Abstract
Ectodomain shedding at the cell surface is a major mechanism to regulate the extracellular and circulatory concentration or the activities of signaling proteins at the plasma membrane. Human meprin β is a 145-kDa disulfide-linked homodimeric multidomain type-I membrane metallopeptidase that sheds membrane-bound cytokines and growth factors, thereby contributing to inflammatory diseases, angiogenesis, and tumor progression. In addition, it cleaves amyloid precursor protein (APP) at the β-secretase site, giving rise to amyloidogenic peptides. We have solved the X-ray crystal structure of a major fragment of the meprin β ectoprotein, the first of a multidomain oligomeric transmembrane sheddase, and of its zymogen. The meprin β dimer displays a compact shape, whose catalytic domain undergoes major rearrangement upon activation, and reveals an exosite and a sugar-rich channel, both of which possibly engage in substrate binding. A plausible structure-derived working mechanism suggests that substrates such as APP are shed close to the plasma membrane surface following an “N-like” chain trace.
Physiological processes in the extracellular milieu and the circulation require finely tuned concentrations of signal molecules such as cytokines, growth factors, receptors, adhesion molecules, and peptidases. Many of these proteins are synthesized as type-I membrane protein variants or precursors consisting of a glycosylated N-terminal ectoprotein, a transmembrane helix, and a C-terminal cytosolic tail. Their localization at the cell surface restricts their field of action to autocrine or juxtacrine processes. However, to act at a distance in paracrine, synaptic, or endocrine events, they have to be released from the plasma membrane into the extracellular space as soluble factors through “protein ectodomain shedding” (1, 2). This entails limited proteolysis and is a major posttranslational regulation mechanism that affects 2–4% of the proteins on the cell surface, occurs at or near the plasma membrane (3), and apparently follows a common release mechanism (2). It may also proteolytically inactivate proteins to terminate their function on the cell surface (4). Peptidases engaged in such processing are “sheddases” and the most studied transmembrane sheddases are members of the adamalysin/ADAMs (4, 5) and matrix-metalloproteinase (MMP) (6) families within the metzincin clan of metallopeptidases (MPs) (7–9). These include ADAM-8, -9, -10, -12, -15, -17, -19, -28, and -33 (1, 4) and membrane-type 1 (MT1)-MMP, MT3-MMP, and MT5-MMP (2, 6, 10). Other confirmed transmembrane sheddases are the aspartic proteinases BACE-1 and -2 (ref. 11 and references therein) and the malarian parasite serine proteinases, PfSUB2, PfROM1, and PfROM4 (12). Distinct sheddases may participate in intercalating processes, with disparate physiological consequences: ADAM-9, -10 (α-secretases), and -17 contribute to the nonamyloidogenic pathway of human amyloid precursor protein (APP) processing, whereas BACE-1 (β-secretase) participates in the amyloidogenic pathway. Whereas the former generates innocuous peptides, the latter gives rise to the toxic β-amyloid peptides believed to be responsible for Alzheimer’s disease (11). In several instances, shedding at the membrane surface is followed by a “regulated intramembrane proteolysis” step within the membrane (1). This is the case for the processing of Notch ligand Delta1 and of APP, both carried out by γ-secretase after action of an α/β-secretase (11), and for signal-peptide peptidase, which removes remnants of the secretory protein translocation from the endoplasmic membrane (13).
Recently, human meprin β (Mβ) was found to specifically process APP in vivo, which may contribute to Alzheimer’s disease (14, 15). It was also reported to activate cell-anchored α-secretase ADAM-10 and to be widely expressed in brain, intestine, kidney, and skin (14, 16–18). Disruption of Mβ in mice affects embryonic viability, birth weight, and renal gene expression profiles (19). The enzyme was further identified as a sheddase or proteolytic regulator at the plasma membrane of interleukin-1β (20), interleukin-18 (21), tumor growth factor α (22), procollagen III (23), epithelial sodium channel (24), E-cadherin (25), tenascin-C (26), and vascular endothelial growth factor A (27). Further substrates include fibroblast growth factor 19 and connective tissue growth factor. Altered expression and activity of the enzyme are associated with pathological conditions such as inflammatory bowel disease (28), tumor progression (29), nephritis (30), and fibrosis (23).
Mβ is a 679-residue secreted multidomain type-I membrane MP that belongs to the astacin family within the metzincins (7, 9, 16, 31, 32). The enzyme is glycosylated and assembles into either disulfide-linked homodimers or heterodimers with the closely related meprin α-subunit (33). Mβ homodimers are essentially membrane bound but may also be shed from the surface by ADAM-10 and -17 (34, 35). To assess function, working mechanism, and activation of Mβ, we analyzed the structure of the major ectoprotein of mature Mβ (MβΔC) and of its zymogen, promeprin β (pMβΔC). With regard to transmembrane sheddases, to date only the structures of the isolated monomeric catalytic domains of ADAM-17 [Protein Data Bank (PDB) access code 1BKC], ADAM-33 (PDB 1R55), MT1-MMP (PDB 1BUV), MT3-MMP (PDB 1RM8), BACE-1 (PDB 1FKN), and BACE-2 (PDB 2EWY) have been described. Accordingly, this is a unique structural report of a multidomain oligomeric transmembrane sheddase. This report has allowed us a better understanding of the structural basis for latency and activation of this MP and to derive a plausible working mechanism for shedding of glycosylated type-I membrane substrates such as APP at the extracellular membrane surface.
Results and Discussion
Multidomain Structure of Promeprin β.
