Abstract
Toxoplasma gondii is an obligate intracellular protozoan pathogen that traffics to the central nervous system (CNS) following invasion of its host. In the CNS, T. gondii undergoes transformation from a rapidly dividing tachyzoite to a long-lived, slow-dividing bradyzoite contained within cysts. The role of extracellular adenosine in T. gondii pathogenesis has not been previously investigated. T. gondii uses host purines such as adenosine for its energy needs, as it is unable to make its own. Here, we show that CD73−/− mice, which lack the ability to generate extracellular adenosine, are protected from T. gondii chronic infection, with significantly fewer cysts and reduced susceptibility to reactivation of infection in the CNS independent of host effector function. Parasite dissemination to the brain was unimpaired in CD73−/− hosts, suggesting that the reduced cyst number is due to impaired parasite differentiation in the CNS. Confirming this, T. gondii tachyzoites formed fewer cysts following alkaline pH stress in astrocytes isolated from CD73−/− mice compared with wild type, and in fibroblasts treated with a CD73 inhibitor. Cyst formation was rescued in CD73−/− astrocytes supplemented with adenosine, but not with adenosine receptor agonist 5′-N-ethylcarboxamidoadenosine. Furthermore, mice lacking adenosine receptors had no defect in cyst formation. Based on these findings, we conclude that CD73 expression promotes Toxoplasma bradyzoite differentiation and cyst formation by a mechanism dependent on the generation of adenosine, but independent of adenosine receptor signaling. Overall, these findings suggest that modulators of extracellular adenosine may be used to develop therapies aimed at defending against human toxoplasmosis.
The protozoan Toxoplasma gondii is an obligate intracellular pathogen that traffics to the central nervous system (CNS) following initial invasion and replication in the gut (1). Infection with T. gondii commonly occurs in humans by ingestion of contaminated meat. In healthy individuals, the parasite forms tissue cysts, which limits its replication but enables the parasite to avoid immune cell-mediated destruction. Reactivation of latent infection in immunocompromised individuals and vertical transmission during pregnancy can lead to severe disease (2, 3). Dissemination of the parasite throughout the host is thought to be mediated by infected immune cells, which transport live parasites to the CNS and skeletal muscle where T. gondii establishes a chronic infection by differentiating into long-lived tissue cysts (4, 5).
Host cell-mediated immunity is the major deterrent against toxoplasmosis (6). The immune response in healthy individuals keeps T. gondii in check so that cyst-containing bradyzoites remain dormant in the CNS for the life of the host without overt clinical symptoms. This delicate balance between host and parasite survival is mediated both by host immune modulators and by T. gondii modification of host factors to promote its survival and transmission and to avoid excessive tissue damage leading to the host’s demise (6–8).
Extracellular adenosine is a purine nucleoside generated by the sequential dephosphorylation of adenosine triphosphate (ATP) by the ectoenzymes CD39 and CD73 (reviewed in ref. 9). CD73 is a GPI-anchored cell surface glycoprotein that catalyzes the final and rate-limiting conversion of adenosine monophosphate (AMP) to adenosine (10). Adenosine mediates its effects by binding to four seven-transmembrane receptors: A1, A2A, A2B, and A3. Adenosine receptors and CD73 are highly expressed on various cell types, including immune cells and CNS-resident cells (11). Extracellular adenosine signaling functions to prevent excessive inflammation by suppressing proinflammatory cytokines, inhibiting leukocyte entry into tissues through down-regulation of adhesion molecules and chemokines, and triggering the production of anti-inflammatory cytokines such as IL-10 (12–14). Furthermore, CD73 expression and downstream adenosine signaling are critical for compensatory responses to tissue ischemia (13, 15, 16). Therefore, extracellular adenosine produced as a result of CD73 acts on adenosine receptors to regulate inflammation and protect against collateral tissue damage. Recent studies from our laboratory showed that CD73 and adenosine receptor expression on choroid plexus epithelial cells mediates T-cell infiltration in the CNS, whereas expression on brain endothelial cells regulates blood–brain barrier function (17, 18).
The role of CD73 in T. gondii infection has not been previously explored. However, work by Blader et al. (19) showed that infection of human fibroblast with T. gondii 2 h postinfection resulted in the up-regulation of genes associated with the immune response, including CD73. T. gondii, like other apicomplexa, needs host purines, such as adenosine as it cannot synthesize its own (20). Two purine salvage pathways have been identified in T. gondii, involving the enzymes hypoxanthine–xanthine–guanine phosphoribosyltransferase and adenosine kinase (AK) (21). T. gondii AK activity is 10-fold higher than other purine salvage enzymes, and adenosine is the preferred source of purines for T. gondii (22). This suggests that host-derived adenosine plays an important role in T. gondii pathogenesis. In this study, we set out to determine whether CD73 is thus important for T. gondii pathogenesis. Interestingly, we found that CD73-knockout mice are less susceptible to chronic T. gondii infection, exhibiting reduced morbidity and mortality and markedly reduced cyst burden in the brain, compared with WT control mice. In an in vitro cell culture model that recapitulated the in vivo model, we found that addition of adenosine, but not activation of adenosine receptors, rescued cyst formation. Our findings suggest that CD73 contributes to T. gondii persistence in the CNS by promoting parasite differentiation.
Results
CD73−/− Mice Are Less Susceptible to Toxoplasmosis.
