Abstract
Parkinson disease (PD) results from the slow, progressive loss of dopaminergic neurons in the substantia nigra. Alterations in α-synuclein (aSyn), such as mutations or multiplications of the gene, are thought to trigger this degeneration. Here, we show that aSyn disrupts mitogen-activated protein kinase (MAPK)-controlled stress signaling in yeast and human cells, which results in inefficient cell protective responses and cell death. aSyn is a substrate of the yeast (and human) polo-like kinase Cdc5 (Plk2), and elevated levels of aSyn prevent Cdc5 from maintaining a normal level of GTP-bound Rho1, which is an essential GTPase that regulates stress signaling. The nine N-terminal amino acids of aSyn are essential for the interaction with polo-like kinases. The results support a unique mechanism of PD pathology.
Keywords: aging, neurodegeneration, proteinopathy
Parkinson disease (PD), which is the second most common neurodegenerative disorder, results from the progressive degeneration of dopaminergic neurons in part of the midbrain called the substantia nigra pars compacta (1). A hallmark of this disease is the formation of protein inclusions (Lewy bodies) in the cytoplasm of affected neurons, and the principal component of Lewy bodies is the protein α-synuclein (αS) (2). Age-dependent accumulation of αS, perhaps due to lysosome dysfunction (3), is thought to trigger sporadic PD, whereas missense mutations (4–6) or multiplication (7) of the αS gene trigger early-onset PD. Because αS molecules in Lewy bodies are phosphorylated at Ser-129 (8), a search has been underway to identify the kinase responsible for this phosphorylation and its role in the pathobiology of αS. Polo-like kinase 2 (Plk2/Cdc5, human/yeast) was recently identified as a suppressor of αS toxicity in yeast, worm, and rat PD models (9), and Plk2 phosphorylates αS in vitro and in vivo at Ser-129 (10).
Plks are highly conserved serine/threonine kinases that possess an N-terminal kinase domain and one or two C-terminal polo-box domains that interact with substrates and direct the kinase to various cellular loci (11). Plks regulate the cell cycle and cytokinesis in dividing cells, whereas their function in nondividing cells, such as neurons, is less well understood. Recent studies have shown that in neurons, Plk2 regulates the activity of two small guanosine triphosphatases (GTPases), Ras and Rap, by phosphorylating their activators (GEFs, guanine nucleotide exchange factors) and inactivators (GAPs, GTPase-activating proteins) (12, 13). In yeast, Cdc5 also regulates the activity of the small GTPase Rho1 by phosphorylating its GEFs (Tus1 and Rom2) and GAP (Sac7) (14).
Rho1, which is a member of the Ras-like family of small GTPases, controls actin organization, cell wall biogenesis, polarized secretion, and cytokinesis in yeast cells. Rho1 is the main signaling node in the yeast cell wall integrity (CWI) pathway, which is a MAPK cascade that helps cells monitor and respond to cell wall stress (15). This pathway is activated by elevated temperatures (37–39 °C), mating pheromone, hypo-osmotic shock, and various compounds (caffeine, calcofluor white, and Congo red) (15). In response to these stresses, membrane-associated Rho1 switches on and then activates protein kinase C (Pkc1). Activated Pkc1 triggers a signaling cascade that activates the transcription factor Rlm1, which controls the transcription of cell wall biogenesis genes.
In this study, the mechanism of toxicity of αS was probed by using yeast, and the findings were validated in human neuroblastoma cells. In yeast, αS decreases the level of GTP-Rho1, which disrupts stress signaling from the membrane to the nucleus and makes cells hypersensitive to stress; αS also disrupts stress signaling in human cells. We propose that αS inhibits Plks from phosphorylating and activating the downstream regulatory proteins Rho GEFs and/or Rho GAPs that, in turn, decreases the total cellular level of GTP-Rho and disrupts stress signaling.
Results
αS Toxicity Depends on the Integrity of Its N Terminus and on Membrane Binding.
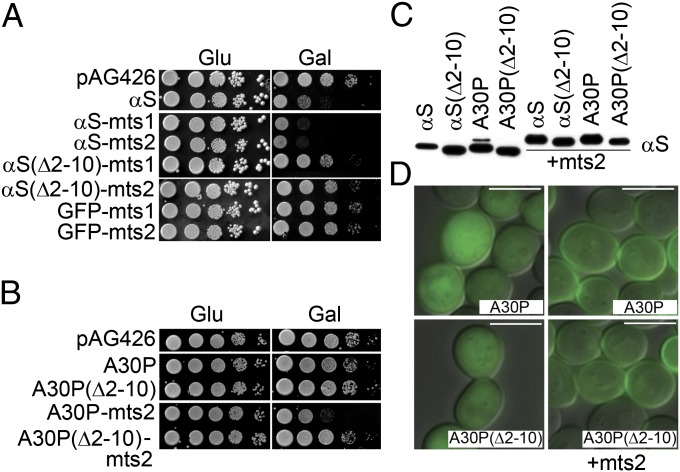
To determine whether αS toxicity is due to membrane binding, two different C-terminal membrane-targeting sequences (mts) that should increase the amount αS on the plasma membrane were tested. Mts1 (SNSVCCTLM) and mts2 (GSGGCCLLS) are derived from the yeast proteins Ste18 and Ras2, respectively; in vivo, these tags are palmitoylated, and the resultant palmitoylated protein localizes to the plasma membrane (16). Cells expressing untagged αS exhibited impaired growth compared with vector control cells, whereas cells expressing αS-mts1 or αS-mts2 exhibited an even more pronounced growth defect than cells expressing the untagged protein (Fig. 1A). Cells expressing GFP-mts1 or GFP-mts2 exhibited nearly the same growth pattern as vector control cells. More dramatic results were obtained with the A30P mutant, which is mainly cytosolic and less toxic than αS in yeast (17). Cells expressing A30P or A30P(∆2–10) showed no growth defects compared with vector control cells, whereas A30P-mts2 but not A30P(∆2–10)-mts2 severely inhibited growth (Fig. 1B). Western blot analysis showed similar expression levels of all proteins (Fig. 1C). Fluorescence and electron microscopy revealed that the mts tag promoted the accumulation of A30P and A30P(∆2–10) at the plasma membrane and the bud neck (Fig. 1D and Fig. S1). Overall, αS/A30P toxicity increases with increased plasma membrane binding. Additionally, the loss of toxicity of the membrane-tethered synucleins upon deletion of only nine N-terminal amino acids indicates that the N terminus of membrane-bound αS inhibits some other membrane protein or membrane-bound αS adopts a toxic conformation only in the context of the full-length protein.
