Abstract
Recent reports show that fewer adolescents believe that regular cannabis use is harmful to health. Concomitantly, adolescents are initiating cannabis use at younger ages, and more adolescents are using cannabis on a daily basis. The purpose of the present study was to test the association between persistent cannabis use and neuropsychological decline and determine whether decline is concentrated among adolescent-onset cannabis users. Participants were members of the Dunedin Study, a prospective study of a birth cohort of 1,037 individuals followed from birth (1972/1973) to age 38 y. Cannabis use was ascertained in interviews at ages 18, 21, 26, 32, and 38 y. Neuropsychological testing was conducted at age 13 y, before initiation of cannabis use, and again at age 38 y, after a pattern of persistent cannabis use had developed. Persistent cannabis use was associated with neuropsychological decline broadly across domains of functioning, even after controlling for years of education. Informants also reported noticing more cognitive problems for persistent cannabis users. Impairment was concentrated among adolescent-onset cannabis users, with more persistent use associated with greater decline. Further, cessation of cannabis use did not fully restore neuropsychological functioning among adolescent-onset cannabis users. Findings are suggestive of a neurotoxic effect of cannabis on the adolescent brain and highlight the importance of prevention and policy efforts targeting adolescents.
Keywords: marijuana, longitudinal, cognition
Cannabis, the most widely used illicit drug in the world, is increasingly being recognized for both its toxic and its therapeutic properties (1). Research on the harmful and beneficial effects of cannabis use is important because it can inform decisions regarding the medicinal use and legalization of cannabis, and the results of these decisions will have major public-health consequences. As debate surrounding these issues continues in the United States and abroad, new findings concerning the harmful effects of cannabis on neuropsychological functioning are emerging.
Accumulating evidence suggests that long-term, heavy cannabis use may cause enduring neuropsychological impairment—impairment that persists beyond the period of acute intoxication (2). Studies of long-term, heavy cannabis users fairly consistently show that these individuals perform worse on neuropsychological tests (2–5), and some (6–8) but not all (9) studies suggest that impairment may remain even after extended periods of abstinence. The magnitude and persistence of impairment may depend on factors such as the quantity, frequency, duration, and age-of-onset of cannabis use (2), as more severe and enduring impairment is evident among individuals with more frequent and prolonged heavy use and a younger age-of-onset (3, 6, 8, 10–16).
The extant evidence base draws on case–control studies of recruited cannabis users and comparison subjects. These studies screen participants for potential confounding factors, such as alcohol and drug dependence, and compare them on neuropsychological test performance after a period of abstinence from cannabis. There are two commonly cited potential limitations of this approach. One is the absence of data on initial, precannabis-use neuropsychological functioning. It is possible that differences in test performance between cannabis users and controls are attributable to premorbid rather than cannabis-induced deficits (17–20). A second limitation is reliance on retrospectively reported quantity, frequency, duration, and age-of-onset of cannabis use, often inquired about years after initiation of heavy use.
A prospective, longitudinal investigation of the association between cannabis use and neuropsychological impairment could redress these limitations and strengthen the existing evidence base by assessing neuropsychological functioning in a sample of youngsters before the onset of cannabis use, obtaining prospective data on cannabis use as the sample is followed over a number of years, and readministering neuropsychological tests after some members of the sample have developed a pattern of long-term cannabis use. To our knowledge, only one prospective, longitudinal study of the effects of cannabis on neuropsychological functioning has been conducted (21), and, in this study, the sample was small and the average duration of regular cannabis use was only 2 y.
In the present study, we investigated the association between persistent cannabis use—prospectively assessed over 20 y—and neuropsychological functioning in a birth cohort of 1,037 individuals. Study members underwent neuropsychological testing in 1985 and 1986 before the onset of cannabis use and again in 2010–2012, after some had developed a persistent pattern of cannabis use. We tested six hypotheses. First, we tested the “cognitive decline” hypothesis that persistent cannabis users evidence greater decline in test performance from childhood to adulthood than nonusers. By examining within-person change in neuropsychological functioning, any effect of premorbid deficits on later (postcannabis-initiation) test performance was nullified. Second, we tested the “specificity” hypothesis to address whether impairment is confined to specific neuropsychological domains or whether it is more global. To test this hypothesis, we administered multiple tests for each of five specific domains, as different tests may be differentially sensitive to cannabis-associated neuropsychological impairment. In conducting our analyses, we tested alternative explanations for the association between persistent cannabis use and neuropsychological functioning by ruling out potential confounding effects of (i) acute or residual cannabis intoxication, (ii) tobacco dependence, (iii) hard-drug dependence (e.g., heroin, cocaine, amphetamines), (iv) alcohol dependence, and (v) schizophrenia. Third, we tested the “education” hypothesis that persistent cannabis users experience neuropsychological decline simply because they have eschewed academics and other opportunities for learning. Recent evidence suggests that staying in school can boost one's intelligence quotient (IQ) (22), and cannabis users tend to receive less schooling than nonusers (23). Therefore, we tested whether the association between persistent cannabis use and neuropsychological decline remained after controlling for years of education. Fourth, we queried third-party informants to test the “everyday cognition” hypothesis that cannabis-induced neuropsychological impairment translates into functional problems in daily life. Fifth, we tested the “developmental vulnerability” hypothesis that individuals who begin cannabis use as adolescents are particularly vulnerable to the effects of persistent cannabis use on neuropsychological functioning, as evidence suggests that cannabis has especially toxic effects on the developing brain (24–31). Sixth, we tested the “recovery” hypothesis that former persistent users who quit or reduce their cannabis use may be able to restore their neuropsychological health.
Results
Do Study Members with More Persistent Cannabis Use Show Greater IQ Decline?
