Abstract
Replicating bacterial chromosomes continuously demix from each other and segregate within a compact volume inside the cell called the nucleoid. Although many proteins involved in this process have been identified, the nature of the global forces that shape and segregate the chromosomes has remained unclear because of limited knowledge of the micromechanical properties of the chromosome. In this work, we demonstrate experimentally the fundamentally soft nature of the bacterial chromosome and the entropic forces that can compact it in a crowded intracellular environment. We developed a unique “micropiston” and measured the force-compression behavior of single Escherichia coli chromosomes in confinement. Our data show that forces on the order of 100 pN and free energies on the order of 105 kBT are sufficient to compress the chromosome to its in vivo size. For comparison, the pressure required to hold the chromosome at this size is a thousand-fold smaller than the surrounding turgor pressure inside the cell. Furthermore, by manipulation of molecular crowding conditions (entropic forces), we were able to observe in real time fast (approximately 10 s), abrupt, reversible, and repeatable compaction–decompaction cycles of individual chromosomes in confinement. In contrast, we observed much slower dissociation kinetics of a histone-like protein HU from the whole chromosome during its in vivo to in vitro transition. These results for the first time provide quantitative, experimental support for a physical model in which the bacterial chromosome behaves as a loaded entropic spring in vivo.
Keywords: chromosome segregation, depletion forces, polymer physics, mother machine, optical trap
Like many other model bacterial organisms such as Bacillus subtilis and Caulobacter crescentus, Escherichia coli has a single circular chromosome. In vivo, the chromosome exists in a highly compacted state, occupying only a subvolume of the micron-sized rod-shaped cell. As early as 1956, Mason and Powelson observed that compact E. coli chromosomes exhibit dynamic morphological changes during DNA replication and segregation before cell division (1). Undoubtedly, the changing chromosome morphologies during the cell cycle reflect a dynamic balance between the polymeric nature of the chromosome and the active and passive forces acting on the chromosome by other molecules inside the cell. It is odd, then, that our understanding of the micromechanical properties of bacterial chromosomes is almost completely lacking, especially considering the detailed knowledge of the molecular factors involved in replication and protein synthesis that influence the chromosome (2). For instance, despite a large number of proposed models (3–17), we have not been able to experimentally answer obvious questions such as how much force is required to maintain the in vivo chromosomes in their compacted state or to segregate them during DNA replication. Can we characterize experimentally the nature of the forces that shape the bacterial chromosome?
For the much smaller (and biochemically simpler) system of viruses and their genomes, we now have a comprehensive understanding of the role of physical properties of viral DNA on its packaging into and ejection from the capsid (18, 19). For the much larger (and more easily manipulable) system of eukaryotic chromosomes and spindles, a combination of biophysical and biochemical methods has begun to unravel their micromechanical properties (20–22). In contrast, despite some of the earlier pioneering biophysical work (23), experimental progress for bacterial chromosomes has lagged behind, because of compound experimental difficulties (ı.e., small in size but complex in molecular and biochemical detail).
In this work, we measured the force-compression curves (rather than more traditional force-stretching curves) of individual whole bacterial chromosomes in confinement. Because the spatial dimensions of the confinement were comparable to the size of the cell, we developed a new experimental system that brings together imaging, microfluidics, and single-molecule manipulation techniques. Importantly, by combining the microfluidic “mother machine” (24) and an optical trap, we developed the micropiston to compress isolated single chromosomes confined in long narrow microchannels.
The measured force-compression curves are consistent with an entropic spring model (11, 17, 25). The quantitative agreement between the data and the model allows us to estimate the force and free energy required to compress the in vitro chromosome to its in vivo size. In stark contrast to the viral DNA in a capsid, our experiments demonstrate that the bacterial chromosome is fundamentally “soft” and can be readily influenced by entropic forces. These experimental results, combined with a physical model, provide quantitative and mechanistic insights into the physical nature of the bacterial chromosome.
Results
Experimental System for Gentle and Synchronous Lysis of Cells in a Confined Space.
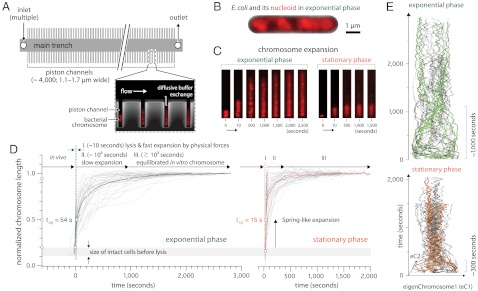
To investigate the micromechanical properties of isolated bacterial chromosomes in confinement, we had to overcome considerable technical challenges imposed by the 1-μm length scale of the E. coli cell. The small size of the cell prevented the use of micropipettes (20) or microneedles (21) that have been successful in probing much larger eukaryotic chromosomes and spindles. Instead, we started from previous work that demonstrated the proof of principle of bacterial chromosome extraction in a microfluidic device (26) and lysis in suspension (27). As illustrated in Fig. 1A, the mother machine (24) was ideally suited for this purpose. The E. coli chromosomes could be extracted and confined directly in long microchannels about as wide as the cells. In brief, the cells were loaded into the microchannels. Then, sucrose buffer and lysozyme were added to the media. Synchronous lysis was achieved when this cell wall digestion buffer was replaced with the relatively dilute HEPES buffer at a specific ionic strength dictated by the concentration of NaCl (23). Experiments were conducted for cells prepared in two different physiological conditions (exponential and stationary phase). To visualize the chromosome, we made use of a previously characterized, fully functional fusion of the fluorescent protein mCherry to one of the most abundant nucleoid-associated proteins, HupA (part of the histone-like HU protein dimer) (28). Thus, morphological and biochemical changes of the chromosomes during the in vivo to in vitro transition were easily observable (Fig. 1B–E).
Fig. 1.
Experimental setup and chromosome dynamics during lysis (100 mM NaCl). (A) Illustration of the device: A main trench connects multiple inlets to one outlet, with thousands of protruding microchannels. Cells were loaded into microchannels (typically one per channel) and lysed, causing rapid expansion of chromosomes. The microchannels containing the chromosomes equilibrated with the reservoir via passive diffusion in seconds (24). (B) Typical E. coli cell and its nucleoid in exponential phase. The nucleoids (in red) occupy a subvolume of the cell. (C) Time-lapse pictures showing expansion and morphological relaxation of representative exponential and stationary phase chromosomes. (D) Normalized chromosome length vs. time shows rapid expansion of chromosomes to half their equilibrium length in tens of seconds. (E) Morphological relaxation analyzed by principal component analysis. Multiple time-lapse chromosome images, such as shown in C, were normalized and projected onto the top eigenmodes [the “eigenChromosomes (eC)”] (representative trajectories of individual chromosomes are in color). Consistent with D, the chromosomes from exponential phase cells show longer relaxation times (approximately 1,000 s) than those from stationary phase cells (approximately 300 s).