Fig. 1.
The integrity of the N terminus determines αS toxicity. (A and B) Growth assay. Yeast cells with indicated plasmids were diluted, spotted onto plates, and grown for 3 d at 30 °C. Gal, galactose; Glu, glucose. (C) Western blot of αS variants. Untagged and tagged variants of αS, αS(∆2–10), A30P, and A30P(∆2–10) were expressed at similar levels (induction time = 8–10 h). Cell extracts were prepared and subjected to SDS/PAGE and Western blotting. (D) GFP-A30P-mts2 localizes to the plasma membrane. Yeast cells transformed with indicated plasmids were induced for 10 h at 30 °C and then imaged by fluorescence microscopy. mts2, membrane-targeting sequence. (Scale bar: 5 μm.)
αS Disrupts the Yeast Cell Wall Integrity Pathway.
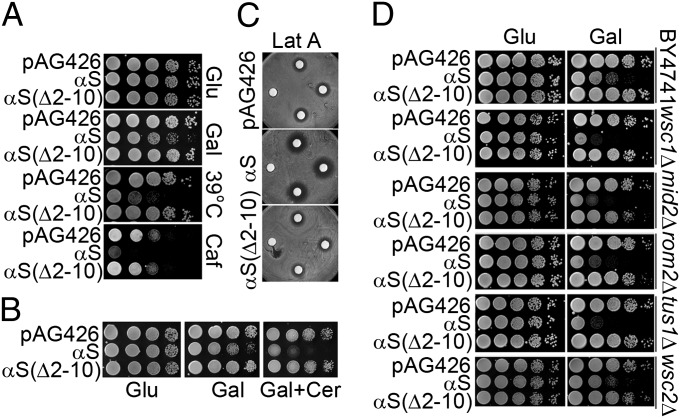
We also discovered that yeast cells expressing αS but not αS(∆2–10) were hypersensitive to elevated temperatures and caffeine, each resulting in impaired growth (Fig. 2A; see SI Text and Fig. S2). On the basis of these findings, we hypothesized that membrane-bound αS disrupts the yeast cell wall integrity (CWI) pathway—the MAPK cascade that helps cells monitor and respond to cell wall stress (15). This hypothesis was tested in various assays.
Fig. 2.
αS disrupts the yeast cell wall integrity pathway. (A) αS disrupts the CWI pathway. Cells with high-dose plasmids (pAG426) were serially diluted, spotted onto the indicated plates, and grown for 3–5 d at 30 °C or 39 °C. Caf, caffeine (8 mM). (B) Growth assay of BY4741 transformed with indicated high-dose pAG plasmids, serially diluted, and spotted onto various plates. Cer, cercosporamide (5 μg/mL). Plates were incubated at 30 °C for 2 or 3 d at 30 °C. (C) A halo assay was used to test the sensitivity of cells expressing high-dose αS or αS(∆2–10) to latrunculin-A. (D) Growth analysis of deletion mutants in the CWI pathway at 30 °C. Cells were serially diluted in successive 10-fold dilutions and spotted onto indicated plates, which were then incubated 2 d at 30 °C.
Cells expressing αS but not αS(∆2–10) were hypersensitive to cercosporamide (Cer), which inhibits Pkc1 (18), and to latrunculin-A (Lat-A), which inhibits F-actin polymerization (19), resulting in impaired growth (Fig. 2 B and C). The hypersensitivity of αS-expressing cells to heat and to these various compounds is consistent with the notion that αS disrupts the CWI pathway.
To probe the CWI pathway for genes with synthetic lethal interactions with αS at 30 °C, 15 deletion mutants in the CWI pathway were tested and enhanced toxicity of αS but not αS(∆2–10) was found in wsc1Δ and mid2Δ and rom2Δ and tus1Δ (Fig. 2D and Fig. S3A). WSC1 and MID2 encode for membrane bound sensors, and ROM2 and TUS1 encode for Rho1 GEFs.One deletion mutant, sac7Δ, was identified that partially protected against αS-induced toxicity at 39 °C (Fig. S3B). SAC7 encodes a Rho1 GAP, and deletion of this gene increases the total cellular level of GTP-Rho1 (20).
