaptic currents (EPSCs), and AMPA/NMDA ratio in both the IL and PL layer 5 (L5) pyramidal neurons after fear acquisition and extinction (see Fig. S2 for corresponding behavioral data and Fig. S7 for representative images of electrode placement). Although we did not observe any modification of the sEPSCs, EPSCs, or AMPA/NMDA ratio in the IL L5 pyramidal neurons from fear-conditioned P23 mice (Fig. 2 B–D), fear-extinguished P23 mice exhibited a significant increase in frequency [F(2, 47) = 6.9, P < 0.01] and amplitude [F(2, 47) = 14, P < 0.001] of sEPSCs, EPSC amplitude [F(2, 27) = 11, P < 0.001], and AMPA/NMDA ratio [F(2, 29) = 7.8, P < 0.01] in the IL L5 pyramidal neurons (Fig. 2 B–D), suggesting that fear extinction involves an enhancement of glutamatergic synaptic transmission in the IL. An increase in AMPA/NMDA ratio [F(2, 29) = 7.8, P < 0.01] suggests that the enhanced excitatory synaptic transmission is primarily mediated by AMPA receptors (Fig. 2D). Unlike the IL neurons, the PL L5 pyramidal neurons from fear-conditioned P23 mice exhibited a significant increase in sEPSC amplitude [F(2, 46) = 6.9, P < 0.01], EPSC amplitude [F(2, 29) = 5.8, P < 0.01], and AMPA/NMDA ratio [F(2, 27) = 3.4, P < 0.05] compared with the control group (Fig. S8 A–C). The fear-extinguished P23 mice showed a significant decrease in sEPSC amplitude, EPSC amplitude, and AMPA/NMDA ratio compared with the fear-conditioned group, suggesting a depotentiation of glutamatergic synaptic transmission after fear extinction (Fig. S8 A–C). Although the PL L5 pyramidal neurons from fear-conditioned P23 mice showed an increase in sEPSC frequency, which was depotentiated in extinguished mice, these effects did not reach statistical significance (Fig. S8A). In contrast to the synaptic plasticity in the IL and PL of P23 mice after fear acquisition and extinction, neither fear conditioning nor extinction trials affected sEPSCs, EPSCs, or AMPA/NMDA ratio in P29 mice (Fig. 3 A–C and Fig. S9 A–C). The lack of modification of glutamatergic synapses in the vmPFC of P29 mice is consistent with the absence of fear extinction during this narrow developmental window. Next, we examined glutamatergic synaptic transmission in the IL and PL L5 pyramidal neurons of adult mice after fear acquisition and extinction. Similar to P23 mice, we did not observe any modification of sEPSCs, EPSCs, or AMPA/NMDA ratio in the adult IL L5 pyramidal neurons after fear conditioning (Fig. 4 A–C). However, we observed an increase in sEPSC amplitude [F(2, 43) = 3.8, P < 0.05], EPSC amplitude [F(2, 24) = 3.7, P < 0.05], and AMPA/NMDA ratio [F(2, 31) = 8.2, P < 0.01] without any effect on sEPSC frequency in fear-extinguished adult mice (Fig. 4 A–C). Unlike the IL neurons, we observed a significant increase in frequency [F(2, 50) = 4.9, P < 0.05] and amplitude [F(2, 50) = 12.7, P < 0.001] of sEPSCs, EPSC amplitude [F(2, 21) = 5.4, P < 0.05], and AMPA/NMDA ratio [F(2, 31) = 4.8, P < 0.05] in the PL L5 pyramidal neurons from the fear-conditioned adult mice, and these potentiating effects were reversed in fear-extinguished mice (Fig. S10 A–C). These results suggest that fear extinction involves an enhancement of glutamatergic synaptic transmission in the IL L5 pyramidal neurons in P23 and adult mice, but not adolescent mice. In addition, fear acquisition involves an enhancement of glutamatergic synaptic transmission at the PL glutamatergic synapses in preadolescent (P23) and adult mice but not in adolescent (P29) mice. The potentiated glutamatergic synapses in the PL undergo depotentiation during fear extinction. Although the basal sEPSC amplitude and EPSC amplitude showed a tendency to increase in P29 mice compared with P23 and adult mice, only sEPSC amplitude in the PL L5 pyramidal neurons reached statistical significance [F(2, 48) = 5, P < 0.05]. Although the mechanism is unclear, this enhanced basal excitatory synaptic transmission might contribute to a lack of plasticity in the P29 mice. Furthermore, the IL sEPSC frequency in the adult mice was significantly higher compared with both the P23 and P29 mice, suggesting a protracted development of spontaneous glutamate release mechanism in the IL [F(2, 41) = 9.3, P < 0.001] (Figs. 2B, 3A, and 4A). In summary, these results suggest that synaptic plasticity in the vmPFC is involved in fear regulation, and the lack of synaptic plasticity in the vmPFC may play a role in altered fear extinction in adolescent mice.