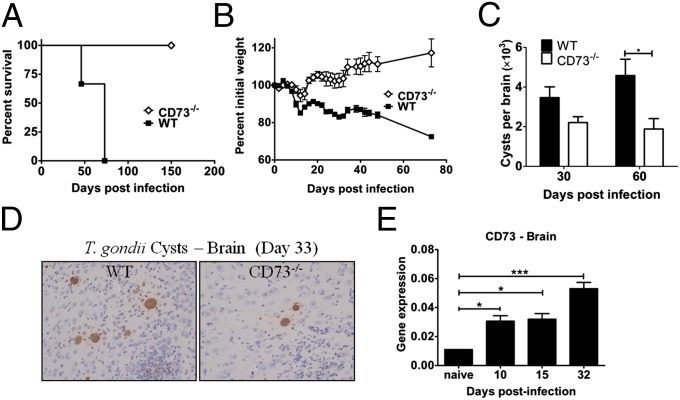
To determine the role of CD73 in Toxoplasma infection, C57BL/6 (WT) and CD73−/− mice (11) were infected with 10 cysts of T. gondii ME49 strain (23) by oral gavage, and then monitored for survival, weight loss, and cyst burden. In WT mice, infection with this dose of parasites allows survival through acute infection (<14 d) and establishment of chronic infection. However, WT mice are susceptible to parasite reactivation in the CNS, and the animals ultimately succumb to toxoplasmic encephalitis. CD73−/− mice were significantly more resistant to chronic T. gondii infection than WT mice (Fig. 1 A–D). Although both WT and CD73−/− mice infected with a low dose of the ME49 strain of Toxoplasma survived the acute infection (Fig. 1A), WT but not CD73−/− mice died during chronic infection, with the onset of morbidity and mortality occurring 8–10 wk postinfection (Fig. 1A). Weight loss monitored during infection confirmed that WT mice exhibited increased susceptibility to toxoplasmosis, losing significantly more weight during both acute and chronic infection (Fig. 1B). In addition, the increased survival observed in CD73−/− mice correlated with decreased parasite burden in the CNS during chronic infection (Fig. 1 C and D). Gene expression analysis showed that CD73 is expressed in the brain and that its expression increased over the course of infection in WT mice (Fig. 1E). Therefore, these results suggest that CD73 promotes parasite persistence in the brain, resulting in increased susceptibility to toxoplasmic encephalitis.
Fig. 1.
CD73−/− mice are resistant to chronic T. gondii infection. WT and CD73−/− mice were infected with 10 cysts of the T. gondii ME49 strain and then monitored for (A) survival and (B) weight loss. (C) Cyst burden in infected mouse brains was quantified by light microscopy. (D) Brain sections from T. gondii infected mice were stained by immunohistochemistry for T. gondii cysts and are shown with representative cyst distribution near the hippocampus from day 33 postinfection (T. gondii cysts, brown; hematoxylin-stained nuclei, blue). (E) CD73 gene expression in WT mice during infection. Expression was normalized to the PGK1 housekeeping gene. Error bars represent the SEM. Significant differences based on two-tailed t tests are displayed (*P < 0.05, **P < 0.01, ***P < 0.005). The data shown are representative of three experiments using three to five mice per group per time point.
CD73−/− and WT Mice Show Similar Levels of T. gondii Parasite Burden in the Brain During the Acute but Not Chronic Stages of Infection.
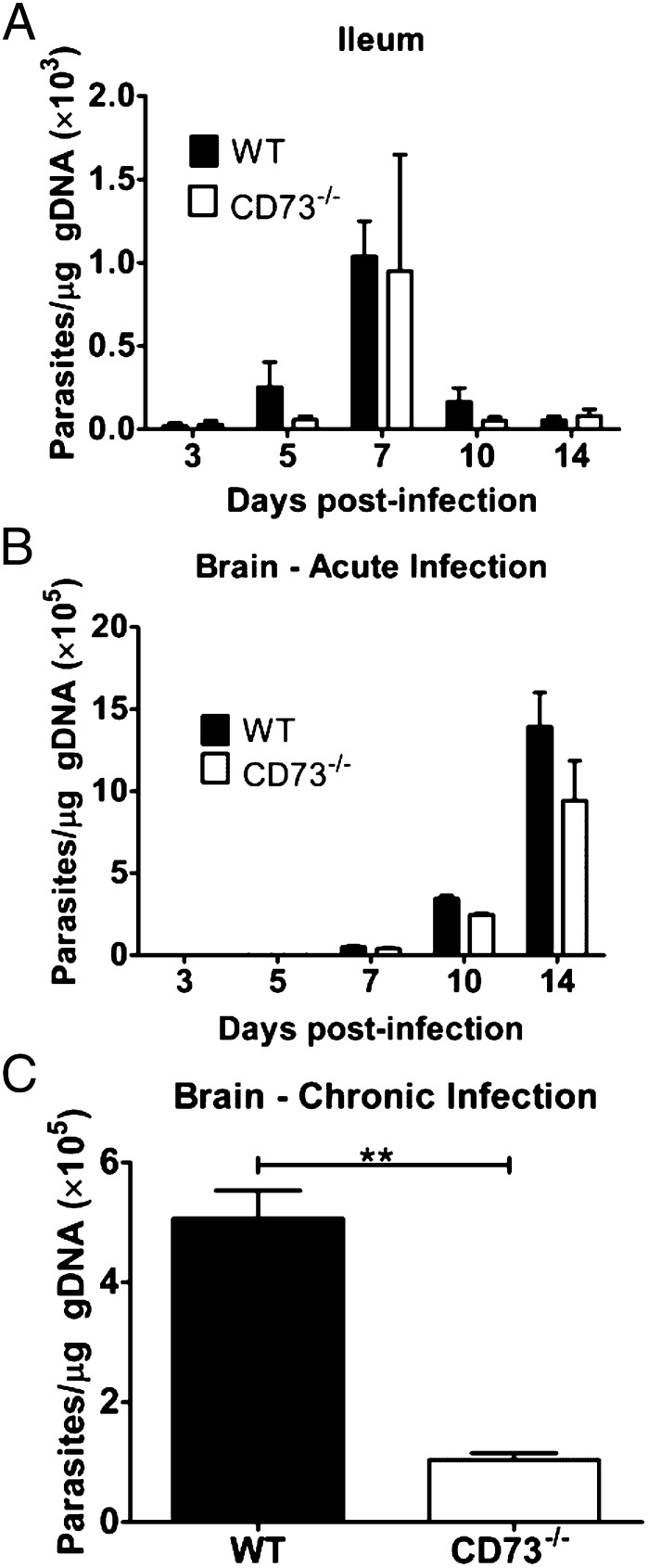
To assess whether the reduced parasite burden in CD73−/− mice could be explained by failure of the parasite to establish infection in the intestinal ileum or to disseminate to the brain following oral infection, WT and CD73−/− mice were infected with 20 ME49 cysts, and then euthanized at various time points postinfection to determine parasite burden in the ileum of the small intestine and in the brain by quantitative real-time PCR. Both strains of mice showed similar kinetics of parasite expansion and clearance during the acute stage of infection in the gut, as well as similar dissemination to the brain (Fig. 2 A and B). In the ileum of WT and CD73−/− mice, T. gondii DNA was consistently detectable by day 3, peaked on day 7, and then decreased significantly by day 14 (Fig. 2A). Parasite dissemination to the brain was detectable by day 7 postinfection, and then increased markedly thereafter (Fig. 2B). Despite the similar initial kinetics of T. gondii dissemination to the brain, PCR results confirmed that CD73−/− mice had significantly reduced parasite burden in the brain during chronic disease compared with WT mice (Fig. 2C). This reduced parasite burden in CD73−/− mice was consistent with their reduced susceptibility to latent infection (Fig. 1).