During the experiments, the biochemical contents of the reservoir main trench were controlled with multiple syringes and a valve. Importantly, this allowed switching from one ambient buffer to another in seconds, without subjecting the chromosomes to direct flow. SI Appendix, Sections I.A.2–4, describes the microfluidic system and lysis protocol in full detail.
Bacterial Chromosomes Show Spring-Like Fast Expansion During Lysis.
Chromosome expansion following cell lysis is a visually striking process (see Movies S1 and S2), starting with an abrupt “explosion” of the chromosome from the cell and continuing with a more gradual equilibration within the new in vitro environment. Fig. 1C shows a typical time series of the expansion dynamics of exponential and stationary phase chromosomes during lysis. Although individual chromosomes exhibited some variation, on average each chromosome showed a 2- to 3-fold expansion to half its equilibrium length in approximately 10 s (Fig. 1D, green and orange lines illustrating the mean chromosome length). After the fast initial expansion, the chromosomes slowly continued to expand to many times their in vivo volumes over a period on the order of 102–103 s before plateauing to their final equilibrium lengths.
The in vitro Chromosomes Show Physiology-Dependent Morphological Dynamics.
The chromosomes exhibited distinct morphologies after lysis, likely reflecting their in vivo physiological state. To quantify the morphological dynamics, we employed principal component analysis, a powerful method commonly used in pattern recognition with a wide range of applications in many fields (29, 30). The basic idea is to extract eigenmodes of the chromosome morphologies from the time-lapse images of each chromosome-containing microchannel. See SI Appendix, Section I.A.7, for full description and analysis.) The top eigenmodes, or the top “eigenChromosomes,” often resembled space-filling helicoids of right-handed, left-handed, or mixed handedness. We can visualize the morphological dynamics in a lower dimensional space by projecting the original time-series of chromosome images onto the eigenChromosomes. The projection coefficients form trajectories moving forward in time. Fig. 1E shows such projections onto the top two eigenChromosomes.
Notice in Fig. 1E that the exponential phase chromosomes show spiral trajectories and converge to the top eigenChromosomes after on the order of 103 s; whereas the stationary phase trajectories decay faster and converge to zero (ı.e., no apparent morphological features) in on the order of 102 s. These physiology-dependent morphological dynamics are also consistent with our observations in Fig. 1 C–D and Movies S1 and S2. That is, the exponential phase chromosomes always look more structured, and they equilibrate more slowly than the stationary phase chromosomes (Fig. 1 C–E). We attribute the differences to different replication topologies, transcription levels, and nucleoid-associated protein activities between the two physiological states (31).
After Lysis, a Histone-Like Protein HU Dissociates Slowly from the Whole Chromosome.
HU is a histone-like protein in bacteria that binds DNA nonspecifically. Phenotypic analyses of HU mutants point to many important roles in global transcription regulation, DNA replication, and DNA repair (31–33). Reported in vitro measurements for key parameters such as the dissociation rate (koff) and the equilibrium dissociation constant (KD) vary more than 10-fold (34, 35).
Our system can directly monitor dissociation of HU from the entire chromosomes during the in vivo to in vitro transition. Fig. 2 shows the normalized integrated fluorescence of a functional subunit HUα-mCherry after lysis. The integrated fluorescence exhibited nonexponential decay for approximately 103 s. This might reflect a wide range of binding affinities across the whole chromosome. Eventually, all chromosomes showed exponential decay. The dissociation rate from this last stage shows that DNA binding of HU can be remarkably stable: 1/koff ≈ 523 min for exponential and 68 min for stationary phase chromosomes at 100 mM NaCl. See SI Appendix, Section I A. 6, for full analysis of KD, kon, and koff at NaCl concentrations ranging from 40 to 200 mM.
Fig. 2.
Dissociation of HU from whole chromosomes at 100 mM NaCl, after start of acquisition phase with negligible photobleaching. (Main) HU dissociates slowly after lysis (data shown from 100 s after lysis). Initially, dissociation is nonexponential, perhaps due to a wide range of HU binding affinities of the whole chromosome. Eventually, the dissociation kinetics reached an exponential decay regime, from which we estimated the off-rates, koff. Dissociated HU that escaped from the channel via diffusion was washed from the device. (Inset) At the moment of lysis, the integrated HU intensity showed an abrupt initial drop due to loss of cytoplasmic HU. Our measurements allowed us to estimate KD and kon. Movie S3 and SI Appendix, Section I A.6, discuss cytoplasmic HU during lysis in more detail.
HU is one of the most abundant nucleoid-associated proteins with high DNA binding affinities (36). Thus, in light of the separation of time scales, we hypothesize that the fast initial chromosome expansion in Fig. 1 C–D is driven mainly by the release of micromechanical energy stored in the in vivo chromosome, rather than by dissociation of nucleoid-associated proteins. We cannot exclude the possibility that fast dissociation of unlabeled nucleoid-associated proteins or Mg2+ contributed to chromosome expansion or modulated the unbinding rates of other nucleoid-associated proteins (37); however, lysed in suspension (27), chromosomes expanded to comparable in vitro volumes in the presence and absence of Mg2+ and adenosine-5′-triphosphate (ATP) (see SI Appendix, Section I D).
The Optical-Trap Micropiston Enables Direct Measurement of the Force-Compression Curves of the Bacterial Chromosome.
Because the chromosome expands like a loaded spring, one should be able to measure its “spring constant.” Because confinement is the major physical constraint that characterizes the bacterial chromosome, it was necessary to measure micromechanical properties of individual chromosomes in confinement. To this end, we developed the micropiston, a unique system that couples optical tweezers and the mother machine. Unlike most prior work in single-molecule manipulation that focuses on pulling or stretching biomolecules, the micropiston enables the direct measurement of force-compression curves of in vitro chromosomes (Fig. 3 and Movie S4).
Fig. 3.
