Abstract
Roots of taro (Colocasia esculenta [L.] Schott cvs Bun-long and Lehua maoli) exuded increasing concentrations of oxalate with increasing Al stress. This exudation was a specific response to excess Al and not to P deficiency. Addition of oxalate to Al-containing solutions ameliorated the toxic effect of Al.
Al toxicity is a major factor limiting plant growth in many regions of the world (Kamprath, 1984), yet some species or cultivars within species are able to grow in Al-toxic soils (Foy, 1988). A mechanism proposed to function in Al tolerance by plants is the exudation of Al-chelating organic acids into the rhizosphere. Evidence that supports this hypothesis is as follows: (a) Al-stimulated exudation of malate occurs in Al-tolerant wheat cultivars (Delhaize et al., 1993; Basu et al., 1994); (b) oxalate is secreted in response to Al by buckwheat, a known Al-accumulating species; (c) citrate exudation is increased by exposure to Al in an Al-tolerant snapbean cultivar (Miyasaka et al., 1991) and in Al-tolerant corn genotypes (Pellet et al., 1995); and (d) overexpression of a bacterial citrate synthase gene in transgenic tobacco increased cytoplasmic citrate production, increased the release of citrate extracellularly, and reduced the inhibition of root growth by Al (de la Fuente et al., 1997).
In addition, exogenous application of organic acids (e.g. malate, citrate, and oxalate) to nutrient solutions containing toxic levels of Al has been shown to protect root growth and reduce Al toxicity in several crop species, such as wheat (Ownby and Popham, 1989; Delhaize et al., 1993; Basu et al., 1994; Ryan et al., 1995; Pellet et al., 1996), sorghum (Shuman et al., 1991), and corn (Pellet et al., 1995). According to Hue et al. (1986), malate is a moderate detoxifier, whereas both citrate and oxalate are strong detoxifiers of Al.
Taro (Colocasia esculenta L.), a tropical root-crop species, is naturally tolerant of excess Al (Miyasaka et al., 1993a). Two taro cultivars, Bun-long and Lehua maoli, are able to grow at high Al concentrations in nutrient solutions (Miyasaka et al., 1993a) that would be toxic to many other crop species. Taro must have a mechanism to avoid Al toxicity, because it does not accumulate high concentrations of Al in its shoots (Miyasaka et al., 1993b).
Taro contains substantial quantities of oxalate (Standal, 1983), as do the majority of higher plants (82 of 93 orders) (Zindler-Frank, 1976). The ubiquity of oxalate in plants has raised questions about its roles. Proposed functions of oxalate include protection from insects and foraging animals through toxicity and/or unpalatability, osmoregulation, and regulation of Ca levels in plant organs and tissues (Libert and Franceschi, 1987).
We propose that the secretion of oxalate is a mechanism by which taro avoids Al toxicity. We characterized the exudation of oxalate from taro roots into the rhizosphere in response to excess Al.
MATERIALS AND METHODS
Tissue-cultured plantlets of two taro (Colocasia esculenta L.) cultivars, Bun-long and Lehua maoli, were obtained from a commercial laboratory (Paradise Propagation, Hilo, HI). Plants were ready for experiments when they were about 10 cm tall, with an average of six leaves and approximately 5-cm roots.
Plantlets were grown in nutrient solution under aseptic conditions to quantitatively estimate their production of organic acids. Microbial contamination was monitored by plating samples onto nutrient agar, and only negligible contamination was detected.
The macronutrient concentrations in a modified Steinberg solution were (in mm): NH4-N, 1.2; NO3-N, 3.6; P, 0.1; K, 1.2; Ca, 1.0; Mg, 0.4; and S, 0.7. The micronutrients were (in μm): Mn, 2; B, 6; Zn, 1; Cu, 0.5; Mo, 0.1; and Fe as Fe-N,N′-ethylenebis[2-(2-hydroxyphenyl)-glycine], 10 (Miyasaka et al., 1993a). All nutrient solutions were autoclaved except AlCl3, which was filter sterilized through 0.2-μm-pore-diameter syringe filters to avoid Al precipitation caused by pH changes during autoclaving.