Table 1 (far right column) shows effect sizes for within-person IQ change from childhood to adulthood as a function of persistent cannabis dependence. In this analysis, each study member served as his or her own control; given that the groups were not equivalent on childhood IQ, we accounted for premorbid IQ differences by looking at IQ change from childhood to age 38 y. Study members with more persistent cannabis dependence showed greater IQ decline. For example, study members who never used cannabis experienced a slight increase in IQ, whereas those who diagnosed with cannabis dependence at one, two, or three or more study waves experienced IQ declines of −0.11, −0.17, and −0.38 SD units, respectively. An IQ decline of −0.38 SD units corresponds to a loss of ∼6 IQ points, from 99.68 to 93.93. Results of analyses for persistent cannabis dependence and persistent regular cannabis use were similar (Table 1).
Table 1.
IQ before and after cannabis use
| N | % male | Age 7–13 full-scale IQ | Age 38 full-scale IQ | Δ IQ effect size* | |
| Persistence of cannabis dependence | |||||
| Never used, never diagnosed | 242 | 38.84 | 99.84 (14.39) | 100.64 (15.25) | 0.05 |
| Used, never diagnosed | 479 | 49.48 | 102.32 (13.34) | 101.25 (14.70) | −0.07 |
| 1 diagnosis | 80 | 70.00 | 96.40 (14.31) | 94.78 (14.54) | −0.11 |
| 2 diagnoses | 35 | 62.86 | 102.14 (17.08) | 99.67 (16.11) | −0.17 |
| 3+ diagnoses | 38 | 81.58 | 99.68 (13.53) | 93.93 (13.32) | −0.38 |
| Persistence of regular cannabis use | |||||
| Never used | 242 | 38.84 | 99.84 (14.39) | 100.64 (15.25) | 0.05 |
| Used, never regularly | 508 | 50.59 | 102.27 (13.59) | 101.24 (14.81) | −0.07 |
| Used regularly at 1 wave | 47 | 72.34 | 101.42 (14.41) | 98.45 (14.89) | −0.20 |
| Used regularly at 2 waves | 36 | 63.89 | 95.28 (10.74) | 93.26 (11.44) | −0.13 |
| Used regularly at 3+ waves | 41 | 78.05 | 96.00 (16.06) | 90.77 (13.88) | −0.35 |
Means (SDs) are presented for child and adult full-scale IQ as a function of the number of study waves between ages 18 y and 38 y for which study members met criteria for cannabis dependence or reported using cannabis on a regular basis (at least 4 d/wk). The last column shows that study members with more persistent cannabis use showed greater IQ decline from childhood to adulthood.
*This coefficient indicates change in IQ from childhood to adulthood, with negative values indicating decreases in IQ. These change scores are in SD units, with values of 0.20, 0.50, and 0.80 reflecting small, medium, and large changes, respectively.
Table 2 expands the analysis by showing results for the subtests of different cognitive abilities that constitute the IQ. Persistent cannabis dependence was associated with greater decline on the majority of the subtests.
Table 2.
IQ subtest changes
| IQ test/subtest | Never used, never diagnosed, n = 242 | Used, never diagnosed, n = 479 | 1 diagnosis, n = 80 | 2 diagnoses, n = 35 | 3+ diagnoses, n = 38 | Linear trend t test* | P |
| Full-scale IQ | 0.05 | −0.07 | −0.11 | −0.17 | −0.38 | −4.45 | <0.0001 |
| Verbal IQ | 0.02 | −0.05 | −0.13 | −0.19 | −0.31 | −4.15 | <0.0001 |
| Information subtest | 0.05 | −0.08 | 0.02 | −0.25 | −0.15 | −2.40 | 0.0168 |
| Similarities subtest | 0.03 | −0.05 | −0.03 | −0.19 | −0.44 | −2.78 | 0.0056 |
| Vocabulary subtest | 0.07 | −0.05 | −0.16 | −0.16 | −0.45 | −3.67 | 0.0003 |
| Arithmetic subtest | −0.05 | −0.07 | −0.05 | 0.00 | 0.06 | −0.73 | 0.47 |
| Performance IQ | 0.08 | −0.08 | −0.09 | −0.08 | −0.42 | −2.84 | 0.0046 |
| Digit symbol coding subtest | 0.15 | −0.09 | −0.17 | −0.23 | −0.62 | −5.60 | <0.0001 |
| Block design subtest | −0.03 | −0.07 | −0.01 | −0.11 | 0.02 | −0.55 | 0.58 |
| Picture completion subtest | −0.01 | −0.08 | 0.08 | 0.05 | 0.15 | 1.18 | 0.24 |
Mean change in IQ subtest scores from childhood to adulthood is presented in SD units as a function of the number of study waves between ages 18 y and 38 y for which a study member met criteria for cannabis dependence. These change scores can be interpreted as effect sizes, with values of 0.20, 0.50, and 0.80 reflecting small, medium, and large effects, respectively. Persistent cannabis dependence was associated with IQ decline for the majority of IQ subtests administered in both childhood and adulthood, i.e., when each study member served as his or her own control.
*To test for a dose–response effect, we conducted an ordinary least-squares regression, estimating the linear trend controlling for sex.
IQ decline was most pronounced among the most persistent cannabis-dependence group (i.e., the 3+ group; n = 38), but the effect of persistent cannabis dependence on IQ decline was not solely attributable to this group. For example, the association between persistent cannabis dependence and full-scale IQ decline was still apparent after excluding the study members with 3+ cannabis-dependence diagnoses from the analysis (t = −2.94, P = 0.0034). Table S1 shows parallel results for persistent regular cannabis use and persistent cannabis dependence.
Is Impairment Specific to Certain Neuropsychological Domains or Is It Global?
