Abstract
Staphylococcal pathogenicity islands (SaPIs) carry superantigen and resistance genes and are extremely widespread in Staphylococcus aureus and in other Gram-positive bacteria. SaPIs represent a major source of intrageneric horizontal gene transfer and a stealth conduit for intergeneric gene transfer; they are phage satellites that exploit the life cycle of their temperate helper phages with elegant precision to enable their rapid replication and promiscuous spread. SaPIs also interfere with helper phage reproduction, blocking plaque formation, sharply reducing burst size and enhancing the survival of host cells following phage infection. Here, we show that SaPIs use several different strategies for phage interference, presumably the result of convergent evolution. One strategy, not described previously in the bacteriophage microcosm, involves a SaPI-encoded protein that directly and specifically interferes with phage DNA packaging by blocking the phage terminase small subunit. Another strategy involves interference with phage reproduction by diversion of the vast majority of virion proteins to the formation of SaPI-specific small infectious particles. Several SaPIs use both of these strategies, and at least one uses neither but possesses a third. Our studies illuminate a key feature of the evolutionary strategy of these mobile genetic elements, in addition to their carriage of important genes—interference with helper phage reproduction, which could ensure their transferability and long-term persistence.
Keywords: temperate phage, small terminase, capsid morphogenesis
Staphylococcal pathogenicity islands (SaPIs) are prototypes of a large family of phage-inducible chromosomal islands (PICIs; formerly referred to as PRCIs) in Gram-positive bacteria (1), and their genomes are organized to take optimal advantage of the temperate phages that they parasitize to carry out their life cycle (1). Specifically, SaPIs reside at specific sites in the chromosome of their host and remain quiescent under the control of a master repressor (2). Upon superinfection by certain phages or upon SOS induction of a resident prophage, SaPIs use specific nonessential phage proteins to lift the repression, enabling the expression of their integrase and excision genes (3–5); following excision, they initiate replication at a SaPI-specific replication origin using SaPI-coded initiator and primase, and continue replication using the host cell replication machinery (6, 7). SaPIs reorganize the phage virion proteins to form small capsids commensurate with their genomes (usually about one-third the size of the phage genome), and they use a SaPI-coded terminase small subunit to package their DNA preferentially (8, 9). This process results in the efficient production of SaPI-specific infectious particles by means of which they are spread not only among staphylococci but also to other bacteria, such as Listeria monocytogenes (10), at very high frequency.
Upon discovery of the first SaPI, SaPI1, it was immediately realized that reproduction of its inducing phage, 80α, was greatly diminished. Indeed, plaque formation was blocked and the phage burst size was greatly reduced, and survival of phage-infected cells was increased (11). It was observed at that time that SaPI1 was packaged in small infectious particles, and it was soon shown that many SaPIs contain two or more capsid morphogenesis (cpm) genes (2, 12, 13) responsible for diverting phage virion proteins to small capsid formation. It was hypothesized that the diversion of phage virion proteins to the formation of these particles was responsible for interference (8).
In this report, we confirm this hypothesis and go beyond it to demonstrate that there are other mechanisms of phage interference. Some SaPIs use one mechanism, some use a second, some use both, and some add a third. We suggest that interference is not simply a consequence of competition between SaPI and phage for “goods and services,” but is a key feature of the SaPI lifestyle.
Results
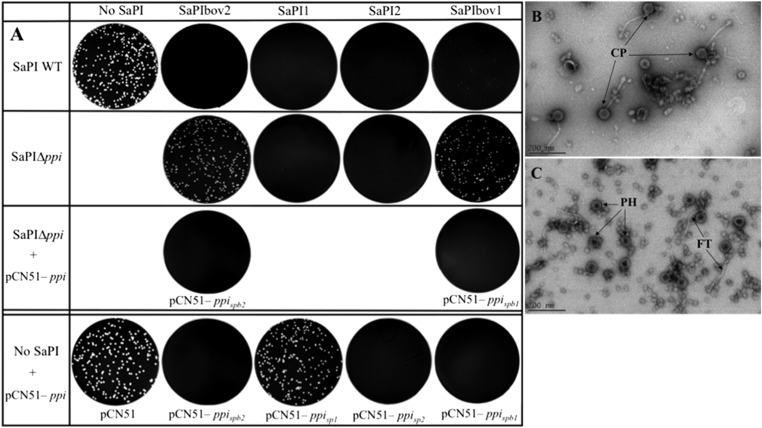
To begin these studies, we questioned whether the SaPI1 interference mechanism was universal, and immediately realized that it could not be, because several of the known SaPIs, including SaPIbov2 (1) and SaPIbov5 (see below), do not contain homologs of the cpm genes or produce small capsids. SaPIbov2 is 27 kb in length, owing to the presence of a 12-kb transposon, and could not be accommodated by small capsids, whose size is presumably determined by capsomere geometry. Nevertheless, SaPIbov2 strongly interferes with helper phage reproduction, blocking plaque formation and causing a 500-fold reduction in helper phage plaque-forming titer (Table 1 and Fig. 1A; note that interference is assessed as a reduction in phage titer or as a reduction in plaque size or number, and that these two parameters do not always match precisely). An earlier study hinted that gene 12 of the closely related 15-kb SaPIbov1 might be involved in phage interference (9). We originally designated this gene pif (phage interference function) (9), but because pif had been used previously (14), we now use ppi for phage packaging interference (see below) and add a subscript to indicate its origin, e.g., ppispb2 denotes the SaPIbov2 version. We cloned ppispb2 gene and tested its role in phage interference. As shown in Fig. 1A, the cloned gene completely blocked 80α plaque formation, and its deletion fully restored plaque formation and increased the 80α plaque titer nearly to that seen with the host strain lacking any SaPI (Table 1). This phenotype was fully complemented by the cloned gene (Fig. 1A). Because the deletion of ppispb2 had no significant effect on the SaPIbov2 life cycle (Table 1), we suggest that Ppi, a SaPI-coded protein, is dedicated to phage interference.
