Abstract
Corticotropin-releasing factor (CRF) is critical for the endocrine, autonomic, and behavioral responses to stressors, and it has been shown to modulate fear and anxiety. The CRF receptor is widely expressed across a variety of cell types, impeding progress toward understanding the contribution of specific CRF-containing neurons to fear dysregulation. We used a unique CRF-Cre driver transgenic mouse line to remove floxed GABA(A)α1 subunits specifically from CRF neurons [CRF-GABA(A)α1 KO]. This process resulted in mice with decreased GABA(A)α1 expression only in CRF neurons and increased CRF mRNA within the amygdala, bed nucleus of the stria terminalis (BNST) and paraventricular nucleus of the hypothalamus. These mice show normal locomotor and pain responses and no difference in depressive-like behavior or Pavlovian fear conditioning. However, CRF-GABA(A)α1 KO increased anxiety-like behavior and impaired extinction of conditioned fear, coincident with an increase in plasma corticosterone concentration. These behavioral impairments were rescued with systemic or BNST infusion of the CRF antagonist R121919. Infusion of Zolpidem, a GABA(A)α1-preferring benzodiazepine-site agonist, into the BNST of the CRF-GABA(A)α1 KO was ineffective at decreasing anxiety. Electrophysiological findings suggest a disruption in inhibitory current may play a role in these changes. These data indicate that disturbance of CRF containing GABA(A)α1 neurons causes increased anxiety and impaired fear extinction, both of which are symptoms diagnostic for anxiety disorders, such as posttraumatic stress disorder.
Keywords: central amygdala, plasticity, HPA axis, short term synaptic depression, knockout
The neuropeptide corticotrophin-releasing factor (CRF) is involved in initiation of the endocrine, autonomic, and behavioral responses to stressors and its dysfunction is implicated in a variety of mood and anxiety disorders (1). Large populations of CRF-containing neurons are located within brain areas crucial for fear and anxiety, including the central amygdala (CeA), bed nucleus of the stria terminalis (BNST), and paraventricular nucleus of the hypothalamus (PVN). Although site-specific and conditional knockout approaches are increasingly being used (2, 3), the broad expression of CRF receptors and the varied cell types in which the CRF peptide is produced (4, 5) make it challenging to identify region and cell-type specific influences of CRF. One way to manipulate specific subpopulations of CRF neurons is to use mice with the Cre recombinase gene driven by the CRF promoter to allow different subgroups of CRFergic cells to be manipulated.
The interaction between CRF and GABA activity in brain structures important for fear and anxiety has been identified as a potential mechanism underlying anxiety disorders (6). CRF administration increases GABA release within the amygdala (7, 8) and causes deficits in GABA(A) receptor-mediated inhibitory transmission (9). Lifelong CRF overexpression leads to changes in GABA receptor subtype expression and sensitivity (10). Furthermore, GABAergic disinhibition or CRF excitation leads to long-term synaptic plasticity and increased excitability of neurons within the amygdala (11, 12). In the BNST, CRF application enhanced GABA(A)-mediated transmission via postsynaptic activation of CRF1 receptors (13). The BNST is rich in GABA and CRFergic input originating from the CeA (14). Furthermore, CRF (15, 16) and GABA (17) signaling in the BNST are involved in anxiety. GABAergic neurons within the BNST also regulate the hypothalamic-pituitary-adrenal axis (HPA) responding to acute stress (18).
The actions of GABA receptors are defined by their component subunits. Research has pointed to an important role for the α1 subunit of the GABA(A) receptor in anxiety and fear memory. Fear training decreases GABA(A)α1 subunit containing neurons (19) and disruption of the GABA(A)α1 subunit receptor leads to enhanced synaptic plasticity in the lateral nucleus of the amygdala and enhanced auditory fear memory (20). GABA(A)α1 neurons can be found within the amygdala (21), BNST, and PVN (22), areas that also strongly express CRF. No studies to date have addressed the role of CRF containing GABA(A)α1 neurons in fear and anxiety disorders.
In the present study we combined mice with the GABA(A)α1 subunit flanked by loxP sites (floxed) with our previously developed CRFp3.0Cre line (23), resulting in a CRF neuron-specific deletion of the GABA(A)α1 gene [CRF GABA(A)α1 KO]. These animals were then examined in a variety of behavioral and physiological tests. Mice with CRF GABA(A)α1 KO were found to have enhanced anxiety-like behavior. Although these mice fear-conditioned normally, their extinction behavior was disrupted compared with littermate controls. Both the disrupted extinction and increased anxiety were rescued with administration of a CRF antagonist delivered systemically or given into the BNST. These animals also showed a lack of responsiveness to BNST administration of a GABA(A)α1 selective benzodiazepine. Electrophysiological findings indicate CRF GABA(A)α1 KO mice have a selective deficit in short-term synaptic plasticity. Taken together, these data show that disrupting this neuronal subtype affects baseline anxiety, fear extinction, and the electrophysiological response properties of these cells.
Results
Expression of Cre, CRF, and GABA(A)α1.
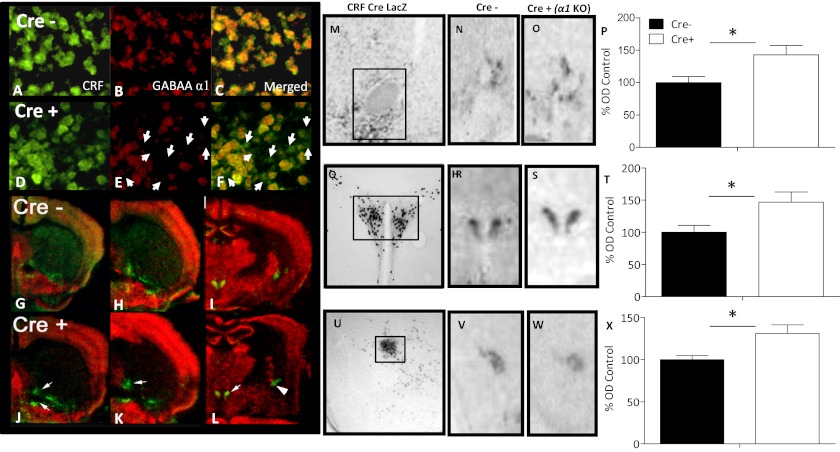
CRF GABA(A)α1 KO and littermate Cre− control mice were compared at baseline using fluorescent in situ hybridization (FISH). Visual inspection of FISH-labeled sections (Fig. 1 A–F) revealed that CRF-expressing neurons from CRF GABA(A)α1 KO mice were more often found without GABA(A)α1 mRNA compared with Cre− controls (Fig. 1). Observation of in situ demonstrated decreased double-labeling in the BNST, PVN, and the CeA in the CRF GABA(A)α1 KO (Fig. 1 G–L) compared with Cre− mice.