Fig. 2.
CD73−/− mice have reduced parasite burden in the brain during chronic T. gondii infection. WT and CD73−/− mice were infected with 20 cysts of the T. gondii ME49 strain and then euthanized at various time points during (A and B) acute and (C) chronic infection to determine parasite burden in the (A) small intestine ileum and (B and C) brain by real-time quantitative PCR. Error bars represent the SEM. Significant differences based on two-tailed t tests are displayed (**P < 0.01). The data shown represent one experiment with three to five mice per group per time point.
CD73−/− and WT Mice Have a Similar Degree of Leukocyte Infiltration into the Brain During T. gondii Chronic Infection.
As previously reported (24), infection with T. gondii in WT mice is associated with infiltration of leukocytes into the brain by day 10 postinfection and remains elevated throughout the chronic stage of disease (Fig. S1, Upper). The absolute number of leukocyte recruitment into the brains of WT and CD73−/− mice was similar (Fig. S1, Upper Left), although a decrease in T cells (as a percentage of total CD45+ cells) was observed in CD73−/− mice early in infection at day 10 (Fig. S1, Upper Center), with a relative increase in CD11b+ cells (Fig. S1, Upper Right). Because overall leukocyte numbers and composition were similar in CD73−/− compared with WT mice during chronic infection, the reduction in parasite burden observed in CD73−/− mice at the same time points (Fig. 1) was likely not due to increased leukocyte recruitment to the CNS.
Brains from T. gondii-Infected CD73−/− and WT Mice Have Similar Immune Effector Gene Expression Kinetics.
We next analyzed gene expression of effector molecules associated with T. gondii infection to determine whether the resistance of CD73−/− mice was mediated by an increased production of these molecules. IFN-γ is a cytokine critical for controlling T. gondii expansion and reactivation in the brain (25–29). In WT and CD73−/− mice, we observed an equally substantial and sustained increase in IFN-γ mRNA expression during the course of T. gondii infection (Fig. S1, Lower Left). Another effector molecule important in the killing and inhibition of T. gondii growth is inducible nitric oxide synthase (iNOS) found in peripheral macrophages and the CNS (30). T. gondii infection led to increased expression of the iNOS gene, NOS2, in WT and CD73−/− mice to a similar extent (Fig. S1, Lower Center). Likewise, no difference was observed during T. gondii infection in the up-regulation of TNF-α mRNA, which is important in the control of toxoplasmosis in synergy with IFN-γ (31), between WT and CD73−/− mice (Fig. S1, Lower Right). These findings suggest that gene expression of effector molecules in the brain is not altered in the absence of CD73 and therefore is not responsible for protecting CD73−/− mice from T. gondii chronic infection.
Infection and Proliferation of T. gondii in Astrocytes Is Not Dependent on CD73 Expression.
To assess whether CD73−/− cells in the CNS were less permissive for T. gondii infection or proliferation, we isolated WT and CD73−/− astrocytes and assessed the ability of the parasite to infect and proliferate in these cells. Astrocytes from WT and CD73−/− neonates were cultured and expanded in vitro and then infected with tachyzoites of the type II T. gondii strain PTG (Fig. S2A). Flow-cytometric analysis of astrocytes from WT mice revealed two distinct populations of cells based on CD73 expression, with ∼40% of the cells being CD73-positive (Fig. 2A). Likewise, the proliferation of tachyzoites of the PTG and ME49 strains of T. gondii, as measured by [3H]uracil incorporation (32), was similar in both WT and CD73−/− infected astrocytes (Fig. S2B). These results indicate that T. gondii infection and replication of tachyzoites in astrocytes is not dependent on CD73 expression.
CD73 Expression Promotes Bradyzoite/Cyst Persistence in the Brain.