Table 1.
Effect of wild-type SaPIs, SaPI mutations, and overexpressed SaPI genes on phage 80α and SaPI titers
| SaPI | SaPI mutation | Plasmid | Phage titer (pfu/mL) × 108† | SaPI titer, TU/mL‡ |
| 520 ± 10 | ||||
| pCN51 | 440 ± 60 | |||
| pCN51-ppispb2 | 2 ± 0.2 | |||
| pCN51-cpmABsp1 | 15 ± 2 | |||
| SaPIbov2 | – | 1 ± 0.1 | 1.1 ± 0.1 × 106 | |
| Δppi | 360 ± 10 | 0.5 ± 0.1 × 106 | ||
| Δppi | pCN51 | 230 ± 30 | 4.2 ± 0.4 × 106 | |
| Δppi | pCN51-ppispb2 | 1 ± 0.5 | 3.6 ± 0.4 × 106 | |
| SaPI1 | – | 40 ± 6 | 2.3 ± 0.4 × 108 | |
| ΔcpmAB | 250 ± 30 | 3.4 ± 0.6 × 108 | ||
| ΔcpmAB | pCN51 | 210 ± 20 | 1.0 ± 0.2 × 108 | |
| ΔcpmAB | pCN51-cpmABsp1 | 8 ± 1.4 | 6.3 ± 0.3 × 107 | |
| SaPIbov1 | – | 49 ± 7 | 4.2 ± 0.1 × 108 | |
| Δppi | 150 ± 20 | 1.1 ± 0.1 × 108 | ||
| ΔcpmAB | 36 ± 7 | 1.6 ± 0.3 × 109 | ||
| Δppi, ΔcpmAB | 210 ± 80 | 1.2 ± 0.2 × 109 | ||
| SaPI2 | – | 3 ± 0.4 | 2.9 ± 0.3 × 108 | |
| Δppi | 3 ± 0.1 | 5.8 ± 0.6 × 108 | ||
| ΔcpmAB¶ | 13 ± 1 | 2.0 ± 0.3 × 109 | ||
| Δppi, ΔcpmAB¶ | 110 ± 10 | 3.1 ± 0.5 × 108 | ||
| SaPIbov5 | – | 390 ± 20 | 4.7 ± 0.2 × 106 |
Values are means ± SD of three independent samples (n = 3). Entries in bold type represent situations showing interference. We considered threefold or greater reduction in phage titer as interference.
†Phage titer of lysate using RN4220 as indicator. pfu, plaque forming units.
‡SaPI titer of lysate using RN4220 as recipient. TU, SaPI Transducing Units.
¶ORF13 deleted along with cpmAB because just cpmAB deletion could not be generated.
Fig. 1.
Ppi interference with phage 80α. (A) Approximately 108 bacteria were infected with phage 80α, plated on phage bottom agar, and incubated 36–48 h at 32 °C. Plates were stained with 0.1% TTC in TSB and photographed. Genes cloned into pCN51 were induced with 1.0 μM CdCl2. (B and C) Electron microscopic analysis of sedimented particles from (B) phage 80α lysate of RN4220 + pCN51 and from a (C) 80α lysate of RN4220 + pCN51 – ppispb2. CP, complete phage 80α particles; FT, free phage tails; PH, phage proheads.