Mechanical compression of equilibrated chromosomes. (A) A polystyrene microbead held by optical tweezers was used to compress the chromosome against the closed channel end. The residual membrane of the cell after lysis was used as a gasket to prevent leakage of the chromosome. (B) (Inset) Raw force-compression data shows two groups of curves that represent one and two nucleoids. The error bars denote the standard deviation (SD) of multiple measurements. When rescaled by the fitted “spring constant” A and the equilibrium length R0 (mean A = -2.04 pN with SD = 1.24 pN, mean R0 = 10.1 μm with SD = 2.2 μm) all data collapsed onto a single master curve. By integrating to the equivalent in vivo size (R/R0 = 0.1, about half the smallest measurement R/R0 ≈ 0.2), we estimated the micromechanical energy stored in the in vivo chromosome to be on the order of 105 kBT.
From the raw force-compression curves (Fig. 3B, Inset), we identified two distinct populations corresponding to one and two nucleoids. Each of these exhibits nonlinear behavior that appears to be asymptotic at high forces.
The next challenge was modeling the data. In general, spatial constraints pose formidable technical barriers to analytical theory. In fact, we found only one theoretical expression for the force-compression behavior of a confined polymer that has been tested against numerical simulations (25). It is the loaded entropic spring model written by some of us and used to explain, among other phenomena, a physical mechanism of chromosome segregation in bacteria:
 |
[1] |
where f is the applied force, A = k/wβ is the rescaled spring constant for the dimensionless spring constant k (w is the width of the piston), β = 1/kBT (kB is the Boltzmann constant and T the absolute temperature), R the measured chromosome length during compression, and R0 the chromosome length under no externally applied force.
An important implication of Eq. 1 is that, if we rescale R by the equilibrium length R0 and f by the spring constant A, all data should collapse onto a single master curve. That is, the force-compression relationship is universal. It can be used to test the polymeric nature of confined chromosomes, independently of molecular details that determine R0 and A. Indeed, as seen in Fig. 3, the chromosome data collapse onto a single curve and are in excellent quantitative agreement with Eq. 1 *. Furthermore, the equilibrium force-compression data and Eq. 1 appear to be consistent with the expansion dynamics of the chromosome during lysis (see SI Appendix, Section II.A).
The quantitative agreement between the data and the model allowed us to calculate the force and free energy required to compress the chromosome back to its in vivo size. Extrapolating our data to stronger compression using Eq. 1, we estimated that about 100 pN force and 105 kBT free energy are required to fully compress the in vitro chromosome to its in vivo size (roughly 1/10 of the equilibrium length).
Depletion (Entropic) Forces by Molecular Crowding Alone Can Compress Chromosomes to in Vivo Size.
The findings presented above raised the question of why a nucleoid occupies only a subvolume of the cell but expands to several times the size of the cell upon lysis (Fig. 1). Three possibilities have been discussed in the literature (31): nucleoid-associated proteins such as HU and H-NS that can hold DNA together, DNA supercoiling, and the entropic effect of molecular crowding. However, most HU remained on the chromosome during the fast initial expansion of the chromosome after lysis (Figs. 1D and 2). Further, recent experiments demonstrated that supercoiling has only a minor effect on the size of in vitro chromosome (38).
Are depletion forces by molecular crowding sufficient to cause compaction of the chromosome in vivo? A typical E. coli cell contains on the order of one million proteins in the cytoplasm (ref. 2, chap. 3). Because each depletant has an effect of order kBT (39), the change in free energy due to molecular crowding available for chromosome compaction may be approximately 106 kBT. This is about one order of magnitude more significant than the estimated approximately 105 kBT free energy stored mechanically in the in vivo chromosomes.
We tested this idea by using high molecular weight polyethylene glycol (PEG 20000) to simulate the crowded cytoplasmic environment in our device (Fig. 4A). Here, the response of the chromosomes to molecular crowding can be monitored in real time by controlling the ambient buffer. The results were striking.
Fig. 4.
Depletion (entropic) forces by molecular crowding induce chromosome compaction. (A) Illustration of depletion interactions. (Upper) When two macro objects (red squares) are in each other’s proximity such that the volume ΔV between them is inaccessible to the depletants (white), there is effective attraction between the macro objects due to random collisions with the depletants. By integrating the ideal gas law over the volume ΔV in the illustration, we obtain the free-energy reduction  , where c is the concentration of the depletants, (Lower) Essentially the same physics applies to long chains; ı.e., depletion interactions can cause collapse of the chains (see SI Appendix, Section II.C). (B) Addition of PEG at a volume fraction comparable to that of cytoplasmic proteins caused full compaction of the equilibrated chromosomes (multiple traces in grey) back to their in vivo volume. Removal of PEG caused the chromosomes to spring back to their equilibrium in vitro volume in about 10 s, comparable to the expansion timescale after initial lysis. The experiment was repeatable for at least 11 successive cycles of PEG addition and removal. (C) Effective size of the chromosomes at different PEG concentrations. (Experiment) Just below the transition point (PEG volume fraction 11–13%), we observed interesting coexistence of compacted and decompacted regions within individual chromosomes (arrows in the snapshots). The areas of the purple and yellow circles represent the relative fraction of the decompacted and compacted regions within the chromosomes, respectively. The fraction of the decompacted chromosomes (purple) drops sharply before transition. (Theory) The solid line shows the mean chromosome length as a function of PEG concentration calculated using Eq. 1 and a modified Odijk theory (23). (The dashed line shows the result of the original Odijk theory.) Our theory predicts collapse of the chromosome at [PEG] = 18% volume fraction, in a reasonable agreement with the data (11–19%). In both our theory and the Odjik theory, the volume of completely collapsed DNA is zero.
, where c is the concentration of the depletants, (Lower) Essentially the same physics applies to long chains; ı.e., depletion interactions can cause collapse of the chains (see SI Appendix, Section II.C). (B) Addition of PEG at a volume fraction comparable to that of cytoplasmic proteins caused full compaction of the equilibrated chromosomes (multiple traces in grey) back to their in vivo volume. Removal of PEG caused the chromosomes to spring back to their equilibrium in vitro volume in about 10 s, comparable to the expansion timescale after initial lysis. The experiment was repeatable for at least 11 successive cycles of PEG addition and removal. (C) Effective size of the chromosomes at different PEG concentrations. (Experiment) Just below the transition point (PEG volume fraction 11–13%), we observed interesting coexistence of compacted and decompacted regions within individual chromosomes (arrows in the snapshots). The areas of the purple and yellow circles represent the relative fraction of the decompacted and compacted regions within the chromosomes, respectively. The fraction of the decompacted chromosomes (purple) drops sharply before transition. (Theory) The solid line shows the mean chromosome length as a function of PEG concentration calculated using Eq. 1 and a modified Odijk theory (23). (The dashed line shows the result of the original Odijk theory.) Our theory predicts collapse of the chromosome at [PEG] = 18% volume fraction, in a reasonable agreement with the data (11–19%). In both our theory and the Odjik theory, the volume of completely collapsed DNA is zero.
