Abstract
The small RNA PcrZ (photosynthesis control RNA Z) of the facultative phototrophic bacterium Rhodobacter sphaeroides is induced upon a drop of oxygen tension with similar kinetics to those of genes for components of photosynthetic complexes. High expression of PcrZ depends on PrrA, the response regulator of the PrrB/PrrA two-component system with a central role in redox regulation in R. sphaeroides. In addition the FnrL protein, an activator of some photosynthesis genes at low oxygen tension, is involved in redox-dependent expression of this small (s)RNA. Overexpression of full-length PcrZ in R. sphaeroides affects expression of a small subset of genes, most of them with a function in photosynthesis. Some mRNAs from the photosynthetic gene cluster were predicted to be putative PcrZ targets and results from an in vivo reporter system support these predictions. Our data reveal a negative effect of PcrZ on expression of its target mRNAs. Thus, PcrZ counteracts the redox-dependent induction of photosynthesis genes, which is mediated by protein regulators. Because PrrA directly activates photosynthesis genes and at the same time PcrZ, which negatively affects photosynthesis gene expression, this is one of the rare cases of an incoherent feed-forward loop including an sRNA. Our data identified PcrZ as a trans acting sRNA with a direct regulatory function in formation of photosynthetic complexes and provide a model for the control of photosynthesis gene expression by a regulatory network consisting of proteins and a small noncoding RNA.
Keywords: α-proteobacteria, protochlorophyllide reductase
It has emerged over the last decade that small noncoding RNAs (sRNAs) have an important impact on gene regulation in bacteria. They affect gene expression by altering the rate of translation and/or the stability of their target mRNAs (1). sRNAs help the bacteria to adapt to various stresses and control metabolic functions, growth, toxin production, or virulence. Photosynthesis is an important process for ATP production in many bacteria. The simultaneous presence of pigments and oxygen can, however, cause the generation of reactive oxygen species, which makes a balanced control of the formation of photosynthetic complexes necessary. Our data reveal that in the anoxygenic phototrophic α-proteobacterium Rhodobacter sphaeroides the small RNA PcrZ (photosynthesis control RNA Z) affects formation of photosynthetic complexes by targeting mRNAs encoding pigment-binding proteins and enzymes for bacteriochlorophyll synthesis.
R. sphaeroides is a facultative photosynthetic bacterium, which forms photosynthetic complexes in response to environmental stimuli. Under high oxygen tension it performs aerobic respiration and expression of photosynthesis genes is repressed. The most important proteins involved in regulation of photosynthesis genes and their action in R. sphaeroides are shown in Fig. 1. The repressor PpsR binds to the upstream region of photosynthesis genes and prevents transcription (2–4). When oxygen tension decreases, the AppA antirepressor protein binds to PpsR and releases it from the DNA, allowing transcription (3, 5, 6). AppA can sense blue light through its N-terminal BLUF domain (5, 7) and redox signals through a heme that is bound to the SCHIC domain (2, 8). As long as oxygen is available, Rhodobacter performs aerobic respiration. However, at intermediate oxygen tension blue light, even at low intensities, prevents AppA from binding to PpsR and photosynthesis genes are repressed (8, 9), reducing the accumulation of the harmful singlet oxygen. The PrrB/PrrA two-component system senses the electron transport through the cbb3 oxidase and induces transcription of photosynthesis genes at very low oxygen tension or in the absence of oxygen (5, 10–13). Furthermore, the FnrL protein activates some photosynthesis genes at low oxygen tension (13) and the PpaA regulator activates some photosynthesis genes under aerobic conditions (14). More recently CryB, a member of a newly described cryptochrome family (15), was shown to affect expression of photosynthesis genes in R. sphaeroides and to interact with AppA (16, 17). Remarkably, the different signaling pathways for control of photosynthesis genes are also interconnected, e.g., the appA gene is controlled by PrrA (18, 19) and a PpsR binding site is located in the ppaA promoter region (20). Thus, a complex network comprising several regulatory proteins controls the formation of the photosynthetic apparatus.
Fig. 1.
Involvement of PcrZ in the regulatory network controlling photosynthesis gene expression. At low oxygen tension PrrA and FnrL activate expression of photosynthesis (PS) genes and at the same time expression of PcrZ. PcrZ counteracts the activation by PrrA and FnrL and represses photosynthesis genes, indicating an incoherent feed-forward loop. Also the appA gene is repressed by PcrZ, reducing the amount of AppA, which further leads to a stronger repression of photosynthesis genes by PpsR.
Recently, several sRNAs were identified in R. sphaeroides, some of which are specifically induced or processed in response to superoxide or singlet oxygen (21, 22). Among the sRNAs identified by RNAseq was PcrZ (RSs2430), which has homologs in all sequenced R. sphaeroides species, but not in other species with sequenced genomes. Here we show that PcrZ has an important role in balanced formation of the photosynthetic apparatus of R. sphaeroides.
Results
Oxygen-Dependent Expression of PcrZ Is Regulated by PrrA.
PcrZ is transcribed from the intergenic region between RSP_0819 encoding a DEAD/DEAH box helicase and RSP_6134 encoding a hypothetical protein (Fig. 2A). The primary transcript is 136 nt in size (21). The levels of the PcrZ primary transcript increase after a shift from high to low oxygen tension by a factor of about 4 (Fig. 2B). A similar oxygen-dependent increase was previously observed for the polycistronic puf and puc transcripts in Rhodobacter species (8, 23). The primary puf transcript encodes proteins for the formation of the reaction center and light-harvesting I (LHI) complex, and the puc operon encodes proteins for the formation of the LHII complex. In addition to the primary PcrZ transcript, processing products were observed that accumulated with increasing incubation time at low oxygen tension (Fig. 2B). After 24 h (1,440 min) of incubation the level of the full-length transcript was even lower than before the transition to low oxygen and a small processing product of about 50 nt had strongly accumulated.
