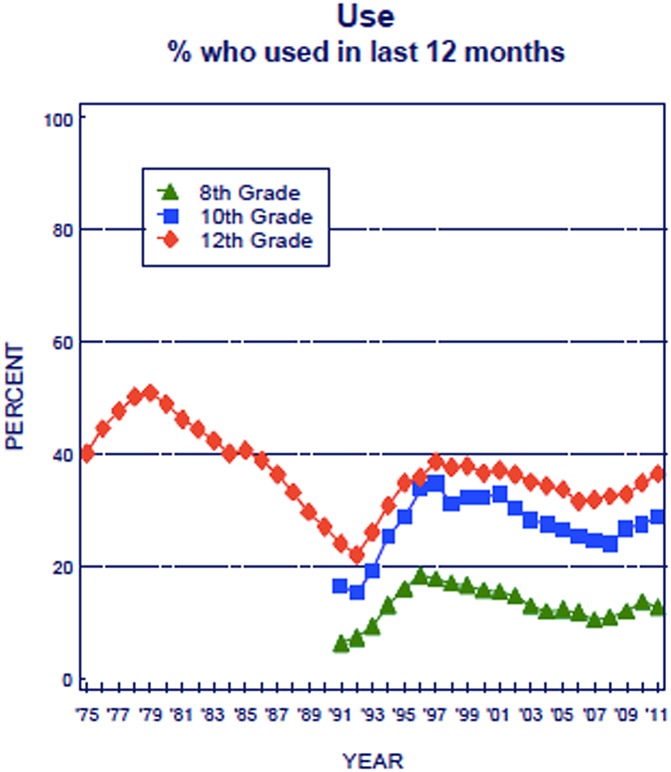
Marijuana use has increased over the past 20 y in the United States, and current trends suggest it may continue to rise. Recent polls in the United States suggest that population acceptance is at an all-time high: 56% support the legalization for recreational use and 70% for medical use (http://healthland.time.com/2012/06/14). A survey of secondary school students in the United States (Monitoring the Future: http://monitoringthefuture.org) suggests a resurgence of marijuana use (Fig. 1): after a decade or more of decline to 22% in 1992, the annual prevalence of use in high school senior students climbed to nearly 40% in 2011, with a parallel decrease in perceived risk of regular use from almost 80% to approximately 45% (1). Although short-term trends reveal some temporary decreases (2), the recent trends of increasing use and acceptance of marijuana over the past 5 y (1) heighten the importance of a scientific basis for understanding effects of marijuana (cannabis).
Fig. 1.
Marijuana: trends in annual use (1).
In PNAS, Meier et al. (3) contribute to this with findings from the 38-y follow-up of the Dunedin Longitudinal Study. The study was initiated in New Zealand in 1972–1973 as a birth cohort (n = 1037) and has generated more than 1,000 publications (http://dunedinstudy.otago.ac.nz/publications) about health and development from infancy to adulthood (with 27 addressing cannabis). Here Meier et al. report, “…persistent cannabis use was associated with neuropsychological decline broadly across domains of functioning” and “…impairment was concentrated among adolescent-onset cannabis users, with more persistent use associated with greater decline.” This is disquieting because many adolescents are engaging in heavy marijuana use. The principal investigator of Monitoring the Future noted, “… one in every fifteen high school seniors today is smoking pot on a daily or near daily basis” (http://www.sampler.isr.umich.edu/2012/research/marijuana). How much concern does this warrant? To put this in perspective, we relate these findings to past work and discuss some questions about cognitive effects of cannabis.
First, we should note the comprehensive scope of the hypotheses tested by Meier et al. They report a global decline in intelligence quotient (IQ) and neuropsychological performance associated with persistent regular cannabis use (4 or more days per week) or dependence (marked by three or more of these DSM-IV symptoms: tolerance, withdrawal, taken in larger amounts than intended, desire to cut back, considerable time spent obtaining, other activities given up, use despite problems). These laboratory effects were corroborated by informant reports of participants’ functional status, remained evident after control for several likely confounds, and did not seem to diminish with limited use of cannabis in adulthood. Importantly, they found that only those who began their cannabis use during adolescence, as opposed to adulthood, were beset with the observed neurocognitive declines.