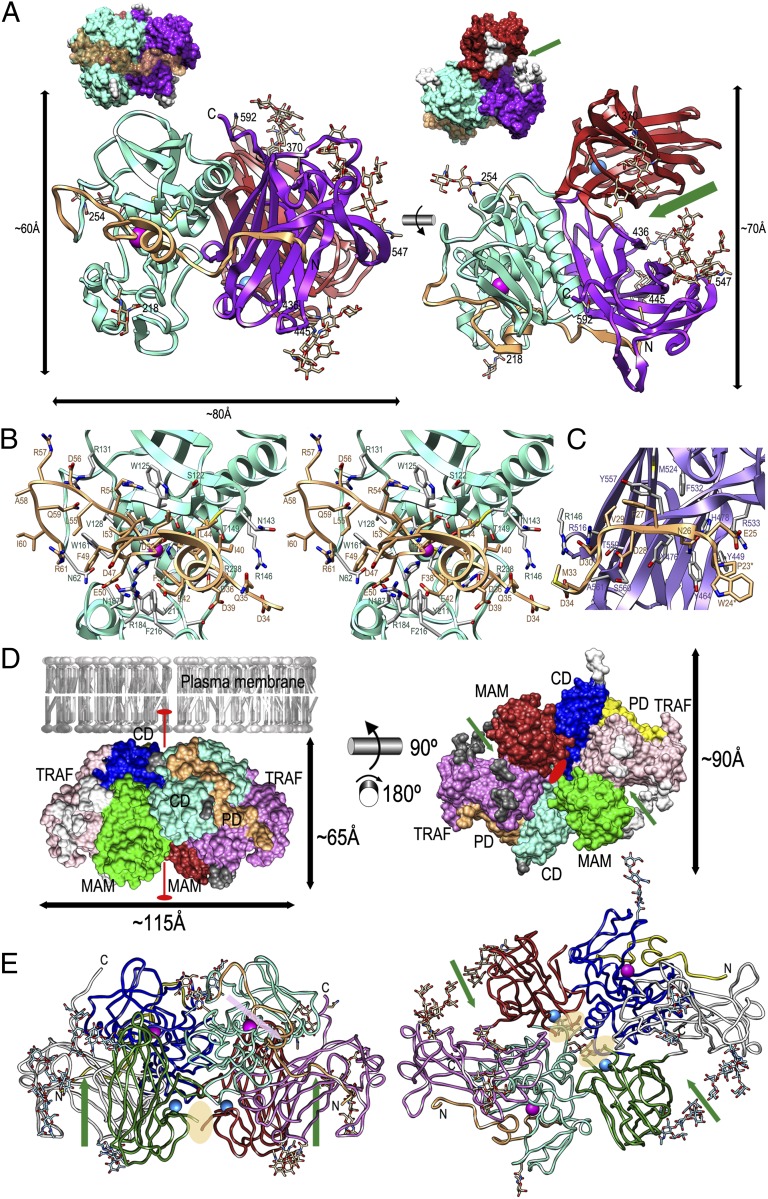
We solved the crystal structures of pMβΔC (with two molecules in the asymmetric unit) and MβΔC (with one molecule) (SI Materials and Methods and Table S1). The pMβΔC monomer has overall dimensions of 80 × 60 × 70 Å (Fig. 1 A–C) and a four-domain architecture (Fig. S2 A and B) spanning an N-terminal propeptide (PD; T23/E25–R61; Mβ residue numbering as superindices according to UniProt Q16820), a catalytic MP domain (CD; N62–L259), a MAM domain (S260–C427), and a C-terminal TRAF domain (P428–S593/Q597). The polypeptide starts on the front surface of TRAF (Fig. 1 A and C) with the first PD residues facing bulk solvent. From E25 to D30, which includes a conserved segment among meprins (F27DVD30), the polypeptide progresses right to left along the TRAF surface in a nearly extended conformation and includes strand β1 (Fig. 1C; see Fig. S1 A and B for nomenclature and extent of regular secondary structure elements), which is engaged in a parallel β-ribbon interaction with TRAF strand β29. In addition, F27 leans toward a hydrophobic pocket generated by Y557, F532, and M524 of TRAF; D28 interacts with S560; V29 with Y557 and R516; and D30 with both R516 and R146, the latter from CD. TRAF residues Y476, H478, and A561 further contribute to binding. This interaction of the N-terminal segment of PD with TRAF, which buries ∼460 Å2, reveals a novel potential exosite on the TRAF surface that would affect genuine substrates at subsites P7′–P10′ when bound at the active-site cleft in reverse orientation to PD (see Zymogenic Determinants in Promeprin β and Activation to Mature Meprin β). Indeed, these positions are conserved among physiological substrates (36). An example is APP, which is cleaved by Mβ at the β-secretase site (M671–D672; APP residue numbers as subindices according to UniProt P05067) in vivo and in vitro to generate amyloidogenic Aβ42 and Aβ41 peptides (14, 15). This process entails that upon Michaelis-complex formation, APP segment D672AEFRHDSGYE682 occupies substrate positions P1′–P11′. The tyrosine in P10′ would spatially overlap with PD residue F27, serine in P8′ with V29, and aspartate in P7′ with D30. Generally, exosites distal from the cleavage site contribute to efficient cleavage and have been previously reported for other peptidases such as thrombin (37) and ADAM family members (38).
Fig. 1.
Structure of promeprin β. (A) The structure of the pMβΔC monomer shown in front (Left) (CD in standard orientation according to ref. 41) and top (Right) reference views. The latter is along the sugar channel (green arrow). Glycans are depicted as stick models and the respective asparagine residues are numbered. The corresponding surface models are depicted above each picture (glycans in white). PD is shown in ochre, CD in aquamarine, MAM in red, and TRAF in purple. The zinc and the sodium ions are shown as magenta and blue spheres, respectively. (B) Close-up view in stereo of A, Left, to highlight residues engaged in PD–CD interactions. (C) Same as B in mono for the interaction between PD and TRAF. The first two residues of the structure (P23–W24) are actually T23–P24 in the natural protein (SI Materials and Methods). (D) pMβΔC dimer superposed with its Connolly surface shown in the front (Left) (in a plane with the membrane) and bottom (Right) dimer reference views. PDs are shown in ochre and yellow, CDs in aquamarine and blue, MAMs in red and green, TRAFs in purple and pink, and sugar moieties in white and gray. The intermolecular twofold axis is shown in red and green arrows point at the sugar channels. (E) Cartoon depicting the dimer as a ribbon in front (Left) and bottom (Right) views as in D. Green arrows run along the sugar channels and a pink arrow highlights the cleft of one CD with the adequate orientation of a substrate. The segment containing the intermolecular disulfide bond between C305 residues is disordered in the zymogen and its approximate position is highlighted by orange ellipses.