Fig. 4B and Movie S5 show that the addition of high-concentration PEG caused sudden collapse of the chromosomes in microchannels. Conversely, removal of the PEG rapidly restored the chromosomes to their expanded conformations. This compaction–decompaction by PEG was completely reversible and repeatable for many cycles, over the course of more than 1 h, similar to the compression-decompression by mechanical force observed in the micropiston experiments.
We repeated the experiments with a wide range of PEG concentrations. Only at or above the PEG volume fraction comparable to that of cytoplasmic proteins (about 12% to 17%) (40) did we observe compaction of the chromosomes back to their in vivo size (Fig. 4C). These results clearly show that molecular crowding alone can cause compaction of the nucleoid in vitro at a sufficiently high (e.g., in vivo) concentration of depletants. In vivo, however, the effects of molecular crowding may be complemented by contributions from nucleoid-associated proteins and supercoiling. Furthermore, because depletion interactions tend to reduce inter-DNA spacing and thus enhance looping, molecular crowding may indirectly enhance binding of nucleoid-associated proteins. Further work is needed to make quantitative predictions in vivo due to significant differences in microscopic detail between PEG and cytoplasmic proteins.
The Compaction–Decompaction Transition in the Microchannels Appears To Be Abrupt.
In the above experiments, we noticed an interesting phase coexistence of compacted and decompacted regions within individual nucleoids (see snapshots with yellow and purple arrows in Fig. 4C). This, together with the fast collapse of the chromosomes in the presence of high-concentration PEG (Fig. 4B and Movie S5), is typical of a first-order phase transition and reminiscent of the coil-globule transition of a stiff chain (41).
To understand the nature of the compaction–decompaction transition, and to explore the extent to which Eq. 1 describes the observed behavior of the bacterial chromosomes, we revised the theoretical approach by Odijk and colleagues (23). That is, we incorporated Eq. 1 to compute the expected size of a linear entropic spring in response to depletion forces in microchannels.
Fig. 4C shows the theoretical predictions against the data. An important prediction of our theory is that the entropic spring (nucleoid) is expected to collapse completely at a concentration of 18% PEG. This compares favorably to the data (11%–19%). Also important, the predicted size of the entropic spring decreases rapidly, albeit continuously, around the transition point. Taken together, the compaction-decompaction transition is consistent with a weakly first-order transition (42) †.
Our conclusion is different from the previous work by Odijk and coworkers, who concluded that the compaction–decompaction transition is second-order (23) (see Fig. 4C for comparison). In SI Appendix, Section II.C, we show that the results critically depend on the form of the force-compression expression used in the theory.
Discussion
Shape Influences the Volume of the in Vitro Chromosome, Consistent with Polymeric Behavior.
When injected into a long narrow tube with open ends, a lump of clay or an agarose gel (polymer gel) maintains constant volume and changes only its shape, whereas a long chain with excluded-volume interactions decreases its containment volume as well (43). The equilibrium volume of the in vitro chromosomes isolated in microchannels is several times smaller than the ones isolated in the absence of confinement (Table 1 and SI Appendix, Section II.B). This shape-dependent decrease in total chromosomal volume is consistent with a polymer model of the bacterial chromosome (11, 17, 44).
Table 1.
Summary of the experimental parameters for nucleoids isolated from a single cell (four chromosome equivalents for our growth conditions)
| V0, nucleoid volume | 25.5 ± 10.7 μm3 (40 mM NaCl) |
| (4 chromosome equivalents) | 27.3 ± 7.4 μm3 (100 mM NaCl) |
| Vfree, unconfined nucleoid volume | 18 μm3 (ref. 38), 27 μm3 (ref. 23) |
| (1.6 chromosome equivalents) | |
| A, rescaled spring constant (fit) | −2.04 pN ± 1.24 pN |
| k, dimensionless constant (fit) | −794 (see Eq. 1) |
| ξ, size of structural unit | 130–440 nm |
| mξ, minimum number of structural units (assumed equal to mcrosslinks) | 63–284 |
| f, force of compaction | on the order of 100 pN |
| F, stored mechanical energy per nucleoid | on the order of 105 kBT |
| τglobal, timescale of global motion of the nucleoid | approximately 10 s |
The Bacterial Chromosome Behaves as a Cross-Linked Polymer.
Because the entropic spring model in Eq. 1 describes the force-compression curves remarkably well, it is plausible to model the bacterial chromosome as a linear or ring-like “string of beads,” in which molecular details determine the spring constant k. In the bare entropic spring model, the absolute value of k should be of order 1 (25). In contrast, the spring constant obtained by fitting our data has the much larger magnitude of k = -794 ± 483 (Fig. 3 and Table 1; see also ref. 15).
The large numerical value of k is a salient feature of cross-linked polymers (43). In particular, based on the recent theoretical results by Metzler et al. (45), we infer that our measured k is directly proportional to the number of cross-links that may tighten large DNA loops (see Fig. 5, lower right). If we interpret the bacterial chromosome as a linear or ring polymer described by Eq. 1, we can estimate the number of cross-links to be 63 or 284 per cell (see Table 1 and SI Appendix, Section II.B). Similarly, we can also estimate the size of individual “beads” comprising the chromosome, which ranges from 130 to 440 nm (see Fig. 5 and SI Appendix, Section II.B), with the former being in a reasonable agreement with the results obtained by Krichevsky and coworkers using fluorescence correlation spectroscopy (38).
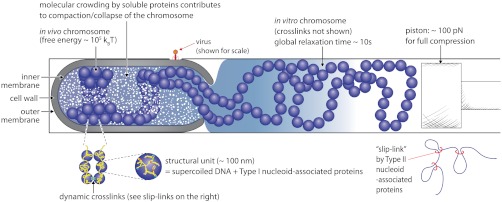
Fig. 5.
Bacterial chromosome model with summary. The in vivo chromosome consists of physical structural units with an effective “bead” size on the order of 100 nm, which are dynamically cross-linked. The effect of dynamic cross-links can be modeled using the idea of a slip-link (lower right) (45). Upon lysis, the structural units remain mostly intact, although the dynamic bridges either break or stretch. In fast-growing cells, we envision that, on ensemble average, the majority of the DNA mass is found near the envelope. See also Fig. 1B. A possible model is that a significant part of the in vivo chromosome is collapsed onto the envelope assisted by molecular crowding (Fig. 4A), with the cross-section of the cell as illustrated above. For slowly-growing cells with a smaller cell diameter, the nucleoid on average may occupy the center of the cell (see figure 2 in ref. 17). In stark contrast to viral DNA, the E. coli chromosome is soft (see Discussion).