We next attempted to identify the stage in the phage reproduction cycle that was inhibited by Ppispb2. A test by quantitative real time-PCR (qPCR) for inhibition of phage replication showed that phage replication was unaffected (Fig. S1A), suggesting that Ppispb2 was targeting a later stage. Although Ppispb2 blocked plaque formation, it did not prevent phage-induced lysis, indicating that the block was between replication and lysis. Accordingly, we compared the particles present in a standard phage lysate with particles present in the lysate of Ppispb2-inhibited phage 80α. A lysate generated by growth of phage 80α on a control strain contained normal intact phage particles (Fig. 1B), and one generated on a strain expressing ppispb2 contained mostly phage tails and proheads (Fig. 1C), indicating that phage maturation was affected. Because proheads do not acquire tails unless they have been filled with DNA (12), it seemed likely that Ppispb2 inhibits the packaging of helper phage DNA.
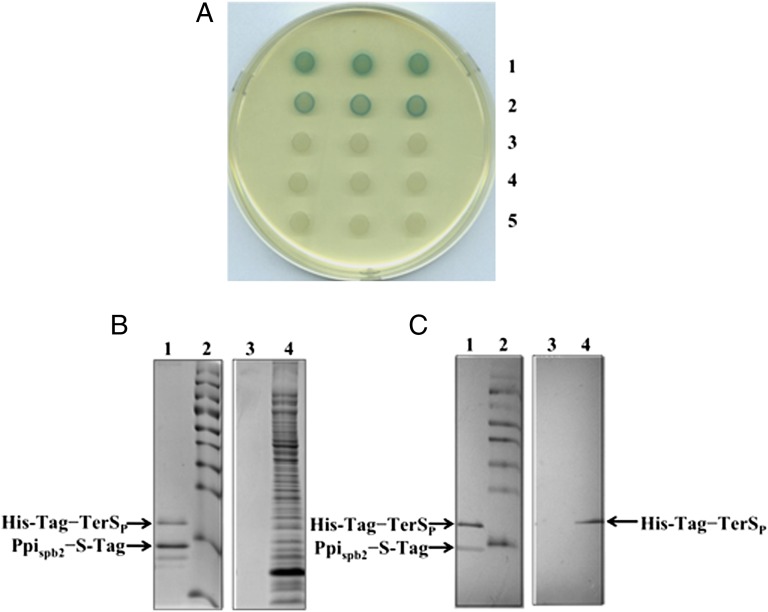
Packaging is initiated by the two-subunit terminase, which recognizes and specifically cleaves target DNA (15–17). Phage and SaPI both use the phage-encoded large (TerL) subunit, and this is complexed with a phage- or SaPI-encoded small subunit (TerSP or TerSS, respectively), which determines packaging specificity (9, 12). It therefore seemed likely that TerSP would be the target of Ppispb2 inhibition. To test this, we isolated phage mutants able to form plaques on a strain containing the cloned ppispb2. Mutants were readily obtained, at a frequency of ∼10−6, and we sequenced the phage small-terminase subunit of 10 of these. In each case there was an amino acid substitution in TerSP; in nine of these, the mutations were at the same site, Q132, and in each of these, the glutamine was replaced by either an arginine or a lysine (Fig. S1B). The 10th mutant had an isoleucine substitution for threonine 106 in the same gene. To confirm that the TerSP mutations were necessary and sufficient to confer the observed phenotype, we replaced the native TerSP gene with the mutated one (Q132K) in an 80α prophage and found that the mutant phage was Ppispb2 resistant (Fig. S2A). We next performed a bacterial two-hybrid test, comparing the WT 80α TerSP with a Ppispb2-resistant TerSP for any interaction with Ppispb2. As shown in Fig. 2A, Ppispb2 bound strongly to the WT 80α TerSP but not to the mutant protein, and it did not interact with the cognate SaPI TerSS. Interaction between Ppispb2 and TerSP was confirmed by a pull-down assay using S-tagged Ppispb2 (Fig. 2 B and C), suggesting that Ppispb2 directly and specifically interacts with TerSP and hence blocks the packaging of phage DNA. Because the mutations were in the C-terminal region of the protein, which, in other phages, is the region of binding to TerL (15, 16), we suggest that Ppispb2 acts by interfering with the interaction of TerSP with TerL, and thus blocks cleavage and packaging of phage DNA.
Fig. 2.
Interactions between Ppispb2 and TerSP (80α). (A) BACTH analysis. Spots in each row represent three independent colonies. Plasmid combinations are numbered as follows: 1, pKT25-zip + pUT18C-zip (positive control); 2, pKT25-TerSP (WT80α) + pUT18-Ppispb2; 3, pKT25-TerSP (Ppispb2-resistant 80α) + pUT18-Ppispb2; 4, pKT25-TerSS (SaPIbov2) + pUT18-Ppispb2; and 5, pKT25 + pUT18 (negative control). Blue color indicates cAMP-dependent lacZ expression following reconstitution of adenlyate cyclase activity by interaction of fusion proteins. (B and C) Pull-down of His-tag–TerSP (80α) coexpressed with Ppispb2–S-tag using S-protein agarose column: lane 1, protein eluted from S-protein agarose beads after binding and washing of cell lysate obtained from cells coexpressing both the proteins; lane 2, prestained markers; lane 3, eluate from S-protein agarose beads after binding and washing of cell lysate obtained from cells expressing His-TerSP (80α); lane 4, cell lysate obtained from cells expressing His-TerSP (80α). (B) Coomassie-stained gel. (C) Western blot of the gel shown in B using Penta-His HRP conjugate and S-protein HRP conjugate antibodies.