Because T. gondii-infected CD73−/− mice have a reduced number of brain cysts compared with WT mice (Fig. 1), we next determined whether CD73 expression is required for T. gondii bradyzoite maturation. We isolated brains of T. gondii-infected WT and CD73−/− mice during acute (day 10 and day 15) and chronic (day 30) infection and determined mRNA expression of the SAG1 (tachyzoite-specific) and BAG1 (bradyzoite-specific) genes by quantitative PCR (qPCR) (33–35). In both WT and CD73−/− mice, tachyzoite SAG1 was highly expressed on day 10 but decreased on days 15 and 30 (Fig. 3A). Conversely, bradyzoite BAG1 was expressed at low levels on day 10 and highly expressed on days 15 and 30 in both WT and CD73−/− mice (Fig. 3B). Interestingly, although BAG1 expression was highly expressed in both mouse strains at day 30 postinfection, mice that lacked CD73 had significantly lower brain BAG1 expression levels compared with WT mice (Fig. 3B). These results suggest that bradyzoites are generated in the brains of CD73−/− mice similar to WT mice but do not persist or proliferate. To test the viability of encysted bradyzoites, we purified cysts from chronically infected WT and CD73−/− mice and liberated bradyzoites using enzymatic digestion. These bradyzoites were used to infect fibroblasts, and their proliferation assessed using [3H]uracil incorporation. We found that bradyzoites isolated from CD73−/− hosts proliferate significantly less than those isolated from WT hosts (Fig. S3). However, in vivo, the few cysts found in CD73−/− hosts were capable of recrudescence if mice were treated with dexamethasone to induce immunosuppression (36) (Fig. S4). Thus, although there is evidence that parasites isolated from CD73−/− mice proliferate less well in vitro, this is not enough to significantly impact the outcome of dexamethasone-induced toxoplasmic encephalitis.
Fig. 3.
Gene expression profile of T. gondii tachyzoites and bradyzoites in the brains of infected WT and CD73−/− mice and in cultured brain astrocytes. (A and B) WT and CD73−/− mice were infected with 10 cysts of the T. gondii ME49 strain and then euthanized at various time points during acute (days 10 and 15) and chronic (day 30) infection to determine the brain expression of the (A) tachyzoite SAG1 and (B) bradyzoite BAG1 genes. The data shown represent one of three experiments using two to five mice per time point. (C) Astrocytes were cultured from neonatal WT and CD73−/− pups and then infected with ME49 tachyzoites to assess spontaneous differentiation of T. gondii in vitro by quantitative real-time PCR analysis of constitutively expressed Tubulin 1, tachyzoite-specific SAG1, and bradyzoite-specific BAG1 and ENO1 genes. Parasite gene expression was normalized to host PGK1 housekeeping gene. Error bars represent the SEM. Significant differences based on two-tailed t tests are displayed (*P < 0.05, **P < 0.01, ***P < 0.0001).
CD73 Promotes Tachyzoite to Bradyzoite Differentiation in Astrocytes.
We next determined whether T. gondii differentiation from tachyzoites to bradyzoites might be impaired in resident CNS cells lacking CD73. Astrocytes from WT and CD73−/− mice were isolated, cultured, and then infected with ME49 tachyzoites (Figs. 3C and 4). After 8 d in culture, we could detect robust expression of constitutive (Tubulin 1), tachyzoite-specific (SAG1), and bradyzoite-specific genes (BAG1 and ENO1) in WT and CD73−/− cell cultures (Fig. 3C). Interestingly, expression of the constitutive gene Tubulin 1 was slightly decreased in CD73−/− infected cells, whereas SAG1, BAG1, and ENO1 expression were significantly reduced in CD73−/− cells compared with WT cells. The reduction in bradyzoite genes (BAG1 and ENO1) in CD73−/− host cells was more pronounced at this time point.
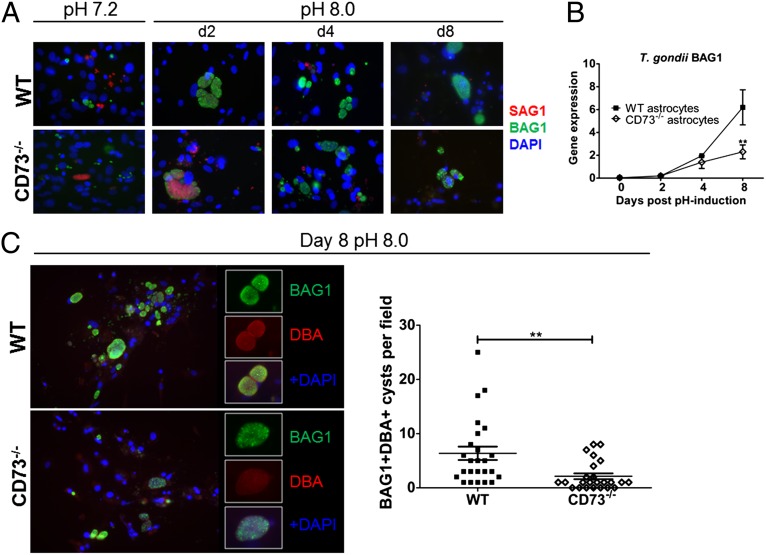
Fig. 4.
Differentiation of T. gondii in cultured astrocytes from WT and CD73−/− mice. Astrocytes from WT and CD73−/− neonates were cultured and infected with T. gondii tachyzoites of the ME49 strain and analyzed for parasite differentiation. (A) Immunofluorescence microscopy of infected astrocytes cultured at neutral pH (7.2; first set of upper and lower panels) or alkaline pH (8.0; second, third, and fourth sets of upper and lower panels) for 2, 4, and 8 d (tachyzoites/SAG1 stained, red; bradyzoites/BAG1 stained, green; nuclei/DAPI stained, blue). (B) Quantification of T. gondii BAG1 gene expression in infected WT and CD73−/− astrocytes normalized to host housekeeping gene PGK1. (C) Visualization and quantification of in vitro cyst formation in cultured WT and CD73−/− astrocytes after 8 d at pH 8.0 as determined by anti-BAG1 (green) and DBA (red) staining; Insets show cysts at higher magnification. Significant differences based on two-tailed t tests are displayed (**P < 0.01).