What constitutes the cross-links is not clear. The nucleoid-associated proteins are the obvious candidates (31). In particular, although not essential for cell viability (46), the MukBEF complex is an interesting possibility because of its ring-like structure (47), role in chromosome organization and segregation (4), and copy number [several hundreds (46, 48), comparable to the number of cross-links we estimated above]. Furthermore, MukB binds to DNA very strongly, shows cooperativity, and acts as a macromolecular clamp (49). Other candidates may include transcription for its direct and indirect effect on chromosome compaction (50).
Based on our discussion above, two types of nucleoid-associated proteins will influence the physical properties of the chromosomes. The first type, which we will refer to as Type I, changes the local physical properties (e.g., by bending), whereas the second type, which we will refer to as Type II, cross-links the DNA at long distance. Most nucleoid-associated proteins appear to be Type I, whereas MukB (and perhaps H-NS) seems to be Type II. Type I nucleoid-associated proteins will influence the excluded-volume interactions between the beads, whereas Type II may change the effective spring constant significantly (e.g., slip-links) (Fig. 5). It will be of interest to measure how the spring constant will change for the cells that lack a specific type of nucleoid-associated protein.
Comparison with Viral DNA in a Capsid Reveals the Soft Nature of the Bacterial Chromosome.
To understand the nature of the forces required to shape and segregate the in vivo bacterial chromosomes, it is useful to highlight the similarities and differences between the viral DNA packaged in a capsid and the bacterial chromosome.
On the surface, both genomes are confined to spaces much smaller than their fully stretched lengths (e.g., 6.6-μm long dsDNA in an approximately 50-nm ϕ29 capsid, and a millimeter-long dsDNA in a micrometer-long E. coli or Bacillus subtilis cell). Also, both virus and bacteria have been shown to package or translocate their entire genome equivalents of DNA using powerful motor proteins that can exert several tens of piconewtons of force [the portal complex for ϕ29 (18) and FtsK/SpoIIIE for E. coli/B. subtilis (51)]. These force scales are consistent with our data in Fig. 3. Extrapolation of this result implies that about 100 pN force is sufficient to fully compress the in vitro chromosome to its in vivo size.
However, if we consider the origin of the internal pressure of the capsid and the cell, the two genomes reveal astonishing differences in their materials properties. For the virus, the 50 atmosphere internal pressure of the capsid is a direct result of the strong bending of and electrostatic repulsions between the stiff, negatively charged dsDNA (18, 19). In stark contrast, the pressure (applied force per μm2 of nucleoid surface area; Fig. 3) required to compress the chromosome to its in vivo size is about a thousand-fold smaller than the approximately 1 atmosphere turgor pressure exerted by the cytoplasm inside the bacterial cell (52). That is, the bacterial chromosome in vivo is fundamentally “soft.”
The softness of the bacterial chromosome, together with the fast timescale of its global motion (Figs. 1 and 4), means that entropic forces can readily and dynamically influence the chromosome, from segregation and organization (11, 13, 17, 44) to compaction (3, 23), as suggested previously and demonstrated in this work.
Conclusions
Chromosomes are the basis of many cellular processes in all living cells, and confinement is the key condition that constrains the physical properties of chromosomes. In this work, we directly measured the micromechanical properties of individual bacterial chromosomes confined in microchannels. We found that the bacterial chromosomes are soft in that the compaction pressures are only approximately 1/1000 of the surrounding turgor pressure inside the cell. Our view is that the softness is important to understanding the dynamic balance between various intracellular forces acting on the chromosomes in vivo. Many of these forces are entropic in nature (e.g., depletion). They can cause fast, global changes in chromosome morphology and size with only tens of piconewtons of force.
Our measurements were possible because of technological development bringing together imaging, optical tweezers, and a microfluidic device. Although pulling on single-molecules has been well established over decades (53), pushing molecules has been a much more formidable challenge with few published examples exclusively focusing on a single DNA molecule (54–57).
Our optical trap micropiston expands the optical tweezer platform to allow for single-chromosome compression, enabling new studies on the biologically important role of the physical properties and spatial organization of confined chromosomes. For example, our approach could be extended to study the properties and interactions of multiple eukaryotic chromosomes. Here, underlying biological principles may be revealed if we understand the forces governing the organization of interphase chromosomes in mammalian nuclei (58) and chromosomal individualization in eukaryotes (59).
In previous work, we suggested how chromosome segregation (demixing) can be driven by physical processes using a confined polymer model (11, 17, 44). Considering the intriguing quantitative agreement between our measurements and the same model (Figs. 3 and 4), physical properties of the bacterial chromosome may indeed provide major driving forces for chromosome segregation in vivo. That is, although the diversity of life is the consequence of evolution, quantitative understanding of the physical properties of biological systems, such as the micromechanical properties of the bacterial chromosomes presented here, may provide deeper insight into the basic processes shared by all life forms.
Materials and Methods
Lysis and Molecular Crowding Experiments.
Polydimethylsiloxane (PDMS) microfluidic channels were cast from a master mold, constructed via standard soft lithography techniques as previously described (24). The PDMS device was passivated with PLL-g-PEG to prevent adsorption of the chromosomes to the inner walls of the device (60). The lysis protocol was derived from ref. (27): E. coli cells expressing HU-mCherry or HU-GFP were grown in liquid LB with varying growth conditions depending on the physiological conditions of interest. The cells were plasmolyzed in 20% sucrose buffer (pH 7.3–7.4) and loaded to the device, approximately one cell per channel. Loaded cells were incubated with lysozyme for digestion of the cell wall. Dilute buffer containing NaCl (varying concentrations from 40 to 200 mM NaCl) was infused to lyse the cells synchronously. After cell lysis, fresh dilute buffer was continuously infused at a reduced flow rate to create a constant chemical environment. During and after lysis, fluorescent HU was imaged to measure the chromosome size, morphology, and occupancy by fluorescently labeled HU. Molecular crowding experiments were performed by cyclic addition and removal of depletant-containing buffer (PEG 20000) using an automated syringe pump system (Harvard Appratus Pump 22, Upchurch valve, LabVIEW). A detailed description of experimental conditions and image analysis is provided in SI Appendix, Section I.A.
