Abstract
The global geographic distribution of subseafloor sedimentary microbes and the cause(s) of that distribution are largely unexplored. Here, we show that total microbial cell abundance in subseafloor sediment varies between sites by ca. five orders of magnitude. This variation is strongly correlated with mean sedimentation rate and distance from land. Based on these correlations, we estimate global subseafloor sedimentary microbial abundance to be 2.9⋅1029 cells [corresponding to 4.1 petagram (Pg) C and ∼0.6% of Earth’s total living biomass]. This estimate of subseafloor sedimentary microbial abundance is roughly equal to previous estimates of total microbial abundance in seawater and total microbial abundance in soil. It is much lower than previous estimates of subseafloor sedimentary microbial abundance. In consequence, we estimate Earth’s total number of microbes and total living biomass to be, respectively, 50–78% and 10–45% lower than previous estimates.
Keywords: deep biosphere, cell enumeration, global microbial biomass, subsurface life
Bacteria and archaea drive many fundamental processes in marine sediment, including oxidation of organic matter, production of methane and other hydrocarbons, and removal of sulfate from the ocean (1–3). Previous studies of subseafloor sediment from ocean margins and the eastern equatorial Pacific Ocean reported high abundances of microbial cells (2). RNA studies indicate that many of these cells are active (4), have a diverse community composition (5, 6), and exhibit high diversity in their anaerobic metabolic activity (5). Cell counts from these environments generally show little variation between sites (2, 7) and decrease logarithmically with sediment depth, although there can be sharp peaks of high cell densities in zones of anaerobic methane-oxidation (2, 8).
In 1998, Whitman et al. (9) estimated subseafloor sedimentary microbial abundance to be 35.5⋅1029 cells, comprising 55–86% of Earth’s prokaryotic biomass and 27–33% of Earth’s living biomass. For their estimates, they assumed the average relationship of cell concentration to depth in six Pacific sites to characterize sedimentary microbial concentrations throughout the world ocean. Based on quantifications of intact phospholipid biomarkers from 15 Pacific Ocean sites and 1 Black Sea site, Lipp et al. (10) subsequently estimated microbial abundance in subseafloor sediment to be 5⋅1030 cells.
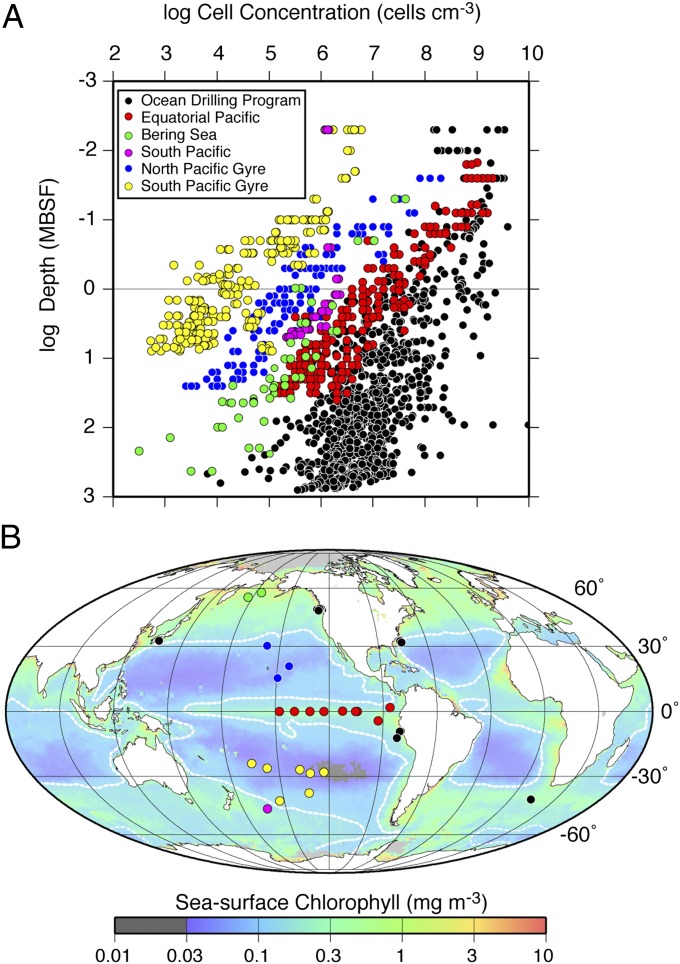
Previously published cell counts are generally from ocean margins and the eastern equatorial Pacific Ocean. Recent counts from the South Pacific Gyre (5) and the North Pacific Gyre are several orders of magnitude lower and show a more rapid decrease with depth (Fig. 1A). In these regions, dissolved oxygen penetrates deeply into the sediment and microbial activity is generally aerobic (5, 11). Metabolic activity per cell is extremely low among the anaerobes of both ocean margins and upwelling regions (12) and the aerobes of the open-ocean gyres (5, 11).
