Abstract
Toll-like receptor 7 (Tlr7) has been linked to systemic lupus disease incidence in humans and mice, but how TLR7 potentiates autoimmunity is unclear. We used a Tlr7 transgenic (tg) mouse model to investigate the cellular and molecular events required to induce spontaneous autoimmunity through increased TLR7 activity. We determined that Tlr7 exerts B-cell–intrinsic effects in promoting spontaneous germinal center (GC) and plasmablast B-cell development, and that these B-cell subsets are dependent on T-cell–derived signals through CD40L and SLAM-associated protein (SAP), but not IL-17. Antigen specificity also factored into TLR7-induced disease, as both a restricted T cell receptor (TCR) specificity and MHC haplotype H2k/k protected Tlr7tg mice from spontaneous lymphocyte activation and autoantibody production. Inflammatory myeloid cell expansion and autoimmunity did not develop in Tlr7tgIgH−/− mice, suggesting either that spontaneous TLR7 activation does not occur in dendritic cells, or, if it does occur, cannot drive these events in the absence of B-cell aid. These data indicate that autoimmune disease in Tlr7tg mice is contingent upon B cells receiving stimulation both through innate pathways and T-cell–derived signals and suggest a codependent relationship between B cells and T cells in the development of autoimmunity.
Keywords: inflammation, SLE, T follicular helper cells
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease with multiple causal factors that induce breakdown in immune tolerance and promote autoimmunity development. It is increasingly apparent that innate pathways, such as Toll-like receptor (TLR) signaling, contribute to spontaneous autoimmunity (1). TLR7 is an intracellular receptor that is expressed in B cells and plasmacytoid dendritic cells (pDCs) and recognizes pathogen-associated molecular patterns in the context of single-stranded viral RNA (2). Tlr7 gene duplication in autoimmune-prone mice potentiates disease incidence and accelerates lupus-like disease development, implicating Tlr7 as a probable susceptibility gene in the generation of autoimmune responses (3, 4). Expression of multiple copies of Tlr7 in an autoimmune-resistant mouse strain also results in spontaneous inflammation and autoimmunity (5), whereas deletion of this gene promotes disease resistance in autoimmune-prone strains (6). Importantly, peripheral blood mononuclear cells from SLE patients display higher levels of Tlr7 expression compared with healthy controls, and a genetic study detected Tlr7 gene duplication in a subset of lupus patients (7, 8). These clinical data highlight the necessity of determining the underlying contribution of TLR7 activation to cellular interactions and signals in SLE development.
Multiple potential mechanisms exist by which TLR7 could exert disease-promoting influence through either pDC- or B-cell–intrinsic signals. TLR7-activated pDCs produce large amounts of IFN-α, a cytokine known to have pathologic consequences in autoimmunity, and could drive disease in Tlr7 transgenic (tg) mice (1). However, extensive B-cell and T-cell activation and autoantibody production in Tlr7tg mice suggest a putative role for B cells and maybe for T cells in disease development, possibly through a mechanism involving GCs.
During a conventional adaptive immune response against an invading pathogen, GCs form under the impetus of coordinated interactions between B- and T cells. A specialized subset of CD4+CXCR5+ T cells called T follicular helper cells (TFH) supports B-cell differentiation in the GC (9). TFH cells provide essential costimulatory signals through CD40L and signaling lymphocytic activation molecule (SLAM) family receptors that promote B-cell survival and class switch recombination (CSR) to IgG antibody isotypes (9). However, conflicting evidence exists as to whether these interactions are vital to the development of an autoimmune response, or whether B cells activated through innate pathways are able to cause autoimmunity independently of T-cell help (10–12).
In some cases of particular relevance to our Tlr7tg system, lupus-like disease occurs in the absence of T-cell–derived signals. A mouse strain tg for B cell activating factor (BAFF), a survival molecule and growth factor for B cells triggered by TLR7 activation, develops activated B cells and autoimmunity independently of T cells (13). Activation, expansion, and differentiation of autoimmune rheumatoid factor-specific B cells from AM14tg mice on the Mrl-lpr/lpr background also occur in the absence of T cells, but are dependent on innate-derived TLR7 and TLR9 signals (14).
With these concerns in mind, in this study we examine the cellular requirements underlying the development of spontaneous autoimmunity in mice with excessive Tlr7 gene expression. We found that excessive Tlr7 preferentially expands GC B cells and plasmablasts, but that these events are dependent on T-cell–derived signals through CD40L and SAP. T-cell antigen specificity and the context of MHC presentation also played a role in the development of autoimmunity. Furthermore, inflammation did not develop in Tlr7tgIgH−/− mice, indicating that inflammatory myeloid cell expansion in Tlr7tg mice occurred downstream of B-cell–derived events. Together, these data suggest that B-cell signaling through innate and adaptive immune receptors works in conjunction with T-cell–B-cell interactions to induce autoimmunity.
Results
Excess Tlr7 Promotes Germinal Center B-Cell and Plasmablast Production in a B-Cell–Intrinsic Manner.