Mechanical Compression Experiments.
The confined chromosomes were manipulated by moving an optically trapped bead (piston head) relative to the chromosome-filled microchannel (piston chamber). The optical trap acts as a soft spring, with bead displacement reporting the force required to compress and decompress the chromosome at various degrees of confinement. These experiments utilize an optical trap setup that integrates a near IR laser (Coherent Compass 1064-4000 M) into an inverted light microscope (Nikon TE2000-U). The microfluidic device is mounted onto a piezo scanning stage (Physik Instruments PI-562.3CD) to move the channels relative to the optical trap. Transmitted light images at 160x total magnification are projected onto a CCD camera (Prosilica GE680) to track the trapped bead and fiducial marks on the stage with a one-dimensional accuracy of 4 nm (61).
Chromosomes in the microfluidic channels were prepared with BSA passivated microspheres (Spherotech, nominal diameter of 1 μm) included with the lysis buffer. Fluorescent images of channels were taken after lysis to identify channels containing potentially compressible chromosomes and a cell ghost at the open end of the channel (empirically, we found that the cell ghost acted as gasket to create a tight seal, preventing chromosome escape during compression). An optically trapped bead was then aligned with the microchannel and moved relative to the channel to repeatedly compress and decompress the chromosome in 500 nm increments. After the experiment, most beads were individually calibrated by measuring fluctuations of a trapped bead (just outside the channel) at low power and fitting with the blur-corrected power spectrum fit (61). A detailed description of the experimental conditions and data analysis is in SI Appendix, Section I.B.
Principal Component Analysis.
Principal component analysis (PCA) is a method that interprets the distribution of data geometrically. To analyze chromosome morphologies using PCA, we built an image stack for each chromosome. For every frame, the bounding box of the chromosome was computed automatically. The chromosome image in the bounding box was rescaled and interpolated to a fixed size of an L × M matrix using a cubic spline algorithm in Igor Pro (WaveMetrics). Further, each pixel intensity was normalized by the total intensity of the bounding box. The results we reported in this work were insensitive to the choice of L and M.
Once a normalized image-stack of chromosome images has been constructed, we performed a standard PCA. Briefly, we computed an average chromosome profile  from the image stack
from the image stack  , and calculated the residual,
, and calculated the residual,  , at each time-index t. The main step of PCA is diagonalization of the covariance matrix
, at each time-index t. The main step of PCA is diagonalization of the covariance matrix
 |
[2] |
where the data matrix  . Because C is symmetric, all its eigenvalues are positive real and its eigenvectors
. Because C is symmetric, all its eigenvalues are positive real and its eigenvectors  form an orthornormal basis that diagonalizes C. The eigenvectors are ordered by eigenvalues, and the resulting ordered set of eigenmodes (
form an orthornormal basis that diagonalizes C. The eigenvectors are ordered by eigenvalues, and the resulting ordered set of eigenmodes ( ) are the principal components.
) are the principal components.
To analyze the morphological dynamics of the chromosome, we projected the original chromosome image  into the principal components by the following simple operation,
into the principal components by the following simple operation,
| [3] |
The projection coefficient, ωi, has a value between -1 and 1 as seen in Fig. 1D. For example, ω1 = +1 means that the system (chromosome) is in the highest eigenmode (first eigenChromosome). SI Appendix, Section I.A.7, provides a full description and the results of PCA of the chromosome expansion data.
Theory.
To predict the size of the in vitro chromosomes in the microchannels at a given PEG concentration, we solved the following two equations simultaneously:
 |
[4] |
 |
[5] |
where V is the envelope volume of the chromosome (in units of μm3), wo and wi are the weight fraction (in units of g/ml) of PEG outside and inside V, and Fchr(V) is the free energy of the confined chain inferred from Eq. 1 (25). The first equation describes mechanical equilibrium across the boundary, and the second equation represents chemical equilibrium of PEG. The constants k2 ≈ 56.75 and k3 ≈ 99.43 are two-body and three-body contributions calculated without the chromosome (23). SI Appendix, Section II, provides detailed information about various theoretical methods used in this work.
Supplementary Material
ACKNOWLEDGMENTS.
S.J. thanks Bela Mulder, Marileen Dogterom, and Sander Tans at Foundation for Fundamental Research on Matter Institute for Atomic and Molecular Physics (FOM Institute AMOLF) for their generous support in the early stage of this work; Wei Lien Dang, Jay Fisher, Peter Galajda, and Nancy Kleckner for helpful discussions; and Jean-Yves Bouet for help with biochemistry and for fruitful discussions. This work was supported by Natural Sciences and Engineering Research Council of Canada (B.-Y.H.), the Rowland Junior Fellows program at Harvard University and Immune Disease Institute/Harvard Medical School/Boston Children's Hospital startup funds (W.W.), and the Bauer Fellows program at Harvard University, the National Institutes of Health Grant P50GM068763, and University of California San Diego startup funds (S.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 15978 (volume 109, number 40).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208689109/-/DCSupplemental.
*Interestingly, Eq. 1 agrees with our chromosome data for a wider range of R/R0 (up to R/R0 ≈ 0.2) than with the numerical data [up to R/R0 ≈ 0.5; (25)]. The difference is perhaps due to the size fluctuations and softness of the chromosomal “beads” (as opposed to the monodisperse hard-spheres of the chain in the simulations). In a polymer-physics language, softer beads will result in more gradual crossover from semidilute to concentrated regimes, in which f ∼ (R/R0)-3.
†Although the theoretical results clearly capture the major features of the data, several assumptions/simplifications made in the theory need to be noted. (i) Depletion interactions have an effect only along the long axis of the microchannel. The transverse dimensions of the nucleoid are fixed by the width of the microchannel. (ii) The chain is a homo-polymer, whereas the chromosome may not be. (iii) The chain interacts only with itself through excluded-volume interactions (i.e., we ignore proteins and other potential biological factors). See SI Appendix, Section II.C) for more details.