Fig. 1.
Subseafloor sedimentary cell counts used for this study. (A) Counted cell concentration vs. depth (mbsf) for the sites used in this study. (B) Site locations overlain on a map of time-averaged sea surface chlorophyll-a (34).
The differences between cell counts from ocean margins and upwelling areas and cell counts from oceanic gyres raise three questions. First, how does the abundance of microbes in subseafloor sediment vary throughout the world ocean? Second, what property or properties are likely to control that variation? Third, how does this variation affect estimates of total subseafloor sedimentary biomass and Earth’s total biomass?
Materials and Methods
To address these questions, we compiled our cell counts from the South Pacific Gyre (5), the North Pacific Gyre, and the eastern equatorial Pacific Ocean with previously published counts from ocean margins and the equatorial Pacific Ocean (Fig. 1B). We limited this compilation to sites with cell counts both above and below 1 m below sea floor (mbsf). To compare the data from different sites, we parameterized the cell distribution at each site by plotting cell abundance against subseafloor sediment depth for each site and then calculating a best-fit maximum likelihood estimate of a power-law function by minimizing the mean squared error (details provided in SI Text) using nontransformed data.
Of the 57 total sites, 34 exhibited a characteristic decrease in cell concentration with correlation coefficients exceeding 0.5 for power-law maximum likelihood regressions. The 23 sites omitted from the study had regression values less than 0.5 due to noisy or erratic cell concentration trends. These noisier data are often explainable by anomalous depositional settings or local geological anomalies [e.g., in the Nankai Trough (13), where cell concentration increases at greater depth due to in situ thermogenic generation of microbial substrates (14); in the Mediterranean Sea, where organic-rich sapropel layers cause elevated cell abundances in certain depth intervals and brine incursions occur at greater depths (15, 16); at the base of continental margins, where mass wasting events alter sediment accumulation rates (17)]. These sites with anomalous cell distributions were omitted from further calculations.
For each of the 34 sites analyzed further, we determined two parameters: (i) cell concentration at 1 mbsf (variable b) and (ii) rate of decrease in cell counts with depth (variable m) (details are provided in SI Text).