αS Decreases the Global Level of GTP-Rho1 by Disrupting Cdc5 Function.
Sakchaisri and colleagues discovered that Cdc5, which localizes to the bud neck (21), phosphorylates Tus1 and that phospho-Tus1 concentrates at the bud neck where it activates Rho1 to trigger cytokinesis (14). These latter authors proposed that Cdc5 also controls the global level of GTP-Rho1 by its ability to phosphorylate and, thus, activate or inactivate Rho1 GEFs or Rho1 GAPs, respectively. Our hypothesis is that αS at high levels saturates Cdc5, which prevents Cdc5 from phosphorylating and activating Rho1 GEFs (or inactivating Rho1 GAPs). The net result is that the global level of GTP-Rho1 decreases, which blocks signaling to the nucleus under conditions that would otherwise activate the CWI pathway. Several different types of assays were used to test this hypothesis.
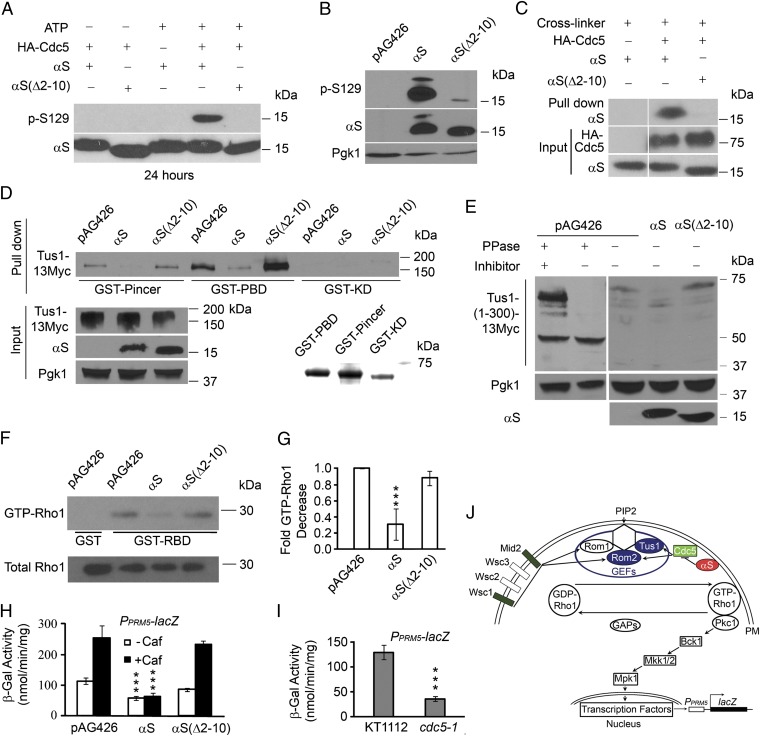
First, to determine whether Cdc5 interacts with αS and αS(∆2–10), kinase and binding (pull-down) assays were conducted with purified recombinant αS and immunoprecipitated hemagglutinin (HA)-tagged Cdc5. In the kinase assay, HA-Cdc5 and αS or αS(∆2–10) (100 μM) were incubated with Mg+2/ATP, and then the reaction mixture was subjected to SDS/PAGE followed by Western blot analysis by using a monoclonal antibody specific for αS Ser-129-(Pi). αS was phosphorylated by HA-Cdc5 at Ser-129, whereas αS(∆2–10) was nearly devoid of phosphorylation at this amino acid (Fig. 3A). In vivo, αS was phosphorylated at Ser-129, whereas αS(∆2–10) was nearly devoid of phosphorylation at this amino acid (Fig. 3B). In the binding assay, we tried without success to pull down αS and αS(∆2–10) by using HA-Cdc5 bound to agarose beads. Instead, a cross-linking assay was used where HA-Cdc5 and αS or αS(∆2–10) (50 μM) were incubated for 1 h at 4 °C with the reversible cross-linker 3,3′-dithiobis-[sulfosuccinimidyl-propionate]. After centrifugation and washing, the sample was subjected to SDS/PAGE followed by Western blot analysis using antibodies against αS and the HA tag (Fig. 3C); this experiment revealed that αS but not αS(∆2–10) cross-linked to HA-Cdc5. Our interpretation is that αS binds to and dissociates from Cdc5 with rapid kinetics and that the 9 N-terminal amino acids facilitate the interaction with Cdc5.
Fig. 3.