The pH of the basal nutrient solution was adjusted to 4.0 with 0.1 m HCl and autoclaved. After the addition of filter-sterilized, 0.09 m AlCl3 solution, pH 3.87, solutions were again adjusted to pH 4.0 using 0.1 m NaOH. The final solution pH was also recorded after harvest.
Before treatment plantlets were rinsed three times each with 15 mL of sterile deionized water to remove substances that may have accumulated on root surfaces during the previous growth period. Plantlets of similar weights were grouped together. All experiments followed a randomized complete-block design to remove variability attributable to plant size, light conditions, and temperature. Vessels were kept in a growth chamber at 22°C to 28°C, 65% RH, 24 h of light, and a light intensity of 45 μmol photons m−2 s−1.
Ion Chromatography
Samples of the solution culture medium were analyzed for oxalate and other organic acids using HPLC (DX 500 chromatography system, Dionex, Sunnyvale, CA) with an anion self-regenerating suppressor (4 mm, model ASRS-I, Dionex). For oxalate analysis, an anion-exchange analytical column and a guard column (both 4 mm, models IonPac AS4A-SC and AG4A-SC, Dionex) were used with an eluent of 22 mm sodium borate and boric acid at a flow rate of 2.0 mL min−1. The columns were also used to reconfirm identification of oxalate and for analyses of other organic acids. For analyses of oxalate, succinate, and malate, 50 mm NaOH was the eluent, and for citrate, 100 mm NaOH was the eluent, both at a flow rate of 1.0 mL min−1. Concentrations of organic acids were determined via measurement of electrical conductivity using a conductivity detector (model CD20, Dionex).
Time Course of Al-Induced Oxalate Exudation
Tissue-cultured plantlets of the taro cvs Bun-long and Lehua maoli were grown in sterile culture for 10 d in the basal nutrient solution with or without 900 μm Al. Four replicates were used in a factorial combination of two Al levels and two taro cultivars. For each treatment two plantlets were grown in 50 mL of treatment solution. Initial fresh weight of cv Bun-long averaged 6.79 ± 0.75 g, and that of cv Lehua maoli was 4.54 ± 0.85 g.
Samples from the growth medium were taken on d 3, 5, 7, and 10 after treatments were imposed. At each sampling time, 1 mL of solution was withdrawn from each growth vessel, diluted with 1 mL of sterile deionized water, and then stored in a freezer until analyses for oxalate.
Dose Response of Al-Induced Oxalate Exudation
Two taro cultivars were exposed to four initial Al levels (0, 300, 600, and 900 μm) in the basal nutrient solution. Treatments were a complete factorial of two taro cultivars and four Al levels, with five replicates. For each treatment three plantlets were grown in a vessel containing 100 mL of treatment solution. Initial fresh weight of cv Bun-long averaged 11.9 ± 0.61 g, and that of cv Lehua maoli averaged 7.3 ± 0.60 g. Solution samples were taken for the analyses of oxalate and other organic acids (succinate, malate, and citrate) 1 week after exposure to the Al treatments.
Amelioration of Al Toxicity by Oxalate
Roots were removed and plantlets were grown in sterile nutrient solution for 2 d to allow healing of wounds. Plantlets were then transferred to seven treatment solutions, with five replicates per treatment. Treatments included basal nutrient solutions and basal nutrient solutions plus 900 μm Al and oxalic acid (0, 300, 600, 900, 1200, and 1500 μm). Each plantlet was grown in 15 mL of solution in a test tube under aseptic conditions. Initial fresh weight of cv Bun-long was 0.40 ± 0.07 g, and that of cv Lehua maoli was 0.98 ± 0.16 g.
At the end of 14 d, when visible differences in root growth attributable to treatments were observed, plantlets were harvested and root length was determined. Relative root length was calculated as the fraction of root length in various treatment solutions divided by that in controls (plants grown in basal nutrient solution only).