References
- 1.Mason DJ, Powelson DM. Nuclear division as observed in live bacteria by a new technique. J Bacteriol. 1956;71:474–479. doi: 10.1128/jb.71.4.474-479.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neidhardt FC. Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd Ed. Washington, DC: American Society for Microbiology; 1996. [Google Scholar]
- 3.Odijk T. Osmotic compaction of supercoiled DNA into a bacterial nucleoid. Biophys Chem. 1998;73:23–29. doi: 10.1016/s0301-4622(98)00115-x. [DOI] [PubMed] [Google Scholar]
- 4.Sawitzke JA, Austin S. Suppression of chromosome segregation defects of Escherichia coli muk mutants by mutations in topoisomerase I. Proc Natl Acad Sci USA. 2000;97:1671–1676. doi: 10.1073/pnas.030528397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dworkin J, Losick R. Does RNA polymerase help drive chromosome segregation in bacteria? Proc Natl Acad Sci USA. 2002;99:14089–14094. doi: 10.1073/pnas.182539899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woldringh CL. The role of co-transcriptional translation and protein translocation (transertion) in bacterial chromosome segregation. Mol Microbiol. 2002;45:17–29. doi: 10.1046/j.1365-2958.2002.02993.x. [DOI] [PubMed] [Google Scholar]
- 7.Yamaichi Y, Niki H. migS, a cis-acting site that affects bipolar positioning of oriC on the Escherichia coli chromosome. EMBO J. 2004;23:221–233. doi: 10.1038/sj.emboj.7600028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bates D, Kleckner N. Chromosome and replisome dynamics in E. coli: Loss of sister cohesion triggers global chromosome movement and mediates chromosome segregation. Cell. 2005;121:899–911. doi: 10.1016/j.cell.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Possoz C, Sherratt DJ. Dancing around the divisome: Asymmetric chromosome segregation in Escherichia coli. Genes Dev. 2005;19:2367–2377. doi: 10.1101/gad.345305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen HJ, Li Y, Youngren B, Hansen FG, Austin SJ. Progressive segregation of the Escherichia coli chromosome. Mol Microbiol. 2006;61:383–393. doi: 10.1111/j.1365-2958.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 11.Jun S, Mulder B. Entropy-driven spatial organization of highly confined polymers: Lessons for the bacterial chromosome. Proc Natl Acad Sci USA. 2006;103:12388–12393. doi: 10.1073/pnas.0605305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marie-Agnès R, et al. The matP/matS site-specific system organizes the terminus region of the E. coli chromosome into a macrodomain. Cell. 2008;135:475–485. doi: 10.1016/j.cell.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 13.Marenduzzo D, Micheletti C, Cook PR. Entropy-driven genome organization. Biophys J. 2006;90:3712–3721. doi: 10.1529/biophysj.105.077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan NL, Marquis KA, Rudner DZ. Recruitment of smc by parB-parS organizes the origin region and promotes efficient chromosome segregation. Cell. 2009;137:697–707. doi: 10.1016/j.cell.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiggins PA, Cheveralls KC, Martin JS, Lintner R, Kondev J. Strong intranucleoid interactions organize the Escherichia coli chromosome into a nucleoid filament. Proc Natl Acad Sci USA. 2010;107:4991–4995. doi: 10.1073/pnas.0912062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ptacin JL, et al. A spindle-like apparatus guides bacterial chromosome segregation. Nat Cell Biol. 2010;12:791–798. doi: 10.1038/ncb2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jun S, Wright A. Entropy as the driver of chromosome segregation. Nat Rev Microbiol. 2010;8:600–607. doi: 10.1038/nrmicro2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith DE, et al. The bacteriophage ϕ29 portal motor can package DNA against a large internal force. Nature. 2001;413:748–751. doi: 10.1038/35099581. [DOI] [PubMed] [Google Scholar]
- 19.Evilevitch A, Lavelle L, Knobler CM, Raspaud E, Gelbart WM. Osmotic pressure inhibition of DNA ejection from phage. Proc Natl Acad Sci USA. 2003;100:9292–9295. doi: 10.1073/pnas.1233721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houchmandzadeh B, Marko JF, Chatenay D, Libchaber A. Elasticity and structure of eukaryote chromosomes studied by micromanipulation and micropipette aspiration. J Cell Biol. 1997;139:1–12. doi: 10.1083/jcb.139.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimamoto Y, Maeda YT, Ishiwata S, Libchaber AJ, Kapoor TM. Insights into the micromechanical properties of the metaphase spindle. Cell. 2011;145:1062–1074. doi: 10.1016/j.cell.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephens AD, Haase J, Vicci L, Taylor RM, II, Bloom K. Cohesin, condensin, and the intramolecular centromere loop together generate the mitotic chromatin spring. J Cell Biol. 2011;193:1167–1180. doi: 10.1083/jcb.201103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunha S, Woldringh CL, Odijk T. Polymer-mediated compaction and internal dynamics of isolated Escherichia coli nucleoids. J Struct Biol. 2001;136:53–66. doi: 10.1006/jsbi.2001.4420. [DOI] [PubMed] [Google Scholar]
- 24.Wang P, et al. Robust growth of Escherichia coli. Curr Biol. 2010;20:1099–1103. doi: 10.1016/j.cub.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jun S, Thirumalai D, Ha BY. Compression and stretching of a self-avoiding chain in cylindrical nanopores. Phys Rev Lett. 2008;101:138101. doi: 10.1103/PhysRevLett.101.138101. [DOI] [PubMed] [Google Scholar]
- 26.Prinz C, Tegenfeldt J, Austin R, Cox E, Sturm J. Bacterial chromosome extraction and isolation. Lab Chip. 2002;2:207–212. doi: 10.1039/b208010a. [DOI] [PubMed] [Google Scholar]
- 27.Wegner AS, Alexeeva S, Odijk T, Woldringh CL. Characterization of Escherichia coli nucleoids released by osmotic shock. J Struct Biol. 2012;178:260–269. doi: 10.1016/j.jsb.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Marceau AH, et al. Structure of the ssb-DNA polymerase III interface and its role in DNA replication. EMBO J. 2011;30:4236–4247. doi: 10.1038/emboj.2011.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turk M, Pentland A. Eigenfaces for recognition. J Cog Neuro. 1991;3:71–86. doi: 10.1162/jocn.1991.3.1.71. [DOI] [PubMed] [Google Scholar]