Zymogenic Determinants in Promeprin β.
At V29 of PD, the chain sharply kinks downward and runs vertically until G32 (Fig. 1C). Here, the chain turns again and progresses horizontally at G32–D36 to approach CD. From there on, the protein folds across the front surface of CD in reverse orientation to a substrate, thus blocking the cleft. This segment includes a helix (α1) perpendicular to the cleft. Altogether, the interaction of PD with CD buries an interface of ∼1,225 Å2 and includes three salt bridges on the prime side of the cleft (D30–R146 and D34–R146) and two more on the nonprime side (R54–E137 and D56–R131). A loop in the central part of the segment enables D52 to chelate in a bidentate manner the catalytic zinc ion from above (Fig. S1C). In the zymogen, this residue replaces the catalytic solvent molecule of mature astacins following an “aspartate-switch” mechanism (39). At R57, the chain turns down and reaches the final maturation point of pMβ, R61–N62. The first residue of CD is buried in the zymogen in an internal cavity framed by F49 and I53 of PD and W161 and Y191 of CD, and its side chain interacts with E50 and S165. Overall, the fold of PD is reminiscent of that of the propeptide of astacin except that in the latter the N terminus is anchored to the catalytic moiety (in the absence of further domains) and helix α1 is rotated by ∼70° around a vertical axis so that it rather parallels the active-site cleft (PDB 3LQ0) (39). This means that the PDs of the two structures are superposable only at F49EGDIKLD56 (F18PEGDIKLR25P in proastacin) (39), which includes the zinc-binding aspartate. This is consistent with sequence similarity among PDs of general astacin family members being restricted to a short consensus sequence, FXGD (X stands for any residue) (32). The short PD of Mβ and other astacins contrasts with the large prosegments found in ADAMs, which actually constitute separate domains capable of inhibiting the CDs in trans (40).
Catalytic Domain in Promeprin β.
The 198-residue CD is a compact ellipsoid reminiscent of a pac-man (Fig. S1 A and B). A deep and narrow active-site cleft, which harbors the catalytic zinc ion at midwidth (Fig. S1C), separates an upper N-terminal and a lower C-terminal subdomain (NTS and CTS, respectively) of similar size when viewed in standard orientation (Fig. S1A) (41). CD is cross-linked by two disulfide bonds within the NTS: C103–C255 connects the C terminus of the domain with a loop, which links helix α2 and strand β3 (Lα2β3), and C124–C144 connects β5 with Lβ6α3 and contributes thus to shaping the upper rim of the active-site cleft on its prime side. NTS harbors a central twisted five-stranded β-sheet (β2–β6) whose lowermost and only antiparallel strand (β5) shapes the upper rim of the active-site cleft. The sheet is decorated on its concave bottom by two helices, the “backing helix” (α2) and the “active-site helix” (α3), which run nearly parallel to the strands of the sheet. Helix α3 includes the first part of a long zinc-binding consensus sequence, H152EXXHXXGXXH162 (Fig. S1C), which is found in astacins but also metzincins in general (7–9, 42, 43). This helix ends at G159, which allows for a sharp turn of the polypeptide chain to enter the CTS. The latter contains the third zinc-binding residue, H162, and the “family-specific” residue of astacins, E163 (43, 44). Also typical for astacins and metzincins, a tight 1,4-β–type “Met turn” is located below the catalytic zinc site, featuring a strictly conserved methionine, M209 (Fig. S1C) (8, 45). The rest of the CTS has little regular secondary structure farther to the major “C-terminal helix” (α4) (Fig. S1 A and B). Of particular interest is that the polypeptide chain is disordered at Y191/D194–S198/L199. This segment corresponds to the “activation domain” in astacins (39).
MAM and TRAF Domains in Promeprin β.
After CD, the polypeptide chain enters the 168-residue MAM domain, which lies behind TRAF and performs no contact with the MP moiety with the exception of some residues near the interdomain junction (Fig. 1A, Right). MAM is a β-sandwich consisting of two five-stranded antiparallel β-sheets rotated away from each other by ∼25°. The sandwich consists of a front sheet twisted by ∼70° (β10-β13-β18-β15-β16; Fig. S1 A and B, Center) and a back sheet twisted by ∼40° and curled (β11-β9+β12-β19-β14-β17), whose second strand (β9+β12) is interrupted by 310-helix η3 and strands β10 and β11. Overall, the β-sandwich is built following a “jelly-roll” architecture made of two four-stranded Greek-key motifs (Fig. S1B, Center, in red and magenta, respectively), in which the second motif is inserted after the first β-ribbon (β9+β12-β13) of the first motif. The jelly roll is decorated by the aforementioned insertion (Fig. S1B, Center, in pink), which includes Lβ10β11—partially undefined in one of the two molecules in the asymmetric unit—and Lβ11β12, the “dimerization loop” (see below). In addition, the pairs C265–C273 and C340–C427 are at adequate distance and geometry for disulfide bonding but, contrary to the SS bonds in CD, the respective Sγ atoms are 2.9 Å apart. We attribute this to a radiation-damage artifact due to the long exposure time required to collect a complete dataset in space group P1. In addition, the η3-β10-β11 insertion contributes to an octahedral cation-binding site tentatively interpreted as a sodium site. The ion is coordinated by the side chains of E268, D298, S300, and D418 and the main-chain oxygen atoms of S266 and F310 (Fig. S1D). Overall, the topology and architecture of this domain are reminiscent of receptor-type tyrosine-protein phosphatase μ (PDB 2V5Y), which belongs to the MAM protein family of adhesive proteins initially identified by bioinformatic searches in meprin α and β, A5 protein, and receptor protein tyrosine phosphatase μ (46). In particular, the MAM domain of tyrosine phosphatase μ was shown to play a major role in homodimerization of the phosphatase ectoprotein and in cell adhesion (47).
