Living microorganisms were first discovered in sediment cores from scientific ocean drilling in the late 1980s when microbiologists observed and counted DNA-stained microbial cells under the microscope. During the following decade, new observations were made from drill sites in the Atlantic and Pacific Oceans and the Mediterranean Sea (1, 2). The combined data on cell numbers from these sites showed a systematic, log-log linear decrease with depth, from approximately 109 cells⋅cm−3 near the sediment surface to 106 cells⋅cm−3 at several hundred meters below seafloor, where the sediment had been deposited many million years ago (2) (Fig. 1). In their 1998 study, Whitman et al. (3) used these data to make a grand estimate of the total number of prokaryotic cells on Earth. Their astonishing conclusion was that 55% of all microbial cells occurred in the deep seabed whereas most of the rest were found in the deep terrestrial subsurface. Even more staggering, the combined deep biospheres appeared to harbor one third of the total living biomass on Earth. The publication of Whitman et al. (3) sparked a rapidly growing interest in the deep biosphere, and the data from this study were repeatedly cited in the literature in the following years. Their biomass estimate was later supported by data on intact polar membrane lipids as indicators of living cells (4). Now, these numbers are being challenged. In PNAS, Kallmeyer et al. (5) conclude that the total cell number for the deep subseafloor biosphere is probably 12-fold lower than the earlier estimate whereas the total biomass may be 74-fold lower. Is the deep biosphere losing significance?
Fig. 1.
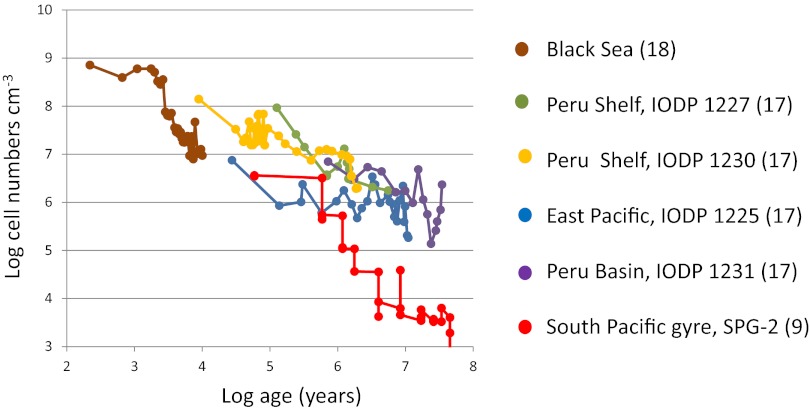
Microbial cells numbers of marine sediments at different subseafloor depths with different sediment ages ranging from 100 y to 100 million years. Data from six sites are plotted as log10 of the cell numbers per cm3 vs. log10 of the sediment age in years. The data illustrate the general decrease in cell numbers with age of the buried organic matter that provides the energy for the deep biosphere. The central North and South Pacific have extremely low sediment burial rates and cell numbers (red symbols), thereby changing the earlier estimates of the deep biosphere to lower cell numbers. IODP, Integrated Ocean Drilling Program.
The low estimates by Kallmeyer et al. (5) do not result from a correction of earlier cell counts. They result from new data from the vast desert regions of the oceans that have been drilled and sampled by microbiologists for the first time (Fig. 1). These regions were not specifically targeted in the earlier Ocean Drilling Program (and the later Integrated Ocean Drilling Program), as the program primarily aimed to explore plate tectonics, paleoceanography, and other dynamic processes that were most strongly expressed along the ocean margins. Microbiologists who joined these geologically motivated drilling expeditions therefore obtained their samples from ocean margin regions with high organic productivity and high sedimentation rates. As a result, the early database was skewed toward sediments with high cell counts.
In stark contrast to the ocean margins, the central gyres of the South and North Pacific have the greatest distance from the continents, the lowest phytoplankton productivity, and the lowest sedimentation rate of anywhere in the world ocean. The red clay covering the basement rock in these regions has extremely low organic carbon content (<0.5% of dry weight), and oxygen penetrates from the seawater and deeply into the seabed, even down to the basaltic crust tens of meters below the seafloor (6, 7). Microbial cells are so few that they cannot be detected by direct microscopy in these sediments. A new method was therefore developed by which the bacteria are first extracted from the sediment, concentrated by density centrifugation to a particle suspension, and then counted (8). With this 100-fold more sensitive procedure, cells turned out indeed to be present, yet in numbers that were 1,000-fold lower than in most other sediments (5, 9). By inclusion of such desert regions in the global compilation, the estimated total cell numbers decreased markedly.