Discussion
Adolescence is a highly conserved developmental stage, both neurobehaviorally and physiologically, during which all mammals must meet evolutionary pressures associated with sexual emergence and transition from dependence on parents to independence (35). Fear learning plays a critical adaptive role in this process as the adolescent leaves the relatively protected and stable family environment and explores a novel and highly variable outside environment. Conservation of the behavioral demands associated with adolescence provides face and construct validity for translational studies of fear learning in this age group. Defining the unique attributes of fear acquisition and extinction during adolescence may have wide clinical implications, as the most common and validated treatment for anxiety disorders involves exposure-based therapy, which relies heavily on extinction principles for reevaluating existing contingencies (1). Although the ontogeny of conditioned fear expression and extinction has primarily been focused on infant and juvenile models (36–39), rodent models have recently started to incorporate older, more intermediate, adolescent ages (23, 25, 40, 41). Previous studies have shown that a temporary suppression of hippocampal-dependent contextual fear memory occurs during adolescence (17), as acquisition and expression of amygdala-dependent cue fear memory remain intact, highlighting a developmental dissociation between contextual and cue fear learning. Developmental differences in fear learning have been previously shown in very young rats and mice. During very early development in preweanling rodents, (<P21), odor-shock conditioning can be modulated by maternal presence (37), and mechanisms of cued extinction learning differ from adult-like extinction via alterations in NMDA receptor requirement (42) and mPFC activity (43). Furthermore, in early development (<P24), auditory cued extinction learning appears to be permanent, resulting in little spontaneous recovery and leading to persistent attenuation of a fragile memory trace, suggestive of memory degradation and permanent memory erasure (24, 44, 45).
Collectively, our studies have demonstrated attenuated extinction learning, at a defined developmental stage, in both mice and humans. Through performing parallel human and mouse studies examining fear acquisition and extinction, we have uncovered similar developmental patterns in fear-extinction learning, lending credibility to the use of a developmental mouse-model system for examining human adolescent fear and anxiety. After confirming that similar developmental patterns in fear-extinction behavior exist for both mice and humans, we were able exploit the mouse model system to probe underlying physiological mechanisms responsible for the attenuated fear-extinction learning observed in adolescence. Earlier studies have shown changes in intrinsic properties of the vmPFC neurons after fear acquisition and extinction (31, 34, 46). However, the specific involvement of the vmPFC excitatory synapses in fear learning or extinction was unclear. Our findings showing potentiation of the PL excitatory synapses after fear acquisition in P23 and adult mice, and their subsequent depotentiation upon extinction, suggest that the PL excitatory synapses dynamically regulate fear expression. More importantly, the simultaneous potentiation of the IL excitatory synapses in adult fear-extinguished mice provides an additional mechanism by which vmPFC excitatory synapses mediate extinction. The PL projects to the basolateral amygdala and might exert excitatory effects on the central amygdala to enhance fear (47, 48), but on depotentiation, the PL L5 pyramidal neurons might reverse this fear-enhancing effect. In addition, the enhanced glutamatergic IL output during fear extinction might facilitate the intercalated cell-mediated feed-forward inhibition of the central amygdala, resulting in decreased fear response (49–53). However, these synaptic plasticity changes in the PL and IL observed in P23 and adult mice are absent in adolescent mice, suggesting that the vmPFC is not similarly engaged in the regulation of learned fear at this age. Given the delayed development of cortical GABAergic transmission, it is plausible that an imbalance in inhibitory synaptic transmission during adolescence interferes with synaptic plasticity in the mPFC (54, 55).
These experiments identify unique synaptic properties in the vmPFC that underlie developmentally regulated differences in fear extinction. During adolescence, there is altered vmPFC synaptic activity and decreased fear-extinction behavior compared with younger and older ages, which may provide insights into the efficacy of treatments for anxiety disorders that rely on extinction mechanisms during this developmental period. In particular, these data suggest that treatment response to exposure-based cognitive behavioral therapy would vary nonlinearly across age, with the poorest response in adolescents, highlighting the importance of optimizing treatment strategies based on age.
Materials and Methods
Human Participants.
Before participating in the study, subjects were screened for exclusion criteria, which included hearing impairment and neurological and psychiatric disorders. All subjects gave written informed consent approved by the Weill Cornell Medical College Institutional Review Board. In addition to the consent given by their legal guardian, minor subjects gave a written assent. All participants were compensated for their participation. Age, sex, and number of subjects are further defined in SI Materials and Methods. Experimental design and behavioral paradigms are further defined in the SI Materials and Methods and as shown previously (18, 56).