αS disrupts stress signaling in yeast. (A) Cdc5 phosphorylates αS at serine-129 (p-S129) in vitro. HA-Cdc5 and recombinant αS or αS(∆2–10) (100 μM) were incubated with Mg+2/ATP, followed by SDS/PAGE, Western blotting, and staining with indicated antibodies. (B) Phosphorylation of αS at S129 in vivo. Shown is Western blot of cell lysates from αS or αS(∆2–10) expressing cells using antibodies against αS. (C) αS cross-links to Cdc5. HA-Cdc5 and recombinant αS or αS(∆2–10) (50 μM) were cross-linked with 3,3′-dithiobis-[sulfosuccinimidylpropionate] and then analyzed by SDS/PAGE, Western blotting and staining with an antibody against αS. (D) Tus1-myc pull-down. Lysates from cdc15-2 cells overexpressing Tus1-myc and αS or αS(∆2–10) were incubated with the indicated GST-Cdc5 constructs. After precipitation, samples were Western blotted and the blots were stained with an anti-myc antibody. Also shown is a blot of the input and a SDS-polyacrylamide gel of the purified GST constructs stained with Coomassie blue. (E) αS inhibits Tus1(1–300) phosphorylation. Lysates from cdc15-2 cells expressing Tus1(1–300)-myc and αS, αS(∆2–10), or the indicated reagents were Western blotted and blots were stained with an anti-myc antibody. PPase, calf intestinal alkaline phosphatases (10 U); inhibitors, EDTA, and Na3VO4. (F) αS decreases GTP-Rho1. cdc15-2 cells with flag-Rho1 and αS plasmids were induced for 8 h at 30 °C. Western blot of cell lysates shows that GST-Pkc1-RBD but not GST pulled down GTP-Rho1. (G) Effect of αS on the ratio of GTP-Rho1 to Rho1 band intensity compared with control cells (pAG426). ***P < 0.001 was determined for αS versus vector (n = 3 independent experiments) by one-way ANOVA, Dunnett’s post hoc analysis. (H) αS inhibits signaling to the nucleus. β-gal activity of cells transformed with high-dose αS plasmid and p1366 plasmid (PRM5::lacZ) that were induced for 10 h (last 6 h ± 20 mM caffeine). ***P < 0.001 was determined for αS versus vector (n = 3) by one-way ANOVA, Dunnett. (I) Cdc5 controls GTP-Rho1. β-gal assay of wild-type and cdc5-1 mutant cells grown to late log phase at 23 °C and then incubated for 40 min at 37 °C (n = 4). ***P < 0.001, determined by a two-tailed Student's t test. (J) Model for how αS disrupts the CWI pathway.
Second, to determine whether αS inhibits the binding of Tus1 to the polo box (14), we used GST attached to three different Cdc5 constructs—the polo box (GST-PBD), the inactive polo box (GST-Pincer), and the kinase domain (GST-KD)—to pull down Tus1. For this assay, BY4741 cdc15-2 cells, which are arrested in anaphase at 37 °C (14), were used. Lysates from cdc15-2 cells expressing Tus1-myc and αS or αS(∆2–10) were incubated with the various purified GST constructs, and then after several washing steps samples were subjected to SDS/PAGE followed by Western blotting with an anti-myc antibody (Fig. 3D). GST-PBD pulled down Tus1-myc from control cells (no αS expression), from cells expressing αS(∆2–10), but not from cells expressing αS. A similar pattern was observed with GST-Pincer, although in general less Tus1-myc was pulled down compared with GST-PBD, consistent with a defect in the polo box. The kinase domain failed to pull down Tus1. Overall, αS but not αS(∆2–10) inhibits the binding of Tus1 to Cdc5.
In complementary experiments, we tested whether αS inhibits the phosphorylation of Tus(1–300)-myc by Cdc5 in cdc15-2 cells. This Tus1 variant is phosphorylated in vivo as indicated by the large electrophoretic mobility shift that occurs upon phosphatase treatment (14) (Fig. 3E, Left). Whether αS alters the mobility of the hyperphosphorylated Tus1(1–300)-myc was tested. Lysates of cdc15-2 cells that expressed Tus1(1–300)-myc and coexpressed αS or αS(∆2–10) were probed with an anti-myc antibody. The high molecular mass band at ∼75 kDa, which we attribute to hyperphosphorylated Tus1(1–300)-myc, occurred in cells expressing αS(∆2–10) and in control cells, but was absent from cells expressing αS (Fig. 3E, Right); instead, a lower molecular mass band was observed. The results are consistent with αS but not αS(∆2–10) partially inhibiting the phosphorylation of Tus1(1–300) by Cdc5.
Third, to determine whether αS alters the total cellular level of GTP-Rho1, a pull-down assay with GST-Pkc1-PBD beads (14) was used. PBD is a Pkc1 domain that specifically binds a GTP-Rho1 molecule. Lysates from the cdc15-2 strain were tested. αS decreased the total cellular level of GTP-Rho1 in cdc15-2 cells compared with vector control cells; whereas, the total cellular level of GTP-Rho1 in αS(∆2–10)-expressing cells was indistinguishable from vector control cells (Fig. 3 F and G).
Fourth, to determine whether the αS disrupts signaling to the nucleus upon activation of the CWI pathway, a reporter plasmid was used in which the bacterial lacZ gene was under the control of the Rlm1-regulated promoter of PRM5 (PRM5::lacZ) (22). The effect of αS-induced transcriptional activation of this reporter was quantified by measuring β-galactosidase (β-gal) activity. Strikingly, for wild-type cells treated without caffeine and with caffeine, αS inhibited β-gal activity by 48% and 75%, respectively, compared with cells expressing αS(∆2–10) or vector control cells (Fig. 3H); this data indicates that membrane-bound but not cytosolic αS inactivates transcription of this reporter gene. A parallel experiment showed that inactivating Cdc5 by using a temperature-sensitive mutant (cdc5-1) decreased β-gal activity compared with wild-type cells (Fig. 3I), which is consistent with Cdc5 controlling the CWI pathway (Fig. 3J). The experiments in Fig. 3 show that αS but not αS(∆2–10) specifically blocks transcription of a gene in the CWI pathway by its ability to inhibit Cdc5. αS also disrupts Cdc5-dependent cell cycle functions; see SI Text and Figs. S4 and S5.