The entire data set on cell numbers is still limited, and a global extrapolation remains difficult and highly dependent on the statistical approach used. Kallmeyer et al. (5) had data available from five times as many sites as Whitmann et al. (3), but they rejected 40% of these as a result of “noisy or erratic cell concentration trends.” By omitting such data, the depth distribution of cell numbers below 0.1 m and down to basement could be simulated for each site by a power-law function and the total depth-integrated cell numbers could be calculated. Interestingly, the cell numbers were inversely correlated to the distance from the continents, which explains why the early estimates based on ocean margin sites were too high. The statistical trends observed in the data were used to calculate areal cell densities in a 1° × 1° gridded map of the world ocean and thereby to estimate a new global number: 2.9 × 1029 cells in the marine deep biosphere (5). Given the great scatter of cell counts and the great diversity in sedimentary environments, such a number still suffers a degree of uncertainty, but it is certainly based on stronger data and more advanced statistical methods than earlier estimates. The effect of rejecting erratic data or of extrapolating to regions for which no data are available is difficult to evaluate for the reader, but the authors carefully explain their approach. A similar statistical analysis is needed for the terrestrial deep biosphere, but the available data are here still insufficient to detect general correlations to environmental properties.
It is unexpected that microbial cells of less than 1 μm size may constitute a large fraction of all living biomass on Earth, including all vascular plants on land and all algae in the sea. The estimate of their biomass, however, depends on the mean cell size and carbon content of the cells. Whereas Whitman et al. (3) assumed a mean carbon content of 86 fg C per cell (1 fg is equal to 10−15 g), Kallmeyer et al. (5) suggest a sixfold lower value of 14 fg C based on microscopic measurements of cells from Integrated Ocean Drilling Program core samples. Their rationale is that microbial cell sizes tend to be smaller under strong nutrient limitation because a larger surface-to-volume ratio of small cells is of competitive advantage because of more efficient substrate uptake and thereby faster growth. The deep biosphere indeed has very low nutrient and energy flux, and the microorganisms have exceedingly slow turnover with generation times of 100 to1,000 y (10, 11). However, it is not obvious that adaptation to extremely low metabolic rate in the highly stable deep biosphere environment should select for small cell size.
The global data compilation of Kallmeyer et al. (5) includes only those microbial provinces in the subseafloor (12) that consist of “normal” sediments deposited over geological time. Although such sediments indeed cover most of the ocean floor, active plate tectonics also create very different environments such as midocean ridges and ridge flanks, subduction zones, and other hotspots of geological and geothermal activity. Chemical alterations are strong in the midocean ridges and continue for 10 to 15 million years in the spreading ridge flanks with the release of reduced iron, sulfur, and H2 (13). These chemical species serve as energy substrates for autotrophic microorganisms that may generate as much as 1012 g cell C globally per year exclusively from the “dark energy” of rock–water reactions (14). The turnover time and the size of this microbial community are not known. If the turnover time were similar to that in deep marine sediments—say, 100 y—the autotrophic community biomass would, at the maximum, be 1014 g C, which is a small fraction of the 4 × 1015 g C estimated by Kallmeyer et al. (5) for the deep biosphere in marine sediments. Another energy source for the crustal biosphere is the slow flow of seawater through the cracked and porous ocean crust beneath the thick sediment cover, which injects oxygen, inorganic ions, and dissolved organic matter into the old basalt. It is unknown how large microbial communities subsist in the basaltic ocean crust.
Although correlations are helpful to calculate global cell numbers, it would be interesting to gain a functional understanding of what really controls the cell numbers in subsurface sediments. Mortality is apparently extremely low, cell turnover takes hundreds of years, and the environment is stable over millions of years with a decreasing energy flux over time, provided by the buried and slowly decaying organic matter. Under such conditions, the community size may be controlled by a fine-tuned balance between the available energy flux from organic matter degradation and the minimum energy requirements of the microbial cells. Here the critical energy requirement is not the biological energy quantum needed to support ATP synthesis, but rather the minimum energy flux per cell. This minimum energy flux appears to be several orders of magnitude lower than the maintenance energy predicted from laboratory cultures. The microorganisms may thus subsist in a physiological state that we currently cannot explain from pure culture studies but for which indications begin to appear from laboratory cultures that have been starved for long periods of time (15).
An alternative explanation to exceedingly low energy flux requirement could be dormancy by which the cells are in a physiologically inactive state (16). Microscopic counts of DNA-stained cells include metabolically active cells and inactive, dormant cells as long as the latter are detectable by fluorescence staining of their DNA. However, for cells locked in an extremely energy-starved and stable environment that steadily decreases in energy flux over millions of years, dormancy would seem to be a dead-end strategy. The cells would be deprived of whatever energy they could have conserved, had they not been dormant, and they would need to spend extra energy on cell repair after awakening. Despite this, some bacteria might still go into the most long-lived dormant state known, the endospore formation. Bacterial endospores are generally impermeable to the DNA stains applied, and spores are therefore not included in the global estimates of microbial cells or biomass. The analysis of a specific component of the spore wall, dipicolinic acid, in sediment samples has recently shown that spores may be as abundant as vegetative cells in the deep biosphere (11). It may be a matter of definition whether they should be added to the global census of cell numbers and living biomass.