Table 3 shows the effects of persistent cannabis dependence on five different areas of mental function assessed at age 38 y. Effects represent mean neuropsychological test performance at age 38 y, adjusted for childhood IQ. Across different areas of mental function, study members with more persistent cannabis dependence generally showed greater neuropsychological impairment. Inspection of the means suggests that the greatest impairments were for the domains of executive functioning and processing speed. To test whether impairment was relatively greater for certain domains, we compared cannabis-associated neuropsychological impairment across the four Wechsler Adult Intelligence Scale-IV (WAIS-IV) indexes (i.e., working memory index, processing speed index, perceptual reasoning index, and verbal comprehension index), which share psychometric properties (i.e., reliability) important for such a test. Using a model-fitting approach, we fitted (i) a model allowing the association between persistent cannabis dependence and age-38 neuropsychological impairment, adjusted for childhood IQ and sex, to vary across the four WAIS-IV indexes and (ii) a model equating this association across the four WAIS-IV indexes. Results showed that associations between persistent cannabis dependence and all four WAIS-IV indexes could be equated without a resultant deterioration in model fit (Δχ2 = 2.13, df = 3, P = 0.55), which suggests that impairment was not statistically significantly different across neuropsychological domains.
Table 3.
Five areas of mental function
| Age 38 y neuropsychological tests | Never used, never diagnosed, n = 242 | Used, never diagnosed, n = 479 | 1 diagnosis, n = 80 | 2 diagnoses, n = 35 | 3+ diagnoses, n = 38 | Linear trend t test* | P |
| Tests of executive functions | |||||||
| WAIS-IV Working Memory Index | 0.01 | 0.03 | −0.16 | −0.03 | −0.16 | −2.16 | 0.0311 |
| Wechsler Memory Scale Months of the Year Backward | 0.24 | 0.01 | −0.38 | −0.23 | −0.63 | −5.24 | <0.0001 |
| Trail-Making Test B Time† | −0.04 | −0.03 | 0.16 | 0.08 | 0.19 | 1.15 | 0.25 |
| CANTAB Rapid Visual Information Processing A Prime (Vigilance) | 0.05 | 0.01 | −0.02 | −0.04 | −0.45 | −2.58 | 0.0100 |
| CANTAB Rapid Visual Information Processing Total False Alarms† | −0.02 | 0.01 | 0.06 | 0.04 | −0.14 | −0.05 | 0.96 |
| Tests of memory | |||||||
| Rey Auditory Verbal Learning Total Recall | 0.11 | 0.06 | −0.26 | −0.22 | −0.48 | −2.65 | 0.0081 |
| Rey Auditory Verbal Learning Delayed Recall | 0.14 | 0.02 | −0.22 | −0.28 | −0.31 | −2.11 | 0.0348 |
| Wechsler Memory Scale Verbal Paired Associates Total Recall | 0.07 | 0.06 | −0.21 | −0.21 | −0.12 | −1.48 | 0.14 |
| Wechsler Memory Scale Verbal Paired Associates Delayed Recall | 0.07 | 0.06 | −0.19 | −0.15 | −0.14 | −1.07 | 0.29 |
| CANTAB Visual Paired Associates Learning First Trial Memory Score | 0.09 | 0.01 | −0.06 | −0.36 | −0.10 | −2.22 | 0.0270 |
| CANTAB Visual Paired Associates Learning Total Errors† | −0.07 | −0.03 | 0.17 | 0.33 | −0.06 | 1.41 | 0.16 |
| Tests of processing speed | |||||||
| WAIS-IV Processing Speed Index | 0.14 | 0.03 | −0.21 | −0.05 | −0.61 | −3.64 | 0.0003 |
| CANTAB Rapid Visual Information Processing Mean Latency† | −0.13 | 0.04 | 0.06 | −0.20 | 0.25 | 1.92 | 0.06 |
| CANTAB Reaction Time 5-Choice Reaction Time† | 0.19 | −0.11 | −0.13 | −0.01 | 0.18 | −0.38 | 0.71 |
| Tests of perceptual reasoning | |||||||
| WAIS-IV Perceptual Reasoning Index | 0.08 | −0.02 | 0.07 | −0.18 | −0.12 | −2.33 | 0.0202 |
| Tests of verbal comprehension | |||||||
| WAIS-IV Verbal Comprehension Index | 0.10 | −0.01 | −0.03 | 0.02 | −0.23 | −3.04 | 0.0025 |
Neuropsychological test scores at age 38 y are shown as a function of the number of study waves between ages 18 y and 38 y for which study members met criteria for cannabis dependence. Scores are standardized means adjusted for baseline (childhood) full-scale IQ assessed before the onset of cannabis use. These means can be interpreted as effect sizes, with values of 0.20, 0.50, and 0.80 reflecting small, medium, and large effects, respectively. Persistent cannabis dependence was associated with impairment in each of the five areas of mental function. CANTAB, Cambridge Neuropsychological Test Automated Battery; WAIS-IV, Wechsler Adult Intelligence Scale-IV.
*To test for a dose–response effect, we conducted an ordinary least-squares regression, estimating the linear trend controlling for childhood full-scale IQ and sex.
†Higher score indicates worse performance.
Is Impairment Attributable to Persistent Cannabis Use or Are There Alternative Explanations?