αS Disrupts Stress Signaling in SH-SY5Y Cells.
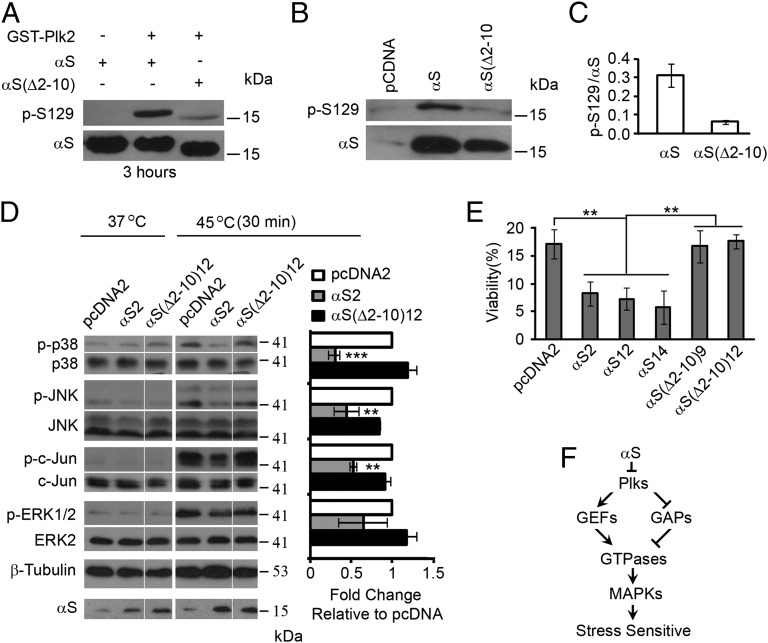
αS has been reported to inhibit the phosphorylation of the p38 MAPK and accelerate the death of mouse neuroblastoma cells in culture (23). Small molecular inhibitors of p38 also trigger cell death (24), including in SH-SY5Y cells (Fig. S6A). Our interpretation is that αS inhibits the phosphorylation of p38 and accelerates cell death because it perturbs upstream interactions between Plk2 with Rho/Ras GEFs and/or Plk2 with Rho/Ras GAPs, which lowers the level of active Rho/Ras and, thus, shuts down this pathway. Because detecting changes in active Rho/Ras levels in human cells, which have ∼56 of these proteins (25), is quite challenging, we probed the phosphorylation of various MAPKs in human neuroblastoma SH-SY5Y cell lines that stably overexpress αS or αS(∆2–10). It was first confirmed that GST-Plk2 phosphorylates αS more efficiently than αS(∆2–10) in vitro (Fig. 4A), and nearly the same results were found in vivo (Fig. 4 B and C). The phosphorylation of p38, c-Jun N-terminal kinase (JNK), and extracellular-signal-regulated kinases 1 and 2 (ERK1/2), which are activated by various stresses (26), and the transcription factor c-Jun was assessed by Western blotting with phospho-specific antibodies after incubating SH-SY5Y cells for 0.5 h at 37 °C or 45 °C. Upon heat stress, αS partially inhibited the phosphorylation of p38, JNK, and c-Jun compared with control cells, whereas αS(∆2–10) had no effect (Fig. 4D and Fig. S6B). We also examined cell viability 24 h after the brief heat shock by using several different SH-SY5Y clones that overexpress αS (clone 2, 12, 14) or αS(∆2–10) (clone 9, 12). αS but not αS(∆2–10) accelerated cell death compared with vector control cells as evidenced by the 59 ± 6% decrease in viability of SH-SY5Y clones overexpressing αS compared with vector control cells and SH-SY5Y clones overexpressing αS(∆2–10) (Fig. 4E). The inhibition of the phosphorylation of p38 by αS but not by αS(∆2–10) in human cells is similar to the inhibition of the CWI pathway by αS in yeast.
Fig. 4.
αS disrupts stress signaling in human cells. (A) Plk2 phosphorylates αS at S129 in vitro. GST-Plk2 and recombinant αS or αS(∆2–10) (50 μM) were incubated with Mg+2/ATP, followed by SDS/PAGE, Western blotting, and staining with antibodies against αS and p-S129-αS. (B) αS is phosphorylated at S129 in SH-SY5Y cells. Cell lysates were Western blotted and stained with indicated antibodies (n = 2). (C) Plot shows the p-S129 to αS ratio. (D) αS inhibits MAPK phosphorylation. SH-SY5Y cells overexpressing αS or αS(∆2–10) were incubated at 37 °C or 45 °C for 30 min; lysates were Western blotted with the indicated antibodies. Bar graph is fold change of the phospho-protein to protein band intensity relative to control cells (pcDNA). Values are means ± SD (n = 3). In each set of three samples αS is compared with vector and to αS(∆2–10). **P < 0.01 and ***P < 0.001 were determined by using a one-way ANOVA with a Tukey post hoc test. (E) Cell viability after heat shock. Cells were heat shocked at 51 °C for 30 min, returned to a 37 °C incubator, and the viability was measured 24 h later by using the MTT assay. Values are means ± SD (n = 3). P value range (**, 0.001 < P < 0.01) of indicated comparisons was determined by using one-way ANOVA with Tukey post hoc test. (F) Model of αS inhibiting Plk interactions with Rho GEFs/GAPs. Inhibition can occur in solution or when αS and Plks concentrate at the plasma membrane.