Animals.
Male, C57BL6/J mice were used for all experiments. To eliminate potential developmentally sensitive, shipping-induced stress effects, breeding pairs of C57BL6/J wild-type mice from Charles River were set up in the colony and monitored daily. Litters were weaned at P21 and males from various litters were randomly combined to eliminate any litter-driven effects on behavior. Mice were housed five per cage in a temperature and humidity controlled vivarium maintained on a 12-h light/dark cycle. Mice had ad libitum access to food and water. Separate cohorts of mice (aged P23–P70) were used for all fear-conditioning, retrieval, and object-placement tasks. All procedures regarding animal care and treatment were in compliance with guidelines established by Weill Cornell Medical College’s Institutional Animal Care and Use Committee and the National Institutes of Health.
Rodent Fear Conditioning.
The fear-conditioning apparatus consisted of a mouse shock-chamber (Coulbourn Instruments) placed in a sound-attenuated box. After a 2-min acclimation period to the conditioning chamber [scented with 0.1% peppermint in 70% (vol/vol) EtOH], mice were fear-conditioned with three tone-shock pairings, consisting of a 30-s presentation of a (5 kHz, 70 dB) tone (CS) that coterminated with a 0.7-mA foot shock (unconditioned stimulus, US) during the last 1.0 s of the tone with an intertrial interval (ITI) of 30 s. After the final tone-shock pairing, mice remained in the conditioning chamber for 1 min before being returned to their home cages. Twenty-four hours after fear conditioning, the extinction procedure began in which mice were exposed to five presentations of the CS in the absence of the US. To eliminate any confounding interactions of contextual fear, tones were presented in a novel context, consisting of a green cylindrical arena [scented with 0.1% lemon in 70% (vol/vol) EtOH]. Tone presentations lasted for 30 s with an ITI of 30 s. After the final tone presentation, mice remained in the conditioning chamber for 1 min before being returned to their home cages. Fear-extinction trials were repeated daily for a total of 4 d of extinction training. Experiments were controlled by a computer using Graphic State software. Mice were videotaped for subsequent analysis by raters blind to behavioral groups. Freezing was defined as the absence of visible movement except that required for respiration (56). The freezing during the initial acclimation period was measured and used as an assay for unconditioned effects on general locomotor activity. Percent time spent freezing was calculated by dividing the amount of time spent freezing during the 30-s tone presentations by the duration of the tone. Extinction trials were binned into early and late trials, with the early trials representing the average of the trials on day 1 of extinction (24 h postconditioning), and late trials representing the average of the trials on day 4 of extinction (96 h postconditioning).
Electrophysiology.
Mice were anesthetized by pentobarbital, perfused intracardially for 2 min with ice-cold artificial cerebrospinal fluid (ACSF) containing: NaCl (118 mM), KCl (2.5 mM), CaCl2 (1 mM), MgSO4 (2 mM), NaH2PO4 (1 mM), and d-glucose (10 mM), pH adjusted to 7.4 with NaHCO3, osmolarity adjusted to 325 mOsm, and aerated by 95% O2/5% CO2. Mice brains were removed and 300-μM slices were prepared using a vibratome (Campden Instruments). Brain slices were kept submerged in a brain slice keeper (Scientific Systems Design) at room temperature for at least 1 h. For the recording of EPSCs, brain slices were placed in a recording chamber continuously perfused with ACSF containing NaCl (118 mM), KCl (2.5 mM), CaCl2 (2 mM), MgSO4 (2 mM), NaH2PO4 (1 mM), and d-glucose (10 mM), pH adjusted to 7.4 with NaHCO3, osmolarity adjusted to 325 mOsm and bubbled with 95% O2/5% CO2 (vol/vol), at 2 mL/min. Recording temperature was maintained at 32 °C using a TC324B in-line solution heater (Warner Instruments). Using video-enhanced infrared differential interference contrast microscopy (Hamamatsu C5405), with an Olympus BX50WI upright microscope fitted with a 40× long working distance water-immersion objective, the PL and IL L5 pyramidal neurons were identified and the stimulating electrodes were placed at the L2/3. sEPSCs and EPSCs were recorded at −60 mV in the presence of GABAA receptor blocker bicuculline (10 μM) using patch electrodes (2-4 MΩ) filled with an intracellular pipette solution consisted of: CsCl (145 mM), Hepes (10 mM), EGTA (0.5 mM), QX-314 (5 mM), GTP (0.2 mM), and MgATP (5 mM), with osmolarity adjusted to 290 mOsm with sucrose, and pH adjusted to 7.4 with CsOH. The AMPA/NMDA ratio was calculated by dividing the peak EPSC at −60 mV by the NMDA receptor current measured at 50 ms after the peak at +40 mV. EPSCs and sEPSCs were analyzed by pClamp 10 (Molecular Devices) and Mini Analysis (Synaptosoft). Recordings were rejected when holding current or series resistance changed by 10% or more.