Downstream of MAM, the 170-residue TRAF domain interacts with the former, burying an interface of ∼650 Å2 (Fig. 1A, Right). TRAF also interacts with CD through a surface spanning ∼950 Å2 in one protomer and ∼1170 Å2 in the other as the polypeptide chain could be traced for four residues more in the latter. TRAF features the second type of all-β structure found in pMβ (Fig. S1 A and B, Right), with a five-stranded front sheet (β21-β22-β23-β29-β28) and a four-stranded back antiparallel sheet (β20-β30-β24-β25) rotated by ∼40° against each other and arranged in a β-sandwich as in MAM. The front sheet is twisted by ∼50°, arched and curled, whereas the back sheet is twisted just by ∼50°. Altogether, the strands are arranged as two Greek-key motifs (Fig. S1B, Right, in purple and violet), in which the second one is inserted between strands 3 (β30) and 4 (β23) of the first one instead of after the first β-ribbon as in MAM (see above). Again contrary to MAM, which features a jelly roll with parallel Greek keys, in TRAF the second Greek key is rotated by ∼180° relative to the first one around an axis perpendicular to the plane of the β-sheets. In TRAF, the double Greek key is decorated with a β-ribbon (β26-β27) after β25, an additional short strand (β28) for the front sheet, and a helix (α5) between β29 and β30 (all in blue in Fig. S1B, Right). The only cysteine found in this domain, C492, is buried and unbound, and the C terminus of the molecule (S593/Q597) protrudes from the top surface of the monomer (Fig. 1A, Left). Overall, Mβ TRAF is structurally similar to tumor-necrosis factor receptor-associated factors 2, 3, and 6 (e.g., PDB 1LB5). These gave rise to the TRAF family, which comprises major mediators of cell activation engaged in homo- and heterodimerization (48).
Glycosylation Sites and “Sugar Channel.”
The pMβΔC and MβΔC structures contained sugar moieties attached to residues N218 and N254 of CTS; N370 of MAM; and N436, N445, N547, and N592 of TRAF. The observed N-glycosylation patterns, which are consistent with those found in other recombinant proteins produced in Trichoplusia ni insect cells (49), are similar to those found in mammalian glycosylation pathways (50) (Fig. S2). Accordingly, it is assumed that the glycosylations, which were able to be modeled to up to 10 hexose moieties at a single site (N547) and a maximum of 26 hexoses per protein monomer (Fig. S1B), represent a bona fide mimic of the authentic glycosylation pattern of the enzyme. Whereas the sugar moieties attached to N218 and N254 point to the bulk solvent and are isolated in the monomer structure, those of the remaining five sites are all oriented toward the interdomain space between MAM and TRAF, although they do not contact each other (Fig. 1A, Right). Given this accumulation, we termed the lumen between MAM and TRAF a sugar channel.
Dimerization of Promeprin β.
Two pMβΔC monomers associate to form a compact ellipsoid with dimensions of 115 × 65 × 90 Å, burying an interface of ∼1,220 Å2 (4.5% of the total monomer surface; Fig. 1 D and E). Superposition of the whole monomers (rmsd of 0.84 Å for 548 common Cα atoms) reveals that whereas PDs, NTSs, and TRAFs perfectly fit together, slight deviations are observed for CTSs and MAMs, which lead to displacements of up to 2.8 Å (measured at S182 Cα) and 2.6 Å (at A410 Cα), respectively. Dimerization occurs through nearly symmetric interactions between CD of one monomer and MAM of the other. Segments involved include the beginning of β2, 310-helix η1, Lη2β8, the sugar moiety attached to N254, and the C-terminal tail of CD; and Lβ9η3, η3, Lη3β10, β13, Lβ13β14, and β18 of MAM. This is consistent with the general oligomerization and protein–protein interaction function of MAM domains (see above). Notably, the dimerization loops of both polypeptide chains point into the center of the particle. They are disordered at segment M302–Q306/G307, which includes residue C305. This residue is engaged in an intermolecular disulfide bond cross-linking the dimer as shown by nonreducing SDS/PAGE of carefully washed and dissolved crystals, thus suggesting a function as a double safeguard rather than a feature indispensable for dimerization. In the particle, the glycans attached at N254 of each monomer interact with each other and contribute thus to a small hydrophilic cluster on the surface (see below). Moreover, the two CDs (with their attached PDs), as well as the sugar channels, are accessible at opposite ends of the particle, which is consistent with a competent conformation for substrate binding (Fig. 1D, Right), and both C termini of the dimer are located on the same face of the particle (Fig. 1 D and E). Given that the complete ectoprotein comprises only a further ∼60 residues, which mainly contribute to a compact EGF domain (C608–C643) before the transmembrane anchor (I653–V673), this face is likely to be membrane proximal and this allowed us to orient the particle with respect to the extracellular plasma membrane surface (Fig. 1D, Left). Further evidence for the consistency of this orientation is based on the proximity of the CDs and their active-site clefts to the membrane surface, which is required if membrane-anchored substrates are to be cleaved close to the membrane.
Activation to Mature Meprin β.