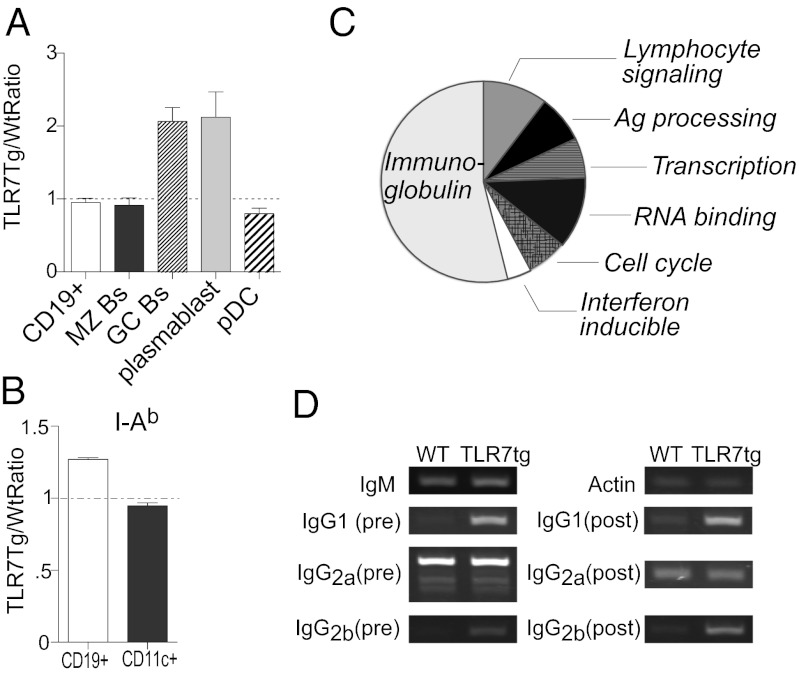
Previous data determined that Tlr7 gene multiplicity alone is able to induce autoimmune pathology in mice (5). Tlr7 is most commonly expressed in B cells and plasmacytoid dendritic cells (pDCs), but it is unknown whether Tlr7 exerts culpable influence in the development of autoimmunity in one or both of these cell types. To address this question, we generated mixed bone marrow (BM) chimeras composed of equal proportions of WT and Tlr7tg cells. We found that B cells and pDCs had equivalent total numbers of cells derived from WT and Tlr7tg origin (Fig. 1A). However, Tlr7tg B cells had increased cell surface MHC Class II expression, indicating a more activated phenotype compared with WT cells, whereas Tlr7tg and WT CD11c+ cells expressed similar levels of this marker (Fig. 1B). We also found that GC and CD138+ plasmablast B-cell subsets skewed preferentially to being of Tlr7tg origin (Fig. 1A). All other B-cell populations examined were equally likely to be derived from WT as Tlr7tg BM (Fig. 1A), indicating that Tlr7 causes a B-cell–intrinsic effect by promoting GC B-cell and plasmablast development.
Fig. 1.
Tlr7 promotes the generation of germinal center and plasmablast B cells through B-cell–intrinsic mechanisms. (A and B) 50:50 mix of Tlr7tg and WT BM was injected into irradiated WT mice; eight weeks later splenic cell populations were examined by flow cytometry. (A) Number of Tlr7tg-derived cells for cell types was expressed as a ratio between Tlr7tg and WT cells. (B) The ratio of Tlr7tg to WT expression of I-Ab on B cells and CD11c+ cells. Dotted line indicates equal proportions of WT and Tlr7tg cells. n = 3 mice/group. (C and D) cDNA derived from follicular B cells of WT or Tlr7tg mice was analyzed by (C) microarray (pie chart segments represent percentages of gene categories that contain genes up-regulated more than twofold in at least three mice) or (D) RT-PCR for Ig transcripts of preisotype switched and postisotype switched Ig genes.
Because we found an increase in the number of GC B cells and plasmablasts in Tlr7tg mice, we next studied gene expression differences in Tlr7tg B cells compared with WT B cells. To do this, we performed microarray analysis on CD9−CD43− follicular B cells from Tlr7tg and WT mice and found several striking changes, including up-regulation of type-1 IFN signature genes as well as antigen processing and presentation genes (Fig. 1C, Table S1). Interestingly, Ig genes comprised 54% of the total number of genes up-regulated more than twofold (Fig. 1C). Ig gene transcription precedes CSR or somatic hypermutation in activated B cells, so Tlr7tg B cells could have enhanced basal or postclass switch transcription of these genes. To investigate this, we analyzed transcription of both germline and class-switched Ig genes and found that Tlr7tg follicular B cells had increased transcription of IgG2b and IgG1, but not IgG2a genes, before and after CSR compared with WT B cells (Fig. 1D).
CD40L or SAP Deficiency Abrogates the Development of Spontaneous Germinal Centers and Autoimmunity in Tlr7tg Mice.