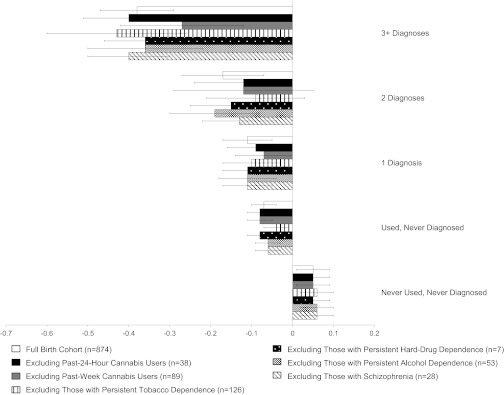
We ruled out six alternative explanations for the observed effects of persistent cannabis use on neuropsychological functioning, namely that these effects could be explained by (i) past 24-h cannabis use, (ii) past-week cannabis use, (iii) persistent tobacco dependence, (iv) persistent hard-drug dependence, (v) persistent alcohol dependence, and (vi) schizophrenia. We recalculated the mean change in full-scale IQ as a function of persistent cannabis dependence, excluding each of the aforementioned groups. We elected to show results just for full-scale IQ for this analysis as well as all subsequent analyses because full-scale IQ captures overall intellectual functioning. Fig. 1 shows that excluding each of these groups of study members did not alter the initial finding; effect sizes, representing within-person IQ change as a function of persistent cannabis dependence, remained virtually the same and remained statistically significant (see Table S2 for IQ subtests). Furthermore, a multivariate regression of the effect of persistent cannabis dependence on full-scale IQ decline, controlling for past 24-h cannabis use, persistent substance dependence (the number of study waves for which study members diagnosed with tobacco, hard-drug, or alcohol dependence), and schizophrenia remained statistically significant (t = −2.20, P = 0.0282).
Fig. 1.
Ruling out alternative explanations. Shown is change in full-scale IQ (in SD units) from childhood to adulthood as a function of the number of study waves between ages 18 y and 38 y for which a study member met criteria for cannabis dependence. Change scores are presented for the full birth cohort and the cohort excluding (i) past 24-h cannabis users, (ii) past-week cannabis users, (iii) those with persistent tobacco dependence, (iv) those with persistent hard-drug dependence, (v) those with persistent alcohol dependence, and (vi) those with lifetime schizophrenia. Persistent tobacco, hard-drug, and alcohol dependence were each defined as dependence at three or more study waves. IQ decline could not be explained by other factors. Error bars = SEs.
Is Impairment Apparent Even After Controlling for Years of Education?
The linear effect of persistent cannabis dependence on change in full-scale IQ was significant before controlling for years of education (t = −4.45, P < 0.0001; Table 2, top row) and remained significant after controlling for years of education (t = −3.41, P = 0.0007). Moreover, although fewer persistent cannabis users pursued education after high school (χ2 = 63.94, P < 0.0001), among the subset with a high-school education or less, persistent cannabis users experienced greater IQ decline (Table 4).
Table 4.
IQ decline after holding education constant
| Sample | Never used, never diagnosed | Used, never diagnosed | 1 diagnosis | 2 diagnoses | 3+ diagnoses | Linear trend t test* | P |
| Full sample | 0.05 (n = 242) | −0.07 (n = 479) | −0.11 (n = 80) | −0.17 (n = 35) | −0.38 (n = 38) | −4.45 | <0.0001 |
| High-school education or less | −0.03 (n = 59) | −0.14 (n = 130) | −0.16 (n = 43) | −0.25 (n = 20) | −0.48 (n = 26) | −3.36 | 0.0009 |
Mean change in full-scale IQ from childhood to adulthood is presented in SD units as a function of the number of study waves between ages 18 y and 38 y for which a study member met criteria for cannabis dependence. These change scores can be interpreted as effect sizes, with values of 0.20, 0.50, and 0.80 reflecting small, medium, and large effects, respectively. Change scores are presented for the full sample and for the sample of study members with a high-school education or less. Persistent cannabis dependence was associated with IQ decline in the full sample and the sample of study members with a high-school education or less.
*To test for a dose–response effect, we conducted an ordinary least-squares regression, estimating the linear trend controlling for sex.
Does Cannabis-Associated Neuropsychological Impairment Translate into Functional Problems in Daily Life?
Informant reports of study members’ neuropsychological functioning were also obtained at age 38 y. Study members nominated people “who knew them well.” These informants were mailed questionnaires and asked to complete a checklist, including whether the study members had problems with their attention and memory over the past year. Table 5 shows mean informant-reported cognitive problems, adjusted for childhood IQ, as a function of persistent cannabis dependence. Informants reported observing significantly more attention and memory problems among those with more persistent cannabis dependence.
Table 5.
Cognitive problems outside the laboratory
| Age 38 y informant reports | Never used, never diagnosed, n = 228 | Used, never diagnosed, n = 457 | 1 diagnosis, n = 71 | 2 diagnoses, n = 31 | 3+ diagnoses, n = 35 | Linear trend t test* | P |
| Informant-reported attention problems† | −0.21 | −0.07 | 0.31 | 0.64 | 0.96 | 7.74 | <0.0001 |
| Informant-reported memory problems† | −0.27 | −0.03 | 0.38 | 0.78 | 0.75 | 7.65 | <0.0001 |
Shown are informant reports of cognitive problems at age 38 y as a function of the number of study waves between ages 18 y and 38 y for which study members met criteria for cannabis dependence. Scores are standardized means adjusted for baseline (childhood) full-scale IQ assessed before the onset of cannabis use. These means can be interpreted as effect sizes, with values of 0.20, 0.50, and 0.80 reflecting small, medium, and large effects, respectively. Cognitive problems among persistent cannabis users were apparent to the “naked-eye.”
*To test for a dose–response effect, we conducted an ordinary least-squares regression, estimating the linear trend controlling for childhood full-scale IQ and sex.
†Higher score indicates worse everday problems.
Are Adolescent Cannabis Users Particularly Vulnerable?