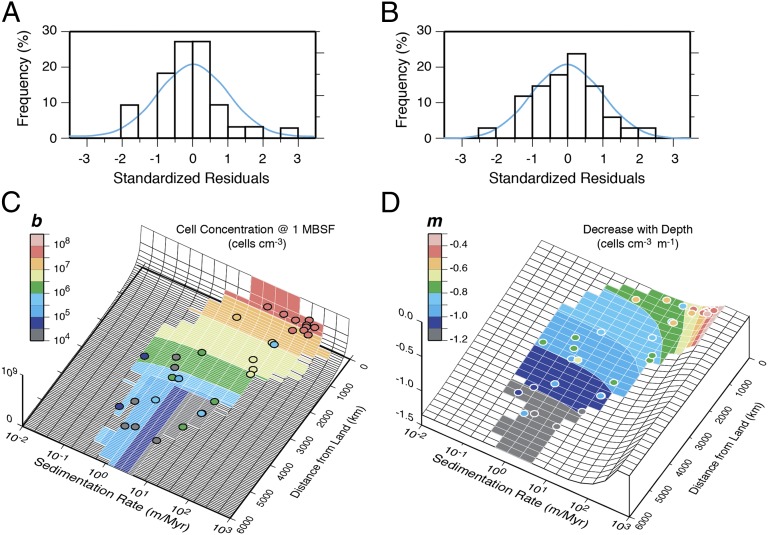
To test possible causes of geographic variation in subseafloor sedimentary cell abundance, we then calculated the correlations of b and m to several oceanographic parameters that vary strongly between ocean margins and midocean gyres (Table 1). Of these individual parameters, mean sedimentation rate is most highly correlated with both b and m. The combination of mean sedimentation rate and distance from land (distance from landmasses greater than 105 km2) explains an even higher percentage of the variance in both b and m (Table 1). The residuals are normally distributed, and our sites span the broad range of sedimentation rate/distance combinations that occur in the world ocean (Fig. 2). Principal component analyses indicate that the addition of any of the remaining variables does not increase the explanation of variance in either b or m.
Table 1.
Percentage of variance for (i) cell count at 1 mbsf (b) and (ii) rate of cell count decrease with depth (m) explained by various parameters
| Variable | Cell count at 1 mbsf (b) | Rate of decrease with sediment depth (m) |
| Mean sedimentation rate | 72 | 42 |
| Distance from land | 58 | 56 |
| Sea-surface chlorophyll | 22 | 4 |
| Gross primary production | 29 | 9 |
| Water depth | 38 | 15 |
| Sea-surface temperature | 10 | 13 |
| Sedimentation rate and water depth | 72 | 45 |
| Sedimentation rate and distance from land | 85 | 62 |
Data sources are provided in SI Text.
Fig. 2.
Distributions of cell abundance at 1 mbsf (b) and the power-law rate of decrease of cell abundance with depth (m) relative to sedimentation rate and distance from land. (A) Distribution of residuals for b. (B) Distribution of residuals for m. The histograms show the distributions of the actual residuals. The blue lines are the probability density functions for normal distributions with the appropriate SDs. (C) Distribution of b vs. sedimentation rate and distance from land. (D) Distribution of m vs. sedimentation rate and distance from land. Colored fields in C and D mark the actual range of combinations of sedimentation rate and distance from land in the world ocean. Note that data used for this model (shown as dots in C and D) occur throughout this range of actual combinations. Dot colors indicate actual values of b and m for each site.
Results
These correlations are consistent with a strong influence of organic matter burial rate on subseafloor sedimentary cell abundance. Burial of organic matter from the surface world is generally inferred to be the primary source of electron donors for microbes in most subseafloor sediment (2, 18). The rate of organic matter oxidation in subseafloor sediment has been described as declining with age according to a power-law function (19) or logarithmically (20). Correlation between concentration of intact phospholipids (a proxy for microbial biomass) and total organic carbon content in subseafloor sediment shows a clear relationship between subseafloor microbial biomass and buried organic matter (10), indicating that the availability of electron donors, with organic matter being the quantitatively most important one, strongly controls microbial activity and abundance.
Factors that affect organic burial rate include the productivity of the overlying ocean, water depth, the flux of organic matter from land, and sedimentation rate (21, 22). Some of these parameters influence organic burial rate directly (mean sedimentation rate), whereas others influence it indirectly, by influencing organic flux to the seafloor (water depth), marine productivity (sea-surface chlorophyll, sea-surface temperature, and gross primary production), or flux of organic matter from land. Organic burial rates have been estimated from many of these properties for most of the world ocean (22, 23). Other potential electron donors include reduced metal [e.g., Fe(II), Mn(II)] and H2 from water radiolysis. However, in the anoxic sediment that constitutes the vast majority of sediment in near-shore regions and open-ocean upwelling systems, sulfate is the predominant external electron acceptor (12); consequently, thermodynamic considerations preclude use of reduced metal as a predominant electron donor (2). H2 from water radiolysis appears likely to be a significant electron donor only in sediment that contains extremely little organic matter, such as the sediment of midocean gyres (5, 24).