To determine whether the differentiation of T. gondii in CD73−/− host cells was also impaired under bradyzoite-inducing conditions, we again cultured astrocytes from WT and CD73−/− hosts and infected them with T. gondii ME49 tachyzoites. At 2 d postinfection, the pH of the culture media was increased from 7.2 to 8.0, to induce cyst formation (37). Confirming our quantitative gene expression data (Fig. 3C), some tachyzoites (SAG1+) spontaneously differentiated to bradyzoites (BAG1+) even before alkaline pH challenge (Fig. 4A, first set of upper and lower panels). Nevertheless, pH induction triggered substantial bradyzoite differentiation, with cyst formation observed after only 2 d in alkaline media (Fig. 4A, second set of upper and lower panels), and mature cysts by 8 d postinduction (Fig. 4A, fourth set of upper and lower panels, and 4C). Mature cysts, defined as positive for the bradyzoite marker BAG1 as well as the cyst wall component that binds Dolichos biflorus agglutinin (DBA) were quantified to compare the ability of astrocytes from WT and CD73−/− mice to support cyst formation in vitro. Significantly more cysts were observed in astrocyte cultures from WT mice compared with those from CD73−/− mice (Fig. 4C). Consistent with this finding, analysis of BAG1 expression at the mRNA level by qPCR revealed significantly higher expression of this bradyzoite T. gondii gene in WT compared with CD73−/− astrocyte cultures (Fig. 4B). These results strongly suggest that CD73 expression is required for the efficient formation or maintenance of cysts in astrocytes.
Exogenous Adenosine Rescues T. gondii Cyst Formation in CD73-Deficient Host Cells.
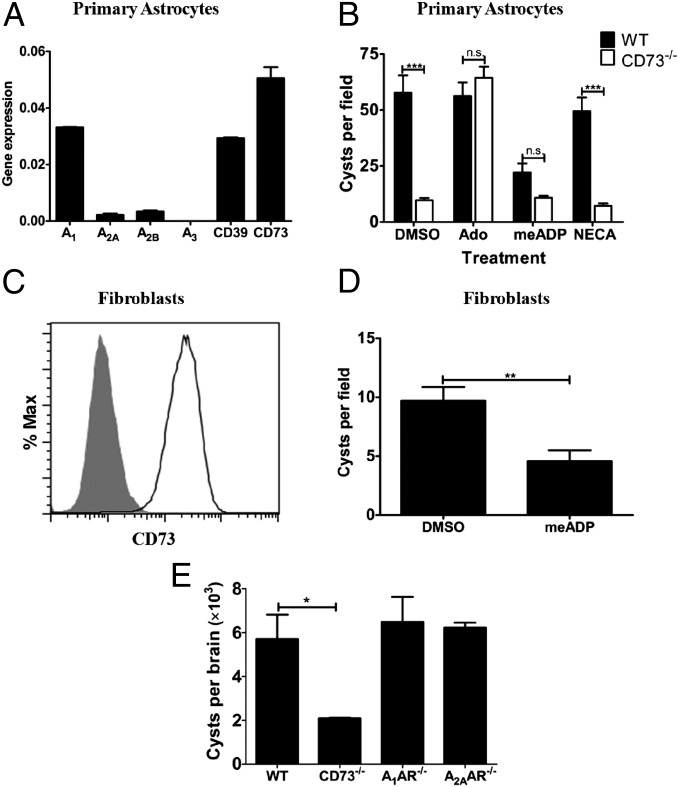
To investigate the mechanism by which CD73 promotes cyst formation or bradyzoite differentiation in vitro, we next determined whether exogenous adenosine, which mediates both adenosine receptor signaling as well as serving as a source of purines for parasites such as T. gondii can rescue the defect in bradyzoite maturation observed in mice and cells lacking CD73. We first determined by real-time PCR that three of the four adenosine receptors are expressed on astrocytes in addition to the ecto-enzymes CD39 and CD73 that generate adenosine from the breakdown of ATP (Fig. 5A). As described above, astrocytes from WT and CD73−/− mice were isolated, cultured, and then infected with ME49 tachyzoites in increased pH medium (from 7.2 to 8.0), to induce cyst formation in the presence of adenosine or vehicle control (DMSO). We observed that exogenous adenosine was able to significantly rescue T. gondii cyst formation in CD73−/− astrocytes to levels similar to WT but not vehicle control (Fig. 5B).
Fig. 5.
Exogenous adenosine rescues T. gondii cyst formation in CD73-deficient host cells independent of adenosine receptor signaling. (A) Gene expression analysis of adenosine receptors (A1, A2A, A2B, A3), CD39, and CD73 on cultured astrocytes. Real-time quantitative PCR results are normalized to the housekeeping gene PGK1. (B) Astrocytes from WT and CD73−/− neonates were cultured and infected with T. gondii tachyzoites of the ME49 strain in the presence or absence of adenosine (50 μM), the broad-spectrum adenosine receptor agonist NECA (10 μM), or the CD73 inhibitor meADP (50 μM). Cyst formation was assessed 12 d after continuous culture at pH 8.0 by immunofluorescence microscopy to quantify DBA binding cysts. (C) The human foreskin fibroblast line Hs27 was assessed for CD73 expression by flow cytometry (unshaded curve, CD73-PE-labeled cells; shaded curve, unstained live cells). (D) Cyst formation was quantified as described above following 8-d culture of T. gondii-infected Hs27 fibroblasts at pH 8.0 in the presence of DMSO or 50 μM meADP. Significant differences based on two-tailed t tests are displayed (**P < 0.01, ***P < 0.001). (E) Quantitation of brain parasite cyst burden in A1AR−/−, A2AAR−/−, CD73−/−, and WT mice infected with 20 cysts of T. gondii ME49 for 30 d (*P < 0.05).