Fig. 2.
PrrA regulates oxygen-dependent expression of PcrZ. (A) Genetic context of the pcrZ gene. The gene RSP_0819 encoding a DEAD/DEAH box helicase ends 91 nt upstream of PcrZ and is preceded by a PrrA consensus promoter. The hypothetical protein RSP_6134 starts 134 nt downstream and is preceded by a FnrL consensus promoter. (B) PrrA-dependent expression of PcrZ shown by Northern blot analysis of total RNA, isolated from R. sphaeroides WT 2.4.1 and PrrA2 (prrA−). Cultures were grown under high-oxygen conditions (HO) and shifted to low-oxygen conditions (LO) at t0. The oxygen tension dropped from 8 to 0.5 mg/L during the first 30 min. A PcrZ-specific oligonucleotide was used as a probe for hybridization. Detection of PcrZ primary transcript (136 nt) and processed fragments (127 nt, 73 nt, and 51–56 nt) is indicated. 5S rRNA served as a loading control.
In R. sphaeroides transcription of photosynthesis genes at low oxygen tension is activated by the response regulator PrrA (24). A sequence with good similarity to the PrrA binding motif (20) is also present upstream of PcrZ (Fig. S1). To analyze the role of PrrA and other regulators of photosynthesis genes in PcrZ expression we performed Northern blots with RNA from strains lacking the regulatory proteins PrrA, PpsR, and FnrL (Fig. 2 and Fig. S2). Strains were grown under high-oxygen, low-oxygen, and phototrophic conditions. Interestingly the abundance and pattern of processing products varied under different growth conditions. Under phototrophic growth smaller processing products were more abundant than under growth in the presence of oxygen (Fig. S2). The lack of PpsR had only little effect on PcrZ expression, whereas lack of FnrL resulted in reduced PcrZ expression under low-oxygen conditions (Fig. S2). Strikingly, PcrZ levels were strongly reduced in the PrrA mutant under high and low oxygen tension (Fig. 2 and Fig. S2). This mutant strain does not grow under phototrophic conditions. These results demonstrate a strong activation of PcrZ transcription by PrrA at high- and low-oxygen conditions and also an activating influence of FnrL on PcrZ transcription under low oxygen tension. We also compared the kinetics of PcrZ and puc mRNA accumulation after reduction of oxygen tension in all three strains (Fig. S2). In the wild-type puc mRNA levels reach a maximum before PcrZ levels.
The binding of PrrA to the PcrZ upstream region was also demonstrated in vitro by gel retardation (Fig. S1). Recombinant PrrA isolated from Escherichia coli resulted in retardation of a DNA fragment spanning this upstream region (Fig. S1) and also bound to the puc promoter (RSP_0314 upstream region) with a known PrrA binding site (20) in the presence of unspecific competitor DNA.
Processing of PcrZ.
In a previous study, RNAseq indicated a size of 136 nt for PcrZ (21). Fig. 3A shows a secondary structure for PcrZ as predicted by Mfold (25). Northern blot analysis using probes against either the 5′ part of PcrZ or its 3′ part revealed that the small processing products are generated from the 5′ part (Fig. 3B). The RNAseq data clearly determined the 5′ end of the primary transcript. To verify its 3′ end and to characterize the processing products, we performed 3′ RACE. Determination of the 3′ end of the full-length transcript confirmed the size of 136 nt. One of the additional minor bands observed on Northern blots could be attributed to a 3′ end at position 127. Further 3′ ends were mapped to positions 73 (minor), 56, 53, and 51 as shown in Fig. 3A. Further minor bands are visible on Northern blots adjacent to these bands, indicating multiple 3′ ends around the determined positions. The 3′ ends at positions 73, 53, and 51 mapped to the basis of predicted stem-loop structures. This mapping may reflect the hindrance of a 3′–5′ exonucleolytic decay by these secondary structures.
Fig. 3.
The stable processing product of PcrZ stems from its 5′ end. (A) Predicted secondary structure of PcrZ by the Mfold program (25). Solid stars indicate the five different 3′ ends, identified by 3′ RACE. Hybridization sites of different oligonucleotide probes used for Northern blot analysis are displayed (5′ probe in gray and 3′ probe in black). (B) Northern blot analysis of total RNA, isolated from R. sphaeroides 2.4.1pRK4352 and 2.4.1pRKPcrZ. Cultures were grown under low-oxygen conditions to an OD660 of 0.8. Two different oligonucleotides, binding near the 5′ and 3′ ends of PcrZ, were used for hybridization. Detection of PcrZ primary transcript (136 nt) and processed fragments (127 nt, 73 nt, and 51–56 nt) is indicated. tmRNA (coding piece), 5S rRNA, and tRNA-Ala oligonucleotides served as internal size markers. (C) Quantification of Northern blot signals from strains 2.4.1pRK4352 (solid bars) and 2.4.1pRKPcrZ (open bars). RNA levels were calculated after normalizing PcrZ signal intensities to 5S rRNA signal intensities (B, 5′ probe). 2.4.1pRK4352 intensities were set to 1 and fold changes of 2.4.1pRKPcrZ were calculated relative to 2.4.1pRK4352.
Full-Length PcrZ but Not a Smaller Processing Product Affects Formation of the Photosynthetic Apparatus.