- 30.Stephens GJ, Johnson-Kerner B, Bialek W, Ryu WS. Dimensionality and dynamics in the behavior of C. elegans. PLoS Comput Biol. 2008;4:e1000028. doi: 10.1371/journal.pcbi.1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stavans J, Oppenheim A. DNA–protein interactions and bacterial chromosome architecture. Phys Biol. 2006;3:R1–10. doi: 10.1088/1478-3975/3/4/R01. [DOI] [PubMed] [Google Scholar]
- 32.Azam TA, Iwata A, Nishimura A, Ueda S, Ishihama A. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J Bacteriol. 1999;181:6361–6370. doi: 10.1128/jb.181.20.6361-6370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dillon SC, Dorman CJ. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat Rev Microbiol. 2010;8:185–195. doi: 10.1038/nrmicro2261. [DOI] [PubMed] [Google Scholar]
- 34.Grove A. Functional evolution of bacterial histone-like HU proteins. Curr Issues Mol Biol. 2010;13:1–12. [PubMed] [Google Scholar]
- 35.Xiao B, Johnson RC, Marko JF. Modulation of HU-DNA interactions by salt concentration and applied force. Nucleic Acids Res. 2010;38:6176–6185. doi: 10.1093/nar/gkq435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azam TA, Ishihama A. Twelve species of the nucleoid-associated protein from Escherichia coli. J Biol Chem. 1999;274:33105–33113. doi: 10.1074/jbc.274.46.33105. [DOI] [PubMed] [Google Scholar]
- 37.Graham JS, Johnson RC, Marko JF. Concentration-dependent exchange accelerates turnover of proteins bound to double-stranded DNA. Nucleic Acids Res. 2011;39:2249–2259. doi: 10.1093/nar/gkq1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romantsov T, Fishov I, Krichevsky O. Internal structure and dynamics of isolated Escherichia coli nucleoids assessed by fluorescence correlation spectroscopy. Biophys J. 2007;92:2875–2884. doi: 10.1529/biophysj.106.095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asakura S, Oosawa F. On interaction between two bodies immersed in a solution of macromolecules. J Chem Phys. 1954;22:1255–1256. [Google Scholar]
- 40.Valkenburg JA, Woldringh CL. Phase separation between nucleoid and cytoplasm in Escherichia coli as defined by immersive refractometry. J Bacteriol. 1984;160:1151–1157. doi: 10.1128/jb.160.3.1151-1157.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Gennes PG. Collapse of a polymer chain in poor solvents. J Phys Lett. 1979;36:55–57. [Google Scholar]
- 42.Binder K. Theory of first-order phase transitions. Rep Prog Phys. 1987;50:783–859. [Google Scholar]
- 43.de Gennes PG. Scaling Concepts in Polymer Physics. Ithaca, NY: Cornell Univ Press; 1979. [Google Scholar]
- 44.Jung Y, et al. Ring polymers as model bacterial chromosomes: Confinement, chain topology, single chain statistics, and how they interact. Soft Matter. 2012;8:2095–2102. [Google Scholar]
- 45.Metzler R, Kantor Y, Kardar M. Force-extension relations for polymers with sliding links. Phys Rev E. 2002;66:022102. doi: 10.1103/PhysRevE.66.022102. [DOI] [PubMed] [Google Scholar]
- 46.Niki H, Jaffe A, Imamura R, Ogura T, Hiraga S. The new gene mukB codes for a 177 kd protein with coiled-coil domains involved in chromosome partitioning of E. coli. EMBO J. 1991;10:183–193. doi: 10.1002/j.1460-2075.1991.tb07935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woo J, et al. Structural studies of a bacterial condensin complex reveal ATP-dependent disruption of intersubunit interactions. Cell. 2009;136:85–96. doi: 10.1016/j.cell.2008.10.050. [DOI] [PubMed] [Google Scholar]
- 48.Petrushenko ZM, Lai C, Rybenkov VV. Antagonistic interactions of kleisins and DNA with bacterial condensin mukB. J Biol Chem. 2006;281:34208–34217. doi: 10.1074/jbc.M606723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cui Y, Petrushenko ZM, Rybenkov VV. MukB acts as a macromolecular clamp in DNA condensation. Nat Struct Mol Biol. 2008;15:411–418. doi: 10.1038/nsmb.1410. [DOI] [PubMed] [Google Scholar]
- 50.Cabrera JE, Cagliero C, Quan S, Squires CL, Jin DJ. Active transcription of rrna operons condenses the nucleoid in Escherichia coli: Examining the effect of transcription on nucleoid structure in the absence of transertion. J Bacteriol. 2009;191:4180–4185. doi: 10.1128/JB.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pease PJ, et al. Sequence-directed DNA translocation by purified FtsK. Science. 2005;307:586–590. doi: 10.1126/science.1104885. [DOI] [PubMed] [Google Scholar]
- 52.Deng Y, Sun M, Shaevitz JW. Direct measurement of cell wall stress stiffening and turgor pressure in live bacterial cells. Phys Rev Lett. 2011;107:158101. doi: 10.1103/PhysRevLett.107.158101. [DOI] [PubMed] [Google Scholar]
- 53.Neuman KC, Nagy A. Single-molecule force spectroscopy: Optical tweezers, magnetic tweezers and atomic force microscopy. Nat Methods. 2008;5:491–505. doi: 10.1038/nmeth.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang J, Du N, Doyle PS. Compression and self-entanglement of single DNA molecules under uniform electric field. Proc Natl Acad Sci USA. 2011;108:16153–16158. doi: 10.1073/pnas.1105547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reccius CH, Mannion JT, Cross JD, Craighead HG. Compression and free expansion of single DNA molecules in nanochannels. Phys Rev Lett. 2005;95:268101. doi: 10.1103/PhysRevLett.95.268101. [DOI] [PubMed] [Google Scholar]
- 56.Liu X, Noble PC, Luo ZP. Direct measurements of the compressive properties of single proteoglycan aggregates. Biochem Biophys Res Commun. 2004;316:313–316. doi: 10.1016/j.bbrc.2004.02.054. [DOI] [PubMed] [Google Scholar]
- 57.Xingfei Z, et al. Direct measurement of compression spring constant of single DNA molecule with AFM. Chin Sci Bull. 2005;50:954–957. [Google Scholar]
- 58.Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2001;2:292–301. doi: 10.1038/35066075. [DOI] [PubMed] [Google Scholar]
- 59.Maeshima K, et al. A two-step scaffolding model for mitotic chromosome assembly. Dev Cell. 2003;4:467–480. doi: 10.1016/s1534-5807(03)00092-3. [DOI] [PubMed] [Google Scholar]
- 60.Lee S, Vörös J. An aqueous-based surface modification of poly(dimethylsiloxane) with poly(ethylene glycol) to prevent biofouling. Langmuir. 2005;21:11957–11962. doi: 10.1021/la051932p. [DOI] [PubMed] [Google Scholar]
- 61.Wong WP, Halvorsen K. The effect of integration time on fluctuation measurements: Calibrating an optical trap in the presence of motion blur. Opt Express. 2006;14:12517–12531. doi: 10.1364/oe.14.012517. [DOI] [PubMed] [Google Scholar]