Activation of meprins requires proteolysis of the N-terminal PD, which is catalyzed by trypsin in the intestinal lumen and kallikrein-related peptidases (KLK-4, -5, and -8) in other tissues (17, 51). Comparison of the zymogenic and mature structures, which were obtained in different crystal forms, reveals that in general the dimers (established in MβΔC between symmetry equivalents) fit together with a global rmsd of 1.2 Å for 1,006 common Cα atoms (of 561 + 554 residues in pMβΔC and 533 + 533 in MβΔC; Fig. S3A). Detailed inspection, however, shows that CTSs undergo major rearrangement upon maturation through a hinge rotation of ∼25° toward the cleft around E163, H210, and G236, which entails a maximum displacement of ∼8 Å (at G183 Cα; Fig. S3 B–D). In this way, the space exposed by PD removal is subsequently occupied in part by the CTS. This closing-hinge motion further causes displacement of the mature N terminus of Mβ and a rotation of its first three residues by ∼180°. Thus, N62 becomes completely buried inside the mature CD moiety and hydrogen bonded through its Nδ2 atom to the side chain of the family-specific residue E163. The latter has the same side-chain conformation as in the zymogen and is likewise bound to K248 through a buried salt bridge. Maturation further entails rigidification of the formerly flexible activation segment (see above) and its upward shift toward the cleft by ∼4 Å (at Y191 Cα). This leads to a competent conformation that enables D197O and S196Oγ to bind the α-amino group of N62 (Fig. S3 C and D). The stiffening of CD contributes to the traceability of MβΔC structure along its entire polypeptide chain (N62–T594). This holds true also for the flexible loop Lβ11β12, which encompasses the intermolecular disulfide bond, although this is based on weak electron density. A further key role in CTS rearrangement is played by W161, whose side chain rotates by ∼180° around its χ2 angle and becomes sandwiched by the ascending side chain of Y191 of the activation segment. Overall, the major rearrangement observed is compatible with the gross particle structure, indicating that the zymogen is already in a preformed conformation adequate for catalysis, which requires a rigid-body rearrangement of a subdomain spanning only one-seventh of the full-length protein for full competence.
Maturation also constricts the active-site cleft and this affects the side chain of Y211, which is engaged in zinc and substrate binding, and catalysis in mature astacin CDs (i.e., the “tyrosine-switch” residue) (52) and some other metzincins such as serralysins and pappalysins (8). In the zymogen, it is pulled away from its competent position by the intercalation of PD helix α1, in particular the side chains of I37–F38 (Fig. 1B), and removal of PD allows Y211 to approach the catalytic ion (replaced in the MβΔC structure with a cadmium; SI Materials and Methods). There is additional electron density on the prime side of the active-site cleft of the mature enzyme—potentially corresponding to a substrate or inhibitor with low occupancy, which was conservatively interpreted as a glycerol molecule. Most noteworthy, R238, engaged in a salt bridge with D36 in the zymogen (see above), becomes reoriented with regard to its side chain and occupies the space of D36–I37 of PD. This arginine is found within a segment mainly engaged in shaping the S1′ pocket in astacins, the “170 loop” (32), and in Mβ it accounts for its preference for acidic residues in this subsite (Fig. S3 and ref. 36). Further inspection of the active-site cleft and the adjacent exosite provided by TRAF (see Multidomain Structure of Promeprin β) reveals that R146 and R516 (to the right of R238 in Fig. 1 B and C), and R184 from Lβ7η2 of CD, which interacts with E42 in the zymogen and becomes reoriented upon maturation (Fig. S3 B–D), could explain the preference of Mβ for acidic side chains also in downstream prime-side subsites (see Multidomain Structure of Promeprin β), pinpointing a unique cleavage specificity among extracellular proteases (36).
Finally, the substrate specificity of Mβ is complementary to that of other physiologically relevant transmembrane sheddases. The most studied ones, ADAM-17, ADAM-10, and MT1-MMP, as well as other ADAM and MMP family members, have specificity for large and medium-sized hydrophobic residues in P1′ as found, for example, in ADAM-17 substrates (53) and the α-secretase site of APP (K687–L688). BACE-2 also cleaves at the α-secretase site (54). Accordingly, Mβ would be the only transmembrane sheddase capable of cleaving before acidic residues in general and at the β-secretase site of APP in particular in addition to BACE-1 (15).
Working Mechanism for Shedding at the Plasma Membrane.
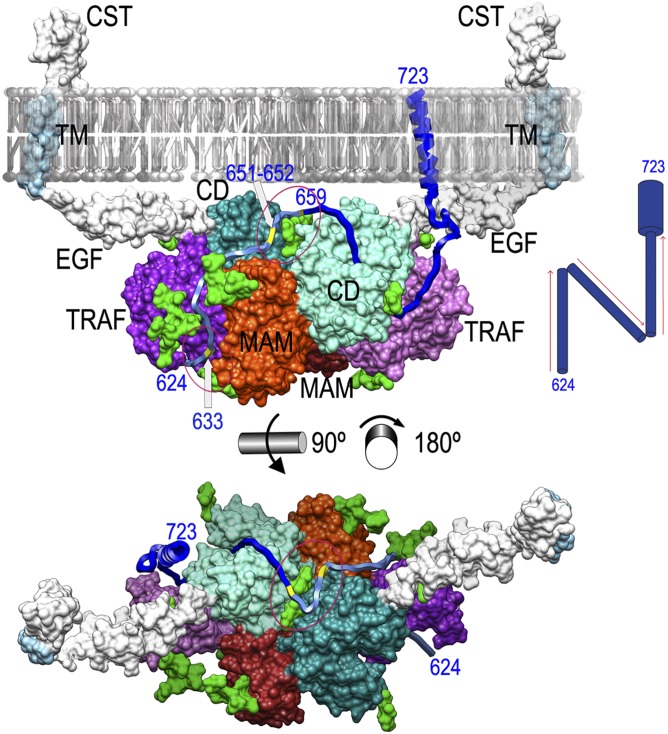
The structures of both pMβΔC and MβΔC reveal a dimer with a membrane-proximal face (see above). We constructed a homology model for the remaining ∼60 residues of the ectoprotein, encompassing an EGF-like domain, the 20-residue transmembrane anchor, and the 27-residue cytosolic tail of each monomer (SI Materials and Methods), which enabled us to propose a molecular mechanism for Mβ-mediated shedding at the plasma membrane (Fig. 2). This is visualized using a tentative model for APP region F624–L723, which includes the final segment of the ectoprotein and the transmembrane helix (55) and, thus, the β-secretase cleavage site (see Multidomain Structure of Promeprin β). Following this model, a substrate chain would be bound at the membrane-distal part (F624–V640 of APP) by the sugar channel of Mβ monomer one, whose two major glycosylations at N547 of MAM and N370 of TRAF would act like a bat-winged saloon door to admit and retain the substrate chain. This would be consistent with the general function of such domains in protein–protein interactions. Downstream, the substrate chain would run along the back surface of the cognate CD until position L650 to approach the interface between CDs within the dimer and, eventually, enter the active-site cleft of Mβ monomer two (Fig. 2). An intramolecular mechanism involving all three domains of one monomer is unlikely as the substrate would have to undergo a long excursion after passing through the sugar channel and the back surface of the cognate CD to reach its active-site cleft with the correct N-to-C polarization (Fig. 1E). The first residue after the β-secretase cleavage site, D672, would be located in the S1′ pocket of Mβ, thus matching its substrate specificity. Farther downstream subsites of the substrate would run across the cleft and the prime-site exosite on the TRAF surface (see Multidomain Structure of Promeprin β) of Mβ monomer two and then turn down to reach the APP transmembrane segment at G700. Generally, the substrate would follow an “N-like” trajectory (Fig. 2, Upper Right) and involve domains from both monomers within the Mβ dimer. In addition, this mechanism would be sugar assisted. The majority of potential shedding substrates (87% of single-pass transmembrane proteins) (56) are glycosylated, and this holds true also for APP and other Mβ substrates. In particular, APP is glycosylated at T633, T651, T652, T659, T663, S667, and Y681 with regard to the segment under inspection here (57). Of these sites, the proposed model predicts that the latter three could be at or close to subsites P5, P7, and P10′; i.e., they would not interact with the enzyme but rather be solvent exposed. By contrast, the glycans attached to T651, T652, and T659 could potentially interact with the site created by the symmetric N254 glycosylations of Mβ, and the one at T633 could interact with that of Mβ N436 (Fig. 2). Overall, this mechanism would be compatible with other type-I transmembrane substrates that are shed at sites at least 20–25 residues above the membrane. The different N-glycans found on the surface of the Mβ particle along the proposed substrate path (Fig. 2) could provide alternative anchor points for the particular sugar moieties of each substrate.