The TerSP proteins fall into seven superfamilies (18). Four tested phages were sensitive to Ppispb2 (Table S1); all of these belong to the terminase_2 superfamily, as do the SaPI TerSS (Fig. S2B). We tested four other staphylococcal phages that encode TerSP proteins not belonging to the terminase_2 superfamily, and all four were resistant to Ppispb2 (Table S1), and were quite dissimilar in the region surrounding position 132 (Fig. S2B). The terminase_2 superfamily phage TerSP proteins lack a conserved C-terminal extension of ∼26 amino acids found in the SaPI TerSS proteins. A test of the possibility that this extra segment is responsible for the indifference of SaPI TerSS to Ppispb2 is in progress.
All known SaPIs have a Ppi homolog that belongs to one of two conserved subsets, either SaPIbov2-like (high similarity to Ppispb2) or SaPI1-like (30% or less similarity to Ppispb2), as shown in Fig. S3A. Within the SaPIbov2 subset, ppispb2 is the strongest inhibitor of 80α, ppispb1 is weaker, and ppisp2 is weakest (Fig. S3B), suggesting the involvement of one or more additional SaPI genes in SaPIbov1 and SaPI2 interference. The ppi genes in the SaPI1 subset had no inhibitory effect on phage 80α or 80 (Fig. 1A and Fig. S4A), but the cloned ppisp1 blocked plaque formation by ϕ12 (Fig. S4B), presumably by inhibiting TerSP of the terminase_4 superfamily, which includes the Panton-Valentine leukocidin-encoding Staphylococcal Leukocytolytic Toxin phages.
Because SaPI1-mediated interference with 80α does not involve ppisp1, we considered the possibility that capsomere diversion was entirely responsible for interference. Accordingly, we deleted the two principle capsid morphogenesis genes, cpmA and cpmB, of SaPI1 and tested this deletion for its effects on 80α plaque formation and phage titer. As shown in Fig. 3A and Table 1, this in-frame deletion virtually eliminated interference with 80α. Additionally, when cloned and overexpressed, the two genes together totally eliminated plaque formation and greatly reduced the phage titer (Fig. 3A and Table 1). To demonstrate the basis of this effect, we analyzed the DNA present in both large- and small-headed particles. As shown in Fig. 3C, in the absence of any SaPI, the particle DNA migrates as a 45-kb band, corresponding to monomeric phage DNA from large-headed particles. However, in the presence of SaPI1 or SaPI2, most of the particle DNA migrates as an 18-kb band, corresponding to monomeric SaPI DNA from small-headed particles, and only a tiny fraction migrates as 45-kb DNA from large-headed particles, accounting for the observed reduction in phage titer. We have previously shown by Southern blotting of such a gel that both of these bands contain both phage and SaPI DNA, and that most of the phage DNA migrates with the SaPI monomers (9). As also shown in Fig. 3C, deletion of cpmAB eliminates the SaPI monomer-sized material, all of the particle DNA migrating as a 45-kb band, which includes both phage- and SaPI-specific DNAs. Therefore, although packaging is sequence-specific and determined by TerS, it is not size-specific: both phage and SaPI DNAs are packaged in capsids of both sizes. Because the complete SaPI genome is packaged in capsids of either size, capsid remodeling has relatively little effect on most SaPI titers. Because the complete phage genome cannot be accommodated in the small capsid, CpmAB-determined capsid morphogenesis results in the packaging of subgenomic and therefore defective phage DNA segments. These results suggest that there are at least two different mechanisms of SaPI-mediated interference with helper phages: Ppi-mediated interference with packaging and Cpm-mediated diversion of capsomere proteins for small capsid formation.
Fig. 3.
Effect of SaPI interference genes on phage 80α. Phage platings were as in Fig. 1A. (A) Effect of SaPI1 CpmAB. (B) Effects of SaPI2 and SaPIbov1 CpmAB and Ppi. Note that ORF13 was always included in deletions of SaPI2 cpmAB because a deletion of the two genes alone could not be constructed. ORF13 does not affect interference. (C) Agarose gel analysis of DNA extracted from sedimented phage particles. Phage 80α was grown on RN4220 and various derivatives of SaPI2 (i; Upper) or SaPI1 (ii; Lower). The resulting lysates were treated with DNase, PEG-precipitated, and phenol extracted. Equivalent amounts of DNA were separated on 0.8% agarose gel for 13 h at 60 V, strained with ethidium bromide, and photographed.