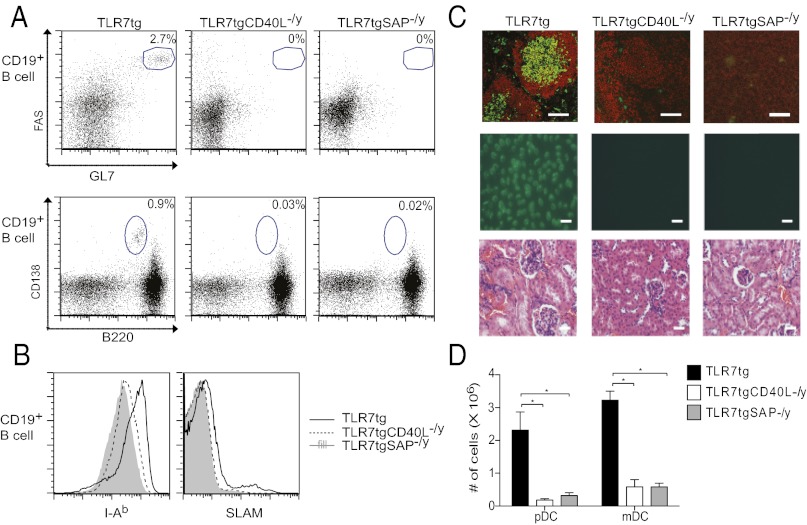
Because TLR7 signaling selectively supported GC B-cell differentiation, we wanted to determine whether GC formation is integral to spontaneous autoimmunity in Tlr7tg mice. To do this, we analyzed Tlr7tgCd40lg−/y mice for disease and found a drastic reduction in spontaneous GC B-cell and plasmablast numbers in these mice compared with Tlr7tg mice by flow cytometry (Fig. 2A) and immunohistochemistry (Fig. 2C). We analyzed B-cell activation markers MHC Class II and SLAM on Tlr7tgCd40lg−/y B cells and found that expression of these molecules was reduced compared with Tlr7tg B cells (Fig. 2B). We also found an absence of two lupus disease cardinal features: antinuclear serum antibodies by antinuclear antibody (ANA) staining, and glomerulonephritis development by kidney section H&E staining (Fig. 2C).
Fig. 2.
CD40L and SAP signals are required for the generation of spontaneous autoimmunity in Tlr7tg mice. At eight weeks of age, spleens, kidneys, and serum were harvested from Tlr7tg, Tlr7tgCd40lg−/y, and Tlr7tgSAP−/y mice. (A) Representative FACS plots showing GC B-cell percentages (Top) or plasmablasts (Bottom) in Tlr7tg (Left), Tlr7tgCd40lg−/y (Center), and Tlr7tgSAP−/y mice (Right). (B) Representative histograms of surface expression of I-Ab (Left) and SLAM (Right) on B cells from Tlr7tg (black line), Tlr7tgCd40lg−/y (dotted line), and Tlr7tgSAP−/y (gray fill) mice. (C) (Top) Frozen spleen sections stained with B220 (red) and PNA (green) in Tlr7tg (Left), Tlr7tgCd40lg−/y (Center), and Tlr7tgSAP−/y mice (Right). Bars = 50 μM. (Middle) Serum ANA tests. Bars = 50 μM. (Bottom) Histological analysis of representative kidney sections stained with H&E. Bars = 50 μM. (D) Absolute numbers of CD11c+B220+ pDCs and CD11c+CD11b+ mDCs. Experiments are representative of n = 3 mice per group repeated two times. *P < 0.05.
Dendritic cells can induce pathogenic inflammation in the development of lupus disease, particularly pDCs that are capable of producing copious quantities of IFN-α (15). Other data suggest that myeloid dendritic cells (mDCs) may also trigger autoimmunity by presenting self-antigens to self-reactive T cells (16, 17). Both of these cell types are known to interact with B- and T cells via CD40–CD40L signaling; thus, we enumerated both mDCs and pDCs in Tlr7tgCd40lg−/y mice and found that the absence of CD40–CD40L signaling eliminated the expansion of both of these dendritic cell (DC) subsets (Fig. 2D).
Because it is possible that lupus-like disease induction in Tlr7tg mice requires either B cells or DCs or both of these subsets to interact with T cells via CD40, we wanted to specifically pinpoint whether B-cell interactions with T cells are required. To do this, we crossed SAP−/y mice with Tlr7tg mice. T-cell SAP signaling downstream of SLAM receptors is essential for productive B-cell activation and prolonged adhesion to B cells, whereas it is dispensable for T-cell–DC interactions (18). Similar to our findings in Tlr7tgCd40lg−/y mice, we saw very few GC B cells, plasmablasts, mDCs, and pDCs (Fig. 2 A, C, and D), ablated production of antinuclear antibodies, and no development of glomerulonephritis in Tlr7tgSAP−/y mice (Fig. 2C). Tlr7tgSAP−/y B cells had even lower MHC Class II expression and comparably low B-cell SLAM expression compared with Tlr7tgCd40lg−/y B cells (Fig. 2B). Collectively, these data indicate that B-cell–T-cell interactions are integral to the development of lupus-like disease in Tlr7tg mice.
Germinal Center Formation and Autoimmunity in Tlr7tg Mice Require Antigen-Specific T-Cell Signals but Not T-Cell–Derived IL-17 Production.