Fig. 2.
Sheddase mechanism of meprin β. Shown is a working model of Mβ function based on the experimental dimer (CDs in aquamarine and turquoise, MAMs in orange and red, TRAFs in mauve and purple, and glycosylation moieties in light green) in front (Upper) view (Fig. 1D, Left) and top (from the membrane surface; Lower) view from the membrane (here the membrane was removed for clarity). The segments present in the Mβ dimer but missing in the experimental MβΔC structure [EGF, transmembrane (TM), and cytosolic tail (CST)] have been computationally modeled (SI Materials and Methods) and are shown in white/light blue. A transmembrane substrate model for APP (segment 624–723) is shown as a blue ribbon. The substrate segment proposed to interact with the dimer partner is depicted in pale blue to highlight that this part of the mechanism is more speculative. APP glycosylation sites relevant for the proposed mechanism are marked in yellow on the ribbon and labeled. (Upper) Red ellipses highlight sugars attached to N436 of the right monomer (lower ellipse) and to N254 of both monomers (upper ellipse). (Lower) Image shows only the latter ellipse. (Upper Right Inset) A possible “N-like” trajectory of the substrate.
Materials and Methods
A detailed description of procedures is provided in SI Materials and Methods. Briefly, pMβΔC was produced by recombinant baculovirus-induced overexpression in insect cells and activated by trypsin as reported in ref. 58. The structure of pMβΔC was solved by a combination of single-wavelength anomalous diffraction and Patterson search. The structure of MβΔC was solved by Patterson search. A model for full-length Mβ was obtained by connecting a homology model for the EGF-like domain with the experimental structure and a modeled transmembrane helix plus cytosolic tail by linkers of stereochemically reasonable conformation.
Supplementary Material
Acknowledgments
We are grateful to the Automated Crystallography Platform at Barcelona Science Park for assistance during crystallization experiments. We acknowledge the help provided by European Molecular Biology Laboratory and European Synchrotron Radiation Facility synchrotron local contacts. This study was supported in part by grants from European, German, Swiss, Spanish, and Catalan agencies (FP7-HEALTH-F3-2009-223101 “AntiPathoGN”; FP7-HEALTH-2010-261460 “Gums&Joints”; FP7-PEOPLE-2011-ITN-290246 “RAPID”; Deutsche Forschungsgemeinschaft Grants DFG Sto185/3-3, BE 4086/1-2, and SFB877 (project A9) and German Cluster of Excellence “Inflammation at Interfaces”; Swiss National Science Foundation Grant 31003A; BIO2009-10334; BFU2012-32862; CSD2006-00015; a JAE postdoctoral contract from Consejo Superior de Investigaciones Cientificas; Fundació “La Marató de TV3” ref. 2009-100732; and 2009SGR1036). Funding for data collection was provided in part by the European Synchrotron Radiation Facility.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4GWM and 4GWN).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211076109/-/DCSupplemental.