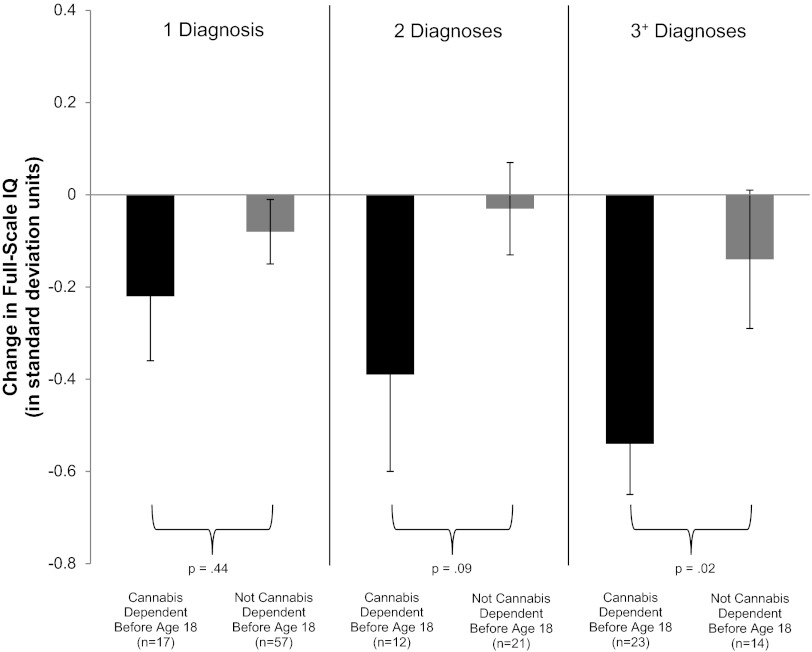
Adolescent-onset users, who diagnosed with cannabis dependence before age 18 y, tended to become more persistent users, but Fig. 2 shows that, after equating adolescent- and adult-onset cannabis users on total number of cannabis-dependence diagnoses, adolescent-onset users showed greater IQ decline than adult-onset cannabis users. In fact, adult-onset cannabis users did not appear to experience IQ decline as a function of persistent cannabis use. Because it might be difficult to develop cannabis dependence before age 18 y, we also defined adolescent-onset cannabis use in terms of weekly use before age 18 y [the correspondence between cannabis dependence before age 18 y and weekly use before age 18 y was not perfect (κ = 0.64)]. Results of this analysis (Fig. S1) were similar.
Fig. 2.
Adolescent vulnerability. Shown is change in full-scale IQ (in SD units) from childhood to adulthood among study members with 1, 2, or 3+ diagnoses of cannabis dependence as a function of age of onset of cannabis dependence. Individuals with adolescent-onset cannabis dependence (black bars) experienced greater IQ decline than individuals with adult-onset cannabis dependence (gray bars). IQ decline of approximately −0.55 SD units among individuals with adolescent-onset cannabis dependence in the 3+ group represents a decline of 8 IQ points. Error bars = SEs.
What Is the Effect of Cessation of Cannabis Use?
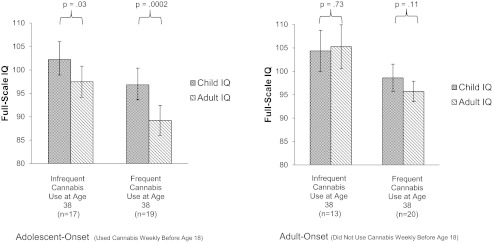
Given that adolescent-onset cannabis users exhibited marked IQ decline and given speculation that this could represent a toxic effect of cannabis on the developing brain, we examined the cessation effect separately within adolescent-onset and adult-onset cannabis users. Fig. 3 shows that, among adolescent-onset persistent cannabis users, within-person IQ decline was apparent regardless of whether cannabis was used infrequently (median use = 14 d) or frequently (median use = 365 d) in the year before testing. In contrast, within-person IQ decline was not apparent among adult-onset persistent cannabis users who used cannabis infrequently (median use = 6 d) or frequently (median use = 365 d) in the year before testing. Thus, cessation of cannabis use did not fully restore neuropsychological functioning among adolescent-onset former persistent cannabis users.
Fig. 3.
Postcessation IQ among former persistent cannabis users. This figure is restricted to persistent cannabis users, defined as study members with two or more diagnoses of cannabis dependence. Shown is full-scale IQ in childhood and adulthood. IQ is plotted as a function of (i) age of onset of at least weekly cannabis use and (ii) the frequency of cannabis use at age 38 y. Infrequent use was defined as weekly or less frequent use in the year preceding testing at age 38 y. Median use among infrequent and frequent adolescent-onset cannabis users was 14 (range: 0–52) and 365 (range: 100–365) d, respectively. Median use among infrequent and frequent adult-onset cannabis users was 6 (range: 0–52) and 365 (range: 100–365) d, respectively. IQ decline was apparent even after cessation of cannabis use for adolescent-onset former persistent cannabis users. Error bars = SEs.
Discussion
Persistent cannabis use over 20 y was associated with neuropsychological decline, and greater decline was evident for more persistent users. This effect was concentrated among adolescent-onset cannabis users, a finding consistent with results of several studies showing executive functioning or verbal IQ deficits among adolescent-onset but not adult-onset chronic cannabis users (8, 10, 14, 15), as well as studies showing impairment of learning, memory, and executive functions in samples of adolescent cannabis users (11–13, 32).
The present study advances knowledge in five ways. First, by investigating the association between persistent cannabis use and neuropsychological functioning prospectively, we ruled out premorbid neuropsychological deficit as an explanation of the link between persistent cannabis use and neuropsychological impairment occurring after persistent use. Second, we showed that the impairment was global and detectable across five domains of neuropsychological functioning. Third, we showed that cannabis-associated neuropsychological decline did not occur solely because cannabis users completed fewer years of education. Fourth, we showed that impairment was apparent to third-party informants and that persistent cannabis use interfered with everyday cognitive functioning. Fifth, we showed that, among adolescent-onset former persistent cannabis users, impairment was still evident after cessation of use for 1 y or more. Collectively, these findings are consistent with speculation that cannabis use in adolescence, when the brain is undergoing critical development, may have neurotoxic effects.