We next asked whether adenosine receptor signaling was important in mediating bradyzoite cyst maturation by treating astrocytes with an adenosine analog, 5′-N-ethylcarboxamido adenosine (NECA). NECA acts as a broad-spectrum adenosine receptor agonist but does not participate in the adenosine purine salvage pathway. Interestingly, we found that NECA had no effect on cyst formation (Fig. 5B). To confirm that the differentiation defect in CD73−/− cells was due to the absence of CD73 activity, we cultured astrocytes from WT and CD73−/− mice with ME49 tachyzoites in the presence of the specific CD73 inhibitor α,β-methylene ADP (meADP), which blocks the enzymatic activity of CD73. As anticipated, meADP had no effect on cyst formation in CD73−/− astrocytes, whereas it significantly inhibited cyst formation in WT astrocytes (Fig. 5B). To determine whether CD73 expression on other cell types also promoted T. gondii cyst formation, we infected the human fibroblast cell line Hs27 (which is commonly used to maintain T. gondii tachyzoites) with T. gondii ME49 under cyst-inducing conditions. Because the cell line expresses CD73 (Fig. 5C), we were able to show that inhibition of CD73 enzymatic activity with meADP led to a twofold reduction in cysts generated under high pH (Fig. 5D), similar to what we observe with primary mouse astrocytes (Fig. 5B). Consistent with this finding, mice lacking A1 or A2A adenosine receptors also exhibited no defect in bradyzoite cyst formation, as their cyst burden was similar that of WT mice (Fig. 5E). All together, these results confirm that CD73 promotes T. gondii bradyzoite differentiation through a mechanism involving adenosine acquisition rather than adenosine receptor signaling.
Discussion
The ability of T. gondii to infect a wide variety of warm-blooded hosts and virtually any nucleated cell poses a challenge in the control of this parasite. One reason that T. gondii is a successful parasite is its ability to differentiate to the slow-growing bradyzoite stage in skeletal muscle and the CNS, allowing the parasite to persist even in the face of a robust immune response (38, 39). In this study, we established that the widely expressed host-derived enzyme, 5′-ecto-nucleosidase (CD73), plays a significant role in T. gondii persistence during chronic infection. In mice infected with T. gondii, we observed a significant increase in CD73 expression in the brain, the major tissue parasitized during the chronic stage of infection. Importantly, CD73−/− mice were resistant to cerebral toxoplasmosis, with increased survival and reduced parasite loads in the brains of infected animals. This protection was observed despite similar kinetics of T. gondii dissemination into the brains of WT and CD73−/− mice, suggesting that CD73 expression promotes T. gondii persistence or survival in situ.
In the CNS, control of T. gondii infection is mediated by infiltrating leukocytes, particularly cytotoxic T lymphocytes that activate parasiticidal and parasitistatic mechanisms in infected cells (6, 8, 26, 40). Recrudescence of infection occurs when there is a breakdown in immunosurveillance or in the absence of key mediators of parasite control, such as IL-12, IFN-γ, iNOS, and TNF-α (6). In CD73−/− mice, protection from cerebral toxoplasmosis was not associated with elevated IFN-γ or other effector molecules such as iNOS and TNF-α in the infected CNS. Moreover, the lack of CD73 on CNS-resident cells did not alter T. gondii’s ability to proliferate.
Although T. gondii can synthesize pyrimidines de novo, it lacks the ability to synthesize purines, including adenosine (20). CD73 mediates the final rate-limiting step in the enzymatic chain that catalyzes the dephosphorylation of extracellular AMP to adenosine from ATP. High levels of adenosine in the extracellular space trigger its transport into cells by means of transporters (41). The transport of adenosine across cell membranes is the first step in the salvage of adenosine by T. gondii (42–44). Although deletion of the major adenosine salvage enzyme AK alone is not lethal and imparts only a modest growth defect (21), whether deletion of AK impairs cyst formation has not been reported. It is possible that, under conditions in which extracellular adenosine levels are diminished or absent such as in mice or cells lacking CD73, there would be an overall deficit in the availability of adequate sources of adenosine. Such a deficit can have an impact on T. gondii’s ability to undergo efficient transformation to the bradyzoite or long-lived cyst stage. Thus, interference with adenosine uptake can be detrimental to T. gondii, as we observed in CD73−/− mice, and in in vitro differentiation assays in glial and fibroblast cells expressing or lacking CD73.
In summary, we showed that CD73−/− mice are protected from T. gondii-induced mortality and morbidity. Ablation of CD73 renders T. gondii incapable of efficiently establishing latent infection as CD73−/− mice have dramatically decreased cyst burden compared with WT mice. We show that glial cells from CD73−/− mice are unable to sustain cyst differentiation or survival. Although addition of adenosine rescues the cyst maturation defect, our results show this is not mediated by adenosine receptor signaling. Furthermore, inactivation of CD73 enzymatic function with a pharmacological inhibitor significantly reduces cyst formation in WT astrocytes and human fibroblasts. Although the precise mechanism of how T. gondii may acquire host adenosine for its survival is not yet clear, these findings clearly indicate that inhibitors to CD73 or adenosine might be important targets for therapy in limiting the growth or survival of T. gondii and other apicomplexan parasites.
Materials and Methods
Mouse Strains and in Vivo Infection.