Fig. 1.
(A–L) In situ hybridization conducted on Cre− and CRF GABA(A)α1 (Cre+) mice under basal conditions. Cre− and Cre+ CRF (green, A and D), GABA(A)α1 and (red, B and E) merged CRF and GABA(A)α1 (C and F) FISH images from the PVN are shown. White arrows (E and F) indicate areas of CRF staining without coexpression of GABA(A)α1 in Cre+ mice. Pseudocolor images from autoradiographic in situ hybridization of CRF (green) and GABA(A)α1 (red) in the BNST (G, H, J, and K), PVN and CeA (I and L) of Cre− and Cre+ mice. Arrows indicate areas of more intense CRF staining in Cre+ animals compared with Cre− mice. (M–X) LacZ staining with X-Gal demonstrates functional Cre activity in the BNST (M), PVN (Q), and CeA (U) of a CRFp3.0CreLacZ mouse. The box indicates the area sampled for data acquisition. Representative expression of CRF from Cre− (N) BNST, (R) PVN, (V) CeA and Cre+ (O) BNST, (S) PVN, and (W) CeA in situ hybridization. Data from densitometry conducted on in situ hybridization shows increased CRF in (P) BNST, (T) PVN, and (X) CeA. Bars represent the mean of the densitometry measurements (± SEM) in all figures. *P < 0.05.
Quantitative analyses were also performed with in situ hybridization (Fig. 1 M–X). Expression levels in the BNST (Fig. 1 M–O), PVN (Fig. 1 Q–S), and CeA (Fig. 1 U–W) from CRF GABA(A)α1 KO (Cre+) and Cre− animals were normalized to the same structure and compared using one-way ANOVA. Results from the BNST (Fig. 1P) [Cre− n = 8; Cre+ n = 6; F(1, 12) = 6.654, P < 0.05]; PVN (Fig. 1T) [Cre− n = 8; Cre+ n = 6; F(1, 12) = 6.464, P < 0.05], and CeA (Fig. 1X) [Cre− n = 7; Cre+ n = 8; F(1, 13) = 7.192, P < 0.05] showed that CRF GABA(A)α1 KO animals expressed significantly more CRF mRNA under baseline conditions.
Anxiety-Like Behavior.
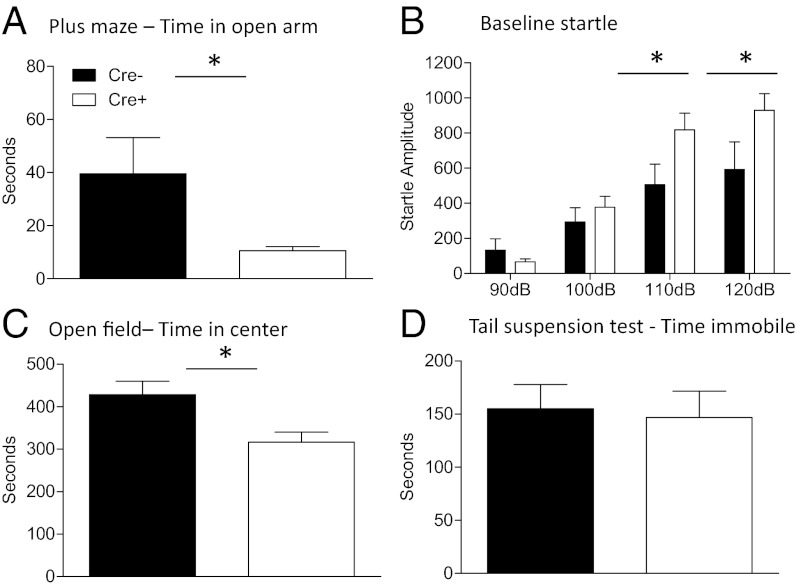
We conducted a series of tests to determine whether baseline differences existed in anxiety-like behavior between Cre− (n = 6) and CRF GABA(A)α1 KO (Cre+, n = 9) mice (Fig. 2). One cohort of animals was examined on the elevated plus maze followed by an open-field test the following day. Time in the open arm of the plus maze was measured over 5 min. As shown in Fig. 2A, CRF GABA(A)α1 KO animals showed significantly decreased time in the open arm compared with Cre− controls [F(1, 13) = 6.920, P < 0.05]. There was no significant difference between Cre− and CRF GABA(A)α1 KO animals in total distance traveled in the plus maze (Fig. S1A), indicating the increased time on the open arm of the plus maze is not because of differences in general locomotor activity [F(1, 13) = 0.055, P > 0.05]. We also compared Cre− and CRF GABA(A)α1 KO animals in time spent in the center of an open field over 15 min (Fig. 2C). CRF GABA(A)α1 KO animals spent significantly less time in the center portion of the open field compared with Cre− mice [F(1, 13) = 8.246, P < 0.05]. However, no significant difference in the total distance traveled was shown between the groups, demonstrating general activity was similar (Fig. S1B) [F(1, 13) = 0.075, P > 0.05].
Fig. 2.