Because ppi could account for at most a part of the observed interference with 80α by SaPIbov1 or SaPI2 (Figs. 1A and 3B and Table 1), we considered the possibility that cpmAB and/or other SaPI genes might also be involved. With SaPIbov1 and SaPI2, this was, indeed, the case, because both cpmAB and ppi are involved. On one hand, cpmABsp2 completely blocks plaque formation in the absence of ppisp2, and ppisp2 blocks plaque formation in the absence of cpmABsp2, suggesting that the two interferences are redundant. On the other hand, ppispb1 has a much stronger effect than cpmABspb1, and the two mechanisms appear to be additive; however, they do not fully account for the observed interference with 80α by SaPIbov1, suggesting that there may be yet a third mechanism. Although SaPIbov1 deletions and subclones have not been very informative in this respect, similar studies with SaPI2 clearly demonstrate the existence of a third interference mechanism. For these experiments, we used phage 80 rather than 80α, because phage 80 does not support the production of small SaPI particles (19) and is not inhibited by any of the ppi genes described above or by cpmABsp2 (Fig. S4A and Fig. 4). Nevertheless, SaPI2 inhibits phage 80 very strongly, completely blocking plaque formation (Fig. 4). This inhibition was largely eliminated by deletion of SaPI2 ORF17, which has homologs in the four other SaPIs analyzed in this study. When expressed from the exogenous promoter Pcad, ORF17 blocks plaque formation by phage 80 but has no effect on 80α, whereas the cloned SaPI2 ppi and cpmAB genes interfere with phage 80α but have no effect on phage 80 (Fig. 4). These results clearly demonstrate three independent mechanisms of phage interference and show additionally that the interference is largely phage-specific.
Fig. 4.
SaPI2 interference with different phages. SaPI2 uses three mechanisms of phage interference: with phage 80 (Left) it uses ORF17, and with phage 80α (Right) Ppi and CpmAB.
According to these results, one would predict the existence of SaPIs that use only that third mechanism. A possible example of this is a recently identified SaPI, SaPIbov5, which lacks cpm genes and contains a ppi gene of the SaPI1 type, which does not affect 80α. SaPIbov5 is induced, packaged, and transduced at high frequency by phage 80α, but does not significantly interfere with it, having no effect on either 80α plaque formation or phage titer (Fig. S4C and Table 1). However, the yield of SaPIbov5 transducing units (SaPI TU) is four orders of magnitude lower than the phage titer, in contrast to SaPI titers within 1–2 orders of magnitude of the helper phage seen for SaPIs that interfere with 80α. If interference is important for SaPI survival, we predicted that SaPIbov5 would survive by interfering with other helper phages, and, indeed, it interferes with ϕ11 (Table 2 and Fig. S4D), but not via ppispb5 (Fig. S5A), perhaps using the same interference mechanism as that used by SaPI2 against phage 80. This interference seems paradoxical, however, because SaPIbov5 virtually eliminates plaque formation by ϕ11 but causes only a ∼3× decrease in phage titer. This apparent paradox was resolved by a one-step phage growth analysis, as shown in Fig. S5B. Although the reduction in burst size paralleled the reduction in overall phage titer, lysis was delayed by ∼15 min. Because phages multiply and form plaques only during growth of the indicator bacteria, plaque size is profoundly affected by burst size and latency, and we suggest that this combination of effects is sufficient to account for the observed effect of SaPIbov5 on ϕ11 plaque formation. Although not tested specifically for this report, we suggest that other examples of disparity between phage titer and plaque size can probably be similarly explained.
Table 2.
Effect of SaPIbov5 on phage ϕ11 and SaPI titers
| SaPI | SaPI mutation | Plasmid | Phage titer (pfu/mL) × 108† | SaPI titer, TU/mL‡ |
| 140 ± 10 | ||||
| SaPIbov5 | 49 ± 5 | 3.8 ± 0.6 × 107 |
Values are means ± SD of three independent samples (n = 3). Entries in bold type represent situations showing interference. We considered threefold or greater reduction in phage titer as interference.
†Phage titer of lysate using RN4220 as indicator. pfu, plaque forming units.
‡SaPI titer of lysate using RN4220 as recipient. TU, SaPI Transducing Units.