The study’s results must be interpreted in the context of its limitations. First, although we were able to rule out a set of plausible alternative explanations for the association between persistent cannabis use and neuropsychological functioning, such as premorbid neuropsychological deficit and hard-drug and alcohol dependence among persistent cannabis users, our data cannot definitively attest to whether this association is causal. For example, there may be some unknown “third” variable that could account for the findings. The data also cannot reveal the mechanism underlying the association between persistent cannabis dependence and neuropsychological decline. One hypothesis is that cannabis use in adolescence causes brain changes that result in neuropsychological impairment. Several lines of evidence support this possibility (24–31, 33, 34). First, puberty is a period of critical brain development, characterized by neuronal maturation and rearrangement processes (e.g., myelination, synaptic pruning, dendritic plasticity) and the maturation of neurotransmitter systems (e.g., the endogenous cannabinoid system), making the pubertal brain vulnerable to toxic insult (33). Second, cannabis administration in animals is associated with structural and functional brain differences, particularly in hippocampal regions, with structural differences dependent on age and duration of exposure to cannabinoids (33). Third, studies of human adolescents have shown structural and functional brain differences associated with cannabis use (26, 29, 35). Alternatively, persistent cannabis users may experience greater neuropsychological decline relative to nonusers because they receive less education. Our results suggest that cannabis-associated neuropsychological decline does not occur solely for this reason, because the association between persistent cannabis use and neuropsychological decline was still apparent after controlling for years of education. Notably, the aforementioned processes are not mutually exclusive and may, in fact, be interrelated. For example, the toxic effects of cannabis on the brain may result in impaired neuropsychological functioning, poor academic performance, and subsequent school dropout, which then results in further neuropsychological decline. In this case, our statistical control for education in the analysis of the association between persistent cannabis use and neuropsychological decline is likely an overcontrol (36).
A second limitation is that we obtained information on past-year cannabis dependence and self-reported frequency of cannabis use with no external validation of use (e.g., biological assays). Validation of cannabis use through laboratory measures could have helped detect cannabis users who did not report use. Underreporting of cannabis use due to concerns about admitting to using an illegal substance is unlikely, however, because study members, interviewed repeatedly over 38 y about a number of illegal activities, have learned to trust the Dunedin Study’s confidentiality guarantee. Moreover, any such misclassification would have mitigated against differences. Third, additional research is needed to define the parameters of use sufficient to produce neuropsychological impairment, such as the quantity, frequency, and age-of-onset of use. Our findings suggest that regular cannabis use before age 18 y predicts impairment, but others have found effects only for younger ages (10, 15). Given that the brain undergoes dynamic changes from the onset of puberty through early adulthood (37, 38), this developmental period should be the focus of future research on the age(s) at which harm occurs. Fourth, additional research is needed to determine whether cannabis-related neuropsychological impairment is reversible. Our finding of neuropsychological difficulties among adolescent-onset former persistent cannabis users who quit or reduced their use for 1 y or more suggests that neuropsychological functioning is not fully restored in this time. Fifth, these findings are limited to a cohort of individuals born in Dunedin, New Zealand in the 1970s. Notably, the prevalence of cannabis dependence is somewhat higher among New Zealanders than Americans (39), but the potency of cannabis obtained from police seizures in New Zealand is similar to that of cannabis in the United States (40, 41).
Increasing efforts should be directed toward delaying the onset of cannabis use by young people, particularly given the recent trend of younger ages of cannabis-use initiation in the United States and evidence that fewer adolescents believe that cannabis use is associated with serious health risk (42). In the present study, the most persistent adolescent-onset cannabis users evidenced an average 8-point IQ decline from childhood to adulthood. Quitting, however, may have beneficial effects, preventing additional impairment for adolescent-onset users. Prevention and policy efforts should focus on delivering to the public the message that cannabis use during adolescence can have harmful effects on neuropsychological functioning, delaying the onset of cannabis use at least until adulthood, and encouraging cessation of cannabis use particularly for those who began using cannabis in adolescence.
Methods
Participants.
Participants are members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of the health and behavior of a complete birth cohort of consecutive births between April 1, 1972, and March 31, 1973, in Dunedin, New Zealand. The cohort of 1,037 children (91% of eligible births; 52% boys) was constituted at age 3 y. Cohort families represent the full range of socioeconomic status in the general population of New Zealand’s South Island and are primarily of white European ancestry. Follow-up assessments were conducted with informed consent at 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, and most recently at 38 y of age, when 96% of the 1,004 living study members underwent assessment in 2010–2012. The Otago Ethics Committee approved each wave of the study. Study members gave informed consent before participating.
Because individuals with missing data at one wave tend to return to the study at some later wave(s), the attrition in the Dunedin Study has not been cumulative, and reasons for missing assessments seem to be idiosyncratic rather than systematic. There was no evidence of differential attrition for cannabis-dependent individuals. For example, the 4% of study members who did not participate at age 38 y were no more likely to have been cannabis dependent at age 18 y than study members who did participate (F = 2.22, P = 0.14).
Measures.
Cannabis use.
Past-year cannabis dependence was assessed with the Diagnostic Interview Schedule (43, 44) at ages 18, 21, 26, 32, and 38 y following criteria for the Diagnostic and Statistical Manual of Mental Disorders (DSM) (45, 46). Cohort members having missing data from three or more of the five study waves (ages 18, 21, 26, 32, and 38 y) were excluded when we defined our cannabis-exposure variables: 97% of living cohort members were studied, composed of 83% of living study members with no missing data points, 11% with one missing data point, and 3% with two missing data points. Our main exposure, persistence of cannabis dependence, was defined as the total number of study waves out of five at which a study member met criteria for cannabis dependence. Study members were grouped according to their number of dependence diagnoses: (i) those who never used cannabis at any study wave and thus could not have become dependent, (ii) those who used cannabis at least once at one or more study waves but never diagnosed, (iii) those who diagnosed at one wave, (iv) those who diagnosed at two waves, and (v) those who diagnosed at three or more waves.
