BIOPHYSICS AND COMPUTATIONAL BIOLOGY Correction for “Folding helical proteins in explicit solvent using dihedral-biased tempering,” by Cheng Zhang and Jianpeng Ma, which appeared in issue 21, May 22, 2012, of Proc Natl Acad Sci USA (109:8139–8144; first published May 9, 2012; 10.1073/pnas.1112143109).
The authors note that on page 8140, right column, first full paragraph, line 5, “(A5E-E6A, E54A-A54E, and R71N-N71R)” should instead appear as “(A5E-E6A, E54A-A55E, and R71N-N73R).”
On page 8141, left column, first paragraph, lines 3–4, “and angles were similar to the native conformation of α3D” should instead appear as “and angles were similar to those in the native conformation of α3D.”
On page 8142, right column, first full paragraph, line 2, “cytochrome ć” should instead appear as “cytochrome c′.”
Lastly, the authors note that Fig. 5 appeared incorrectly. The corrected figure and its legend appear below. These errors do not affect the conclusions of the article.
Fig. 5.
The logarithm of the packing-chirality distribution 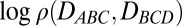 of S-386 at 300 K with representative conformations at the four most populated clusters. The colors of the four helices are blue, green, orange, and red, sequentially, from N to C terminus.
of S-386 at 300 K with representative conformations at the four most populated clusters. The colors of the four helices are blue, green, orange, and red, sequentially, from N to C terminus.