Anxiety measures in CRF GABA(A)α1 KO (Cre+) and Cre− mice. (A) Cre+ (white bar) mice spent less time in the open arm of the plus maze compared with Cre− (black bar) mice. (B) Baseline startle response was significantly increased at the higher decibel levels (110 and 120 dB) for Cre+ compared with Cre− mice. (C) Time in the center of the open field was attenuated in Cre+ mice compared with Cre−. (D) No difference between groups was shown in a test of depression-like behavior using the tail suspension test. *P < 0.05.
Animals also showed increased anxiety behavior when baseline startle was measured (Fig. 2B). Animals were exposed to 90-, 100-, 110-, and 120-dB noise bursts. No significant difference was seen at 90 dB [F(1, 13) = 1.512, P > 0.05] or 100 dB [F(1, 13) = 1.250, P > 0.05]. At higher decibel levels, 110 [F(1, 13) = 6.207, P < 0.05] and 120 dB [F(1, 13) = 5.601, P < 0.05] CRF GABA(A)α1 KO mice showed higher startle amplitude compared with Cre− animals further supporting disruption of CRF GABA(A)α1 as important for anxiety behavior. CRF Cre mice without floxed GABA(A)α1 KO showed no difference in anxiety measures compared to controls, indicating that the presence of the promotor alone did not effect anxiety behavior (Fig. S2 A–C).
Depression-Like Behavior.
Because CRF has been shown to play a role in depression, mice that were previously tested in baseline startle were assessed for differences in the tail suspension test, a common measure of depressive-like behavior. Using time spent immobile as a measure of depressive behavior, we found no significant differences between Cre− and CRF GABA(A)α1 KO mice [F(1, 13) = 0.054, P > 0.05], suggesting these mice do not have a depressive phenotype (Fig. 2D).
Fear Conditioning and Extinction.
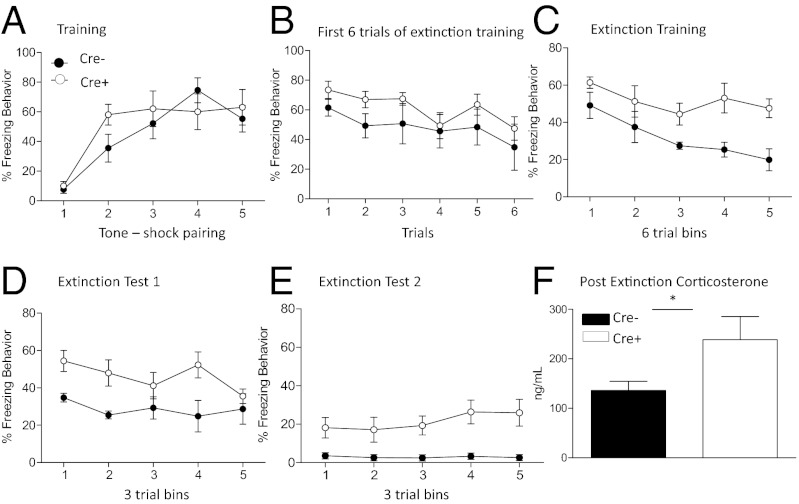
Fear conditioning and extinction were conducted with Cre− (n = 5) and CRF GABA(A)α1 KO (Cre+, n = 8) mice (Fig. 3). Animals were trained with five tone-shock pairings. Repeated-measures ANOVA with Trial and Genotype as factors showed that there was a significant main effect for Trial [F(4, 44) = 17.766, P < 0.05] indicating freezing increased over time during training. There was no interaction between Trial and Genotype [F(4, 44) = 1.896, P > 0.05] nor was there any difference between groups during training [F(1, 11) = 0.341, P > 0.05] or in shock reactivity (Fig. S3) [t(11) = 0.408, P = 0.52] between groups, indicating that Cre− and CRF GABA(A)α1 KO animals fear-conditioned equivalently and there were no differences in shock sensitivity.
Fig. 3.
Fear conditioning, extinction, and corticosterone in CRF GABA(A)α1 KO (Cre+) and Cre− mice. (A) Cre− (black circles) and Cre+ (white circles) animals showed no difference in response to tone-shock pairings during training. (B) During extinction training there were no significant differences in freezing between the groups during the first six extinction trials. (C) When the entire extinction session was analyzed, Cre+ mice showed significantly more freezing compared with Cre− animals. Extinction test (D) 1 and (E) 2 show Cre+ animals continue to demonstrate significantly impaired extinction compared with Cre− controls. (F) Cre+ animals show significantly increased corticosterone levels when measured after extinction. *P < 0.05.
Animals were extinguished with 30 tone presentations in the absence of a foot shock. To test for differences in memory consolidation, a repeated-measures ANOVA was conducted on freezing behavior during the first six tone presentations (Fig. 3B), with Trial and Genotype as factors. There was no significant main effect [F(4, 44) = 2.504, P > 0.05], interaction [F(4, 44) = 0.472, P > 0.05], or group effect [F(1, 11) = 4.178, P > 0.05], indicating no effect on fear memory consolidation. The same analysis was conducted across the complete 30-tone extinction session. Data were separated into five blocks of six trials with Block and Genotype entered as factors (Fig. 3C). Data from the extinction training session revealed a significant main effect for Block [F(4, 44) = 4.162, P < 0.05] with no Block-by-Genotype interaction [F(4, 44) = 0.846, P > 0.05]. The significant effect on Block indicates animals froze significantly less over extinction trials. We also found a significant between-subjects effect of Genotype [F(1, 11) = 21.834, P < 0.05] driven by CRF GABA(A)α1 KO mice freezing significantly more than Cre− mice during extinction training.
We conducted 2 d of 15-tone extinction tests. For both days the data were analyzed as during extinction training, except that data were separated into five blocks of three trials. Repeated-measures ANOVA on extinction test day 1 with Block and Genotype as factors showed there was a significant main effect of Block [F(4, 44) = 4.498, P < 0.05], Block-by-Genotype interaction [F(4, 44) = 7.177, P < 0.05] and a between-subjects group effect [F(1, 11) = 7.696, P < 0.05] (Fig. 3D).