Meier et al. mention that other studies have arrived at somewhat different conclusions about the impact of cannabis use on neurocognitive functioning, and we expand that comparison here. Acute effects often include declines in several aspects of neurocognition, but there is controversy about the lasting (nonacute) impact of cannabis use (4). For example, Pope et al. (5) reported that current (but not former) heavy cannabis users performed more poorly than controls only on measures of verbal memory at baseline and 1 and 7 d after supervised abstinence, but not after 28 d. Fried and colleagues (6, 7) found only current heavy cannabis smokers to show declines in IQ, memory, and processing speed (but not other abilities) at ages 17–20 y relative to their baseline performance at ages 9–12 y. Adult monozygotic twins discordant for history of cannabis use showed significant differences on only one of more than 50 neuropsychological measures (8). A metaanalysis on nonacute effects of cannabis use reported significant (but modest) adverse effects of cannabis only on measures of episodic memory (9). These findings differ in two important ways from those reported by Meier et al.: they suggest that detrimental effects of cannabis may be specific to some neurocognitive functions (rather than general) and that cannabis-associated deficits may recover with abstinence (rather than persist).
What new questions or reinterpretations of the literature are suggested by the findings of Meier et al.? The most obvious issue is related to the degree of exposure. Both adolescent onset and almost 2 decades of persistent cannabis use may be needed to obtain the magnitude and pervasiveness of long-term neuropsychological deficits reported by Meier et al. If the trends of increased adolescent onset and public acceptance of cannabis use continue, a larger segment of the general population may meet these criteria in the future. A less obvious issue is the degree of control for mental health and education, which may be required to isolate the effects reported by Meier et al. Other conditions may accompany persistent regular use or dependence and have independent adverse influences on neurocognition that may mask or complicate the documentation of pervasive and long-term effects of cannabis.
What are other notable merits of the study? The general strengths of the Dunedin Longitudinal Study should be emphasized and not underestimated. For example, the representativeness of the sample (all births at Dunedin’s Queen Mary Hospital between April 1972 and March 1973 and still living in Otago at 3 y of age) avoids classic problems of bias, such as the ascertainment bias and negative dependence of independent events in clinical samples described by Berkson (10) and the specification bias associated with missing data described by Heckman (11). Additionally, the use of third-party informants to document significantly poorer functional outcomes manifested by persistent cannabis users is a valuable addition that elucidates the clinical significance of the observed neuropsychological and intellectual effects and should help others understand their “real-world” impact.
What needs to be addressed in more detail? The interconnectedness of mental health problems, persistent cannabis use, and neuropsychological functioning demands more careful attention, and the literature (12, 13) suggests the value of controlling for more than just schizophrenia (as did Meier et al.). Similarly, potential sex differences require closer scrutiny, given accumulating evidence that nonacute or persistent cannabis use may have differing effects on neurocognitive functioning of males and females (14–17). Sex differences in brain cannabinoid receptor density, endocrine modulation of endocannabinoid activity, tolerance, and tetrahydrocannabinol metabolism may all contribute to these differences (18, 19). These factors may vary with age and maturation differences and interact with the onset of cannabis use and its neurocognitive effects. Persistence of regular use and dependence depended on sex, with males representing approximately 80% of the most persistent group. Meier et al. controlled for sex in their analyses, but this evaluation was underpowered by the relatively small numbers of females (n = 24 to n = 7) in the subgroups with a history of persistent regular cannabis use or dependence.
The sample size issue was addressed in the description of the history of the Dunedin Longitudinal Study (http://dunedinstudy.otago.ac.nz/about-us), which stated that a contributing factor to the choice of the cohort size (n ∼1,000) was to allow for studies of groups of children with common disorders down to a prevalence rate of approximately 3% (or n ∼30). Some of the previous 27 publications that addressed cannabis also had subgroups near this minimum size, which imposes some obvious and understandable limitations. For example, Arseneault et al. (20) reported that marijuana dependence, present in approximately 9.5% of the 961 participants (n = 91), was a risk factor (odds ratio = 3.8) for violent behavior. By self-report or court convictions, violent behavior was present in 9.6% of 961 participants (n = 92), with approximately one-third (n = 31) manifesting marijuana dependence. Caspi et al. (21) reported that cannabis use had adolescent onset in 26% of the cohort and adult onset in 19%, and that adolescent-onset cannabis use was a risk factor for developing adult psychosis for the subset with a functional variant (the valine158 allele of the COMT gene that affects metabolism of the neurotransmitter dopamine). As defined by these studies, schizophreniform disorder was present in 3.6% of 803 participants (n = 29).
How did Meier et al. address the important issue of small numbers in some subgroups? To increase statistical power, they used regression analysis that evaluated the relationship across the subgroup of participants with regular use or dependence and repeated the analysis, and the basic statistical findings were unchanged. A very valuable contribution of the Meier et al. report is that it provides the basis for a clear and important hypothesis that should be tested in larger birth cohorts that are in progress (e.g., the Danish National Birth Cohort of 100,000; http://www.dnbc.dk) or planned (e.g., the US National Children’s Study of 100,000; http://www.nationalchildrensstudy.gov). Will those high school seniors who are “smoking pot on a daily or near daily basis”—and continue this into adulthood—have significantly reduced mental abilities?