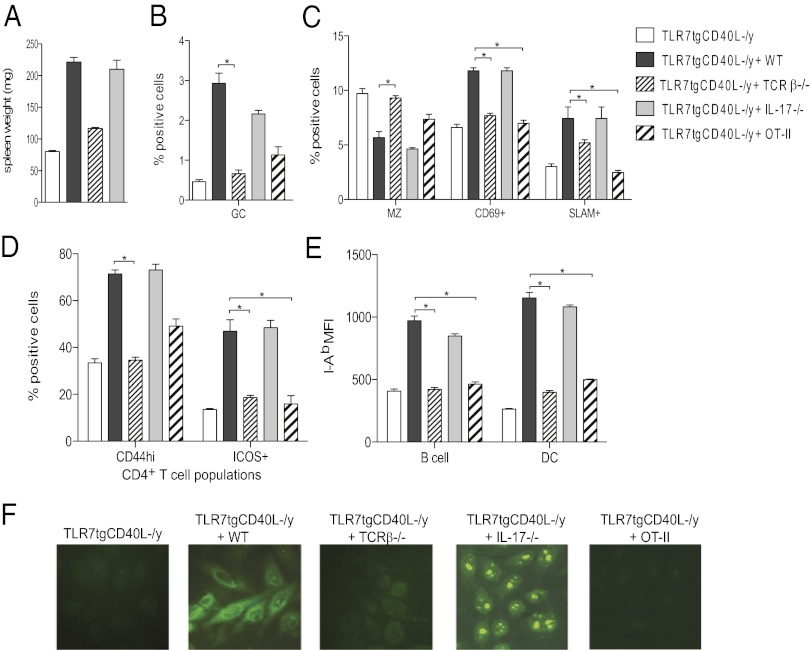
To determine whether T cells provide CD40L-dependent help to B cells, we generated 1:1 mixed BM chimeras by transferring Tlr7tgCd40lg−/y + TCRβ−/− BM into irradiated WT hosts. In this scenario, TCRβ−/− BM restores all costimulatory signals to Tlr7tgCd40lg−/y mice except those that are T-cell–derived because TCRβ−/− mice have a developmental block at the CD4−CD8− double negative thymocyte stage (19). When we examined these chimeras we observed that the spleens weighed significantly less than spleens from Tlr7tgCd40lg−/y + WT mixed BM chimeras (Fig. 3A). The number of GC B cells and activated B-cell subsets expressing high levels of CD69 and SLAM were also reduced (Fig. 3 B and C). Tlr7tg mice display diminution of the marginal zone (MZ) B-cell subset (5), but Tlr7tgCd40lg−/y or Tlr7tgCd40lg−/y + TCRβ−/− BM reconstituted this population in WT mice (Fig. 3C), indicating that T-cell–derived signals may contribute to contraction of the MZ B-cell compartment as well.
Fig. 3.
Spontaneous autoimmunity in TLR7tg mice requires antigen-specific T-cell help but not IL-17. Irradiated WT mice received BM from Tlr7tgCd40lg−/y, or an equal mix of BM from Tlr7tgCd40lg−/y and the indicated mouse strain, except for OT-II cells, which were used at a 4:1 ratio. (A) Measured spleen weight. Percentages of (B) FAS+GL7+ B cells; (C) splenic CD21hiCD23low MZ B cells, activated CD69+ B cells, and activated SLAM+ B cells; (D) ICOS+ or CD44+ CD4+ T cells; (E) mean fluorescence intensity (MFI) of I-Ab, SLAM+ B cells or DCs; and (F) ANA+ serum autoantibodies.
Tlr7tgCd40lg−/y + TCRβ−/− BM chimeras also had fewer activated CD4+ T cells expressing CD44 or ICOS and fewer B cells and DCs expressing high levels of I-Ab than Tlr7tgCd40lg−/y + WT BM reconstituted mice (Fig. 3 D and E). Additionally, WT mice transplanted with Tlr7tgCd40lg−/y + TCRβ−/− BM tested negative for serum autoantibodies by ANA staining, similar to Tlr7tgCd40lg−/y BM reconstituted mice, whereas serum from Tlr7tgCd40lg−/y + WT BM chimeras indicated the presence of both cytoplasmic and nucleolar autoantibodies (Fig. 3F). Collectively these data suggest an integral role for T cells in the development of autoimmunity in Tlr7tg mice.
IL-17 is a primarily CD4+ T-cell–derived cytokine implicated in autoimmune disease development, including lupus, in some mouse models and humans (20, 21). Because our previous microarray from C57BL/6Yaa mice showed IL-17 family member transcriptional up-regulation, we wanted to determine whether IL-17 played a role in Tlr7tg autoimmunity by generating 1:1 Tlr7tgCd40lg−/y + IL-17−/− BM transplants (3). We found equivalent spleen weights, spontaneous activation of B cells and T cells, and serum ANA staining in these mice compared with Tlr7tgCd40lg−/y + WT BM chimeras (Fig. 3 A–F), indicating that IL-17 was dispensable for disease incidence in Tlr7tg mice.
Self-antigen-dependent activation of T cells is purported to be one of the driving forces behind autoimmune responses (10, 22, 23). If T-cell antigen specificity is important for spontaneous autoimmunity induction, then restricting the repertoire to an irrelevant antigen would prevent disease occurrence in Tlr7tg mice. To explore this possibility, we created Tlr7tgCd40lg−/y + OT-II (an ovalbumin-specific TCR tg) mixed BM chimeras. We examined the splenic populations of these mice, compared them with Tlr7tgCd40lg−/y + WT BM chimeras, and found significantly lower percentages of CD69+ and SLAM+ activated B-cell populations (Fig. 3C) and CD4+ICOS+ T cells (Fig. 3D). Tlr7tgCd40lg−/y + OT-II BM chimeras also had reduced I-Ab MFI on B cells and DCs, negative serum ANA staining (Fig. 3 E and F), as well as lower percentages of GC B cells and CD4+CD44hi cells compared with Tlr7tgCd40lg−/y + WT BM chimeras (Fig. 3 B and D). These data indicate a requirement for T-cell receptor antigen specificity in Tlr7tg autoimmune responses.