Discussion
This study has provided evidence for the following mechanism of αS pathology: high expression levels of αS prevent Plks from activating Rho regulatory proteins (GEFs/Gaps), which, in turn, leads to a decrease in the total cellular level of GTP-Rho. αS is toxic is because it inhibits Plks and disrupts signaling (Fig. 4F), not because it is phosphorylated by Plks at Ser-129. Although αS-Ser-129-Pi may aggregate and forms inclusions, whether it is toxic to cells is debatable.
Deleting the first nine N-terminal residues of αS causes a loss of toxicity in yeast and human cells compared with the full-length protein, and that the lack of toxicity of αS(Δ2–10) is a consequence of its failure to interact with Plks (Figs. 3 A–I and 4 A–E), not to its failure to interact with the plasma membrane. For instance, for the two proteins forced into the yeast plasma membrane, A30P-mts and A30P(Δ2–10)-mts, the former is extremely toxic, whereas the latter is not (Fig. 1 A–C). Strikingly, in both yeast and human SH-SY5Y cells αS but not αS(∆2–10) blocks a MAPK pathway and accelerates death due to heat shock (Figs. 2 A–D and 4 D and E and Fig. S2 B–E). A recent report showed that, in contrast to yeast, deleting the first 10 N-terminal residues has no effect on αS membrane binding, aggregation, and cell viability in human SH-SY5Y cells (27). Specifically, αS and αS(Δ2–11) each bind to the plasma membrane and each exhibit identical toxicity when stably expressing SH-SY5Y cells are treated with a proteasome inhibitor. Although αS and the N-terminal deletion mutant are equally toxic in the proteasomal inhibition assay, the two proteins have unequal toxicities in the heat shock assay; thus, whether αS and the N-terminal deletion mutant have the same or different toxicities depends on the cellular pathway.
The efficiency by which Plks phosphorylate αS at Ser-129 was recently shown to depend on the N terminus of αS. Specifically, αS(103–140) fails to be phosphorylated by all four Plks (Plk1–4) tested, whereas the intact protein is efficiently phosphorylated (28). Our results show that deletion of only 9 N-terminal residues abolishes the ability of Cdc5/Plk2 to phosphorylate αS in vitro and in vivo (Figs. 3 A and B and 4 A–C). Our interpretation of our findings is that membrane-bound αS/A30P are extremely toxic because they inhibit membrane-bound Cdc5, which, in turn, decreases the level of GTP-Rho1, and the membrane-bound deletion mutants are not toxic because they cannot interact with Cdc5. Whether the N terminus of αS/A30P directly binds to the polo-box of Cdc5/Plk2 or whether a unique conformation, which binds to the polo-box, exists only in full-length protein cannot be determined at this time. Whether membrane-associated or soluble, Plks that interact with Rho GEFs and Rho GAPs should be subject to inhibition and consequent disruption of cell signaling by αS.
We propose that αS does not inhibit Plk2 under normal cellular conditions because its concentration is not high enough. Were the concentration of αS to increase with age, however, then its inhibition of Plk2-GEF/GAP interactions would proportionally increase, and cells would become more and more sensitive to stress (Fig. 4F). An increase in the concentration of neuronal αS can come from an age-dependent decline in the function of the lysosome or the proteasome (3). In our model, decreasing the concentration of αS, increasing the concentration of Plk2 (9), or blocking the binding of αS to Plk2 should rescue αS toxicity. A therapeutic strategy for PD is to block the binding of αS to Plks without interfering with the binding of other substrates. Our mechanism explains how soluble, monomeric αS can become toxic to cells with age.
Materials and Methods
Yeast Strains and Media.
For additional methods, see SI Materials and Methods. Yeast strains and plasmids used in this study are given in Tables S1 and S2, respectively. Synthetic complete drop-out media were prepared as described in ref. 29. Raffinose and galactose were used in the noninducing and inducing media, respectively. Drop-out media were purchased from Sigma-Aldrich and United States Biological, and, unless otherwise noted, all chemicals were purchased from Sigma-Aldrich. Yeast cells were grown with shaking at 30 °C, or, for heat stress experiments, at 39 °C.
Growth Assay.
Yeast cells transformed with various plasmids were grown overnight in liquid raffinose medium and then diluted (to OD600 = 0.2) into liquid galactose medium and incubated for 6–8 h. Cultures were normalized to the same OD, serially diluted in 10-fold increments, spotted (10 μL) onto solid plates, and incubated for 2–6 d at 30 °C. Assays using latrunculin A and β-galactosidase are described in SI Materials and Methods.
Western Blot Analysis.