Role of Low P in Oxalate Exudation
Al can induce P deficiency in plants through its complexation of phosphate ions, and plants are known to respond to P deficiency by exudation of organic acids (Lipton et al., 1987). To investigate whether oxalate exudation was related to low P or to Al stress, tissue-cultured plantlets were grown under sterile conditions in the presence or absence of 900 μm Al and three levels of P (0, 0.3, and 100 μm). Treatments were a complete factorial combination of two cultivars, two Al levels, and three P levels. Each treatment was replicated five times, with one plantlet grown in a culture tube containing 15 mL of nutrient solution per replicate. The initial weight of cv Bun-long averaged 1.6 ± 0.2 g, and that of cv Lehua maoli averaged 2.2 ± 0.61 g. One week after exposure to treatments, solution samples were collected and analyzed for oxalate.
Statistical Analyses
Statistical analyses of the data were conducted using SAS (Statistical Analysis Systems Institute, 1982). In the time-course trial, analysis of variance was used for a repeated-measures design. In the other experiments, two-way or three-way analysis of variance was used. In the dose-response experiment, single degree-of-freedom contrasts were used to evaluate linear, quadratic, and cubic effects of Al on oxalate exudation. A P value of 0.05 or less was considered statistically significant.
RESULTS
Time Course of Al-Induced Oxalate Exudation
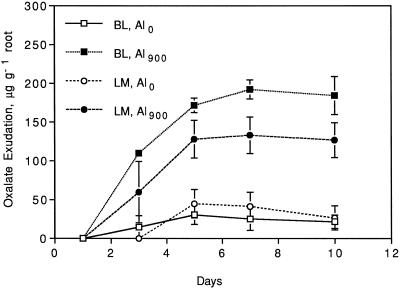
In the control treatments, both cultivars released only small concentrations of oxalate, and the exudation changed only slightly over time (Fig. 1). The addition of 900 μm Al significantly stimulated oxalate excretion from roots of both taro cultivars (P = 0.0001), with a linear increase in the cumulative amount up to 7 d after initiation of treatment (Fig. 1). No significant difference in oxalate exudation was found between the cultivars (P = 0.12).
Figure 1.
Exudation of oxalate from roots (on a fresh-weight basis) of taro cvs Bun-long (BL) and Lehua maoli (LM) at Al levels of 0 (Al0) and 900 (Al900) μm over a period of 10 d.
Final solution pH was in the range of 3.5 to 4.2, with no significant differences in final solution pH attributable to cultivars or Al levels (P = 0.56 and 0.47, respectively).
Dose Response of Al-Induced Oxalate Exudation
Increasing Al concentrations from 0 to 900 μm significantly increased exudation of oxalate into the rhizosphere from both cultivars (P = 0.0001; Fig. 2). No significant cultivar difference was detected in oxalate exudation in response to increasing Al levels (P = 0.65; Fig. 2). Accumulation of oxalate in the growth medium was linear for cv Bun-long over the range of solution Al levels tested (P = 0.0002; Fig. 2), whereas for cv Lehua maoli, the increase of oxalate concentration with increased Al levels followed a cubic trend (P = 0.0043; Fig. 2).
Figure 2.
Effect of increasing total Al levels in nutrient solution on the exudation of oxalate from roots (on a fresh-weight basis) of taro cvs Bun-long (BL) and Lehua maoli (LM) over a period of 7 d.
No detectable amounts of citrate, malate, or succinate were found in the nutrient solution. The final solution pH ranged from 3.8 to 4.2 for cv Bun-long and from 3.6 to 3.8 for cv Lehua maoli, with a significant difference attributable to cultivar (P = 0.0001), but none attributable to Al treatment (P = 0.08).