Supplementary Material
Acknowledgments
This work was supported by the Sackler Institute (B.J.C.); the DeWitt-Wallace Fund of the New York Community Trust (F.S.L.); the Irma T. Hirschl/Monique Weill-Caulier Trust (F.S.L.); the International Mental Health Research Organization (F.S.L.); the Burroughs Wellcome Foundation (F.S.L.); the Pritzker Consortium (F.S.L. and C.E.G.); National Institutes of Health Grants HD055177 (to B.J.C. and S.S.P.), MH079513 (to B.J.C. and F.S.L.), NS052819 (to F.S.L.); and Swiss National Science Foundation Grant PBGEP3-123643 (to S.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206834109/-/DCSupplemental.
References
- 1.Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- 2.Liberman LC, Lipp OV, Spence SH, March S. Evidence for retarded extinction of aversive learning in anxious children. Behav Res Ther. 2006;44:1491–1502. doi: 10.1016/j.brat.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Monk CS, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merikangas KR, et al. Service utilization for lifetime mental disorders in U.S. adolescents: Results of the National Comorbidity Survey-Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2011;50:32–45. doi: 10.1016/j.jaac.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman DL, et al. Psychiatric disorder in a birth cohort of young adults: Prevalence, comorbidity, clinical significance, and new case incidence from ages 11 to 21. J Consult Clin Psychol. 1996;64:552–562. [PubMed] [Google Scholar]
- 7.Pollack MH, et al. Relationship of childhood anxiety to adult panic disorder: Correlates and influence on course. Am J Psychiatry. 1996;153:376–381. doi: 10.1176/ajp.153.3.376. [DOI] [PubMed] [Google Scholar]
- 8.Kim-Cohen J, et al. Prior juvenile diagnoses in adults with mental disorder: Developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry. 2003;60:709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- 9.Merikangas KR, et al. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller MB, et al. Chronic course of anxiety disorders in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1992;31:595–599. doi: 10.1097/00004583-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Lévesque J, et al. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 12.Galvan A, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hare TA, et al. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey BJ, et al. The storm and stress of adolescence: Insights from human imaging and mouse genetics. Dev Psychobiol. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 17.Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc Natl Acad Sci USA. 2011;108:1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soliman F, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 20.Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- 21.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 22.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 23.McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: Effects of D-cycloserine. Neuropsychopharmacology. 2010;35:2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gogolla N, Caroni P, Lüthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Li S, Richardson R. Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cereb Cortex. 2011;21:530–538. doi: 10.1093/cercor/bhq116. [DOI] [PubMed] [Google Scholar]
- 26.Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- 27.Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- 28.Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 30.Hefner K, et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spear LP. Adolescent brain development and animal models. Ann N Y Acad Sci. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- 36.Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci. 1993;107:887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- 37.Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JH, Richardson R. The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: Unlearning as a potential mechanism for extinction early in development. J Neurosci. 2008;28:1282–1290. doi: 10.1523/JNEUROSCI.4736-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: Implications for consolidation, infantile amnesia, and hippocampal system function. Behav Neurosci. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- 40.Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen H, et al. A critical role for alpha4betadelta GABAA receptors in shaping learning deficits at puberty in mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langton JM, Kim JH, Nicholas J, Richardson R. The effect of the NMDA receptor antagonist MK-801 on the acquisition and extinction of learned fear in the developing rat. Learn Mem. 2007;14:665–668. doi: 10.1101/lm.692407. [DOI] [PubMed] [Google Scholar]
- 43.Kim JH, Hamlin AS, Richardson R. Fear extinction across development: The involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JH, Richardson R. Extinction in preweanling rats does not involve NMDA receptors. Neurobiol Learn Mem. 2010;94:176–182. doi: 10.1016/j.nlm.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Kim JH, Richardson R. A developmental dissociation in reinstatement of an extinguished fear response in rats. Neurobiol Learn Mem. 2007;88:48–57. doi: 10.1016/j.nlm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 47.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 48.Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 49.Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 50.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 51.Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 54.Chattopadhyaya B, et al. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kilb W. Development of the GABAergic system from birth to adolescence. Neuroscientist. 2011 doi: 10.1177/1073858411422114. 10.1177/1073858411422114. [DOI] [PubMed] [Google Scholar]
- 56.Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.