References
- 1.Blobel CP. ADAMs: Key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 2.Arribas J, Merlos-Suárez A. Shedding of plasma membrane proteins. Curr Top Dev Biol. 2003;54:125–144. doi: 10.1016/s0070-2153(03)54007-8. [DOI] [PubMed] [Google Scholar]
- 3.Pandiella A, Bosenberg MW, Huang EJ, Besmer P, Massagué J. Cleavage of membrane-anchored growth factors involves distinct protease activities regulated through common mechanisms. J Biol Chem. 1992;267:24028–24033. [PubMed] [Google Scholar]
- 4.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saftig P, Reiss K. The “A Disintegrin And Metalloproteases” ADAM10 and ADAM17: Novel drug targets with therapeutic potential? Eur J Cell Biol. 2011;90:527–535. doi: 10.1016/j.ejcb.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Murphy G, Nagase H. Localizing matrix metalloproteinase activities in the pericellular environment. FEBS J. 2011;278:2–15. doi: 10.1111/j.1742-4658.2010.07918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomis-Rüth FX. Structural aspects of the metzincin clan of metalloendopeptidases. Mol Biotechnol. 2003;24:157–202. doi: 10.1385/MB:24:2:157. [DOI] [PubMed] [Google Scholar]
- 8.Gomis-Rüth FX. Catalytic domain architecture of metzincin metalloproteases. J Biol Chem. 2009;284:15353–15357. doi: 10.1074/jbc.R800069200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stöcker W, et al. The metzincins—topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci. 1995;4:823–840. doi: 10.1002/pro.5560040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbolina MV, Stack MS. Membrane type 1-matrix metalloproteinase: Substrate diversity in pericellular proteolysis. Semin Cell Dev Biol. 2008;19:24–33. doi: 10.1016/j.semcdb.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dislich B, Lichtenthaler SF. The membrane-bound aspartyl protease BACE1: Molecular and functional properties in Alzheimer’s disease and beyond. Front Physiol. 2012;3:1–16. doi: 10.3389/fphys.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olivieri A, et al. Juxtamembrane shedding of Plasmodium falciparum AMA1 is sequence independent and essential, and helps evade invasion-inhibitory antibodies. PLoS Pathog. 2011;7:e1002448. doi: 10.1371/journal.ppat.1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weihofen A, Binns K, Lemberg MK, Ashman K, Martoglio B. Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science. 2002;296:2215–2218. doi: 10.1126/science.1070925. [DOI] [PubMed] [Google Scholar]
- 14.Jefferson T, et al. Metalloprotease meprin β generates nontoxic N-terminal amyloid precursor protein fragments in vivo. J Biol Chem. 2011;286:27741–27750. doi: 10.1074/jbc.M111.252718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bien J, et al. The metalloprotease meprin β generates amino terminal truncated Aβ-peptide species. J Biol Chem. 2012 doi: 10.1074/jbc.M112.395608. 10.1074/jbc.M112.395608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterchi EE, Stöcker W, Bond JS. Meprins, membrane-bound and secreted astacin metalloproteinases. Mol Aspects Med. 2008;29:309–328. doi: 10.1016/j.mam.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker-Pauly C, et al. The α and β subunits of the metalloprotease meprin are expressed in separate layers of human epidermis, revealing different functions in keratinocyte proliferation and differentiation. J Invest Dermatol. 2007;127:1115–1125. doi: 10.1038/sj.jid.5700675. [DOI] [PubMed] [Google Scholar]
- 18.Schütte A, Lottaz D, Sterchi EE, Stöcker W, Becker-Pauly C. Two α subunits and one β subunit of meprin zinc-endopeptidases are differentially expressed in the zebrafish Danio rerio. Biol Chem. 2007;388:523–531. doi: 10.1515/BC.2007.060. [DOI] [PubMed] [Google Scholar]
- 19.Norman LP, Jiang W, Han X, Saunders TL, Bond JS. Targeted disruption of the meprin β gene in mice leads to underrepresentation of knockout mice and changes in renal gene expression profiles. Mol Cell Biol. 2003;23:1221–1230. doi: 10.1128/MCB.23.4.1221-1230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzog C, et al. Meprin A and meprin alpha generate biologically functional IL-1beta from pro-IL-1beta. Biochem Biophys Res Commun. 2009;379:904–908. doi: 10.1016/j.bbrc.2008.12.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerjee S, Bond JS. Prointerleukin-18 is activated by meprin β in vitro and in vivo in intestinal inflammation. J Biol Chem. 2008;283:31371–31377. doi: 10.1074/jbc.M802814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergin DA, et al. Activation of the epidermal growth factor receptor (EGFR) by a novel metalloprotease pathway. J Biol Chem. 2008;283:31736–31744. doi: 10.1074/jbc.M803732200. [DOI] [PubMed] [Google Scholar]
- 23.Kronenberg D, et al. Processing of procollagen III by meprins: New players in extracellular matrix assembly? J Invest Dermatol. 2010;130:2727–2735. doi: 10.1038/jid.2010.202. [DOI] [PubMed] [Google Scholar]
- 24.Bondarava M, Li T, Endl E, Wehner F. α-ENaC is a functional element of the hypertonicity-induced cation channel in HepG2 cells and it mediates proliferation. Pflugers Arch. 2009;458:675–687. doi: 10.1007/s00424-009-0649-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huguenin M, et al. The metalloprotease meprinbeta processes E-cadherin and weakens intercellular adhesion. PLoS ONE. 2008;3:e2153. doi: 10.1371/journal.pone.0002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambort D, et al. Specific processing of tenascin-C by the metalloprotease meprinbeta neutralizes its inhibition of cell spreading. Matrix Biol. 2010;29:31–42. doi: 10.1016/j.matbio.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Schütte A, Hedrich J, Stöcker W, Becker-Pauly C. Let it flow: Morpholino knockdown in zebrafish embryos reveals a pro-angiogenic effect of the metalloprotease meprin α2. PLoS ONE. 2010;5:e8835. doi: 10.1371/journal.pone.0008835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vazeille E, et al. Role of meprins to protect ileal mucosa of Crohn’s disease patients from colonization by adherent-invasive E. coli. PLoS ONE. 2011;6:e21199. doi: 10.1371/journal.pone.0021199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lottaz D, et al. Enhanced activity of meprin-α, a pro-migratory and pro-angiogenic protease, in colorectal cancer. PLoS ONE. 2011;6:e26450. doi: 10.1371/journal.pone.0026450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oneda B, et al. Metalloprotease meprin β in rat kidney: Glomerular localization and differential expression in glomerulonephritis. PLoS ONE. 2008;3:e2278. doi: 10.1371/journal.pone.0002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bond JS, Beynon RJ. The astacin family of metalloendopeptidases. Protein Sci. 1995;4:1247–1261. doi: 10.1002/pro.5560040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomis-Rüth FX, Trillo-Muyo S, Stöcker W. Functional and structural insights into astacin metallopeptidases. Biol Chem. 2012;393 doi: 10.1515/hsz-2012-0149. 10.1515/hsz-2012-0149. [DOI] [PubMed] [Google Scholar]
- 33.Bertenshaw GP, Norcum MT, Bond JS. Structure of homo- and hetero-oligomeric meprin metalloproteases. Dimers, tetramers, and high molecular mass multimers. J Biol Chem. 2003;278:2522–2532. doi: 10.1074/jbc.M208808200. [DOI] [PubMed] [Google Scholar]
- 34.Hahn D, et al. Phorbol 12-myristate 13-acetate-induced ectodomain shedding and phosphorylation of the human meprinbeta metalloprotease. J Biol Chem. 2003;278:42829–42839. doi: 10.1074/jbc.M211169200. [DOI] [PubMed] [Google Scholar]
- 35.Jefferson T, et al. The substrate degradome of meprin metalloproteases reveals an unexpected proteolytic link between meprin β and ADAM10. Cell Mol Life Sci. 2012;29 doi: 10.1007/s00018-012-1106-2. 10.1007/s00018-012-1106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker-Pauly C, et al. 2011. Proteomic analyses reveal an acidic prime side specificity for the astacin metalloprotease family reflected by physiological substrates. Mol Cell Proteomics 10:M111.009233.