Data from the second extinction test (Fig. 3E) showed no significant main effect of Block [F(4, 44) = 0.959, P > 0.05] nor a Block-by-Genotype interaction [F(4, 44) = 1.266, P > 0.05]; however, there was a significant between-subjects group effect [F(1, 11) = 7.475, P < 0.05], again driven by CRF GABA(A)α1 KO mice freezing significantly more than Cre− mice during extinction training. CRF Cre mice without floxed GABA(A)α1 KO showed no difference in fear training or extinction compared to controls, indicating that the presence of the promotor did not effect extinction behavior (Fig. S2 D–F). Altogether these data suggest CRF GABAα1 KO disrupts extinction of conditioned fear.
Extinction and Plasma Hormone Measurement.
Corticosterone was also measured after extinction training in CRF GABA(A)α1 KO (Cre+, n = 7) and Cre− (n = 7) animals to assess if the HPA axis was engaged differentially by CRF GABA(A)α1 KO. A one-way ANOVA revealed a significant difference between the groups [F(1, 13) = 7.612, P < 0.05]. Cre+ animals showed a significant increase in corticosterone after extinction compared with Cre− controls (Fig. 3F). These findings suggest increased corticosterone levels may underlie the disrupted extinction seen in CRF GABA(A)α1 KO mice.
Systemic Administration of the CRF Antagonist R121919 and Anxiety.
To assess whether the differences found in anxiety may be mediated by increased activation of CRF, we gave the CRF1 receptor antagonist R121919 40 min before anxiety testing. If overactivation of CRF underlies the disruptions in anxiety behavior we would expect administration of an antagonist to ameliorate these effects.
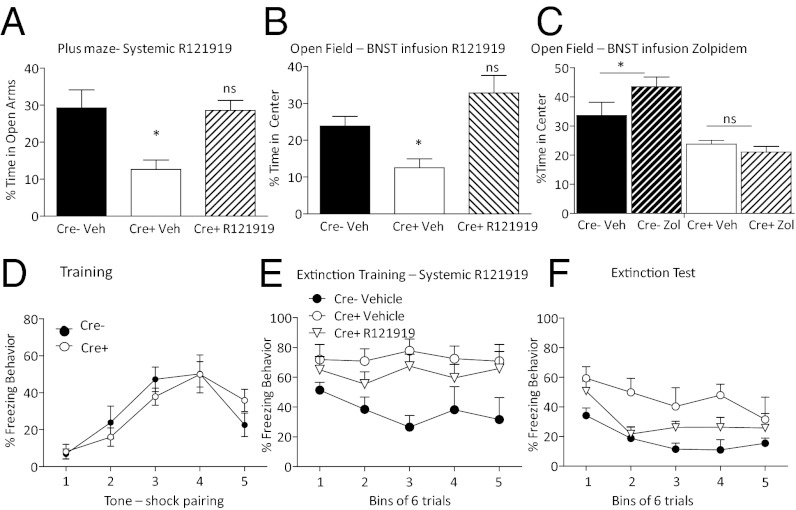
One-way ANOVA conducted on the percentage of total time spent in the open arm of the elevated plus maze after R121919 injection showed a significant difference between groups (Fig. 4A) (F = 8.467, P < 0.05). Planned comparisons between Cre− (n = 4) and CRF GABA(A)α1 KO (n = 6) mice given vehicle replicated our finding by showing CRF GABA(A)α1 KO spent significantly decreased time in the open arms (P < 0.05). In contrast, planned comparisons between CRF GABA(A)α1 KO animals given R121919 (n = 5) and Cre− vehicle controls showed no significant difference in percentage of time spent in the open arms (P > 0.05). One-way ANOVA conducted on overall activity level during the testing session revealed no significant difference (Fig. S4.) [F(2, 12) = 0.268, P > 0.05] suggesting that decreasing CRF in mice with augmented CRF returns anxiety to control levels.
Fig. 4.
Reversal of anxiogenic and extinction deficit phenotype. (A) R121919 given to CRF GABA(A)α1 KO (Cre+) animals (striped bar) systemically before plus-maze testing significantly increased time spent in the open arm. Cre+ animals given vehicle (white bar) showed a significant decrease in percent time spent in the open arms of the elevated plus maze compared with Cre− vehicle-infused mice. In contrast, R121919-injected Cre+ mice showed no difference from Cre− control (ns, not significant). (B) Infusion of R121919 into the BNST increased time spent in the open area of the open field in Cre+ mice. (C) Cre+ animals do not show a difference in open-field exploration when given an Infusion of Zolpidem into the BNST, whereas Zolpidem increased time spent in the center of an open field in Cre− animals. *P < 0.05. (D) Neither Cre+ nor Cre− mice show a significant difference in Pavlovian fear conditioning. (E) Systemic injection of R121919 to Cre+ animals (▽) before extinction training rescues the extinction deficit seen during extinction training. (F) Twenty-four hours later during extinction testing, we continue to see disrupted extinction in the Cre+ vehicle mice (○); however, the R121919-injected Cre+ animals show no significant difference from Cre− vehicle-injected (●) animals. Cre+ vehicle-infused (○) animals again demonstrate significantly disrupted fear extinction.
Intra-BNST Infusion of R121919 and Anxiety.
We targeted the BNST for local infusion of the CRF antagonist R121919 because of its involvement in anxiety behavior. Like systemic administration of R121919, BNST directed infusion just before the open-field test reverses the increase in anxiety in the knockout mouse. One-way ANOVA showed that there was a significant difference between groups in percent time spent in the open area during the open-field test (Fig. 4B) [F(2, 20) = 6.748, P < 0.05]. Planned comparisons showed that Cre− vehicle-infused mice (n = 9) spent significantly more of the test session in the center of the open field (P < 0.05) compared with CRF GABA(A)α1 KO vehicle-infused animals (n = 6). In contrast, planned comparisons show that Cre− vehicle-infused and CRF GABA(A)α1 KO R121919-infused animals (n = 8) did not significantly differ in time spent in the center of the open field (P > 0.05).
Intra-BNST Infusion of Zolpidem and Anxiety.