Female C57BL/6 WT and CD73-knockout mice (11), provided by Linda Thompson (Oklahoma Medical Research Foundation, Oklahoma City, OK), were bred in specific pathogen-free conditions at Cornell University. Adenosine receptor A1 and A2A knockout mice were a gift from Jurgen Schnermann (NIH/NIDDK, Bethesda, MD) and Jiang-Fan Chen (Boston University School of Medicine, Boston, MA), respectively. All animal experiments were approved by Cornell University’s Institutional Animal Care and Use Committee in accordance with guidelines provided by the National Institutes of Health Office of Laboratory Animal Welfare (OLAW). For chronic T. gondii infection, mice were inoculated per os with 10 ME49 cysts and monitored for weight loss, survival, and parasite burden. For determination of parasite cyst burden, T. gondii-infected mice were euthanized and their brains were harvested and homogenized. Brain homogenates were centrifuged at 250 × g for 10 min and resuspended in PBS, and aliquots were mixed with an equal volume of Lugol’s iodine for counting cysts using light microscopy.
Parasite Strains and Maintenance.
Quantitation of Gene Expression by qPCR.
Gene expression from tissues or cultured astrocytes was quantified as described in SI Materials and Methods using primers listed in Table S1.
Quantitation of T. gondii by qPCR.
T. gondii Differentiation and Immunofluorescence Microscopy.
To assess T. gondii differentiation in vitro (37), astrocytes isolated from WT and CD73−/− neonates (as described in ref. 45 and detailed in SI Materials and Methods) were grown to confluence on sterile coverslips in 24-well tissue culture plates. The cells were then infected at a multiplicity of infection of 1:2 with ME49 tachyzoites for 2 d at pH 7.2 in complete DMEM with 3% (vol/vol) FCS at 37 °C and 5% CO2. The medium was then replaced with complete DMEM–3% FCS at pH 8.0 to induce bradyzoite differentiation (replaced every 3 d to maintain alkaline conditions), and the cells were cultured at 37 °C in air. In some cases, the medium was supplemented with adenosine (50 μM), NECA (10 μM), meADP (50 μM), or vehicle (DMSO) during the T. gondii differentiation before fixing and staining the cells at 12 d after alkaline pH challenge. For the differentiation time course, the cells were analyzed at 2, 4, and 8 d after pH increase. Cells were fixed in 2% paraformaldehyde for 20 min at room temperature, then blocked and permeabilized with 10% normal rabbit serum and 0.2% Triton X-100 in PBS with 1% BSA. Intracellular staining was performed overnight at 4 °C with rabbit anti-BAG1 (J. P. Dubey, USDA, Beltsville, MD) followed by goat anti-rabbit conjugated to Alexa Fluor 488 or Texas Red X (Invitrogen) and either DBA conjugated to rhodamine (Vector Labs) or mouse anti-SAG1-FITC. Coverslips were washed and mounted with Vectashield Mounting Medium with DAPI (Vector Labs). Images were obtained on a Zeiss Axio Imager M1 fluorescent microscope using AxioVision software. For quantitation of cyst numbers, 25 adjacent fields per slide were scanned and the number of BAG1+DBA+ cysts was enumerated.
Data Analysis.
Statistical analyses were performed with GraphPad Prism software. Two-tailed Student’s t tests or two-way ANOVA with Bonferroni posttests were used to compare differences between groups. Kaplan–Meier survival curves were compared with the Mantel–Cox log rank test. Differences with a value of P < 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Dr. J. P. Dubey for the kind gift of the anti-BAG1 antibody. This work was supported by National Institutes of Health Grants R01 NS053011 (to M.S.B.), R01 NS05301-04S4 (to D.A.M.), and R01 AI50617 (to E.Y.D.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205589109/-/DCSupplemental.
References
- 1.Kim K, Weiss LM. Toxoplasma gondii: The model apicomplexan. Int J Parasitol. 2004;34:423–432. doi: 10.1016/j.ijpara.2003.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland GN, et al. Ocular toxoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1988;106:653–667. doi: 10.1016/0002-9394(88)90697-6. [DOI] [PubMed] [Google Scholar]
- 3.Falangola MF, Petito CK. Choroid plexus infection in cerebral toxoplasmosis in AIDS patients. Neurology. 1993;43:2035–2040. doi: 10.1212/wnl.43.10.2035. [DOI] [PubMed] [Google Scholar]
- 4.Lambert H, Barragan A. Modelling parasite dissemination: Host cell subversion and immune evasion by Toxoplasma gondii. Cell Microbiol. 2010;12:292–300. doi: 10.1111/j.1462-5822.2009.01417.x. [DOI] [PubMed] [Google Scholar]
- 5.Courret N, et al. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107:309–316. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Däubener W, Hadding U. Cellular immune reactions directed against Toxoplasma gondii with special emphasis on the central nervous system. Med Microbiol Immunol (Berl) 1997;185:195–206. doi: 10.1007/s004300050031. [DOI] [PubMed] [Google Scholar]
- 7.Gavrilescu LC, Denkers EY. IFN-gamma overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J Immunol. 2001;167:902–909. doi: 10.4049/jimmunol.167.2.902. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Kang H, Kikuchi T, Suzuki Y. Gamma interferon production, but not perforin-mediated cytolytic activity, of T cells is required for prevention of toxoplasmic encephalitis in BALB/c mice genetically resistant to the disease. Infect Immun. 2004;72:4432–4438. doi: 10.1128/IAI.72.8.4432-4438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Schetinger MR, Morsch VM, Bonan CD, Wyse AT. NTPDase and 5′-nucleotidase activities in physiological and disease conditions: New perspectives for human health. Biofactors. 2007;31:77–98. doi: 10.1002/biof.5520310205. [DOI] [PubMed] [Google Scholar]