To learn more about the role of PcrZ we overexpressed this sRNA in R. sphaeroides wild type. Overexpression was confirmed by Northern blot (Fig. 3 B and C). Especially the processing products with sizes of 51–56 nt were much more abundant than in a control strain. The constitutive PcrZ overexpression strain 2.4.1pRKPcrZ showed a color, which was significantly lighter than that of the control strain 2.4.1pRK4352. This is documented by decreased absorbances of cell extracts (Fig. 4A) and by a total bacteriochlorophyll content, which was only about 22% of that of the control strain (Fig. 4B). A reduction of the levels of photosynthetic complexes and of the bacteriochlorophyll content upon PcrZ overexpression was also observed in a strain lacking Hfq (Fig. S3), implying that Hfq is not required for PcrZ function. The doubling time of the R. sphaeroides strain was not influenced by overexpressing PcrZ.
Fig. 4.
PcrZ reduces levels of photosynthetic complexes. (A) Absorption spectra of R. sphaeroides whole-cell extracts. Three independent cultures of each strain were grown under low-oxygen conditions to an OD660 of 0.8. The absorbance was measured from 500 nm to 900 nm. One representative spectrum of 2.4.1pRK4352 (solid dashed line), 2.4.1pRKPcrZ (shaded dashed line), and 2.4.1pRKPcrZ-51nt (shaded line) is shown. Peaks at 800 and 850 nm correspond to the light-harvesting complex II (B800–850) and the light-harvesting complex I (B870) of the photosynthetic apparatus. (B) Relative bacteriochlorophyll content of R. sphaeroides strains 2.4.1pRK4352 (solid bar), 2.4.1pRKPcrZ-51nt (shaded bar), and 2.4.1pRKPcrZ (open bar) grown under low-oxygen conditions to an OD660 of 0.8. The relative bacteriochlorophyll content was calculated on the basis of the absorbance at 770 nm after acetone-methanol (7:2) extraction of 4 mL cells, normalized to the OD660. Results from three independent experiments are shown with error bars depicting the SE of mean.
We also overexpressed the 51-nt processing fragment of PcrZ (Fig. S4) to see whether the effect on formation of photosynthetic complexes is caused by the full-length transcript or rather by the processing products, which accumulate over time. Spectra from strain 2.4.1pRKPcrZ-51nt were identical to those of the control strain (Fig. 4A) and only slight variation in the bacteriochlorophyll content was observed (Fig. 4B). We conclude that the full-length PcrZ transcript is responsible for repression of photosynthesis complex formation and therefore reflects the functional unit.
Total RNA from the control strain 2.4.1pRK4352 and from the PcrZ overexpression strain was used to study the effect of PcrZ on the transcriptome of R. sphaeroides. Microarrays (Agilent) were designed (26) that comprise DNA oligonucleotides representing 4,372 ORFs and 144 genes for sRNAs that were identified in our group (21, 22). The microarray analysis revealed that PcrZ affects the expression of a small subset of genes (4.8%), whereas 95.2% of the genes did not pass the selection criteria (Materials and Methods). The majority of genes affected by PcrZ showed lower expression levels in the overexpression strain (217 genes with an expression change of ≤0.7-fold). Only 7 genes showed a higher expression (≥1.75), when PcrZ was overexpressed. This was the case for RSP_4246, a SinR-like protein of unknown function in R. sphaeroides, for three transporters (RSP_1613, RSP_3297, and RSP_3386) and for RSP_7386 encoding asparagine synthase. For most of the affected genes the change in expression was quite small (0.5- to 2-fold). Many of the down-regulated genes are involved in the formation of the photosynthetic apparatus (Table 1). These genes include the puf and puc operons, several genes for bacteriochlorophyll synthesis, the crtA and crtD genes for carotenoid synthesis, and appA. Because AppA functions as an antagonist of PpsR, which represses photosynthesis genes, a lower expression of the appA gene could indirectly cause a stronger repression of other photosynthesis genes by PpsR. Additional genes with lower expression in the strain with increased PcrZ levels encode cytochrome c2; cytochrome b562; and transcriptional regulators of the LuxR, TetR, and LysR families. Both cytochromes are involved in cyclic photosynthetic electron transport.
Table 1.