H2k Haplotype and B-Cell Deficiency Render Tlr7tg Mice Resistant to Spontaneous Lymphocyte Activation and Autoimmune Disease.
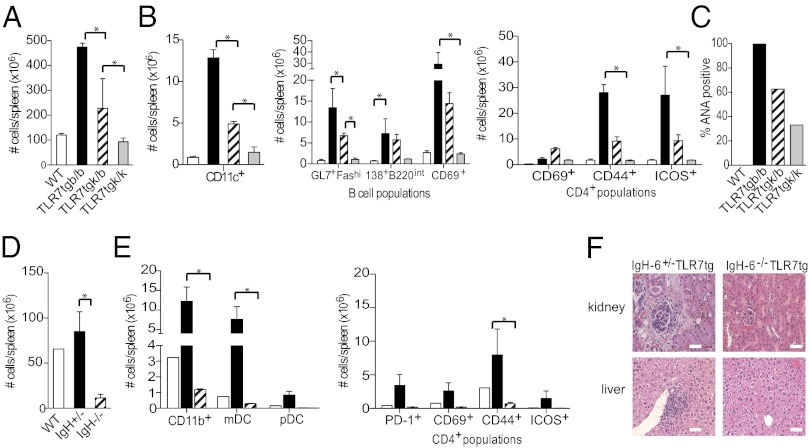
Because we found T-cell receptor antigen specificity made a difference to autoimmunity development in Tlr7tg mice, we wanted to determine whether there were also MHC restrictions for antigen presentation to T cells. We engineered C57BL/6 Tlr7tg mice homozygous or heterozygous for H2k MHC alleles to determine whether MHC haplotypes change the autoimmune susceptibility of Tlr7tg mice. Overall, we found an H2k allele dose-dependent decrease in spontaneous autoimmune phenotype in Tlr7tg mice. Tlr7tgH2b/b mice had ∼4.5-fold higher average splenocyte numbers than Tlr7tgH2k/k mice, whereas Tlr7tgH2k/b mice displayed an intermediate phenotype (Fig. 4A). Tlr7tgH2k/k mice had drastically reduced CD11c+ cell populations compared with Tlr7tgH2b/b mice, whereas Tlr7tgH2k/b again exhibited an intermediate phenotype (Fig. 4B). We determined the cell numbers in different B- and T-cell splenic populations in the three groups of mice, and the same trends held true for GC B cells, plasmablasts, and activated CD69+ B-cell subsets, as well as CD69+, CD44+, and ICOS+ activated CD4+ T-cell subsets (Fig. 4B). Lastly, Tlr7tgH2b/b mouse serum exhibited a higher prevalence of ANAs, whereas fewer Tlr7tgH2k/b mice had ANA positive serum, and Tlr7tgH2k/k serum displayed little presence of ANAs (Fig. 4C). These data suggest that MHC molecules do influence lupus disease susceptibility in Tlr7tg mice.
Fig. 4.
H-2k/k haplotype or B-cell deficiency in Tlr7tg mice confers protection against spontaneous autoimmunity. At 12 weeks of age, livers, kidneys, spleens, and serum were harvested from denoted groups of mice. (A) Total spleen cell counts in WT (white bars), Tlr7tgH2b/b (black bars), Tlr7tgH2k/b (hatched bars), and Tlr7tgH2k/k mice (gray bars). (B) Total numbers of indicated cell types from groups designated in (A): CD11c+ cells (Left), indicated B-cell populations (center panel), and CD4+ T-cell populations (right panel), n = 3 mice/group. (C) Percentage of ANA positive serum, n = 3 mice/group. (D) Total splenocyte cell counts in WT (white bars), Tlr7tgIgH+/− (black bars), and Tlr7tgIgH−/− mice (hatched bars). (E) Total numbers of CD11b+ cells, CD11b+CD11c+ mDCs, and CD11c+B220+ pDCs (Left), and indicated CD4+ cell populations (Right) from groups described in D, n = 2 mice/group, repeated two times. (F) Representative H&E stained kidney and liver sections. (Scale bar, 50 μm.)
B-cell autoreactivity correlates to the development of autoimmunity in humans and mouse models, and most likely is a control element of disease. However, in some murine disease models, other cell types drive autoimmunity (10, 24, 25). Thus, we wanted to determine whether cells other than B cells are able to induce inflammation and pathology in Tlr7tg mice. We intercrossed IgH−/− mice with Tlr7tg mice to render them deficient in B cells and examined splenic immune cell populations as well as kidney and liver pathology. We found a dramatic reduction in spleen cellularity of Tlr7tgIgH−/− mice compared with both WT and Tlr7tgIgH+/− littermate control spleens (Fig. 4D).
Monocyte, granulocyte, and CD11b+ precursor cell population expansion is associated with many autoimmune models as well as human disease (15). We examined these populations in Tlr7tgIgH−/− mice to determine whether myeloid cells still expand in the absence of B cells. Whereas Tlr7tgIgH+/− mice maintained a much larger population of CD11b+ cells in contrast with WT mice, Tlr7tgIgH−/− animals had twofold fewer CD11b+ cells than WT and 10-fold fewer cells than Tlr7tgIgH+/− mice (Fig. 4E). Tlr7tgIgH−/− mice also had dramatically reduced numbers of mDCs and pDCs compared with Tlr7tgIgH+/− mice (Fig. 4E).