To assess whether CRF GABA(A)α1 KO shifted the effectiveness of benzodiazepines, we administered Zolpidem (0.25 mg/mL, 0.5 μL), a GABA(A)α1-preferring benzodiazepine-site agonist into the BNST of Cre− and CRF GABA(A)α1 KO animals. One-way ANOVA indicated a significant between-groups effect (Fig. 4C) [F(3, 19) = 13.427, P < 0.05]. Planned comparisons between Cre− vehicle (n = 5) and Cre− Zolpidem (n = 5) groups showed a significant increase in percentage time exploring the center area of the open field (P < 0.05) for Cre− Zolpidem-infused mice. Planned comparisons revealed Zolpidem was unable to alter anxiety behavior in the CRF GABA(A)α1 KO mice (vehicle, n = 7; and Zolpidem, n = 6, P > 0.05). These findings indicate sensitivity to benzodiazepines is altered with CRF GABA(A)α1 KO in the BNST.
Systemic CRF Antagonist and Extinction.
To determine whether the CRF system mediates the disruption in fear extinction, we administered R121919 systemically 40 min before extinction training. Cre− (n = 4) and CRF GABA(A)α1 KO (n = 11) animals previously run in plus-maze were trained with five tone-shock pairings (Fig. 4D). A repeated-measures ANOVA was run on training data with Trial and Genotype as factors. A significant within-subjects effect for Trial was found [F(4, 52) = 13.241, P < 0.05], indicating increased freezing over the training session across conditions. There was no significant Trial-by-Genotype interaction [F(4, 52) = 0.929, P > 0.05] or Genotype effect [F(1, 13) = 0.012, P > 0.05]. The lack of a difference during training replicates our previous finding that CRF GABA(A)α1 KO disruption does not affect acquisition of a fear memory.
The following day, 40 min after animals received a systemic infusion of R121919, mice were exposed to 30 tones during extinction training. A repeated-measures ANOVA with Block and Drug as factors was conducted on the freezing data from extinction training grouped into five blocks of six tones each (Fig. 4E) and revealed no significant main effect for Block [F(4, 48) = 0.599, P > 0.05] and no Block-by-Drug interaction [F(8, 48) = 0.940, P > 0.05]. However, there was a significant between-subjects group effect [F(1, 1) = 4.898, P < 0.05]. Tukey post hoc analysis showed that Cre− animals that received vehicle showed significantly less freezing behavior than CRF GABA(A)α1 KO animals that received vehicle (P < 0.05), replicating our earlier findings. A repeated-measures ANOVA was run on data from extinction testing 24 h later. We found a significant effect of Block [F(4, 48) = 9.081, P < 0.05] but no significant Block-by-Group effect [F(8, 48) = 1.011, P > 0.05]. A between-subjects group effect was found [F(1, 12) = 3.876, P < 0.05] and follow-up Tukey post hoc analysis showed this effect was because of a difference between Cre− and CRF GABA(A)α1 KO vehicle-injected mice (P < 0.05). There was no difference between Cre− vehicle-injected and CRF GABA(A)α1 KO R121919-injected mice (P > 0.05). These data suggest that CRF neuronal disinhibition results in CRF receptor overactivation as a mechanism for the disrupted extinction seen in Cre+ animals.
Single-Cell RT-PCR.
BNST neurons from CRF GABA(A)α1 KO mice were screened for their expression of the mRNA transcripts GABA(A) α1, α2, and β1 subunits and for 18S rRNA (a ubiquitous housekeeping ribosomal RNA). Here, we used the fact that CRF GABA(A)α1 KO neurons express Cre to selectively drive expression of an mCherry fluorophore in CRF neurons following local injection of a floxed mCherry AAV viral vector (see Materials and Methods). Using this combined methodology, and floxed GABA(A)α1mice as well as floxed-stop GFP reporter mice, we were able to identify cells that were CRF+/GABA(A)α1 KO compared with CRF+/GABA(A)α1 wild-type.
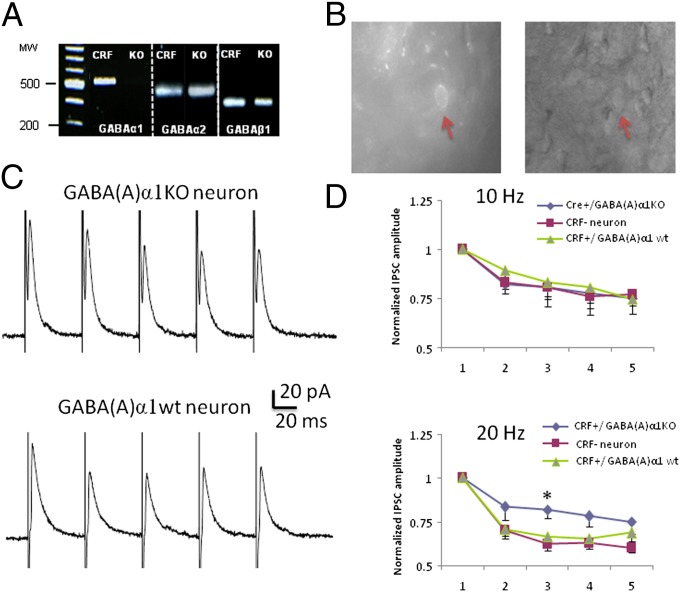
Cytosolic mRNA was pulled from five visually identified CRF-mCherry neurons from the BNST in CRF GABA(A)α1 KO mice and screened for 18S rRNA (Fig. 5A). Three out of five neurons expressed 18S rRNA. These three neurons were then screened for GABA(A)α1, α2 and β1 transcripts. As expected, none of these putative CRF neurons expressed GABA(A)α1 transcripts; however, they did express GABA(A)α2 and β1 transcripts indicating our deletion of GABA(A)α1 did not result in gross disruption in GABA(A)α2 and β1 subunit expression in these neurons. We also screened for GABA(A)α1 transcripts in 10 CRF neurons recorded from control CRF-Cre-GFP mice (23) and found that 8 of these neurons expressed GABA(A)α1 mRNA, confirming that mouse BNST CRF neurons normally express GABA(A)α1 mRNA transcripts.