For yeast experiments, lysate preparation and Western blotting were carried out as described (30). Fifty micrograms of total protein was loaded per well. For mammalian cell culture experiments, cells were cultured, harvested, and lysed in RIPA buffer [50 mM Tris at pH 7.2, 150 mM NaCl, 1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, 10 mM NaF, 1 mM Na3VO4, and protease inhibitor mixture (1:1,000; Sigma-Aldrich)]. All steps were carried out at 4 °C. Lysates were sonicated for 10 s and centrifuged at 14,000–15,000 × g for 10 min. Protein concentration was determined by bicinchoninic acid assay (Pierce). Equivalent amounts of protein were separated on a 7.5–12% (wt/vol) or 4–20% (wt/vol) SDS–polyacrylamide gel (Bio-Rad) and transferred to polyvinylidene difluoride membrane. Membranes were blocked for nonspecific binding and then incubated with primary antibodies at the recommended dilutions followed by the appropriate secondary antibody conjugated to horseradish peroxidase. Immunoreactive bands were visualized by using enhanced chemiluminescence solution (Pierce). Membranes were stripped and blotted for tubulin as a loading control. Bands were quantified by densitometry using the Photoshop (Adobe) histogram function. Antibodies and methods for analyzing the effect of αS on the phosphorylation of Tus1(1–300) are given in SI Materials and Methods.
GTP-Rho1 Pull Down.
The 3Flag-Rho1 labeled strain transformed with pAG426, pAG426-αS, or pAG426-αS(∆2–10) was pregrown in noninducing medium and then induced in galactose medium for 8 h. Lysates were prepared and incubated with either Pkc1-RBD (David Pellman, Harvard University, Boston) bound to GST agarose beads or GST agarose beads as a control, as described in ref. 14. After several washes with PBS, the beads were analyzed for retention of GTP-Rho1 by SDS/PAGE followed by Western blotting with an anti-Flag antibody. Total Rho1 was used as loading control.
GST-Cdc5 Pull Down.
The cdc15-2 strain with integrated TUS1-13-myc transformed with pAG426, pAG426-αS, or pAG426-αS(Δ2–10) was pregrown in sucrose medium at 23 °C and then diluted (OD600 = 0.2) into galactose medium and induced overnight at 23 °C. The cultures were shifted to 37 °C to arrest the cell cycle at anaphase for 4 h and then released by returning to 23 °C. After a 1-h recovery, the cells were harvested and lysed. A sample of supernatant (3 mg of total protein) was mixed with an aliquot of GST-PBD, GST-Pincer, or GST-KD bound to agarose beads. After overnight incubation at 4 °C, the beads were washed, boiled for 5 min at 95 °C, and centrifuged. Samples were subjected to SDS/PAGE followed by Western blotting. A mouse monoclonal anti-myc (9E10) (gift from Kelly Tatchell, Shreveport, LA) was used as the primary antibody.
Kinase and Cross-Linking Assays.
HA-Cdc5 was immunoprecipitated from a yeast cell lysate as follows. BY4741 cells transformed with HA-Cdc5 expressing plasmid p384 (Angela Amon, Massachusetts Institute of Technology, Cambridge, MA) or empty vector were grown, harvested, and lysed with glass beads. The supernatant was incubated with anti-HA antibody (2367P; Cell Signaling) for 10 h, then protein-A-agarose beads were added, and the solution was incubated for another 2 h. The kinase assay consisted of 200-μL samples of 0.1 mM αS or αS(∆2–10), 10 mM MgCl2, 1 mM ATP, and 20 μL of HA-Cdc5 bound agarose beads (or controls beads with bound antibody but no Cdc5) in 20 mM Tris⋅Cl (pH 7.4). After 24 h at 30 °C, the reaction mixture was centrifuged and the supernatant was subjected to SDS/PAGE followed by Western blotting with an antibody specific for phospho-Ser-129 αS (Epitomics). The cross-linking assay consisted of 200-μL samples containing 0.5 mM αS or αS(∆2–10), 0.5 mM DTSSP (3,3′-dithiobis-[sulfosuccinimidylpropionate]) (Pierce), and 20 μL of the agarose beads prepared as above in 50 mM Hepes (pH 7.4). After 1-h incubation at 4 °C, the reaction was stopped by adding 50 mM Tris. After several washes, the beads were subjected to SDS/PAGE followed by Western blot analysis with an antibody against αS.
The Plk2 kinase assay was conducted in 15 μL of 20 mM Tris⋅Cl buffer (pH 7.4) containing 0.05 mM purified αS or αS(∆2–10), 10 mM MgCl2, 1 mM ATP, and 0.13 μg/mL human GST-Plk2 (PV4204; Invitrogen). After 3-h incubation at 30 °C, the reaction mixture was halted by adding 15 μL of 2× SDS/PAGE loading buffer and boiled for 5 min. The supernatant was subjected to SDS/PAGE followed by Western blotting with the phospho-specific αS antibody.
Mammalian cell culture.
SH-SY5Y human neuroblastoma cells (ATCC) were maintained in a 1:1 mixture of EMEM (ATCC) and Ham’s F-12 medium (Invitrogen) supplemented with 10% FBS (Biowest), 1% penicillin-streptomycin solution in a humidified incubator, 5% CO2 at 37 °C. Stably transfected cell lines (SI Materials and Methods) were kept in the same medium supplemented with 200 μg/mL G418.
Fluorescence microscopy.