Selection of PcrZ-responsive genes in R. sphaeroides
| Category and RSP no.* | Gene | Ratio† | Description |
| Photosynthesis | |||
| RSP_0294 | 0.53 | Magnesium-protoporphyrin IX monomethyl ester cyclase | |
| RSP_1574 | 0.61 (0.47) | Cytochrome b562 | |
| RSP_1565 | appA | 0.65 (0.37) | AppA, antirepressor of PpsR, sensor of blue light |
| RSP_0261 | bchY | 0.74 (0.28) | Chlorophyllide reductase |
| RSP_0280 | bchJ | 0.49 | Bacteriochlorophyll synthase, 23-kDa subunit |
| RSP_0272 | crtA | 0.59 | Spheroidene monooxygenase |
| RSP_0266 | crtD | 0.58 | Methoxyneurosporene dehydrogenas |
| RSP_0296 | cycA | 0.65 | Cytochrome c2 |
| RSP_0283 | ppaA | 0.67 (0.46) | Regulatory protein, PpaA |
| RSP_6256 | pucA | 0.58 | LHII α, light-harvesting B800/850 protein |
| RSP_6158 | puc2A | 0.68 (0.52) | Light-harvesting complex α-subunit |
| RSP_0273 | bchI | 0.57 | Magnesium chelatase, ChlI subunit |
| RSP_0275 | bchO | 0.52 (0.52) | Magnesium chelatase |
| RSP_0291 | puhA | 0.75 | Reaction center H protein |
| RSP_0290 | 0.61 | Light-harvesting 1 (B870) complex assembly | |
| RSP_0289 | bchM | 0.62 | Mg-protoporphyrin IX methyl transferase |
| RSP_0288 | bchL | 0.68 | Light-independent protochlorophyllide reductase iron protein |
| RSP_0286 | bchB | 0.63 | Light-independent protochlorophyllide reductase subunit B |
| RSP_0285 | bchN | 0.78 (0.43) | Light-independent protochlorophyllide reductase subunit N |
| RSP_0314 | pucB | 0.62 | LHII β, light-harvesting B800/850 protein |
| RSP_0315 | pucC | 0.49 | Light-harvesting 1 (B870) complex assembly |
| RSP_0258 | pufA | 0.55 | LHI α, light-harvesting B875 protein |
| RSP_6108 | pufB | 0.49 | LHI β, light-harvesting B875 subunit |
| Transcriptional regulators | |||
| RSP_1435 | 0.62 | Regulatory protein TetR family | |
| RSP_2027 | 0.68 | Transcriptional regulator LysR family | |
| RSP_3324 | 0.53 (0.49) | Transcriptional regulator LuxR family | |
| RSP_4246 | 2.49 (5.71) | Putative SinR-like protein | |
| Transporter | |||
| RSP_1613 | 1.76 | TRAP-T family transporter, DctP subunit | |
| RSP_3297 | 1.73 | ABC branched-chain amino acid transporter | |
| RSP_3386 | 1.88 | TRAP-T family transporter, periplasmic binding protein | |
| Replication | |||
| RSP_0674 | 0.62 | DNA polymerase III subunit-δ | |
| Metabolism | |||
| RSP_7386 | 1.98 | Asparagine synthase (glutamine hydrolyzing) |
*Genes with RSP numbers in boldface type are a possible PcrZ target as predicted by IntaRNA (27). RSP_0273-0275, RSP_0285-0291, RSP_0314-0315, and RSP_6108-0258 respectively belong to same operons.
†Expression level of selected genes (2.4.1pRKPcrZ vs. 2.4.1pRK4352) is shown. Numbers defined by underline did not pass our selection criteria (≥1.75 or ≤0.7). Numbers in parentheses depict the results from real-time RT-PCR validations.
Surprisingly, many genes for tRNAs or sRNAs seemed to be less expressed in the overexpression strain. This was also true for PcrZ itself, which we demonstrated to be overexpressed by Northern blots. We conclude that the microarray data did not give reliable results for small structured RNAs, maybe due to different efficiencies of the two labels (Cy3/Cy5). This assumption was also supported by an analysis of total RNA on urea gels. Despite the lower expression levels as indicated in the microarray, there was no significant change in the level of tRNAs (Fig. S5).
We applied real-time RT-PCR for selected genes to verify the microarray data. The lower expression levels of appA, ppaA, puc2A, bchO, bchY, bchN, RSP_1574, and RSP_3324 as well as the higher expression level of RSP_4246 in strain 2.4.1pRKPcrZ were confirmed. The fold change was mostly larger in the real-time RT-PCR than in the microarray dataset (Table 1).
Target Prediction and Confirmation.
We assumed that PcrZ may directly target mRNAs for photosynthesis genes. An IntaRNA search (27, 28) suggested a base pairing between PcrZ and several mRNAs that showed altered expression levels in the microarray analysis (Table S1). When only putative targets with predicted energy values of less than −16 kcal/mol were considered, the predicted interacting nucleotides mapped preferentially to the three stem-loop structures in the 5′ part of PcrZ. For one predicted target (bchN) the region of base pairing to PcrZ included the translational start site, and for others the predicted site of hybridization was in the coding region (e.g., puc2A). Targeting of the coding sequence was previously demonstrated for MicC in Salmonella (29). To test the putative interaction of PcrZ with some of these mRNAs we established an in vivo reporter system for R. sphaeroides, which is also applicable to other α-proteobacteria. In this two-plasmid system potential target mRNAs are translationally fused to the lacZ gene on plasmid pPHU4352, thereby expressing the target constitutively from a 16S rRNA promoter. The sRNA counterpart is expressed from the identical promoter on plasmid pBBR4352. We used this system to investigate sRNA–mRNA interaction more directly and to prove that altered mRNA levels, as monitored by microarray, impact translation.