Fig. 5.
Single neuron RT-PCR and electrophysiological recordings. (A) Image of an ethidium bromide-stained electrophoresis-separated agarose gel in which GABA(A)α1 mRNA is expressed in CRF-CRE-GFP (with wild-type GABA α1) and not expressed in CRF-CRE-GABA(A)α1 KO mice. We tested for expression of two other GABA(A) receptors types (α2 and β1) and found the mRNA of both receptors are expressed in CRF-CRE-GFP and CRF-CRE-GABA(A)α1 KO mice. MW represents molecular weight marker (magnification, 1×). (B) Representative image of recording site from CRF-expressing neurons identified with the floxed mCherry AAV. (C and D) GABA(A)α1 KO affects short term plasticity of inhibitory transmission. Trains of five pulses at 10 or 20 Hz were delivered to induce IPSCs and determine short-term plasticity. (C) Representative traces show the IPSCs of a CRF Cre+ [GABA(A)α1 KO] neuron and mCherry Cre− [GABA(A)α1 wild-type] neuron following five pulses at 20 Hz. (D) At 10 Hz, the short-term plasticity is not affected by GABA(A)α1 KO, whereas at 20 Hz, a reduction of short-term synaptic depression is observed in CRF GABA(A)α1 KO neurons compared with control neurons [mCherry− (CRF−) and CRF expressing neurons with wild-type GABA(A) α1].
Potential Cellular Function of GABA(A)α1.
We investigated the consequences of the GABA(A)α1 KO on the intrinsic properties of BNST CRF neurons in an in vitro slice preparation. Here, we used the same mCherry AAV vector to identify CRF GABA(A)α1 KO neurons and compared their basic membrane properties with that of mCherry− neurons. The physiological properties of CRF GABA(A)α1 KO mCherry+ neurons were not significantly different from neighboring CRF GABA(A)α1 KO mCherry− neurons with the exception that they had a more hyperpolarized resting membrane potential (Table S1). We then compared the properties of visualized CRF GABA(A)α1 KO mCherry+ neurons to those of CRF neurons recorded from CRF-Cre-GFP mice. No significant difference was found between these two groups of type III neurons, suggesting CRF GABA(A)α1 KO did not change the intrinsic physiological properties of CRF neurons.
We next examined the effect of the GABA(A)α1 KO on inhibitory postsynaptic currents (IPSCs) evoked in visually identified neurons by focal electrical stimulation of the BNST. No significant difference were observed across groups for the mean IPSC amplitude evoked by low-frequency (0.2 Hz) stimulation [CRF GABA(A)α1 KO mCherry+ = 71.7 ± 14.4 pA, n = 7; CRF GABA(A)α1 KO mCherry− = 76.9 ± 13.3 pA, n = 6; CRF-Cre-GFP+ = 62 ± 3.4 pA, n = 15; F(2, 15) = 0.60 P > 0.05], or for the IPSC reversal potential [CRF GABA(A)α1 KO mCherry+ = −65.0 ± 1.2 mV; CRF GABA(A)α1 KO mCherry− = −64.6 ± 1.5 mV; P > 0.05]. We then examined the effect of the GABA(A)α1 KO on short-term synaptic plasticity using a train of five stimuli delivered at 10 or 20 Hz. In all neurons recorded, the amplitude of the evoked IPSC gradually reduced during repetitive stimulation (Fig. 5C). At 10 Hz, a two-way ANOVA indicated there was no significant difference in short-term synaptic depression elicited in CRF-expressing, GABA(A)α1 mCherry+ KO neurons, CRF-CreGFP+ neurons, or non-CRF–expressing mCherry− neurons [F(2, 70) = 0.5, P > 0.05]. However, at 20-Hz a two-way ANOVA indicated a significant difference among these three groups of neurons [F(2, 80) = 8.15, P < 0.01]. Bonferroni post hoc tests indicated that CRF GABA(A)α1 KO mCherry+ neurons showed a smaller short-term synaptic depression in comparison with CRF GABA(A)α1 KO mCherry− neurons and CRF-Cre-GFP+ neurons (Fig. 5 C and D). These results suggest that the GABA(A)α1 subunit contributes to a frequency selective modulation of short-term synaptic plasticity in BNST CRF neurons.
Discussion
The present study used a Cre-expressing transgenic mouse in which Cre is driven by a CRF-specific promoter to delete GABA(A)α1 only within CRF-expressing neurons [CRF GABA(A)α1 KO]. Radioactive and fluorescent in situ hybridization show CRF GABA(A)α1 KO increased expression of CRF within the PVN, CeA, and BNST. This type of knockdown provides a unique way to model disinhibition of CRF-containing neurons that have been shown to contribute to anxiety disorders (24, 25). We found that CRF GABA(A)α1 KO leads to increased anxiety and a persistent deficit in fear extinction. We also found that enhanced anxiety and disrupted fear extinction were rescued with application of the CRF antagonist R121919 systemically and when given into the BNST, indicating that directly attenuating CRF restores disrupted behavior to levels comparable to mice without the deletion.
We also found a significant increase in corticosterone after fear extinction. This finding suggests that the extinction phenotype of these animals does have a HPA axis contribution. Glucocorticoids (corticosterone in rodents and cortisol in humans) facilitate fear memory extinction (26–29). Because we did not measure baseline corticosterone, we cannot say whether this increase was a result of extinction training deficits or existed throughout fear training and fear extinction. However, if corticosterone was increased during fear memory formation or at baseline in the CRF GABA(A)α1 KO animals we would predict our knockouts would acquire fear faster than controls (e.g. ref. 30), whereas CRF GABA(A)α1 KO acquired fear during training equivalently compared to controls.