The deep biosphere has become widely known, not only for its high cell numbers and large biomass but also for the fascinating slow life and its important interactions with the geosphere. Kallmeyer et al. (5) now estimate that there are fewer microorganisms all together in the global deep biosphere. However, the importance of these deeply buried communities for driving carbon and nutrient cycling and catalyzing a multitude of reactions between rocks, sediments, and fluids is not challenged. Neither is the persisting enigma of slow life beneath the surface of Earth. The deep biosphere is still alive.
Acknowledgments
The Center for Geomicrobiology, Aarhus University, is supported by the Danish National Research Foundation, the German Max Planck Society, and the European Research Council.
Footnotes
The author declares no conflict of interest.
See companion article on page 16213.
References
- 1.Parkes RJ, Cragg BA, Fry JC, Herbert RA, Wimpenny JWT. Bacterial biomass and activity in deep sediment layers from the Peru margin. Philos Trans R Soc Lond A. 1990;331:139–153. [Google Scholar]
- 2.Parkes RJ, Cragg BA, Wellsbury P. Recent studies on bacterial populations and processes in subseafloor sediments: A review. Hydrogeol J. 2000;8:11–28. [Google Scholar]
- 3.Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: The unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipp JS, Morono Y, Inagaki F, Hinrichs KU. Significant contribution of Archaea to extant biomass in marine subsurface sediments. Nature. 2008;454:991–994. doi: 10.1038/nature07174. [DOI] [PubMed] [Google Scholar]
- 5.Kallmeyer J, Pockalny R, Adhikari RR, Smith DC, D’Hondt S. Global distribution of microbial abundance and biomass in subseafloor sediment. Proc Natl Acad Sci USA. 2012;109:16213–16216. doi: 10.1073/pnas.1203849109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer JP, et al. Oxygen penetration deep into the sediment of the South Pacific gyre. Biogeosciences. 2009;6:1467–1478. [Google Scholar]
- 7.Røy H, et al. Aerobic microbial respiration in 86-million-year-old deep-sea red clay. Science. 2012;336:922–925. doi: 10.1126/science.1219424. [DOI] [PubMed] [Google Scholar]
- 8.Kallmeyer J, Smith DC, D'Hondt SL, Spivack AJ. New cell extraction procedure applied to deep subsurface sediments. Limnol Oceanogr Methods. 2008;6:236–245. [Google Scholar]
- 9.D’Hondt S, et al. Subseafloor sedimentary life in the South Pacific Gyre. Proc Natl Acad Sci USA. 2009;106:11651–11656. doi: 10.1073/pnas.0811793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jørgensen BB, D’Hondt S. Ecology. A starving majority deep beneath the seafloor. Science. 2006;314:932–934. doi: 10.1126/science.1133796. [DOI] [PubMed] [Google Scholar]
- 11.Lomstein BA, Langerhuus AT, D’Hondt S, Jørgensen BB, Spivack AJ. Endospore abundance, microbial growth and necromass turnover in deep sub-seafloor sediment. Nature. 2012;484:101–104. doi: 10.1038/nature10905. [DOI] [PubMed] [Google Scholar]
- 12.Schrenk MO, Huber JA, Edwards KJ. Microbial provinces in the subseafloor. Annu Rev Mar Sci. 2010;2:279–304. doi: 10.1146/annurev-marine-120308-081000. [DOI] [PubMed] [Google Scholar]
- 13.Bach W, Edwards KJ. Iron and sulfide oxidation within the basaltic ocean crust: Implications for chemolithoautotrophic microbial biomass production. Geochim Cosmochim Acta. 2003;67:38712–3887. [Google Scholar]
- 14.Edwards KJ, Becker K, Colwell F. The deep, dark energy biosphere: Intraterrestrial life on Earth. Annu Rev Earth Planet Sci. 2012;40:551–568. [Google Scholar]
- 15.Finkel SE. Long-term survival during stationary phase: Evolution and the GASP phenotype. Nat Rev Microbiol. 2006;4:113–120. doi: 10.1038/nrmicro1340. [DOI] [PubMed] [Google Scholar]
- 16.Lennon JT, Jones SE. Microbial seed banks: The ecological and evolutionary implications of dormancy. Nat Rev Microbiol. 2011;9:119–130. doi: 10.1038/nrmicro2504. [DOI] [PubMed] [Google Scholar]
- 17.D’Hondt S, et al. Distributions of microbial activities in deep subseafloor sediments. Science. 2004;306:2216–2221. doi: 10.1126/science.1101155. [DOI] [PubMed] [Google Scholar]
- 18.Leloup J, et al. Diversity and abundance of sulfate-reducing microorganisms in the sulfate and methane zones of a marine sediment, Black Sea. Environ Microbiol. 2007;9:131–142. doi: 10.1111/j.1462-2920.2006.01122.x. [DOI] [PubMed] [Google Scholar]