- 37.Bode W, Turk D, Karshikov A. The refined 1.9-A X-ray crystal structure of D-Phe-Pro-Arg chloromethylketone-inhibited human α-thrombin: Structure analysis, overall structure, electrostatic properties, detailed active-site geometry, and structure-function relationships. Protein Sci. 1992;1:426–471. doi: 10.1002/pro.5560010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao W, et al. Rearranging exosites in non-catalytic domains can redirect the substrate specificity of ADAMTS proteases. J Biol Chem. 2012;287:26944–26952. doi: 10.1074/jbc.M112.380535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guevara T, et al. Proenzyme structure and activation of astacin metallopeptidase. J Biol Chem. 2010;285:13958–13965. doi: 10.1074/jbc.M109.097436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moss ML, et al. The ADAM10 prodomain is a specific inhibitor of ADAM10 proteolytic activity and inhibits cellular shedding events. J Biol Chem. 2007;282:35712–35721. doi: 10.1074/jbc.M703231200. [DOI] [PubMed] [Google Scholar]
- 41.Gomis-Rüth FX, Botelho TO, Bode W. A standard orientation for metallopeptidases. Biochim Biophys Acta. 2012;1824:157–163. doi: 10.1016/j.bbapap.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Bode W, Gomis-Rüth FX, Stöckler W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the ‘metzincins’. FEBS Lett. 1993;331:134–140. doi: 10.1016/0014-5793(93)80312-i. [DOI] [PubMed] [Google Scholar]
- 43.Stöcker W, Gomis-Rüth FX, Bode W, Zwilling R. Implications of the three-dimensional structure of astacin for the structure and function of the astacin family of zinc-endopeptidases. Eur J Biochem. 1993;214:215–231. doi: 10.1111/j.1432-1033.1993.tb17915.x. [DOI] [PubMed] [Google Scholar]
- 44.Bode W, Gomis-Rüth FX, Huber R, Zwilling R, Stöcker W. Structure of astacin and implications for activation of astacins and zinc-ligation of collagenases. Nature. 1992;358:164–167. doi: 10.1038/358164a0. [DOI] [PubMed] [Google Scholar]
- 45.Goulas T, Arolas JL, Gomis-Rüth FX. Structure, function and latency regulation of a bacterial enterotoxin potentially derived from a mammalian adamalysin/ADAM xenolog. Proc Natl Acad Sci USA. 2011;108:1856–1861. doi: 10.1073/pnas.1012173108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beckmann G, Bork P. An adhesive domain detected in functionally diverse receptors. Trends Biochem Sci. 1993;18:40–41. doi: 10.1016/0968-0004(93)90049-s. [DOI] [PubMed] [Google Scholar]
- 47.Aricescu AR, et al. Structure of a tyrosine phosphatase adhesive interaction reveals a spacer-clamp mechanism. Science. 2007;317:1217–1220. doi: 10.1126/science.1144646. [DOI] [PubMed] [Google Scholar]
- 48.Zapata JM, et al. A diverse family of proteins containing tumor necrosis factor receptor-associated factor domains. J Biol Chem. 2001;276:24242–24252. doi: 10.1074/jbc.M100354200. [DOI] [PubMed] [Google Scholar]
- 49.Ailor E, et al. N-glycan patterns of human transferrin produced in Trichoplusia ni insect cells: Effects of mammalian galactosyltransferase. Glycobiology. 2000;10:837–847. doi: 10.1093/glycob/10.8.837. [DOI] [PubMed] [Google Scholar]
- 50.Harrison RL, Jarvis DL. Protein N-glycosylation in the baculovirus-insect cell expression system and engineering of insect cells to produce “mammalianized” recombinant glycoproteins. Adv Virus Res. 2006;68:159–191. doi: 10.1016/S0065-3527(06)68005-6. [DOI] [PubMed] [Google Scholar]
- 51.Ohler A, Debela M, Wagner S, Magdolen V, Becker-Pauly C. Analyzing the protease web in skin: Meprin metalloproteases are activated specifically by KLK4, 5 and 8 vice versa leading to processing of proKLK7 thereby triggering its activation. Biol Chem. 2010;391:455–460. doi: 10.1515/BC.2010.023. [DOI] [PubMed] [Google Scholar]
- 52.Grams F, et al. Structure of astacin with a transition-state analogue inhibitor. Nat Struct Biol. 1996;3:671–675. doi: 10.1038/nsb0896-671. [DOI] [PubMed] [Google Scholar]
- 53.Maskos K, et al. Crystal structure of the catalytic domain of human tumor necrosis factor-α-converting enzyme. Proc Natl Acad Sci USA. 1998;95:3408–3412. doi: 10.1073/pnas.95.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barrett PJ, et al. The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science. 2012;336:1168–1171. doi: 10.1126/science.1219988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuhn P-H, et al. Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J. 2012;31:3157–3168. doi: 10.1038/emboj.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halim A, et al. Site-specific characterization of threonine, serine, and tyrosine glycosylations of amyloid precursor protein/amyloid beta-peptides in human cerebrospinal fluid. Proc Natl Acad Sci USA. 2011;108:11848–11853. doi: 10.1073/pnas.1102664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Becker C, et al. Differences in the activation mechanism between the alpha and beta subunits of human meprin. Biol Chem. 2003;384:825–831. doi: 10.1515/BC.2003.092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.