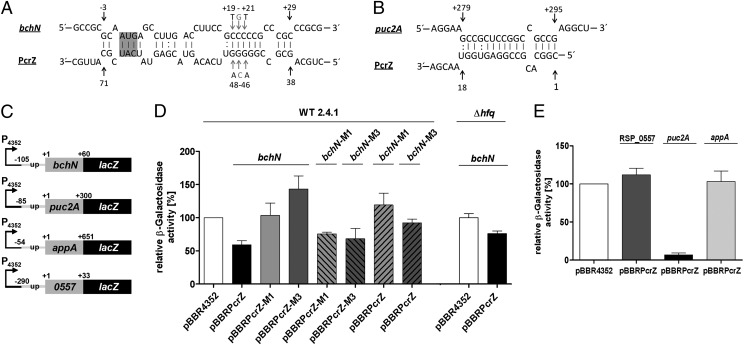
We chose the mRNA bchN for the in vivo assay with a predicted interaction site from −3 to +29 with respect to its start codon (Fig. 5A). bchN is part of the bchFNBHL operon and encodes the N-subunit of the light-independent protochlorophyllide oxidoreductase (DPOR). This DPOR complex is responsible for protochlorophyllide reduction to chlorophyllide (30, 31). Fig. 5D clearly shows that β-galactosidase activity from the bchN::lacZ reporter plasmid decreases when PcrZ is overexpressed on plasmid pBBRPcrZ. Insertion of single (M1) or triple (M3) base exchanges into the predicted bchN binding region of PcrZ at positions 47 or 46–48, respectively, led to β-galactosidase activities comparable to those in the pBBR4352 control. This result indicates a hindrance of PcrZ-bchN interaction. When compensatory M1 or M3 mutations are inserted into bchN at positions +20 or +19–21, respectively, binding to the corresponding mutated PcrZ counterparts is restored. This restoration is reflected by decreased β-galactosidase activity. When the reporter constructs harboring the M1 or M3 mutations were present together with wild-type PcrZ, the β-galactosidase level was not significantly lower than in the control. To further elucidate the role of Hfq in PcrZ function we also tested the effect of PcrZ on expression of the bchN::lacZ reporter in an hfq− background. The presence of PcrZ still resulted in reduced β-galactosidase activity, but the reduction was not as pronounced as in the wild-type strain.
Fig. 5.
PcrZ directly targets bchN and puc2A. (A) Predicted PcrZ-bchN duplex structure. Single- and triple-nucleotide exchanges of PcrZ and bchN are indicated by shaded arrows. Position +1 of the mRNA refers to the A of the translational start codon (shaded). For PcrZ position 1 refers to the 5′ nucleotide. (B) Predicted PcrZ-puc2A duplex structure. (C) Schematic picture of mRNA::lacZ fusions. 16S rRNA promoter is depicted as P4352. “+1” indicates the first nucleotide of the corresponding start codon. (D) Relative β-galactosidase activity of the lacZ-based in vivo reporter system. Activity in the control strains containing the bchN::lacZ, bchN-M1::lacZ, or bchN-M3::lacZ reporter fusions and plasmid pBBR4352 is set to 100% (open bar). Activities for all other combinations are calculated in relation to their respective control. Activity in the strain containing the reporter fusion and plasmid pBBRPrcZ is indicated by a solid bar. Bars with light shading and bars with dark shading represent strains overexpressing PrcZ with single (M1) or triple (M3) mutations, respectively. Compensatory mutations of bchN in the reporter plasmid are represented by dashed bars. (E) Relative β-galactosidase activity of the lacZ-based in vivo reporter system. Activity in the control strains containing the reporter fusion and plasmid pBBR4352 is set to 100% (open bar). Activity of strains with plasmid pBBRPcrZ and the RSP_0557::lacZ reporter (bar with dark shading), the puc2A::lacZ reporter (solid bar), and the appA::lacZ reporter (bar with light shading) is shown. For each strain, three independent biological experiments with technical duplicates were performed. SDs are depicted by error bars. β-Galactosidase assays were performed as described previously (42).
Furthermore, we tested the puc2A target, which was predicted to interact from position +279 to +295 (relative to the start codon) with PcrZ (Fig. 5B). puc2A is part of the puc2BA operon that encodes α- and β-subunits of the light-harvesting complex II (32). The puc2A::lacZ reporter plasmid showed a strong reduction of β-galactosidase activity in the presence of elevated PcrZ levels (Fig. 5E). This reduction identifies puc2A mRNA as a direct target of PcrZ. AppA was another putative direct interaction partner of PcrZ as predicted by IntaRNA (Table S1). However, in our reporter system PcrZ had no significant effect on expression of the appA::lacZ reporter, indicating that the effect on appA mRNA levels is indirect.
The RSP_0557::lacZ reporter plasmid served as a nontarget control and accordingly is not affected by PcrZ levels. We also tested a sinR::lacZ reporter, but β-galactosidase activity was too low to give reliable data.
To test whether the amount of target mRNAs influences PcrZ processing, we also performed Northern blot analyses with RNA isolated from R. sphaeroides strain App11 and Rhodobacter capsulatus strain 37b4 expressing PcrZ from a plasmid. App11 has the appA gene deleted and due to the strong repression by PpsR expresses photosynthesis genes at strongly reduced levels (6). R. capsulatus wild-type strains do not harbor a gene for PcrZ. In both strains we observed the identical processing products and the same extent of PcrZ processing as in R. sphaeroides strain 2.4.1 (Fig. S3).
Discussion
Facultative photosynthetic bacteria like R. sphaeroides show a high metabolic versatility and respond to environmental impacts by adapting their metabolism. Formation of photosynthetic complexes is induced by decreasing oxygen tension and is inhibited by light at intermediate oxygen tension. The regulation of photosynthesis genes in Rhodobacter has been intensively studied in the past and a regulatory network involving several regulatory proteins and parts of the electron transport chain emerged (10, 13, 33–35), which is partly depicted in Fig. 1. Our results reveal that this regulatory network also comprises the small RNA PcrZ and that the full-length PcrZ RNA directly targets photosynthesis genes.
Our data show that PcrZ undergoes processing leading to stable products, which are derived from the 5′ part of the sRNA. Although most interactions of PcrZ with its targets are predicted to involve this 5′ part (Table S1), the fifty-one 5′ nucleotides of PcrZ are not sufficient for its biological function, suggesting that more nucleotides contribute to interaction with the target mRNAs, as predicted for bchN. It is also conceivable that additional nucleotides are required for interaction with a protein that strengthens the sRNA–mRNA interaction. A previous study revealed that PcrZ does not interact with Hfq, which serves this function in many known sRNA–mRNA interactions (22). The Hfq-independent function of PcrZ was confirmed by the fact that PcrZ also reduces pigment protein complexes in a mutant lacking Hfq. Furthermore, an effect of PcrZ on bchN::lacZ expression was observed in our reporter system also in the absence of Hfq.