We determined the cell surface phenotype of CD4+ T cells in Tlr7tgIgH−/− mice and found similar CD4+ T-cell expression of PD-1, CD69, CD44, and ICOS compared with WT CD4+ T cells, which indicated a more naive phenotype than Tlr7tgIgH+/− CD4+ T cells (Fig. 4E). In addition, whereas glomerulonephritis and liver inflammation were readily evident in H&E stained sections of Tlr7tgIgH+/− mice, Tlr7tgIgH−/− exhibited no pathology in either tissue (Fig. 4F). Collectively, these data indicate that B cells are required for development of spontaneous lupus-like disease in Tlr7tg mice, as well as for concomitant expansion of inflammatory monocytes, granulocytes, and pDCs.
Discussion
In an autoimmune response it is likely that synergy between innate immune receptors, such as TLR7, and antigen receptors, such as B-cell receptors, is pivotal to reach an activation threshold that overcomes tolerance. A B-cell–intrinsic role for TLR7 signaling in the development of systemic autoimmunity is unproven up to this point, although evidence from Tlr7 deficient mice and Tlr7tg mice suggests that triggering of this innate receptor in B cells may significantly contribute to disease incidence (3, 4, 6, 14). Our current studies confirm the need for duality in B-cell receptor signaling through both innate pathways and T-cell–derived costimulatory signals to cause autoimmunity in Tlr7tg mice.
Expression of multiple copies of Tlr7 in mixed BM chimeras led to preferential expansion of GC B cells and plasmablasts in Tlr7tg-derived B cells (Fig. 1A), indicating that spontaneous TLR7 signaling exerted a B-cell–intrinsic effect. It is possible that DCs expressing multiple copies of Tlr7 may present antigen to T cells and preferentially skew the T-cell repertoire to differentiate into TFH cells that promote GC and plasmablast B cells. However, CD11c+ cells did not expand in our mixed BM chimera system (Fig. 1A), nor did they spontaneously up-regulate MHC II (Fig. 1B), suggesting that DC functions, important for initiation of inflammatory responses in general, are dependent on humoral immune activation and may play a secondary role in our spontaneous Tlr7tg model of autoimmunity. Previous research suggests that pDC IFN-α production up-regulates TLR7 and promotes B-cell plasmablast differentiation and class switch to IgG2a (1, 26). However, Tlr7tg B cells had increased transcription of IgG1 and IgG2b genes rather than IgG2a, suggesting that this phenotype is IFN-α independent (Fig. 1C). Additionally, multiple copies of Tlr7 in mice enhanced expression of genes associated with antigen processing and presentation in B cells, perhaps increasing the likelihood of autoreactive B cells to present self-antigen to T cells and to garner T-cell support in the GC (Fig. 1B). Together, these data suggest that Tlr7 exerts B-cell–specific effects that influence B-cell fate and development of spontaneous autoimmunity.
High levels of Tlr7 alone could not drive spontaneous B-cell activation, GC formation, and autoantibody production in the absence of costimulatory CD40L or SLAM-family receptor signals (Fig. 2). Interestingly, DC population expansion also depended on costimulatory signals as Tlr7tg mice deficient in Cd40lg or SAP had significantly reduced numbers of these cells compared with Tlr7tg controls. These data are in contrast with those found in an Mrl/Mp-lpr/lpr mouse model of autoimmunity wherein Cd40lg gene deletion results in diminution of kidney disease, pathogenic IgG rheumatoid factors, and anti-dsDNA antibodies, but B cells are still able to class switch and produce snRNP antibodies (12). However, our findings do correlate with data from other autoimmune-prone strains lacking CD40L or SAP—Cd40lg deficiency in New Zealand black (NZB) models of spontaneous autoimmunity results in reduced IgG autoantibodies and glomerulonephritis scores (11). Additionally, the autoimmune phenotype in Sanroque mice can be reduced when these mice are crossed to an SAP deficient background (10). These data collectively indicate the importance of costimulatory signals delivered to B cells during the development of autoimmunity.
The contribution of T cells to B-cell autoantibody production is the subject of much debate, but it is most likely that T cells, particularly TFH cells, are a source of vital costimulatory signals for GC B cells (9). However, T-cell deletion in a variety of autoimmune-prone mouse strains has met with mixed results. T-cell deficiencies in NZB, MRL/Mp-lpr/lpr, and B6.56R mice reduce B-cell activation and/or organ disease, but have varying success in preventing autoantibody production (27–29). BAFFtg mice develop autoimmunity independently of T cells and it has been suggested that this occurs through a TLR7-dependent mechanism (13). However, our data from mixed BM chimeras of TCRβ−/− BM + Tlr7tgCd40lg−/y BM support an integral role for T-cell help in the generation of autoimmunity in Tlr7tg mice (Fig. 3), indicating that perhaps other pathways along with TLR7 may be involved in the BAFFtg mice. We also determined that IL-17, a cytokine that is attributed with being a key contributor to autoreactive GC development in autoimmune BXD2 mice and ectopic central nervous system GC in a model of experimental autoimmune encephalomyelitis (30, 31), is not essential for lymphocyte activation or autoantibody production in Tlr7tg mice. In contrast, restricting the T-cell repertoire to a non-self-antigen, ovalbumin, showed that T-cell antigen specificity was critical to activation of lymphocytes and production of autoantibodies in Tlr7tg mice (Fig. 3), adding further weight to the importance of T-cell specificity as a checkpoint in maintaining peripheral tolerance.