We also assessed CRF GABA(A)α1 KO on depressive-like behavior using the tail suspension test. Work has shown altered levels of multiple GABA(A) receptor subunits within the prefrontal cortex of depressed suicide victims compared with nondepressed controls (31). The same study showed CRF was decreased in some parts of the prefrontal cortex of depressed individuals. Our findings show no differences in one measure of depressive-like behavior, the tail suspension test. Thus, our findings do not support CRF GABA(A)α1 KO as underlying one measure of depressive-like behavior. However, more comprehensive studies are needed to exclude a role for this neuronal subtype in depression.
The GABA(A) receptor is the target of a number of clinically important drugs, including benzodiazepines (32, 33). Zolpidem is a GABA(A)α1-preferring benzodiazepine-site agonist. We infused Zolpidem into the BNST, where it effectively reduced anxiety in Cre− controls, as predicted. In contrast, when given into the BNST of CRF GABA(A)α1 KO mice, Zolpidem was ineffective at reducing anxiety. CRF GABA(A)α1 KO animals continued to show increased anxiety, even with BNST infusion of Zolpidem. This shift in effectiveness of Zolpidem may be due to the overall decrease in the number of GABA(A)α1 subunits within the BNST, because decreased GABAergic tone in this structure has been linked with heightened anxiety (17) and decreased Zolpidem effectiveness (34). However, the mechanism of this shift in effectiveness remains to be determined.
It is unclear if there is compensatory up-regulation of other subunits of the GABA(A) receptor in response to the CRF GABA(A)α1 KO. With any developmental knockout, there is concern that compensatory alterations in a multitude of other genes may occur. Single-cell RT-PCR shows, qualitatively, that α2 and β1 are both expressed within GABAA neurons lacking α1. In a mouse line without α1-subunit expression, compensation of other GABA(A) subunits was found, along with other compensatory changes (34, 35). It is unclear whether our cell-type specific disruption would have less dramatic compensatory effects. Determining compensation that occurs in these mice may offer interesting insights into GABA(A) receptor subunit dynamics, especially since the deletion is only within CRF containing neurons. This is an important and interesting question that requires future study.
Electrophysiological analysis demonstrated that compared with animals that expressed GFP in CRF neurons, CRF GABA(A)α1 KO mice showed reduced short-term synaptic depression. Short-term synaptic depression indicates a decrease in the probability of neurotransmitter release caused by recurring presynaptic activation (36) and may play a role in neural coding of information (37). While these findings are intriguing, it remains to be determined whether disrupted short-term synaptic depression in the BNST underlies our behavioral effects.
Disinhibition mediated by GABA or CRF-mediated hyperexcitability within the amygdala may act as a mechanism underlying disorders of anxiety (6). Work to date has been unable to address which subtypes of CRFergic neurons contribute to dysregulation of anxiety. Global deletion of the GABA(A) receptor α1 subunit enhances auditory fear memory (20) or has no affect on anxiety (38). In the present work, when we specifically delete the GABA(A) α1 subunit in CRF neurons, we show enhanced anxiety and disrupted fear memory extinction, both of which are hallmarks of anxiety disorders, such as posttraumatic stress disorder. We also showed that the behavioral disruption in these mice was normalized by a CRF antagonist and sensitivity to a selective α1-acting benzodiazepine was disrupted. Finally, we demonstrated alterations in GABAergic responses in CRF-expressing neurons within BNST using in vitro electrophysiological recordings. These data indicate that increased CRF expression within this subtype of neurons leads to disrupted anxiety and fear extinction that can be rescued with pharmacological disruption of CRF.
This model of subtype-specific deletion of CRF-containing neurons adds to our understanding of the mechanism through which CRFergic circuits contribute to anxiety-related psychological disorders. These findings also highlight the contribution cell-type specific genetic approaches can make to the functional dissection of circuits underlying complex behavior. Further understanding of cell-specific modulation of CRF neurons may also provide novel, more specific targets for therapeutic intervention into disorders of anxiety and fear.
Materials and Methods
Further details beyond these materials and methods are provided in SI Materials and Methods. Experimental methods were approved by the Institutional Animal Use and Care Committee of Emory University. The transgenic mouse was made by crossing the CRFp3.0Cre transgenic mouse (23) with the GABA(A) α1 floxed mouse (39, 40). For stereotaxic surgery, mice were anesthetized and received bilateral microinjections of 0.5 μL of Cre-dependent floxed mCherry reporter virus via Hamilton microsyringe lowered to the following coordinates from bregma, based on the mouse brain atlas of ref. 41: AP = +0.5, ML = ±2.1, DV = −4.2 or cannulae were lowered to the same BNST coordinates for later drug delivery.
Staining Procedures.
X-Gal staining was performed as in ref. 23. In situ hybridization was conducted as in ref. 42.
Behavior.
Elevated plus maze.
Open-arm entries, the total number of arm entries, and the total time spent in the open arms was recorded over 5 min.
Open field.
The center zone was located 6 cm from the perimeter of the chamber walls. Activity was monitored and data was collected over 10 min.
Baseline startle.
After 5-min acclimation, animals were given 10 startle stimuli at four stimulus intensities (90, 100, 110, 120 dB) with an interstimulus interval of 30 s in pseudorandom sequence and mean startle amplitudes were calculated.
Tail suspension test.
Animals were suspended by the tail for 6 min. Time spent immobile was recorded by an observer blind to the genotype of the animals.
Fear conditioning, shock reactivity, and extinction.
All groups received five conditioned-stimulus tones (30 s, 6 kHz, 74 dB) coterminating with unconditioned-stimulus shocks (500 ms, 0.6 mA) with a 5-min intertrial interval (ITI). Shock reactivity data were collected from movement occurring during training. Extinction training and testing was conducted using 15 or 30 conditioned-stimulus tones (30 s, 6 kHz, 74 dB) in a novel context.
Single-Cell RT-PCR and Electrophysiological Recordings.
The procedure used to determine mRNA transcript expression in single cells has been described in detail elsewhere (23, 43). BNST slices were obtained as previously described (44). ChR2mCherry CRE+ neurons were selected under fluorescent illumination. Whole-cell patch clamp recordings were obtained with standard techniques (44).
Statistical Analysis.