Because the 51-nt PcrZ processing product does not affect formation of photosynthetic complexes, processing of PcrZ releases its inhibitory effect on photosynthesis gene expression. Interestingly, the processing pattern of PcrZ differs under different growth conditions, but the reasons for this remain to be elucidated as well as the enzymes involved in processing. The extent of PcrZ processing is not determined by the amount of target mRNAs as demonstrated by expressing PcrZ in an R. sphaeroides appA mutant or in R. capsulatus. It should be noted that the amount of the full-length PcrZ transcript is low under anaerobic, phototrophic growth conditions, when high expression of photosynthesis genes is required for ATP generation and there is no risk of production of singlet oxygen.
Our data revealed an influence of the FnrL regulatory protein on PcrZ expression, although no sequence with good similarity to known FnrL binding sites (20) is present upstream of the pcrZ gene. Whereas the level of PcrZ is similar in the wild type and the FnrL mutant under high oxygen tension, decrease of oxygen tension failed to significantly induce PcrZ in the mutant. How FnrL influences PcrZ expression remains to be elucidated. A strain lacking the transcriptional repressor PpsR showed similar expression levels and induction of PcrZ to those in the wild type, excluding a major role of the AppA/PpsR system on PcrZ expression.
Like many of the photosynthesis genes PcrZ is under control of a PrrA-dependent promoter. After reduction of oxygen tension the sensor kinase PrrB phosphorylates PrrA (36, 37), which consequently activates its targets genes. The activation of photosynthesis genes is counteracted by the simultaneously increased transcription of PcrZ. PcrZ decreases expression of some mRNAs for photosynthesis genes and also decreases appA transcript levels, which consequently leads to stronger repression of photosynthesis genes by PpsR. The effect of PcrZ on transcript levels of target mRNAs is most likely due to effects on stability but needs further investigation. Whereas our data demonstrate that bchN and puc2A are direct targets of PcrZ, this was not confirmed for appA, suggesting that PcrZ affects appA mRNA levels indirectly. We conclude that the function of PcrZ is to balance the response of photosynthesis genes to redox signals. Such fine-tuning of regulatory networks by the action of sRNAs is widely used by bacteria, e.g., for regulation of iron homeostasis or carbon metabolism (38). Because PrrA directly activates photosynthesis genes and at the same time PcrZ, which negatively affects photosynthesis gene expression, this action constitutes an incoherent feed-forward loop as frequently found in bacteria (39). However, until now examples for such regulatory loops including an sRNA have been rare (40). Two dynamical features of incoherent feed-forward loops were proposed on the basis of mathematical modeling: a transient pulse of gene expression and acceleration of the dynamics of target genes. Indeed after a transition from high to low oxygen tension puf and puc genes show a transient strong increase in expression. Fig. 1 integrates PcrZ into the regulatory network controlling photosynthesis genes in R. sphaeroides. Remarkably, PcrZ is exclusively found in species of R. sphaeroides and even R. capsulatus, which shares many of the regulatory proteins to control photosynthesis genes, lacks a homolog of this sRNA. It appears that early in evolution facultative photosynthetic bacteria acquired proteins for a major control of photosynthesis genes but that at least some mechanisms for fine-tuning developed later in evolution and may be species specific. Despite the fact that PcrZ is most likely restricted to R. sphaeroides, it can be expected that sRNAs play an important role in controlling the formation of the photosynthetic apparatus also in other bacteria.
Materials and Methods
Bacterial strains used in this study are listed in Table S2. Details on their construction are given in SI Materials and Methods. R. sphaeroides and R. capsulatus strains were cultivated at 32 °C in a malate minimal-salt medium (41) under continuous shaking at 140 rpm. For phototrophic growth, strains were cultured without agitation in sealed flat glass bottles filled to the top with medium and illuminated with 60 W⋅m−2 of white light. Conditions of high oxygen tension (8 mg/L soluble O2) were applied by cultivation of strains in beaked flasks. For oxygen-shift experiments, R. sphaeroides precultures were grown under high-oxygen conditions overnight to an OD660 of 0.8–0.9. Precultures were diluted to an OD660 of 0.4 and grown under low oxygen tension (0.5 mg/L soluble O2). For the lacZ-based in vivo reporter system, all cultures were grown under high oxygen tension. E. coli strains (Table S2) were cultured in Luria–Bertani broth at 37 °C with continuous shaking at 180 rpm.
Microarray analysis was performed as described before (26). In brief, total RNA of strains 2.4.1pRKPcrZ and 2.4.1pRK4352 was chemically labeled with Cy3 and Cy5, respectively. Multiarray analysis was performed with the Bioconductor package Limma for R. On the basis of calculated MA plots, genes were considered reliable if the average signal intensity [A-value: 1/2 log2(Cy3 × Cy5)] was ≥ 12. To filter out potentially insignificant changes among genes that passed the reliability criterion, a cutoff value was applied; i.e., those genes were retained whose average expression value of 2.4.1pRKPcrZ (a) compared with the average value of the control treatment 2.4.1pRK4352 (b) was either a ≥ 1.75b or a ≤ 0.7b. Microarray data are deposited in the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/; accession no. GSE37381).