Discussion
Interference with phage propagation has been observed in a variety of situations and by a variety of mechanisms (aside from classical superinfection immunity and restriction modification). Among these strategies are interference with late phage T7 mRNA translation by the pifA and pifB genes of plasmid F (14), mutual interference between λ and P2, owing to inactivation of RecBC by λ (20), and interference with several phages by SP02, possibly by a similar mechanism (21); a rather artificial mechanism is interference with one phage by the cloned homolog of one of its essential genes (22) or by phage genome fragments (defective interfering particles) (23). A hitherto unknown interference mechanism is the incorporation during phage infection of short segments of phage DNA by chromosomally located CRISPRs (clustered regularly interspaced short palindromic repeats) that are used to prime synthesis of interfering small RNAs that block phage development (24, 25). In this study we describe a unique mechanism of phage interference in which phage DNA packaging is blocked by Ppi. All SaPIs sequenced to date encode a Ppi homolog (1) and presumably use this mechanism of phage interference. Two classes of Ppi’s have been identified by sequence analysis and have been shown by genetics to interfere correspondingly with two different sets of phages. Class I (prototype SaPIbov2) interferes with phages 80α and ϕ11, and class II (prototype SaPI1) interferes with ϕ12. No SaPI encodes more than one Ppi; however, SaPIs may use other interference mechanisms in lieu of or in addition to the Ppi-mediated one for certain phages, and these mechanisms may be specific for different phages. Each of the five SaPIs studied here interferes with at least one known phage; therefore, phage interference may be a universal feature of the PICIs, such as the SaPIs. Moreover, a tBLASTn search with prototypes of either of the Ppi classes identifies only the Ppi homologs in the PICIs plus hypothetical proteins in pathogenicity islands (probably PICIs) of a few other Gram-positive organisms—Eubacterium limosum KIST612, Aerococcus viridans ATCC 11563, Clostridium bolteae ATCC BAA-613. Thus, the Ppi mechanism of phage interference may be unique to such elements.
A key SaPI feature that sharply differentiates it from its helper phages is the presence of a SaPI-coded TerSS, which differs from the phage TerSP by a 26-residue C-terminal extension. Ppispb2 binds to the phage small-terminase subunit TerSP, specifically blocking the packaging of phage DNA (Figs. 1C and 2); it does not bind the SaPI TerSS (Fig. 2A). The precise mechanism of this interference has yet to be determined; one possibility is that Ppispb2 binding to TerSP could abolish its DNA binding activity; alternatively, it could prevent the binding of TerSP to TerL. Tests to distinguish between these possibilities and to determine whether the C-terminal extension of TerSS is responsible for Ppispb2 resistance are in progress.
We have also found that SaPIs use at least two other mechanisms of phage interference: one resulting from the diversion of capsid proteins to the formation of small, SaPI-sized capsids, and the other resulting from the expression of SaPI2 ORF17, whose mechanism remains to be determined. Each of the five different SaPIs studied here interferes with at least one of the four bacteriophages tested.
Interference with infecting bacteriophages is a widespread strategy that has evolved primarily or exclusively to benefit host bacteria (14, 20–25). Here, however, we have a tripartite system consisting of host, SaPI, and phage, of which the first two clearly share in the benefits, and we suspect that the third does also because interference is so widespread and well-conserved. The importance of interference is underlined by the fact that SaPIs have evolved multiple mechanisms (Table S2). The question then arises of why SaPIs interfere with helper phage reproduction. One possibility is that interference helps the SaPI to keep pace with the phage during successive infection cycles. As noted, there is a vast excess of SaPI monomer-sized DNA in particles isolated from a typical WT SaPI1 or SaPI2 lysate, but not from a cpmAB mutant (Fig. 3C). Thus, SaPI1 and SaPI2 cause the phage to waste much of its DNA by dead-end packaging in small capsids—a very neat way for the SaPI to ensure that it would keep pace with the phage. We note additionally that, as is typical of bacteriophages, virion proteins (and therefore capsids) are in great excess so that SaPI and phage do not automatically compete for packaging. In other words, without a specific interference mechanism, SaPI maturation does not adversely affect phage maturation, as can be seen in Table 1. A second major advantage could be a reduction in the probability that an organism receiving a SaPI will be killed by a coinfecting phage; this would enhance the survival of SaPIs in the population (although it would not be reflected in the single-round transduction assays reported here). A third major advantage is that an interfering SaPI sharply increases the frequency of host cell survivors that are not lysogens (11).
Fascinating questions on the evolutionary history of the SaPIs are raised by these studies. Why don’t helper phages avoid the SaPI-mediated interference either by mutating the antirepressor protein (3) or by mutating TerSP? For the 27-kb, transposon-carrying SaPIbov2, which came first: acquisition of the transposon or loss of the cpm genes? Assuming that the C-terminal extension in the TerSS proteins is responsible for Ppi resistance, was it acquired along with or before the acquisition of ppi, and what was its source, or that of ppi? Is the Ppi mechanism of interference used in other contexts in the bacterial world? Implicit in these questions is the view that SaPIs are independently evolving entities that have “borrowed” certain phage genes but explicitly excluded others. This behavior provides them with an optimal existence as satellites/parasites, which they have fine-tuned by developing multiple interference functions that target different helper phages.