Data were analyzed using the one-way ANOVA or repeated measures with follow-up planned comparisons or Tukey post hoc tests when appropriate.
Supplementary Material
Acknowledgments
We thank Aaron Jasnow, Jasmeer Chhatwal, and Scott Heldt for mice and reagents used in these studies. Support was provided by the National Institutes of Health (Grants DA019624 and F32MH090785), the National Science Foundation (GRFP DGE-0234618), the Burroughs Wellcome Fund, and National Institutes of Health/National Center for Research Resources base Grant P51RR000165 (to the Yerkes National Primate Research Center).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119261109/-/DCSupplemental.
References
- 1.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 2.Regev L, et al. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry. 2011;16:714–728. doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- 3.Regev L, Tsoory M, Gil S, Chen A. Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol Psychiatry. 2012;71:317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 4.Kapcala LP, Dicke JA. Brain corticotropin-releasing hormone receptors on neurons and astrocytes. Brain Res. 1992;589:143–148. doi: 10.1016/0006-8993(92)91174-d. [DOI] [PubMed] [Google Scholar]
- 5.Sawchenko PE, Swanson LW. Localization, colocalization, and plasticity of corticotropin-releasing factor immunoreactivity in rat brain. Fed Proc. 1985;44:221–227. [PubMed] [Google Scholar]
- 6.Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8:209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- 7.Bagosi Z, Jászberényi M, Szabó G, Telegdy G. The effects of CRF and the urocortins on [3H]GABA release from the rat amygdala—An in vitro superfusion study. Brain Res Bull. 2008;75:15–17. doi: 10.1016/j.brainresbull.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Nie Z, et al. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- 9.Rainnie DG, et al. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinkers CH, et al. Lifelong CRF overproduction is associated with altered gene expression and sensitivity of discrete GABA(A) and mGlu receptor subtypes. Psychopharmacology (Berl) 2012;219:897–908. doi: 10.1007/s00213-011-2423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- 12.Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- 13.Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2006;51:1013–1022. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahuque LL, et al. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: Role of CRF receptor subtypes. Psychopharmacology (Berl) 2006;186:122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sajdyk T, Johnson P, Fitz S, Shekhar A. Chronic inhibition of GABA synthesis in the bed nucleus of the stria terminalis elicits anxiety-like behavior. J Psychopharmacol. 2008;22:633–641. doi: 10.1177/0269881107082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29:7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiltgen BJ, et al. The alpha1 subunit of the GABA(A) receptor modulates fear learning and plasticity in the lateral amygdala. Front Behav Neurosci. 2009;3:37. doi: 10.3389/neuro.08.037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marowsky A, Fritschy JM, Vogt KE. Functional mapping of GABA A receptor subtypes in the amygdala. Eur J Neurosci. 2004;20:1281–1289. doi: 10.1111/j.1460-9568.2004.03574.x. [DOI] [PubMed] [Google Scholar]
- 22.Heldt SA, Ressler KJ. Forebrain and midbrain distribution of major benzodiazepine-sensitive GABAA receptor subunits in the adult C57 mouse as assessed with in situ hybridization. Neuroscience. 2007;150:370–385. doi: 10.1016/j.neuroscience.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin EI, et al. A novel transgenic mouse for gene-targeting within cells that express corticotropin-releasing factor. Biol Psychiatry. 2010;67:1212–1216. doi: 10.1016/j.biopsych.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemeroff CB, Vale WW. The neurobiology of depression: Inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. [PubMed] [Google Scholar]
- 25.Bale TL. Sensitivity to stress: Dysregulation of CRF pathways and disease development. Horm Behav. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Cai W-H, Blundell J, Han J, Greene RW, Powell CM. Postreactivation glucocorticoids impair recall of established fear memory. J Neurosci. 2006;26:9560–9566. doi: 10.1523/JNEUROSCI.2397-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- 28.Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y. Administration of corticosterone after memory reactivation disrupts subsequent retrieval of a contextual conditioned fear memory: Dependence upon training intensity. Neurobiol Learn Mem. 2008;89:178–184. doi: 10.1016/j.nlm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Blundell J, Blaiss CA, Lagace DC, Eisch AJ, Powell CM. Block of glucocorticoid synthesis during re-activation inhibits extinction of an established fear memory. Neurobiol Learn Mem. 2011;95:453–460. doi: 10.1016/j.nlm.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 31.Merali Z, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24:1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 33.Whiting PJ, McKernan RM, Wafford KA. Structure and pharmacology of vertebrate GABAA receptor subtypes. Int Rev Neurobiol. 1995;38:95–138. doi: 10.1016/s0074-7742(08)60525-5. [DOI] [PubMed] [Google Scholar]
- 34.Kralic JE, et al. GABA(A) receptor alpha-1 subunit deletion alters receptor subtype assembly, pharmacological and behavioral responses to benzodiazepines and zolpidem. Neuropharmacology. 2002;43:685–694. doi: 10.1016/s0028-3908(02)00174-0. [DOI] [PubMed] [Google Scholar]
- 35.Sur C, et al. Loss of the major GABA(A) receptor subtype in the brain is not lethal in mice. J Neurosci. 2001;21:3409–3418. doi: 10.1523/JNEUROSCI.21-10-03409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 37.Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci USA. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudolph U, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 39.Vicini S, et al. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heldt SA, Ressler KJ. Amygdala-specific reduction of alpha1-GABAA receptors disrupts the anticonvulsant, locomotor, and sedative, but not anxiolytic, effects of benzodiazepines in mice. J Neurosci. 2010;30:7139–7151. doi: 10.1523/JNEUROSCI.0693-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 2nd ed. San Diego: Academic; 2001. [Google Scholar]
- 42.Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J Neurosci. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hazra R, et al. A transcriptomic analysis of type I-III neurons in the bed nucleus of the stria terminalis. Mol Cell Neurosci. 2011;46:699–709. doi: 10.1016/j.mcn.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo JD, et al. Presynaptic muscarinic M(2) receptors modulate glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2012;62:1671–1683. doi: 10.1016/j.neuropharm.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.