Fluorescent images of yeast cells were acquired with an Olympus AX70 microscope equipped with an Olympus UPlanFl 100×/1.35 N.A. objective and a CoolSNAP HQ CCD camera (Roper Scientific). For GFP detection, a Chroma 41001 filter was used (excitation 480/40 nm, emission 535/50 nm; Chroma Technology). Image analysis, filter wheels, shutters, and z axis stepping motor were under the control of imaging software Slidebook 4.0 (Intelligent Imaging Innovations). Images were acquired at room temperature.
Statistical analysis.
P values were determined by an unpaired, two-tailed Student’s t test when comparing two samples, by one-way ANOVA with a Dunnett post hoc test when comparing more than two samples to a control, or by one-way ANOVA with a Tukey post hoc test when comparing multiple samples. Experimental values are means ± SD of typically three to five independent experiments. Microsoft Excel and KaleidaGraph were used for the statistical tests.
Supplementary Material
Acknowledgments
We thank Angelika Amon, David Levin, Susan Lindquist, David Pellman, and Kelly Tatchell for strains and plasmids. This work was supported by National Institutes of Neurological Disorders and Stroke Grant NS057656 and a grant by the Parkinson’s Disease Resource of Northwest Louisiana (to S.N.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206286109/-/DCSupplemental.
References
- 1.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 2.Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 3.Mazzulli JR, et al. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 5.Krüger R, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 6.Zarranz JJ, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 7.Singleton AB, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JP, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 9.Gitler AD, et al. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inglis KJ, et al. Polo-like kinase 2 (PLK2) phosphorylates alpha-synuclein at serine 129 in central nervous system. J Biol Chem. 2009;284:2598–2602. doi: 10.1074/jbc.C800206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archambault V, Glover DM. Polo-like kinases: Conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10:265–275. doi: 10.1038/nrm2653. [DOI] [PubMed] [Google Scholar]
- 12.Pak DT, Sheng M. Targeted protein degradation and synapse remodeling by an inducible protein kinase. Science. 2003;302:1368–1373. doi: 10.1126/science.1082475. [DOI] [PubMed] [Google Scholar]
- 13.Lee KJ, et al. Requirement for Plk2 in orchestrated ras and rap signaling, homeostatic structural plasticity, and memory. Neuron. 2011;69:957–973. doi: 10.1016/j.neuron.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida S, et al. Polo-like kinase Cdc5 controls the local activation of Rho1 to promote cytokinesis. Science. 2006;313:108–111. doi: 10.1126/science.1126747. [DOI] [PubMed] [Google Scholar]
- 15.Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivasa SP, Bernstein LS, Blumer KJ, Linder ME. Plasma membrane localization is required for RGS4 function in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1998;95:5584–5589. doi: 10.1073/pnas.95.10.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003;302:1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sussman A, et al. Discovery of cercosporamide, a known antifungal natural product, as a selective Pkc1 kinase inhibitor through high-throughput screening. Eukaryot Cell. 2004;3:932–943. doi: 10.1128/EC.3.4.932-943.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarmola EG, Somasundaram T, Boring TA, Spector I, Bubb MR. Actin-latrunculin A structure and function. Differential modulation of actin-binding protein function by latrunculin A. J Biol Chem. 2000;275:28120–28127. doi: 10.1074/jbc.M004253200. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida S, Bartolini S, Pellman D. Mechanisms for concentrating Rho1 during cytokinesis. Genes Dev. 2009;23:810–823. doi: 10.1101/gad.1785209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakchaisri K, et al. Coupling morphogenesis to mitotic entry. Proc Natl Acad Sci USA. 2004;101:4124–4129. doi: 10.1073/pnas.0400641101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung US, Sobering AK, Romeo MJ, Levin DE. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol Microbiol. 2002;46:781–789. doi: 10.1046/j.1365-2958.2002.03198.x. [DOI] [PubMed] [Google Scholar]
- 23.Iwata A, Maruyama M, Kanazawa I, Nukina N. alpha-Synuclein affects the MAPK pathway and accelerates cell death. J Biol Chem. 2001;276:45320–45329. doi: 10.1074/jbc.M103736200. [DOI] [PubMed] [Google Scholar]
- 24.Nemoto S, Xiang J, Huang S, Lin A. Induction of apoptosis by SB202190 through inhibition of p38beta mitogen-activated protein kinase. J Biol Chem. 1998;273:16415–16420. doi: 10.1074/jbc.273.26.16415. [DOI] [PubMed] [Google Scholar]
- 25.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 26.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: Signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 27.Vamvaca K, Lansbury PT, Jr, Stefanis L. N-terminal deletion does not affect α-synuclein membrane binding, self-association and toxicity in human neuroblastoma cells, unlike yeast. J Neurochem. 2011;119:389–397. doi: 10.1111/j.1471-4159.2011.07431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mbefo MK, et al. Phosphorylation of synucleins by members of the Polo-like kinase family. J Biol Chem. 2010;285:2807–2822. doi: 10.1074/jbc.M109.081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burke D, Dawson D, Stearns T. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2000. pp. 103–174. [Google Scholar]
- 30.Lee YJ, Wang S, Slone SR, Yacoubian TA, Witt SN. Defects in very long chain fatty acid synthesis enhance alpha-synuclein toxicity in a yeast model of Parkinson’s disease. PLoS ONE. 2011;6:e15946. doi: 10.1371/journal.pone.0015946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.