For description of additional procedures and information on strains, oligonucleotides, and plasmids see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Fabian Billenkamp for help with construction of the in vivo reporter system and for guidance through the IntaRNA program and Kerstin Haberzettl, Angelika Balzer, and Katrin Müller for technical assistance. We also thank Gregor Langen for support on the Agilent scanner. This work was supported by the Deutsche Forschungsgemeinschaft.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE37381).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207067109/-/DCSupplemental.
References
- 1.Waters LS, Storz G. Regulatory RNAs in bacteria. Cell. 2009;136:615–628. doi: 10.1016/j.cell.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moskvin OV, Kaplan S, Gilles-Gonzalez MA, Gomelsky M. Novel heme-based oxygen sensor with a revealing evolutionary history. J Biol Chem. 2007;282:28740–28748. doi: 10.1074/jbc.M703261200. [DOI] [PubMed] [Google Scholar]
- 3.Masuda S, Bauer CE. AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacter sphaeroides. Cell. 2002;110:613–623. doi: 10.1016/s0092-8674(02)00876-0. [DOI] [PubMed] [Google Scholar]
- 4.Gomelsky M, Kaplan S. Genetic evidence that PpsR from Rhodobacter sphaeroides 2.4.1 functions as a repressor of puc and bchF expression. J Bacteriol. 1995;177:1634–1637. doi: 10.1128/jb.177.6.1634-1637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braatsch S, Gomelsky M, Kuphal S, Klug G. A single flavoprotein, AppA, integrates both redox and light signals in Rhodobacter sphaeroides. Mol Microbiol. 2002;45:827–836. doi: 10.1046/j.1365-2958.2002.03058.x. [DOI] [PubMed] [Google Scholar]
- 6.Gomelsky M, Kaplan S. appA, a novel gene encoding a trans-acting factor involved in the regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1995;177:4609–4618. doi: 10.1128/jb.177.16.4609-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomelsky M, Klug G. BLUF: A novel FAD-binding domain involved in sensory transduction in microorganisms. Trends Biochem Sci. 2002;27:497–500. doi: 10.1016/s0968-0004(02)02181-3. [DOI] [PubMed] [Google Scholar]
- 8.Han Y, Meyer MH, Keusgen M, Klug G. A haem cofactor is required for redox and light signalling by the AppA protein of Rhodobacter sphaeroides. Mol Microbiol. 2007;64:1090–1104. doi: 10.1111/j.1365-2958.2007.05724.x. [DOI] [PubMed] [Google Scholar]
- 9.Metz S, Jäger A, Klug G. In vivo sensitivity of blue-light-dependent signaling mediated by AppA/PpsR or PrrB/PrrA in Rhodobacter sphaeroides. J Bacteriol. 2009;191:4473–4477. doi: 10.1128/JB.00262-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Happ HN, Braatsch S, Broschek V, Osterloh L, Klug G. Light-dependent regulation of photosynthesis genes in Rhodobacter sphaeroides 2.4.1 is coordinately controlled by photosynthetic electron transport via the PrrBA two-component system and the photoreceptor AppA. Mol Microbiol. 2005;58:903–914. doi: 10.1111/j.1365-2958.2005.04882.x. [DOI] [PubMed] [Google Scholar]
- 11.Oh JI, Kaplan S. The cbb3 terminal oxidase of Rhodobacter sphaeroides 2.4.1: Structural and functional implications for the regulation of spectral complex formation. Biochemistry. 1999;38:2688–2696. doi: 10.1021/bi9825100. [DOI] [PubMed] [Google Scholar]
- 12.Kim YJ, et al. Dominant role of the cbb3 oxidase in regulation of photosynthesis gene expression through the PrrBA system in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 2007;189:5617–5625. doi: 10.1128/JB.00443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeilstra-Ryalls J, et al. Control of photosystem formation in Rhodobacter sphaeroides. J Bacteriol. 1998;180:2801–2809. doi: 10.1128/jb.180.11.2801-2809.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomelsky L, et al. Identification and in vivo characterization of PpaA, a regulator of photosystem formation in Rhodobacter sphaeroides. Microbiology. 2003;149:377–388. doi: 10.1099/mic.0.25972-0. [DOI] [PubMed] [Google Scholar]
- 15.Geisselbrecht Y, et al. CryB from Rhodobacter sphaeroides: A unique class of cryptochromes with new cofactors. EMBO Rep. 2012;13:223–229. doi: 10.1038/embor.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrischk AK, et al. A cryptochrome-like protein is involved in the regulation of photosynthesis genes in Rhodobacter sphaeroides. Mol Microbiol. 2009;74:990–1003. doi: 10.1111/j.1365-2958.2009.06912.x. [DOI] [PubMed] [Google Scholar]
- 17.Metz S, et al. Interaction of two photoreceptors in the regulation of bacterial photosynthesis genes. Nucleic Acids Res. 2012;40:5901–5909. doi: 10.1093/nar/gks243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eraso JM, et al. Role of the global transcriptional regulator PrrA in Rhodobacter sphaeroides 2.4.1: Combined transcriptome and proteome analysis. J Bacteriol. 2008;190:4831–4848. doi: 10.1128/JB.00301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomelsky L, et al. Hierarchical regulation of photosynthesis gene expression by the oxygen-responsive PrrBA and AppA-PpsR systems of Rhodobacter sphaeroides. J Bacteriol. 2008;190:8106–8114. doi: 10.1128/JB.01094-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao L, et al. Combining microarray and genomic data to predict DNA binding motifs. Microbiology. 2005;151:3197–3213. doi: 10.1099/mic.0.28167-0. [DOI] [PubMed] [Google Scholar]