In corroboration with our finding that T-cell antigen specificity was crucial to preventing autoimmunity, we found that disease incidence in Tlr7tg mice depended on the context of antigen presentation (Fig. 4). Previous studies suggest that some mouse strains expressing H2d and H2k MHC haplotypes are less prone to developing autoimmune disease because they express the protective MHC II I-E genes contained within the same locus (32–34). This may be the case in Tlr7tg mice as well, but there are many possibilities of how MHC restrictions could be affecting disease development in these mice, such as alterations in antigen presentation, changes to thymic repertoire selection, or autoantibody selection. We observed a protective gene dosage effect in Tlr7tg mice expressing one versus two alleles of H2k. Tlr7tg mice homozygous for H2k displayed a phenotype very similar to WT mice, indicating a reduced propensity to develop autoimmunity. Whether these results are due to changes to the manner of antigen presentation or the type of antigen that is presented remains to be seen.
Given the results from our current study, we attributed a great deal of importance to the role that B- and T-cell interactions play in autoimmunity in Tlr7tg mice. Our data showing a lack of lymphocyte activation and tissue pathology in Tlr7tgIgH−/− mice strengthened this conclusion. The pDC compartment paucity was particularly striking, as these cells express Tlr7 and can produce large quantities of IFN-α. SLE incidence in humans is linked to a strong IFN-α response, and it is thought that IFN-α produced by pDCs drives disease in humans and mice, causing activation of lymphocytes and release of inflammatory mediators (1). However, in the absence of B cells in Tlr7tg mice, inflammatory cell populations did not expand, indicating that spontaneous TLR7 activation in pDCs cannot foment inflammation without a corresponding humoral response.
From the data presented here, we can conclude that spontaneous autoimmunity and inflammation in Tlr7tg mice is driven at least in part by B-cell–intrinsic events, but that B cells require costimulatory support from T cells to break tolerance. In turn, T cells require antigen to be presented in a capacity that favors an autoimmune response. These results likely reflect the circumstances that lead to lupus disease in humans, that not one cell type or causal agent induces autoimmunity, but rather that disease results from a complex interplay of inflammatory gene expression, synergistic innate and adaptive immune signals, and cooperative interactions between multiple cell types.
Materials and Methods
Mice.
C57BL/6 Tlr7tg mice were engineered as previously described (5). Tlr7tg mice were crossed with C57BL/6 Cd40lg−/y (35), Ighmtm1Cgn (IgH−/−) (36), and C57BL/6 H2k/k mice obtained from Jackson Labs, as well as C57BL/6 Sh2d1a−/y (SAP−/y) mice (37). All animals were kept in specific pathogen-free caging and all experiments were conducted under guidelines from the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee.
BM Chimeras.
BM cells isolated from femur and tibia bones of CD45.2+ Tlr7tg and CD45.1+ WT mice were mixed at a 1:1 ratio and injected i.v. via tail vein into 8–10-wk-old C57BL/6 recipient mice that had been irradiated with 940 rad one day before bone marrow transfer (BMT). Eight weeks after BMT, tissues and serum were harvested for analysis. The same scheme was used to make 1:1 BM chimeras using CD45.1+CD45.2+ Tlr7tgCd40lg−/y and CD45.2+ WT, TCRβ−/−, IL-17−/−, or OT-II mice.
Immunohistochemistry.
Tissues were embedded in tissue freezing medium and samples frozen at −80 °C. Frozen tissue sections (6 mm thick) were prepared, fixed in acetone for 10 min, and blocked with blocking serum using the Vectastatin ABC-AP kit (Vector Labs). Antibodies were diluted with 1% BSA/PBS and incubated for 1 h. Slides were washed with PBS for 5 min, two times. Slides were mounted with Vectashield (Vector Labs) and visualized using a Zeiss 510 Meta confocal microscope.
Histology.
Kidney and liver from selected mice were fixed in neutral buffered formalin, embedded in paraffin, and sections stained with hematoxylin and eosin. Immunofluorescence staining was performed on spleens from Tlr7tg, Tlr7tgCd40lg−/−, and Tlr7tgSAP−/− mice. Frozen sections were stained with biotinylated-anti-peanut agglutinin antibody, followed by SA-Alexa 488 and Alexa 647 anti-B220.
Microarray.
cDNA synthesis and labeling of follicular B cells purified from mice aged 8–10 wk old was conducted as mentioned previously (3), as were hybridizations (38) using an Mmca probe set from the National Institute of Allergy and Infectious Diseases (NIAID) Microarray Facility. Images were analyzed using GenePix Pro-5.0.1.38 (Molecular Devices) and uploaded data were analyzed using the mAdb program (http://madb.niaid.nih.gov/).
Real-Time PCR.
Real-time PCR (RT-PCR) was performed on CD9−CD43− B-cell total RNA using iQ SYBR Green Supermix (Biorad) in a Bio-Rad iCycler. Semiquantitative RT-PCR for Ig gene transcription was conducted using previously reported primers (39).
Flow Cytometry Antibodies.