Amelioration of Al Toxicity by Oxalate
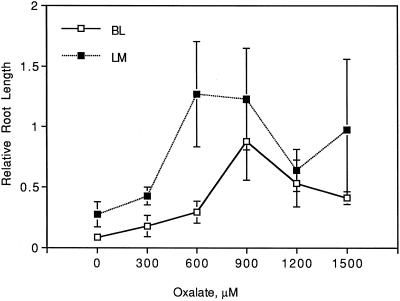
Exogenously added oxalate up to 900 μm significantly increased relative root lengths for both taro cultivars grown in the presence of 900 μm Al (linear effect: P = 0.03; Fig. 3). Oxalate concentrations greater than 900 μm began to reverse the ameliorative effect on Al-induced inhibition of root growth (quadratic effect: P = 0.03; Fig. 3). Taro cv Lehua maoli had significantly longer relative root lengths compared with cv Bun-long (P = 0.01; Fig. 3). For cv Lehua maoli, an oxalate concentration of 600 μm was sufficient to restore relative root length to that of the controls, whereas for cv Bun-long, 900 μm oxalate was required to restore relative root length to 88% of the controls.
Figure 3.
Effect of exogenously applied oxalate on relative root lengths of taro cvs Bun-long (BL) and Lehua maoli (LM) grown at 900 μm Al over a period of 14 d.
The final solution pH for cv Bun-long varied from 3.9 to 4.2, and that of cv Lehua maoli ranged from 3.6 to 4.1, with a significant difference attributable to cultivar (P = 0.004).
Role of Low P in Oxalate Exudation
There were no significant differences in oxalate exudation between P treatments for either cultivar, whereas the presence of Al in solution significantly increased exudation of oxalate (Table I). Much higher concentrations of oxalate were exuded by cv Bun-long in response to 900 μm Al relative to cv Lehua maoli, resulting in a significant interaction between cultivar and Al treatments (Table II).
Table I.
Effect of P and Al levels on the exudation of oxalate from roots of two taro cultivars over 7 d
| cv | Oxalate Exudation
|
||
|---|---|---|---|
| 0 μm P | 0.3 μm P | 100 μm P | |
| μg g−1 root fresh wt | |||
| Bun-long | |||
| 0 μm Al | 48.2 (9.9) | 64.2 (12.7) | 59.6 (14.0) |
| 900 μm Al | 535.9 (111.3) | 693.7 (148.8) | 523.6 (96.6) |
| Lehua maoli | |||
| 0 μm Al | 32.1 (16.4) | 19.8 (8.7) | 30.7 (6.7) |
| 900 μm Al | 188.2 (25.1) | 284.2 (97.8) | 284.2 (123.7) |
Values are means (±se).
Table II.
Analysis of variance probabilities for P, Al, and cultivar effects on exudation of oxalate
| P | 0.4200 |
| Al | 0.0001 |
| Cultivar | 0.0001 |
| P × Al | 0.4300 |
| P × cultivar | 0.6400 |
| Al × cultivar | 0.0005 |
| P × Al × cultivar | 0.7100 |
| Replicate | 0.0150 |
Final solution pH ranged from 3.9 to 5.7 for cv Bun-long and from 3.7 to 4.5 for cv Lehua maoli, with significant differences attributable to cultivar (P = 0.0001). P treatments did not significantly affect solution pH (P = 0.91), but treatments with added Al had significantly lower solution pH (P = 0.0001). Final solution pH levels greater than 4.3 were found only in treatment solutions not containing Al.
DISCUSSION
Al-stimulated oxalate exudation from roots of two taro cultivars was found within 3 d after the initiation of Al treatments (Fig. 1). The increase in cumulative oxalate concentration was linear up to 7 d after exposure to Al. Our long-term results confirm the findings of Ma et al. (1997), in which short-term exposure of buckwheat roots to AlCl3 induced oxalic acid excretion in 30 min and a linear increase in the amount excreted over 12 h.
Increasing Al levels in solution significantly increased oxalate concentrations released from roots of two taro cultivars (Fig. 2). Again, our results agree with those of Ma et al. (1997), who showed that Al-induced exudation of oxalate by buckwheat increased with increasing external Al levels.