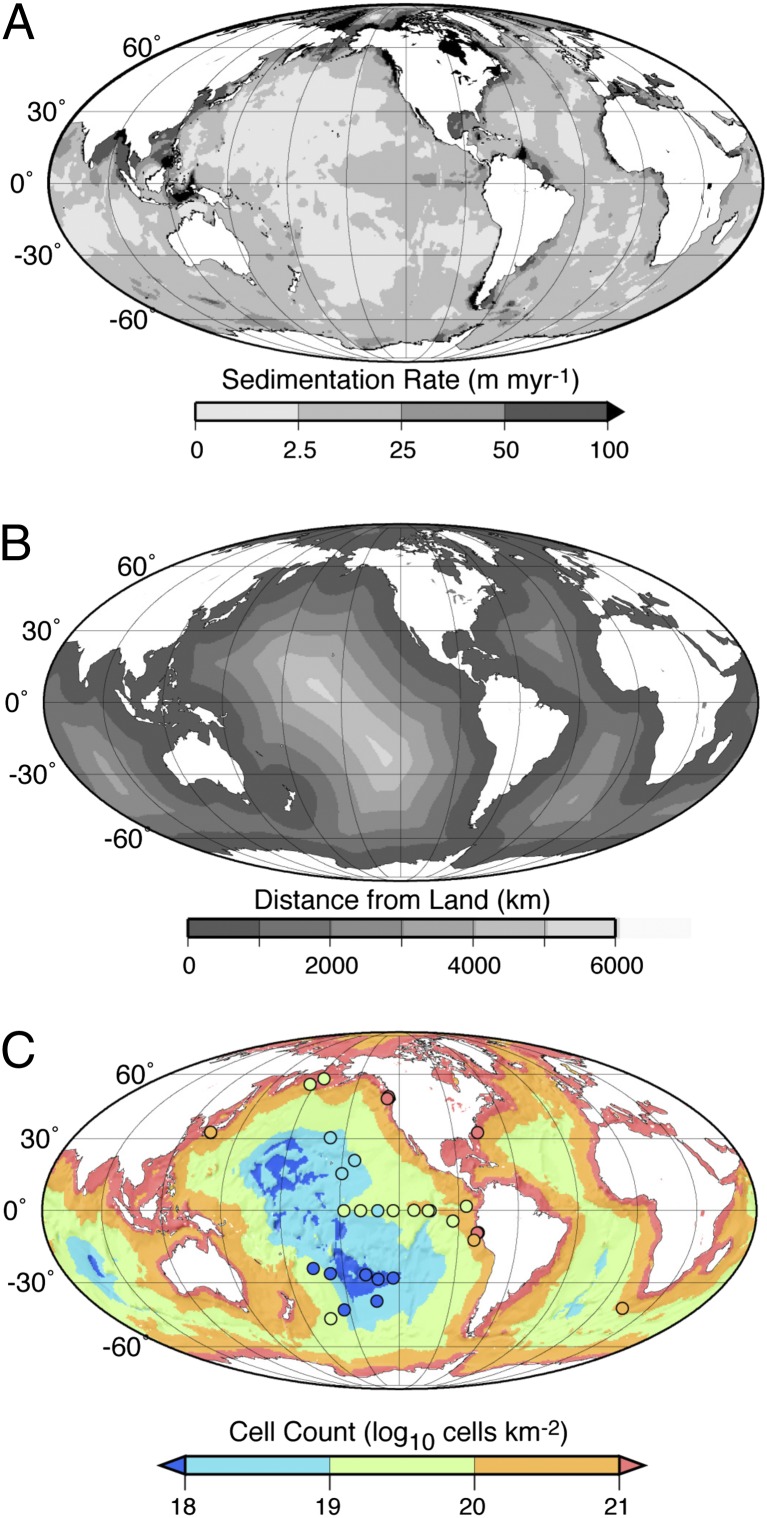
To build a global map of subseafloor sedimentary cell abundance, we used global maps of mean sedimentation rate and distance from land (Fig. 3 A and B) to create global maps of the distributions of b and m (SI Text). These distributions of b and m were then combined with global distributions of marine sediment thickness (25) to integrate cell abundance over the entire sediment column in each 1°-by-1° grid of the world ocean (Fig. 3C). The maximum sediment thickness used in this calculation for any grid was 4,000 m; this depth is the approximate average depth of the 122 °C isotherm, the upper temperature limit for presently known microbial life (26), assuming an average geothermal gradient of ∼30 °C km−1. If the known temperature limit to life rises, this maximum depth will have little effect on global maps of subseafloor cell distributions, because 97% (by volume) of marine sediment is shallower than 4,000 mbsf.