- 21.Berghoff BA, Glaeser J, Sharma CM, Vogel J, Klug G. Photooxidative stress-induced and abundant small RNAs in Rhodobacter sphaeroides. Mol Microbiol. 2009;74:1497–1512. doi: 10.1111/j.1365-2958.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- 22.Berghoff BA, et al. Contribution of Hfq to photooxidative stress resistance and global regulation in Rhodobacter sphaeroides. Mol Microbiol. 2011;80:1479–1495. doi: 10.1111/j.1365-2958.2011.07658.x. [DOI] [PubMed] [Google Scholar]
- 23.Gregor J, Zeller T, Balzer A, Haberzettl K, Klug G. Bacterial regulatory networks include direct contact of response regulator proteins: Interaction of RegA and NtrX in Rhodobacter capsulatus. J Mol Microbiol Biotechnol. 2007;13:126–139. doi: 10.1159/000103604. [DOI] [PubMed] [Google Scholar]
- 24.Eraso JM, Kaplan S. prrA, a putative response regulator involved in oxygen regulation of photosynthesis gene expression in Rhodobacter sphaeroides. J Bacteriol. 1994;176:32–43. doi: 10.1128/jb.176.1.32-43.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peuser V, Metz S, Klug G. Response of the photosynthetic bacterium Rhodobacter sphaeroides to iron limitation and the role of a Fur orthologue in this response. Environ Microbiol Rep. 2011;3:397–404. doi: 10.1111/j.1758-2229.2011.00245.x. [DOI] [PubMed] [Google Scholar]
- 27.Smith C, Heyne S, Richter AS, Will S, Backofen R. Freiburg RNA Tools: A web server integrating INTARNA, EXPARNA and LOCARNA. Nucleic Acids Res. 2010;38((Web Server issue)):W373–W377. doi: 10.1093/nar/gkq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busch A, Richter AS, Backofen R. IntaRNA: Efficient prediction of bacterial sRNA targets incorporating target site accessibility and seed regions. Bioinformatics. 2008;24:2849–2856. doi: 10.1093/bioinformatics/btn544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeiffer V, Papenfort K, Lucchini S, Hinton JC, Vogel J. Coding sequence targeting by MicC RNA reveals bacterial mRNA silencing downstream of translational initiation. Nat Struct Mol Biol. 2009;16:840–846. doi: 10.1038/nsmb.1631. [DOI] [PubMed] [Google Scholar]
- 30.Reinbothe C, et al. Chlorophyll biosynthesis: Spotlight on protochlorophyllide reduction. Trends Plant Sci. 2010;15:614–624. doi: 10.1016/j.tplants.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Nomata J, Swem LR, Bauer CE, Fujita Y. Overexpression and characterization of dark-operative protochlorophyllide reductase from Rhodobacter capsulatus. Biochim Biophys Acta. 2005;1708:229–237. doi: 10.1016/j.bbabio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Zeng X, Choudhary M, Kaplan S. A second and unusual pucBA operon of Rhodobacter sphaeroides 2.4.1: Genetics and function of the encoded polypeptides. J Bacteriol. 2003;185:6171–6184. doi: 10.1128/JB.185.20.6171-6184.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregor J, Klug G. Oxygen-regulated expression of genes for pigment binding proteins in Rhodobacter capsulatus. J Mol Microbiol Biotechnol. 2002;4:249–253. [PubMed] [Google Scholar]
- 34.Wu J, Bauer CE. RegB/RegA, a global redox-responding two-component system. Adv Exp Med Biol. 2008;631:131–148. doi: 10.1007/978-0-387-78885-2_9. [DOI] [PubMed] [Google Scholar]
- 35.Zeilstra-Ryalls JH, Kaplan S. Aerobic and anaerobic regulation in Rhodobacter sphaeroides 2.4.1: The role of the fnrL gene. J Bacteriol. 1995;177:6422–6431. doi: 10.1128/jb.177.22.6422-6431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue K, Kouadio JL, Mosley CS, Bauer CE. Isolation and in vitro phosphorylation of sensory transduction components controlling anaerobic induction of light harvesting and reaction center gene expression in Rhodobacter capsulatus. Biochemistry. 1995;34:391–396. doi: 10.1021/bi00002a002. [DOI] [PubMed] [Google Scholar]
- 37.Oh JI, Kaplan S. Redox signaling: Globalization of gene expression. EMBO J. 2000;19:4237–4247. doi: 10.1093/emboj/19.16.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards GR, Vanderpool CK. Molecular call and response: The physiology of bacterial small RNAs. Biochim Biophys Acta. 2011;1809:525–531. doi: 10.1016/j.bbagrm.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mangan S, Itzkovitz S, Zaslaver A, Alon U. The incoherent feed-forward loop accelerates the response-time of the gal system of Escherichia coli. J Mol Biol. 2006;356:1073–1081. doi: 10.1016/j.jmb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Gogol EB, Rhodius VA, Papenfort K, Vogel J, Gross CA. Small RNAs endow a transcriptional activator with essential repressor functions for single-tier control of a global stress regulon. Proc Natl Acad Sci USA. 2011;108:12875–12880. doi: 10.1073/pnas.1109379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drews G. Mikrobiologisches Praktikum. Heidelberg: Springer; 1983. [Google Scholar]
- 42.Hübner P, Willison JC, Vignais PM, Bickle TA. Expression of regulatory nif genes in Rhodobacter capsulatus. J Bacteriol. 1991;173:2993–2999. doi: 10.1128/jb.173.9.2993-2999.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.