Because there were some study members who used cannabis on a regular basis but never met full criteria for a diagnosis of cannabis dependence, we repeated analyses using persistent regular cannabis use as the exposure. At each of the five study waves between ages 18–38 y, study members self-reported the total number of days (0–365) they used cannabis over the preceding year. Persistence of regular cannabis use was defined as the total number of study waves out of five at which a study member reported using cannabis 4 d/wk or more (the majority of days in a week). Study members were grouped as those who (i) never used cannabis, (ii) used but never regularly, (iii) used regularly at one wave, (iv) used regularly at two waves, and (v) used regularly at three or more waves. Correspondence between cannabis dependence and regular cannabis-use groups was high but not perfect (weighted κ = 0.77).
The Dunedin Study uses past-year reporting to maximize validity and reliability of recall. A potential consequence is that individuals could have experienced dependence only during a gap between the Study’s five 12-mo assessment windows and gone uncounted. Our “net” of 1-y assessments at ages 18, 21, 26, 32, and 38 y captured all but four of the cohort members who reported receiving treatment for a drug-use problem between assessment windows. Three of the four were hard-drug and alcohol dependent, and the remaining person sought counseling for cannabis use only as part of a child custody dispute. As these four cohort members reported cannabis use but not dependence, they were classified as “used but never diagnosed.”
Neuropsychological functioning.
Intelligence was assessed in childhood at ages 7, 9, 11, and 13 y, before the onset of cannabis use (only seven study members reported trying cannabis by age 13 y), and again in adulthood at age 38 y. We report comparison of the Wechsler Intelligence Scale for Children-Revised (WISC-R) (47) and the WAIS-IV (48), both with M = 100 and SD = 15. At age 38 y, additional neuropsychological tests were administered, including the Wechsler Memory Scale-III (WMS-III) (49), the Trail-Making Test (50), the Cambridge Neuropsychological Test Automated Battery (CANTAB) (51), and the Rey Auditory Verbal Learning Test (52). Because the sample is a representative birth cohort, it formed its own norms. Table S3 provides further details about each test. Each study member attended the research unit for an 8-h day of assessments. All testing occurred in the morning in two 50-min counterbalanced sessions.
Informant reports of study members’ neuropsychological functioning were also obtained at age 38 y. Study members nominated people who knew them well. These informants were mailed questionnaires and asked to complete a checklist, including whether the study members had problems with their attention and memory over the past year. The informant-reported attention problems scale consisted of four items: “is easily distracted, gets sidetracked easily,” “can’t concentrate, mind wanders,” “tunes out instead of focusing,” and “has difficulty organizing tasks that have many steps” (internal consistency reliability = 0.79). The informant-reported memory problems scale consisted of three items: “has problems with memory,” “misplaces wallet, keys, eyeglasses, paperwork,” and “forgets to do errands, return calls, pay bills” (internal consistency reliability = 0.64).
Control variables.
Past 24-h cannabis use and past-week cannabis use were assessed at age 38 y on the day of neuropsychological testing. Persistent DSM (45, 46) tobacco, hard-drug, and alcohol dependence were assessed over the same 20-y period during which cannabis dependence was assessed, and the number of study waves during which study members diagnosed was counted and used as covariates. For Fig. 1, persistent dependence was defined as having been diagnosed at three or more study waves. Research diagnoses of lifetime schizophrenia (53) are also reported.
Statistical Analysis.
First, for the IQ test and subtests (47, 48) administered in both childhood and adulthood, change scores were created by subtracting the precannabis childhood IQ averaged across ages 7, 9, 11 and 13 y (or, for the seven members who reported trying cannabis by age 13 y, ages 7, 9, and 11 y) from postcannabis adulthood IQ. Negative scores indicate IQ decline. Ordinary least-squares linear regression was used to test whether persistent cannabis use (entered as a five-level independent variable, with each study member receiving a score ranging from 1 to 5) predicted amount of IQ change. Second, for the neuropsychological tests administered only in adulthood, ordinary least-squares linear regression, including full-scale childhood IQ as a covariate, was used to test whether persistent cannabis use predicted neuropsychological test performance in adulthood (i.e., residualized change scores).
Tables 2–5 present the t tests associated with the regression coefficient testing the linear effect of persistent cannabis use on change in neuropsychological functioning, under the hypothesis that more persistent cannabis use predicts greater decline in neuropsychological functioning. Change scores are presented in SD units as a function of persistence of cannabis use. These scores can be interpreted as effect sizes, with values of 0.20, 0.50, and 0.80 reflecting small, medium, and large change, respectively (54). Sex was included as a covariate in all statistical tests.
Supplementary Material
Acknowledgments
We thank the Dunedin Study members, their families, the Dunedin Multidisciplinary Health and Development Research Unit staff, and study founder Phil Silva. The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council. This research received support from UK Medical Research Council Grants G0100527 and MR/K00381X/1, US National Institute on Aging Grant AG032282, US National Institute of Mental Health Grant MH077874, and US National Institute on Drug Abuse Grant P30 DA023026. Additional support was provided by the Jacobs Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 15970.
See Author Summary on page 15980 (volume 109, number 40).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206820109/-/DCSupplemental.