Fig. 3.
Global distribution of subseafloor sedimentary cell abundance. (A) Geographic distribution of sedimentation rate (27). (B) Geographic distribution of distance from shore (35). (C) Geographic distribution of integrated number of cells (derived from b, m, and sediment thickness). Dot colors indicate numbers of cells calculated for actual sites (log10 cells/km2).
Because cell concentration varies by as much as four orders of magnitude at any given depth and sediment thickness varies by two to three orders of magnitude throughout the ocean, total cell number integrated over the entire sedimentary column varies by ca. five orders of magnitude between sites (Fig. 3C). Depth-integrated cell abundance is highest at continental margins and lowest in midocean gyres.
Integrating over the world’s ocean area, we estimate the total number of cells in subseafloor sediment to be 2.9⋅1029 cells. A bootstrap exercise to check our analytical solution yielded a median value of 3.3⋅1029 cells, with the first SDs at 1.2⋅1029 and 8.0⋅1029 cells (see SI Text for details).
The geographic distribution of subseafloor sedimentary cells varies greatly from continental margins to the open ocean. Although the world’s ocean shelves (water depth <150 m) cover only ∼7% of the total oceanic area, they harbor 33% of the total cells in subseafloor sediment. In comparison, the oligotrophic (<0.14 mg/m3 of chlorophyll-a) oceanic gyres cover about 42% of the world ocean and contain 10% of the total cells.
Discussion
Our estimate of total cell abundance in subseafloor sediment (2.9⋅1029) is 92% lower than the previous standard estimate (35.5 × 1029) (9). It is also ∼70% lower than other estimates of around 10⋅1029 (7, 10). The reasons for this difference are twofold. First, our database is more geographically diverse than those of the previous studies. In particular, our database includes gyre areas with extremely low cell abundances. Second, like the most recent study (10) but unlike the previous studies (7, 9), we used estimates of actual sediment thickness throughout the world ocean derived from geophysical data (25, 27).
Comparison of our subseafloor sedimentary estimate to previous estimates of microbial abundance in other environments should be treated with caution because uncertainties on the estimates for other environments are either not quantified or extremely large. This said, our results suggest that the number of subsurface prokaryotes may roughly approximate the total number of prokaryotes in surface environments. Our estimate of total microbial abundance in subseafloor sediment (2.9⋅1029) is roughly equal to the estimates of Whitman et al. (9) for the total number of prokaryotes in seawater (1.2⋅1029) and in soil (2.6⋅1029). It also approximates their lower bound estimate for total microbial abundance in the terrestrial subsurface (2.5⋅1029; their upper bound is 25⋅1029).
Our more recent estimate of subseafloor sedimentary cell abundance significantly decreases the estimate of Earth’s total prokaryote population. Combining our subseafloor sedimentary estimate with the estimates of Whitman et al. (9) for prokaryote numbers in seawater, soil, and the terrestrial subsurface decreases the estimated number of Earth’s total number of prokaryotes by 50–78% (from 41.8–64.3⋅1029 cells to 9.2–31.7⋅1029 cells).