Addition of oxalate to solutions containing 900 μm Al ameliorated the Al-induced inhibition of root elongation in both taro cultivars (Fig. 3). Similar amelioration of Al toxicity by exogenously applied oxalate was found for sorghum (Shuman et al., 1991) and cotton (Hue et al., 1986). The reversal of the ameliorative effect of oxalate at concentrations greater than 900 μm (Fig. 3) could be caused by a toxic effect of oxalate alone on root elongation. A detrimental effect of oxalate was found in sorghum, in which 10 μm oxalate decreased the root length of seedlings by 80% in the absence of Al (Shuman et al., 1991).
Taro cultivars exuded oxalate specifically in response to excess Al and not to deficient levels of P. P levels in solution had no significant effect on oxalate exudation during a 1-week growth period (Table I). Our results confirm the findings of Ma et al. (1997), in which P deficiency did not induce oxalate excretion by buckwheat.
In previous studies using both soil and nutrient solution, cv Lehua maoli was found to be Al tolerant compared with cv Bun-long at Al levels ≥ 890 μm (Miyasaka et al., 1993a; Calisay, 1996). These cultivar differences were confirmed in our experiment with exogenous application of oxalate, in which cv Lehua maoli was found to have significantly longer relative root lengths compared with cv Bun-long in the presence of 900 μm Al (Fig. 3). These results validate the growth of taro plantlets in a sterile, nutrient medium as a means to assay for cultivar differences.
Taro cv Bun-long exuded significantly greater concentrations of oxalate relative to cv Lehua maoli at 900 μm Al (Table I). These results do not agree with previous research in which Al-tolerant genotypes of corn (Pellet et al., 1995) and wheat (Delhaize et al., 1993; Basu et al., 1994) exuded more Al-chelating organic acids into the rhizosphere compared with Al-sensitive genotypes in the presence of excess Al. Our results indicate that a second Al-tolerance mechanism must operate in cv Lehua maoli exposed to very high Al levels.
In previous research, water-soluble oxalate concentrations decreased within roots of taro exposed to Al (Calisay, 1996). In our experiment using 900 μm Al, cv Bun-long exuded oxalate at 5.1 g kg−1 root dry weight and cv Lehua maoli exuded oxalate at 3.1 g kg−1 root dry weight. In an experiment using 890 μm Al (Calisay, 1996), the water-soluble oxalate concentration in roots decreased 2.0 and 2.2 g kg−1 root dry weight for cvs Bun-long and Lehua maoli, respectively. The greater concentrations of oxalate exuded from taro roots compared with the decrease in water-soluble oxalate levels within taro roots might have been caused by differences in the size of the root systems or to de novo synthesis of oxalate.
To our knowledge, this report is the first to demonstrate that a non-Al-accumulating crop species exudes oxalate into the rhizosphere in response to excess Al. Our findings demonstrate that there is no linkage between exudation of oxalate and internal accumulation of an Al-oxalate complex. Because high levels of Al in the edible portions of a plant may be detrimental to the health of consuming humans or animals, it is important to establish that exudation of oxalate by roots does not result in increased accumulation of Al in shoots.
Our data are consistent with the hypothesis that oxalate is excreted by taro into the rhizosphere, where it detoxifies Al, and that an additional mechanism of Al tolerance is found at very high Al levels. Our tissue-culture assay provides an ideal method to further characterize these Al-tolerance mechanisms, as shown by the correlation between Al tolerance of taro cultivars in soil and the tissue-culture medium.
ACKNOWLEDGMENTS
The authors thank Dr. Michael Tanabe (University of Hawaii-Hilo) for helpful suggestions and sharing of equipment, Joanne Lichty for generous sharing of laboratory facilities, and Drs. William Sakai (University of Hawaii-Hilo) and Chung-shih Tang for useful comments and insights concerning these experiments.