Antibodies against the following antigens were used for flow cytometric analysis of splenocytes: B220, CD19, SLAM, GL7, FAS, IgG2a, IgG1, IgG2b, CD138, CD45.1, CD45.2, CD4, CD69, CD45RB, CD44, CD11b, Ly6C, Gr1, ICOS, CXCR5 from BD Biosciences and CD62L, CD11c, PDCA-1, PD-1, and Fc block (CD16/32) from eBioscience. Flow cytometry was conducted using an FACS Caliber or LSR2 (BD Biosciences) and data were analyzed using FlowJo (Tree Star).
Serological Analysis.
Serum was tested for autoantibody production using an ANA test using Hep-2 cells fixed on slides (MBL-Bion) as per manufacturer instructions. Briefly, sera were diluted 1:200 and applied to slide, incubated for 30 min, and after two washes, a goat anti-mouse IgG secondary antibody was used at a 1:1,000 dilution to identify autoreactive sera. Slides were washed two times, and Vectashield mounting medium applied to slide.
Statistical Analyses.
Statistical analysis of data by one-way ANOVA coupled with Tukey’s multiple comparison posttest was performed using Prism software 5 (GraphPad Software). In some cases a two-tailed paired Student’s t test was used. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Wenjie Zheng and Dr. Mary J. Mattapallil for technical help with experiments. We also thank Dr. Pam Schwartzberg for the gift of SAP-deficient mice. This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209372109/-/DCSupplemental.
References
- 1.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: Emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 3.Pisitkun P, et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 4.Subramanian S, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci USA. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deane JA, et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen SR, et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 7.García-Ortiz H, et al. Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Ann Rheum Dis. 2010;69:1861–1865. doi: 10.1136/ard.2009.124313. [DOI] [PubMed] [Google Scholar]
- 8.Komatsuda A, et al. Up-regulated expression of Toll-like receptors mRNAs in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin Exp Immunol. 2008;152:482–487. doi: 10.1111/j.1365-2249.2008.03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 10.Linterman MA, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pau E, Chang NH, Loh C, Lajoie G, Wither JE. Abrogation of pathogenic IgG autoantibody production in CD40L gene-deleted lupus-prone New Zealand Black mice. Clin Immunol. 2011;139:215–227. doi: 10.1016/j.clim.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, et al. Autoimmune lpr/lpr mice deficient in CD40 ligand: Spontaneous Ig class switching with dichotomy of autoantibody responses. J Immunol. 1996;157:417–426. [PubMed] [Google Scholar]
- 13.Groom JR, et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Boruchov AM, et al. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest. 2005;115:2914–2923. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhodapkar KM, et al. Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc Natl Acad Sci USA. 2005;102:2910–2915. doi: 10.1073/pnas.0500014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mombaerts P, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 20.Crispín JC, Kyttaris VC, Terhorst C, Tsokos GC. T cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:317–325. doi: 10.1038/nrrheum.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183:3160–3169. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakaguchi S, Sakaguchi N. Thymus and autoimmunity: Capacity of the normal thymus to produce pathogenic self-reactive T cells and conditions required for their induction of autoimmune disease. J Exp Med. 1990;172:537–545. doi: 10.1084/jem.172.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: Positive feedback in systemic autoimmune disease. Nat Rev Immunol. 2001;1(2):147–153. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 24.Okazaki T, et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J. Exp. Med. 2011;208(2):395–407. doi: 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finkelman FD, et al. Regulation by interferon alpha of immunoglobulin isotype selection and lymphokine production in mice. J Exp Med. 1991;174:1179–1188. doi: 10.1084/jem.174.5.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen SY, et al. The natural history of disease expression in CD4 and CD8 gene-deleted New Zealand black (NZB) mice. J Immunol. 1996;157:2676–2684. [PubMed] [Google Scholar]
- 28.Peng SL, et al. Pathogenesis of autoimmunity in alphabeta T cell-deficient lupus-prone mice. Clin Exp Immunol. 1998;111:107–116. doi: 10.1046/j.1365-2249.1998.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsao PY, Jiao J, Ji MQ, Cohen PL, Eisenberg RA. T cell-independent spontaneous loss of tolerance by anti-double-stranded DNA B cells in C57BL/6 mice. J Immunol. 2008;181:7770–7777. doi: 10.4049/jimmunol.181.11.7770. [DOI] [PubMed] [Google Scholar]
- 30.Hsu HC, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 31.Peters A, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35:986–996. doi: 10.1016/j.immuni.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibnou-Zekri N, Iwamoto M, Fossati L, McConahey PJ, Izui S. Role of the major histocompatibility complex class II Ea gene in lupus susceptibility in mice. Proc Natl Acad Sci USA. 1997;94:14654–14659. doi: 10.1073/pnas.94.26.14654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang D, et al. Dissection of the role of MHC class II A and E genes in autoimmune susceptibility in murine lupus models with intragenic recombination. Proc Natl Acad Sci USA. 2004;101:13838–13843. doi: 10.1073/pnas.0405807101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathis DJ, Benoist C, Williams VE, 2nd, Kanter M, McDevitt HO. Several mechanisms can account for defective E alpha gene expression in different mouse haplotypes. Proc Natl Acad Sci USA. 1983;80:273–277. doi: 10.1073/pnas.80.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, et al. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura D, Roes J, Kühn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 37.Czar MJ, et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci USA. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaupp CJ, Jiang G, Myers TG, Wilson MA. Active mixing during hybridization improves the accuracy and reproducibility of microarray results. Biotechniques. 2005;38:117–119. doi: 10.2144/05381MT01. [DOI] [PubMed] [Google Scholar]
- 39.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.