Conversion of total cell abundance to total microbial biomass requires an estimate of carbon content per cell, which, in turn, depends on cell volume and the amount of carbon per cell. Microbial cell volume varies by many orders of magnitude (28); however, as a general rule, cells react to nutrient limitation by size reduction (29). Organic matter becomes increasingly recalcitrant with depth (3); therefore, microbial cells in deep subseafloor environments have to adapt to low availability of organic electron donors and organic nutrients. It is thus reasonable to assume that cells will be rather on the small side of the size spectrum, which has profound implications when converting cellular abundance into biomass.
The absolute lower limit for cell size is determined by the minimum amount of macromolecules (e.g., DNA, RNA, ribosomes, proteins) necessary to maintain functionality. Based on the molecular size of a minimum set of these macromolecules (30), the calculated minimum cellular volume would be in the range of 0.014–0.06 μm3; for a spherical cell, this would translate into a diameter of 0.3–0.5 μm. As a general trend, cells with diameters and volumes below 0.2 μm and 0.05 μm3, respectively, are predominantly rod-shaped (29) because of a higher surface-to-volume ratio compared with spherical cells. Because there is a minimum set of macromolecules that any cell needs for functioning and survival, small cells tend to have a higher C content per cell volume than larger cells (31).
There are no previously published studies of actual cell size distributions in deep subseafloor sediment. Preliminary flow cytometry studies on subseafloor samples and our own observations suggest cell widths and lengths in the range of 0.25–0.7 μm and 0.2–2.1 μm, respectively (see SI Text for details). Other studies assumed average spherical cell diameters of 0.5 μm (10) or volumes of 0.21 μm3 (32) for calculating subseafloor biomass. Assuming that the majority of subseafloor cells have a diameter of 0.25–0.7 μm and cell shapes vary between spherical and short rods, cell volumes range from 0.008 to 0.718 μm3, with an average of 0.042 μm3. Using an allometric model (33) that acknowledges the higher C content of smaller cells (31), the carbon content per cell would be ∼14 fg C cell−1 with minimum and maximum estimates of 5 and 75 fg C cell−1, respectively, which is rather to the low end of previously used values: 18 fg C cell−1 (10), 65.1 fg C cell−1 (32), and 86 fg C cell−1 (9).
Based on our estimates of total subseafloor sedimentary cell abundance and cellular carbon content, our calculation of subseafloor microbial biomass amounts to 4.1 petagram (Pg), with minimum and maximum estimates of 1.5–22 Pg C, respectively. This estimate is significantly lower than the previous estimate of 303 Pg C of Whitman et al. (9) and somewhat lower than the estimates of 90 Pg of Lipp et al. (10) and 60 Pg of Parkes et al. (32).
This result significantly decreases the estimate of Earth’s total living biomass. Using published estimates (9) for the total carbon content of plants and nonsubseafloor prokaryotes, Earth’s total (plant + prokaryote) biomass is reduced from 915 to 1,108 Pg C down to 614 to 827 Pg C (average = 713 Pg C). Subseafloor sedimentary biomass comprises only 0.18–3.6% (average = 0.6%) of the total.
Supplementary Material
Acknowledgments
The authors thank the crews and shipboard scientific parties of our RV Roger Revelle cruise to the South Pacific Gyre (Knox-02RR) and our RV Knorr cruise (195-III) to the equatorial Pacific Ocean and North Pacific Gyre. We also thank the co-chiefs, science party, and crew of Integrated Ocean Drilling Program Expedition 323 to the Bering Sea, especially Emily A. Walsh, Heather Schrum, Nils Risgaard-Petersen, and Laura Wehrmann, for taking cell count samples. We thank Jeremy Collie for advice regarding data analysis. J.K. and R.R.A. are supported through the Forschungsverbund GeoEnergie by the German Federal Ministry of Education and Research. Expeditions Knox02-RR and Knorr 195-III were funded, respectively, by the Ocean Drilling Program and Biological Oceanography Program of the US National Science Foundation (Grants OCE-0527167 and OCE-0752336). R.P., D.C.S., and S.D. were funded by National Science Foundation Grants OCE-0527167, OCE-0752336, and OCE-0939564. This is contribution 136 of the Center for Dark Energy Biosphere Investigations.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 15976.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203849109/-/DCSupplemental.