Footnotes
This research was supported by the U.S. Department of Agriculture under the Cooperative State Research, Education and Extension Service (special grant agreement no. 94-34135-0646), managed by the Pacific Basin Administrative Group. This is journal series no. 4363 of the College of Tropical Agriculture and Human Resources, University of Hawaii-Manoa.
LITERATURE CITED
- Basu U, Godbold D, Taylor GJ. Aluminum resistance in Triticum aestivum associated with enhanced exudation of malate. J Plant Physiol. 1994;144:747–753. [Google Scholar]
- Calisay MG (1996) Differential response of taro (Colocasia esculenta L.) cultivars to aluminum toxicity. PhD thesis. University of Hawaii, Honolulu
- de la Fuente JM, Ramirez-Rodriguez V, Cabrera-Ponce JL, Herrera-Estrella L. Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science. 1997;276:1566–1568. doi: 10.1126/science.276.5318.1566. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 1993;103:695–702. doi: 10.1104/pp.103.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy CD. Plant adaptation to acid, aluminum-toxic soils. Commun Soil Sci Plant Anal. 1988;19:959–987. [Google Scholar]
- Hue NV, Craddock GR, Adams F. Effect of organic acids on aluminum toxicity in subsoils. Soil Sci Soc Am J. 1986;50:28–34. [Google Scholar]
- Kamprath EJ (1984) Crop responses to lime on soils in the tropics. In F Adams, ed, Soil Acidity and Liming, Ed 2. Agronomy Monograph No. 12. American Society of Agronomy, Madison, WI, pp 349–368
- Libert B, Franceschi VR. Oxalate in crop plants. J Agric Food Chem. 1987;35:926–938. [Google Scholar]
- Lipton DS, Blanchar RW, Blevins DG. Citrate, malate, and succinate concentration in exudates from P-sufficient and P-stressed Medicago sativa L. seedlings. Plant Physiol. 1987;85:315–317. doi: 10.1104/pp.85.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Matsumoto H. Detoxifying aluminum with buckwheat. Nature. 1997;390:569–570. [Google Scholar]
- Miyasaka SC, Buta JG, Howell RK, Foy CD. Mechanism of aluminum tolerance in snapbeans. Plant Physiol. 1991;96:737–743. doi: 10.1104/pp.96.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka SC, Webster CM, Hue NV. Differential response of two taro cultivars to aluminum. I. Plant growth. Commun Soil Sci Plant Anal. 1993a;24:1197–1211. [Google Scholar]
- Miyasaka SC, Webster CM, Okazaki EN. Differential response of two taro cultivars to aluminum. II. Plant mineral concentrations. Commun Soil Sci Plant Anal. 1993b;24:1213–1229. [Google Scholar]
- Ownby JD, Popham HR. Citrate reverses the inhibition of wheat root growth caused by aluminum. J Plant Physiol. 1989;135:588–591. [Google Scholar]
- Pellet DM, Grunes DL, Kochian LV. Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.) Planta. 1995;196:788–795. [Google Scholar]
- Pellet DM, Papernik LA, Kochian LV. Multiple aluminum-resistance mechanisms in wheat: roles of root apical phosphate and malate exudation. Plant Physiol. 1996;112:591–597. doi: 10.1104/pp.112.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Malate efflux from root apices and tolerance to aluminum are highly correlated in wheat. Aust J Plant Physiol. 1995;22:531–536. [Google Scholar]
- Shuman LM, Wilson DO, Ramseur EL. Amelioration of aluminum toxicity to sorghum seedlings by chelating agents. J Plant Nutr. 1991;14:119–128. [Google Scholar]
- Standal BR (1983) Nutritive value. In JK Wang, ed, Taro: A Review of Colocasia esculenta and Its Potentials. University of Hawaii Press, Honolulu, pp 141–147
- Statistical Analysis Systems Institute (1982) SAS User's Guide: Statistics. SAS Institute, Cary, NC
- Zindler-Frank E. Oxalate biosynthesis in relation to photosynthetic pathway and plant productivity: a survey. Z Pflanzenphysiol. 1976;80:1–13. [Google Scholar]