References
- 1.Murray RM, Morrison PD, Henquet C, Di Forti M. Cannabis, the mind and society: The hash realities. Nat Rev Neurosci. 2007;8:885–895. doi: 10.1038/nrn2253. [DOI] [PubMed] [Google Scholar]
- 2.Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: A review. Curr Drug Abuse Rev. 2008;1:81–98. doi: 10.2174/1874473710801010081. [DOI] [PubMed] [Google Scholar]
- 3.Solowij N, et al. Marijuana Treatment Project Research Group Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- 4.Pope HG, Jr, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275:521–527. [PubMed] [Google Scholar]
- 5.Fletcher JM, et al. Cognitive correlates of long-term cannabis use in Costa Rican men. Arch Gen Psychiatry. 1996;53:1051–1057. doi: 10.1001/archpsyc.1996.01830110089011. [DOI] [PubMed] [Google Scholar]
- 6.Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 7.Solowij N. Cannabis and Cognitive Functioning. Cambridge, UK: Cambridge Univ Press; 1998. [Google Scholar]
- 8.Pope HG, Jr, et al. Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 9.Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- 10.Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychol Addict Behav. 2011 doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007;26:309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- 12.Medina KL, et al. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. 2007;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solowij N, et al. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl) 2011;216:131–144. doi: 10.1007/s00213-011-2203-x. [DOI] [PubMed] [Google Scholar]
- 14.Ehrenreich H, et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- 15.Fontes MA, et al. Cannabis use before age 15 and subsequent executive functioning. Br J Psychiatry. 2011;198:442–447. doi: 10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- 16.Messinis L, Kyprianidou A, Malefaki S, Papathanasopoulos P. Neuropsychological deficits in long-term frequent cannabis users. Neurology. 2006;66:737–739. doi: 10.1212/01.wnl.0000201279.83203.c6. [DOI] [PubMed] [Google Scholar]
- 17.Giancola PR, Tarter RE. Executive cognitive functioning and risk for substance abuse. Psychol Sci. 1999;10:203–205. [Google Scholar]
- 18.Tarter RE, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- 19.Ersche KD, et al. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 20.Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana—a comparison with pre-drug performance. Neurotoxicol Teratol. 2005;27:231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Brinch CN, Galloway TA. Schooling in adolescence raises IQ scores. Proc Natl Acad Sci USA. 2011;109:425–430. doi: 10.1073/pnas.1106077109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwood LJ, et al. Cannabis use and educational achievement: Findings from three Australasian cohort studies. Drug Alcohol Depend. 2010;110:247–253. doi: 10.1016/j.drugalcdep.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- 25.Solowij N, Pesa N. [Cognitive abnormalities and cannabis use] Rev Bras Psiquiatr. 2010;32(Suppl 1):S31–S40. [PubMed] [Google Scholar]
- 26.Wilson W, et al. Brain morphological changes and early marijuana use: A magnetic resonance and positron emission tomography study. J Addict Dis. 2000;19:1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- 27.O’Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol. 2004;18:502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- 28.Rubino T, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- 29.Ashtari M, et al. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res. 2011;45:1055–1066. doi: 10.1016/j.jpsychires.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan GCK, Hinds TR, Impey S, Storm DR. Hippocampal neurotoxicity of Delta9-tetrahydrocannabinol. J Neurosci. 1998;18:5322–5332. doi: 10.1523/JNEUROSCI.18-14-05322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider M, Schömig E, Leweke FM. Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addict Biol. 2008;13:345–357. doi: 10.1111/j.1369-1600.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- 32.Hanson KL, et al. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jager G, Ramsey NF. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: An overview of animal and human research. Curr Drug Abuse Rev. 2008;1:114–123. doi: 10.2174/1874473710801020114. [DOI] [PubMed] [Google Scholar]
- 34.Scallet AC. Neurotoxicology of cannabis and THC: A review of chronic exposure studies in animals. Pharmacol Biochem Behav. 1991;40:671–676. doi: 10.1016/0091-3057(91)90380-k. [DOI] [PubMed] [Google Scholar]
- 35.Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: A cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49:561–572, 572, e1–e3. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meehl PE. High school yearbooks: A reply to Schwarz. J Abnorm Psychol. 1971;77:143–148. doi: 10.1037/h0031999. [DOI] [PubMed] [Google Scholar]
- 37.Giedd JN, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 38.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Moffitt TE, et al. How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychol Med. 2010;40:899–909. doi: 10.1017/S0033291709991036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLaren J, Swift W, Dillon P, Allsop S. Cannabis potency and contamination: A review of the literature. Addiction. 2008;103:1100–1109. doi: 10.1111/j.1360-0443.2008.02230.x. [DOI] [PubMed] [Google Scholar]
- 41.Hall W, Swift W. The THC content of cannabis in Australia: Evidence and implications. Aust N Z J Public Health. 2000;24:503–508. doi: 10.1111/j.1467-842x.2000.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 42.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. 2011. Monitoring the Future National Survey Results on Drug Use, 1975-2010: Secondary School Students (Institute for Social Research, University of Michigan, Ann Arbor, MI), Vol I.
- 43.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 44.Robins LN, Cottler L, Bucholz KK, Compton W. Diagnostic Interview Schedule for DSM-IV. St Louis, MO: Washington Univ School of Medicine; 1995. [Google Scholar]
- 45.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd Ed, Revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 46.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 47.Wechsler D. Manual for the Wechsler Intelligence Scale for Children—Revised. New York: Psychological Corporation; 1974. [Google Scholar]
- 48.Wechsler D. Wechsler Adult Intelligence Scale. 4th Ed. San Antonio, TX: Pearson Assessment; 2008. [Google Scholar]
- 49.Wechsler D. Wechsler Memory Scale. 3rd Ed. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 50.Army Individual Test Battery . Manual and Directions for Scoring. Washington, DC: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- 51.Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: Discussion paper. J R Soc Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- 52.Lezak MD. Neuropsychological Assessment. 4th Ed. New York: Oxford Univ Press; 2004. [Google Scholar]
- 53.Reichenberg A, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: A 30-year study. Am J Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]