References
- 1.Hinrichs K-U, et al. Biological formation of ethane and propane in the deep marine subsurface. Proc Natl Acad Sci USA. 2006;103:14684–14689. doi: 10.1073/pnas.0606535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Hondt SL, et al. Distributions of microbial activities in deep subseafloor sediments. Science. 2004;306:2216–2221. doi: 10.1126/science.1101155. [DOI] [PubMed] [Google Scholar]
- 3.Jørgensen BB. Mineralization of organic matter in the sea bed—The role of sulphate reduction. Nature. 1982;296:643–645. [Google Scholar]
- 4.Schippers A, et al. Prokaryotic cells of the deep sub-seafloor biosphere identified as living bacteria. Nature. 2005;433:861–864. doi: 10.1038/nature03302. [DOI] [PubMed] [Google Scholar]
- 5.D’Hondt S, et al. Subseafloor sedimentary life in the South Pacific Gyre. Proc Natl Acad Sci USA. 2009;106:11651–11656. doi: 10.1073/pnas.0811793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inagaki F, et al. Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments on the Pacific Ocean Margin. Proc Natl Acad Sci USA. 2006;103:2815–2820. doi: 10.1073/pnas.0511033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkes RJ, Cragg BA, Wellsbury P. Recent studies on bacterial populations and processes in subseafloor sediments: A review. Hydrogeology Journal. 2000;8(1):11–28. [Google Scholar]
- 8.Parkes RJ, et al. Deep sub-seafloor prokaryotes stimulated at interfaces over geological time. Nature. 2005;436:390–394. doi: 10.1038/nature03796. [DOI] [PubMed] [Google Scholar]
- 9.Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: The unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipp JS, Morono Y, Inagaki F, Hinrichs K-U. Significant contribution of Archaea to extant biomass in marine subsurface sediments. Nature. 2008;454:991–994. doi: 10.1038/nature07174. [DOI] [PubMed] [Google Scholar]
- 11.Røy H, et al. Aerobic microbial respiration in 86-million-year-old deep-sea red clay. Science. 2012;336:922–925. doi: 10.1126/science.1219424. [DOI] [PubMed] [Google Scholar]
- 12.D’Hondt S, Rutherford S, Spivack AJ. Metabolic activity of subsurface life in deep-sea sediments. Science. 2002;295:2067–2070. doi: 10.1126/science.1064878. [DOI] [PubMed] [Google Scholar]
- 13.Moore GF, Taira A, Klaus A. 2001. Proceedings of the Ocean Drilling Program—Initial Reports (Ocean Drilling Program, College Station, TX), Vol 190.
- 14.Horsfield B, et al. Living microbial ecosystems within the active zone of catagenesis: Implications for feeding the deep biosphere. Earth Planet Sci Lett. 2006;246(1–2):55–69. [Google Scholar]
- 15.Cragg BA, Law KM, Cramp A, Parkes RJ. 1998. The response of bacterial populations to sapropels in deep sediments of the Eastern Mediterranean. Proceedings of the Ocean Drilling Program—Scientific Results, eds Robertson AHF, Emeis K-C, Richter C, Camerlenghi A (Ocean Drilling Program, College Station, TX), Vol 160, pp 303–308.
- 16.Cragg BA, Law KM, O’Sullivan GM, Parkes RJ. 1999. Bacterial profiles in deep sediments of the Alboran Sea, western Mediterranean, Sites 976–978. Proceedings of the Ocean Drilling Program—Scientific Results, eds Zahn R, Comas MC, Klaus A (Ocean Drilling Program, College Station, TX), Vol 161, pp 433–438.