Three final points are important to make. First, the subgroups without a diagnosis of dependence or any report of persistent use should also be considered. As shown in Table 1 from Meier et al., most individuals in the study (85.8%) did not ever report regular cannabis use. Those who reported nonregular use (50.6% of the total) showed no decline in IQ or neuropsychological performance. Only 14.2% of the total sample was assigned to one of the three subgroups defined by degree of regular use of cannabis. This raises questions about the proportion of cannabis users in the general population to whom these findings most readily apply and highlights the need to interpret the findings in the context of a specific pattern of cannabis use rather than to all cannabis use, per se. Second, the magnitude of the reported decline in IQ (approximately 6 points) is approximately the same as the estimated decline in IQ associated with a low-level blood lead difference of <1–10 μg/dL (22). Both of these adverse environmental effects on cognition are especially of interest and importance because they are potentially preventable. Third, the knowledge of adverse neurocognitive effects of persistent regular use or dependence of cannabis may not be sufficient to result in decisions by affected individuals to stop using cannabis, as suggested by experience with other substances in widespread use (e.g., alcohol and tobacco) that also have adverse effects. This is expressed in one of the symptoms in the Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DSM-IV) for cannabis dependence (“the substance is used despite knowledge of persistent or recurrent physical or psychological problems caused by the substance”). Along with the findings of Meier et al., this underscores the need for prevention and intervention efforts related to adolescent onset and persistent regular use of cannabis.
Footnotes
References
- 1.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. 2012 Monitoring the future national results on adolescent drug use: Overview of key findings, 2011 (Institute for Social Research, The University of Michigan, Ann Arbor, MI) [Google Scholar]
- 2.Kuntsche E, et al. Decrease in adolescent cannabis use from 2002 to 2006 and links to evenings out with friends in 31 European and North American countries and regions. Arch Pediatr Adolesc Med. 2009;163:119–125. doi: 10.1001/archpediatrics.2008.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meier MH, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychol Rev. 2007;17:347–361. doi: 10.1007/s11065-007-9036-8. [DOI] [PubMed] [Google Scholar]
- 5.Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- 6.Fried P, Watkinson B, James D, Gray R. Current and former marijuana use: Preliminary findings of a longitudinal study of effects on IQ in young adults. CMAJ. 2002;166:887–891. [PMC free article] [PubMed] [Google Scholar]
- 7.Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana—a comparison with pre-drug performance. Neurotoxicol Teratol. 2005;27:231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Lyons MJ, et al. Neuropsychological consequences of regular marijuana use: A twin study. Psychol Med. 2004;34:1239–1250. doi: 10.1017/s0033291704002260. [DOI] [PubMed] [Google Scholar]
- 9.Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. J Int Neuropsychol Soc. 2003;9:679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- 10.Berkson J. Limitations of the application of fourfold table analysis to hospital data. Biometr Bull. 1946;2:47–53. [PubMed] [Google Scholar]
- 11.Heckman JJ. Sample selection bias as a specification error. Econometrica. 1979;47:153–163. [Google Scholar]
- 12.Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 13.Pope HG., Jr Cannabis, cognition, and residual confounding. JAMA. 2002;287:1172–1174. doi: 10.1001/jama.287.9.1172. [DOI] [PubMed] [Google Scholar]
- 14.Medina KL, et al. Prefrontal cortex morphometry in abstinent adolescent marijuana users: Subtle gender effects. Addict Biol. 2009;14:457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McQueeny T, et al. Gender effects on amygdala morphometry in adolescent marijuana users. Behav Brain Res. 2011;224:128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King GR, et al. Altered brain activation during visuomotor integration in chronic active cannabis users: Relationship to cortisol levels. J Neurosci. 2011;31:17923–17931. doi: 10.1523/JNEUROSCI.4148-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J Int Neuropsychol Soc. 2012;18:678–688. doi: 10.1017/S1355617712000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López HH. Cannabinoid-hormone interactions in the regulation of motivational processes. Horm Behav. 2010;58:100–110. doi: 10.1016/j.yhbeh.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Viveros MP, et al. The endocannabinoid system in critical neurodevelopmental periods: Sex differences and neuropsychiatric implications. J Psychopharmacol. 2012;26:164–176. doi: 10.1177/0269881111408956. [DOI] [PubMed] [Google Scholar]
- 20.Arseneault L, Moffitt TE, Caspi A, Taylor PJ, Silva PA. Mental disorders and violence in a total birth cohort: Results from the Dunedin Study. Arch Gen Psychiatry. 2000;57:979–986. doi: 10.1001/archpsyc.57.10.979. [DOI] [PubMed] [Google Scholar]
- 21.Caspi A, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: Longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 22.Lanphear BP, et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]