Materials and Methods
Strains and Plasmids.
Bacterial strains, plasmids, and primers used in this study are listed in Tables S3–S5. Growth media (26) and conditions are described in SI Materials and Methods. Staphylococcal temperate phages Φ80, 80α, Φ11, Φ12, Φ55, Φ187, ΦNM1, and ΦNM4 were used in this study.
Molecular Methods.
All general DNA manipulations (i.e., digestion, ligation, etc.) were carried out by standard methods (27). Oligonucleotides used for this work are listed in Table S5. All restriction enzymes and T4 DNA ligase were purchased from New England Biolabs. Primers were obtained from Integrated DNA Technologies Inc., and DNA sequencing was performed by Macrogen.
Cloning, Expression, and Pull-Down Assay.
Escherichia coli BL21 (DE3) cells were transformed with plasmid pGR00020 and plasmid pGR00021 for expression analysis. Plasmid pGR00020 expresses the TerSP protein fused with His-tag [His-tag–TerSP (80α)], and plasmid pGR00021 coexpresses the His-TerSP (80α) as well as the Ppispb2 protein fused with S-tag (28) (Ppispb2– S-tag). See SI Materials and Methods for further details.
Mutant Construction.
In-frame deletion mutants were generated using the allelic exchange vector pMAD (29) as described previously (2). Details are given in SI Materials and Methods.
Phage Infection and Induction.
Staphylococcus aureus strains (in triplicates) were inoculated in CYGP (Casamino acids Yeast extract Glycerophosphate) broth at an initial OD600 = 0.1 (∼1 × 108 cfu/mL) and grown at 37 °C and 225 rpm. Cultures were adjusted to OD600 = 1.0 (∼1 × 109 cfu/mL) with CYGP broth and diluted 1:1 with phage buffer, infected either with phage 80α at a multiplicity of infection (MOI) of 3 or with phage 80 at an MOI of 2. Induction methods are detailed in SI Materials and Methods.
Plaque Formation Assay on Different SaPI-Containing Strains.
Known number of phage particles e.g., phage 80α (∼200–300 phages), phage 80 (∼300 phages), phage Φ11 (∼500 phages), and Φ12 (104 phages) were mixed with ∼108 cells (100 μL culture adjusted to OD600 = 1.0 ∼1 × 109 cfu/mL) of RN4220 and its derivative strains. This mixture was incubated at RT for 15 min, subsequently mixed with 3 mL of phage top agar (26) and immediately poured on phage bottom agar (26) plate with or without 1.0 μM CdCl2. Plates were incubated at 32 °C for 36–48 h and stained with 0.1% TTC (Difco) in TSB broth (30).
Phage and SaPI Titer Measurement.
Phage lysates with or without SaPI were filter sterilized and serially diluted in phage buffer (26). Please see SI Materials and Methods for further details.
Electron Microscopy, Bacterial Two-Hybrid Assays, and qPCR.
Please see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Eric W. Roth for his support and help with electron microscopy at New York University Langone Medical Center, Office of Collaborative Science Microscopy Core facility. This work was supported by National Institutes of Health Grants R01AI022159 (to R.P.N. and J.R.P.), and R21 AI067654 and R56 AI081837 (to G.E.C.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204615109/-/DCSupplemental.
References
- 1.Novick RP, Christie GE, Penadés JR. The phage-related chromosomal islands of Gram-positive bacteria. Nat Rev Microbiol. 2010;8:541–551. doi: 10.1038/nrmicro2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Úbeda C, et al. SaPI mutations affecting replication and transfer and enabling autonomous replication in the absence of helper phage. Mol Microbiol. 2008;67:493–503. doi: 10.1111/j.1365-2958.2007.06027.x. [DOI] [PubMed] [Google Scholar]
- 3.Tormo-Más MÁ, et al. Moonlighting bacteriophage proteins derepress staphylococcal pathogenicity islands. Nature. 2010;465:779–782. doi: 10.1038/nature09065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Úbeda C, et al. Sip, an integrase protein with excision, circularization and integration activities, defines a new family of mobile Staphylococcus aureus pathogenicity islands. Mol Microbiol. 2003;49:193–210. doi: 10.1046/j.1365-2958.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- 5.Maiques E, et al. Role of staphylococcal phage and SaPI integrase in intra- and interspecies SaPI transfer. J Bacteriol. 2007;189:5608–5616. doi: 10.1128/JB.00619-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Úbeda C, Barry P, Penadés JR, Novick RP. A pathogenicity island replicon in Staphylococcus aureus replicates as an unstable plasmid. Proc Natl Acad Sci USA. 2007;104:14182–14188. doi: 10.1073/pnas.0705994104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Úbeda C, Tormo-Más MÁ, Penadés JR, Novick RP. Structure-function analysis of the SaPIbov1 replication origin in Staphylococcus aureus. Plasmid. 2012;67:183–190. doi: 10.1016/j.plasmid.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruzin A, Lindsay J, Novick RP. Molecular genetics of SaPI1—a mobile pathogenicity island in Staphylococcus aureus. Mol Microbiol. 2001;41:365–377. doi: 10.1046/j.1365-2958.2001.02488.x. [DOI] [PubMed] [Google Scholar]