- 17.Cragg BA, et al. 1995. The impact of fluid and gas venting on bacterial populations and processes in sediments from the Cascadia Margin Accretionary System (Sites 888–892) and the geochemical consequences. Proceedings of the Ocean Drilling Program—Scientific Results, eds Carson B, Westbrook GK, Musgrave RJ, Suess E (Ocean Drilling Program, College Station, TX), Vol 146, pp 399–413.
- 18.Jørgensen BB. Bacteria and marine biogeochemistry. In: Schulz HD, Zabel M, editors. Marine Geochemistry. Berlin: Springer; 2000. pp. 173–207. [Google Scholar]
- 19.Middelburg JJ. A simple rate model for organic matter decomposition in marine sediments. Geochim Cosmochim Acta. 1989;53:1577–1581. [Google Scholar]
- 20.Rothman DH, Forney DC. Physical model for the decay and preservation of marine organic carbon. Science. 2007;316:1325–1328. doi: 10.1126/science.1138211. [DOI] [PubMed] [Google Scholar]
- 21.Berger WH, Wefer G. Export production: Sseasonality and intermittency, and paleoceanographic implications. Global and Planetary Change. 1990;89:245–254. [Google Scholar]
- 22.Jahnke RA. The global ocean flux of particulate organic carbon: Areal distribution and magnitude. Global Biogeochem Cycles. 1996;10(1):71–88. [Google Scholar]
- 23.Seiter K, Hensen C, Zabel M. Benthic carbon mineralization on a global scale. Global Biogeochem Cycles. 2005 doi: 10.1029/2004GB002225. [DOI] [Google Scholar]
- 24.Blair CC, D’Hondt S, Spivack AJ, Kingsley RH. Radiolytic hydrogen and microbial respiration in subsurface sediments. Astrobiology. 2007;7:951–970. doi: 10.1089/ast.2007.0150. [DOI] [PubMed] [Google Scholar]
- 25.Divins DL. NGDC Total Sediment Thickness of the World’s Oceans and Marginal Seas. Boulder, CO: National Oceanic and Atmospheric Administration; 2008. [Google Scholar]
- 26.Takai K, et al. Cell proliferation at 122 degrees C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc Natl Acad Sci USA. 2008;105:10949–10954. doi: 10.1073/pnas.0712334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laske G, Masters G. A global digital map of sediment thickness. Eos, Transactions, American Geophysical Union. 1997 78(46)(Suppl):S41E-1 (abstr) [Google Scholar]
- 28.Schulz HN, Jorgensen BB. Big bacteria. Annu Rev Microbiol. 2001;55:105–137. doi: 10.1146/annurev.micro.55.1.105. [DOI] [PubMed] [Google Scholar]
- 29.Velimirov B. Nanobacteria, ultramicrobacteria and starvation forms: A search for the smallest metabolizing bacterium. Microbes Environ. 2001;16(2):67–77. [Google Scholar]
- 30.Himmelreich R, et al. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 1996;24:4420–4449. doi: 10.1093/nar/24.22.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romanova N, Sazhin A. Relationships between the cell volume and the carbon content of bacteria. Oceanology (Moscow) 2010;50:522–530. [Google Scholar]
- 32.Parkes RJ, et al. Deep bacterial biosphere in Pacific Ocean sediments. Nature. 1994;371:410–413. [Google Scholar]
- 33.Simon M, Azam F. Protein-content and protein-synthesis rates of planktonic marine-bacteria. Mar Ecol Prog Ser. 1989;51(3):201–213. [Google Scholar]
- 34.Gregg WW, Casey NW, McClain CR. Recent trends in global ocean chlorophyll. Geophys Res Lett. 2005 doi: 10.1029/2004gl021808. [DOI] [Google Scholar]
- 35.Wessel P, Smith WHF. New, improved version of the Generic Mapping Tools released. Eos, Transactions, American Geophysical Union. 1998;79:579. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.