- 9.Úbeda C, et al. Specificity of staphylococcal phage and SaPI DNA packaging as revealed by integrase and terminase mutations. Mol Microbiol. 2009;72:98–108. doi: 10.1111/j.1365-2958.2009.06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Novick RP. Phage-mediated intergeneric transfer of toxin genes. Science. 2009;323:139–141. doi: 10.1126/science.1164783. [DOI] [PubMed] [Google Scholar]
- 11.Lindsay JA, Ruzin A, Ross HF, Kurepina N, Novick RP. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol Microbiol. 1998;29:527–543. doi: 10.1046/j.1365-2958.1998.00947.x. [DOI] [PubMed] [Google Scholar]
- 12.Poliakov A, et al. Capsid size determination by Staphylococcus aureus pathogenicity island SaPI1 involves specific incorporation of SaPI1 proteins into procapsids. J Mol Biol. 2008;380:465–475. doi: 10.1016/j.jmb.2008.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damle PK, et al. The roles of SaPI1 proteins gp7 (CpmA) and gp6 (CpmB) in capsid size determination and helper phage interference. Virology. 2012 doi: 10.1016/j.virol.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison TG, Malamy MH. T7 translational control mechanisms and their inhibiton by F factors. Nat New Biol. 1971;231:37–41. doi: 10.1038/newbio231037a0. [DOI] [PubMed] [Google Scholar]
- 15.Catalano CE. The terminase enzyme from bacteriophage lambda: A DNA-packaging machine. Cell Mol Life Sci. 2000;57:128–148. doi: 10.1007/s000180050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao VB, Feiss M. The bacteriophage DNA packaging motor. Annu Rev Genet. 2008;42:647–681. doi: 10.1146/annurev.genet.42.110807.091545. [DOI] [PubMed] [Google Scholar]
- 17.Casjens SR. The DNA-packaging nanomotor of tailed bacteriophages. Nat Rev Microbiol. 2011;9:647–657. doi: 10.1038/nrmicro2632. [DOI] [PubMed] [Google Scholar]
- 18.Marchler-Bauer A, et al. CDD: A Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011;39:225–229. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christie GE, et al. The complete genomes of Staphylococcus aureus bacteriophages 80 and 80α—implications for the specificity of SaPI mobilization. Virology. 2010;407:381–390. doi: 10.1016/j.virol.2010.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghisotti D, Zangrossi S, Sironi G. An Escherichia coli gene required for bacteriophage P2-λ interference. J Virol. 1983;48:616–626. doi: 10.1128/jvi.48.3.616-626.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasbin RE, Ganesan AT, Young FE. Bacteriophage interference in Bacillus subtilis 168. J Virol. 1974;13:916–921. doi: 10.1128/jvi.13.4.916-921.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson G, Widner W, Xin WN, Feiss M. Interference with phage λ development by the small subunit of the phage 21 terminase, gp1. J Bacteriol. 1991;173:2733–2738. doi: 10.1128/jb.173.9.2733-2738.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enea V, Horiuchi K, Turgeon BG, Zinder ND. Physical map of defective interfering particles of bacteriophage f1. J Mol Biol. 1977;111:395–414. doi: 10.1016/s0022-2836(77)80061-2. [DOI] [PubMed] [Google Scholar]
- 24.Barrangou R, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 25.Deveau H, Garneau JE, Moineau S. CRISPR/Cas system and its role in phage-bacteria interactions. Annu Rev Microbiol. 2010;64:475–493. doi: 10.1146/annurev.micro.112408.134123. [DOI] [PubMed] [Google Scholar]
- 26.Novick RP. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1989. [Google Scholar]
- 28.Raines RT, McCormick M, Van Oosbree TR, Mierendorf RC. The S.Tag fusion system for protein purification. Methods Enzymol. 2000;326:362–376. doi: 10.1016/s0076-6879(00)26065-5. [DOI] [PubMed] [Google Scholar]
- 29.Arnaud M, Chastanet A, Débarbouillé M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl Environ Microbiol. 2004;70:6887–6891. doi: 10.1128/AEM.70.11.6887-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pattee PA. Use of tetrazolium for improved resolution of bacteriophage plaques. J Bacteriol. 1966;92:787–788. doi: 10.1128/jb.92.3.787-788.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.