- 11.Thompson LF, et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haskó G, Cronstein BN. Adenosine: An endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Okusa MD, et al. A(2A) adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am J Physiol Renal Physiol. 2000;279:F809–F818. doi: 10.1152/ajprenal.2000.279.5.F809. [DOI] [PubMed] [Google Scholar]
- 14.Haskó G, Németh ZH, Vizi ES, Salzman AL, Szabó C. An agonist of adenosine A3 receptors decreases interleukin-12 and interferon-gamma production and prevents lethality in endotoxemic mice. Eur J Pharmacol. 1998;358:261–268. doi: 10.1016/s0014-2999(98)00619-0. [DOI] [PubMed] [Google Scholar]
- 15.Linden J. Molecular approach to adenosine receptors: Receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 16.Grenz A, et al. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 17.Mills JH, et al. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105:9325–9330. doi: 10.1073/pnas.0711175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carman AJ, Mills JH, Krenz A, Kim DG, Bynoe MS. Adenosine receptor signaling modulates permeability of the blood-brain barrier. J Neurosci. 2011;31:13272–13280. doi: 10.1523/JNEUROSCI.3337-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blader IJ, Manger ID, Boothroyd JC. Microarray analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J Biol Chem. 2001;276:24223–24231. doi: 10.1074/jbc.M100951200. [DOI] [PubMed] [Google Scholar]
- 20.Schwartzman JD, Pfefferkorn ER. Toxoplasma gondii: Purine synthesis and salvage in mutant host cells and parasites. Exp Parasitol. 1982;53:77–86. doi: 10.1016/0014-4894(82)90094-7. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhary K, et al. Purine salvage pathways in the apicomplexan parasite Toxoplasma gondii. J Biol Chem. 2004;279:31221–31227. doi: 10.1074/jbc.M404232200. [DOI] [PubMed] [Google Scholar]
- 22.Krug EC, Marr JJ, Berens RL. Purine metabolism in Toxoplasma gondii. J Biol Chem. 1989;264:10601–10607. [PubMed] [Google Scholar]
- 23.Denkers EY, Caspar P, Hieny S, Sher A. Toxoplasma gondii infection induces specific nonresponsiveness in lymphocytes bearing the V beta 5 chain of the mouse T cell receptor. J Immunol. 1996;156:1089–1094. [PubMed] [Google Scholar]
- 24.Conley FK, Jenkins KA. Immunohistological study of the anatomic relationship of toxoplasma antigens to the inflammatory response in the brains of mice chronically infected with Toxoplasma gondii. Infect Immun. 1981;31:1184–1192. doi: 10.1128/iai.31.3.1184-1192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: The major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 26.Sher A, Denkers EY, Gazzinelli RT. Induction and regulation of host cell-mediated immunity by Toxoplasma gondii. Ciba Found Symp. 1995;195:95–104, discussion 104–109. doi: 10.1002/9780470514849.ch7. [DOI] [PubMed] [Google Scholar]
- 27.Scharton-Kersten TM, et al. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 28.Suzuki Y, Sa Q, Gehman M, Ochiai E. Interferon-gamma- and perforin-mediated immune responses for resistance against Toxoplasma gondii in the brain. Expert Rev Mol Med. 2011;13:e31. doi: 10.1017/S1462399411002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki Y. Host resistance in the brain against Toxoplasma gondii. J Infect Dis. 2002;185(Suppl 1):S58–S65. doi: 10.1086/337999. [DOI] [PubMed] [Google Scholar]
- 30.Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sibley LD, Adams LB, Fukutomi Y, Krahenbuhl JL. Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J Immunol. 1991;147:2340–2345. [PubMed] [Google Scholar]
- 32.Pfefferkorn ER, Pfefferkorn LC. Specific labeling of intracellular Toxoplasma gondii with uracil. J Protozool. 1977;24:449–453. doi: 10.1111/j.1550-7408.1977.tb04774.x. [DOI] [PubMed] [Google Scholar]
- 33.Soete M, Fortier B, Camus D, Dubremetz JF. Toxoplasma gondii: Kinetics of bradyzoite-tachyzoite interconversion in vitro. Exp Parasitol. 1993;76:259–264. doi: 10.1006/expr.1993.1031. [DOI] [PubMed] [Google Scholar]
- 34.Bohne W, Gross U, Ferguson DJ, Heesemann J. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol Microbiol. 1995;16:1221–1230. doi: 10.1111/j.1365-2958.1995.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 35.Ferguson DJ. Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host. Int J Parasitol. 2004;34:347–360. doi: 10.1016/j.ijpara.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 36.Djurković-Djaković O. Murine model of drug-induced reactivation of Toxoplasma gondii. Acta Protozool. 2001;40:99. [Google Scholar]
- 37.Soête M, Camus D, Dubremetz JF. Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Exp Parasitol. 1994;78:361–370. doi: 10.1006/expr.1994.1039. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan WJ, Jr, Jeffers V. Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiol Rev. 2012;36:717–733. doi: 10.1111/j.1574-6976.2011.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camus D, Zalis MG, Vannier-Santos MA, Banic DM. The art of parasite survival. Braz J Med Biol Res. 1995;28:399–413. [PubMed] [Google Scholar]
- 40.Suzuki Y, et al. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am J Pathol. 2010;176:1607–1613. doi: 10.2353/ajpath.2010.090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deussen A, Stappert M, Schäfer S, Kelm M. Quantification of extracellular and intracellular adenosine production: Understanding the transmembranous concentration gradient. Circulation. 1999;99:2041–2047. doi: 10.1161/01.cir.99.15.2041. [DOI] [PubMed] [Google Scholar]
- 42.Schwab JC, Afifi Afifi M, Pizzorno G, Handschumacher RE, Joiner KA. Toxoplasma gondii tachyzoites possess an unusual plasma membrane adenosine transporter. Mol Biochem Parasitol. 1995;70:59–69. doi: 10.1016/0166-6851(95)00005-l. [DOI] [PubMed] [Google Scholar]
- 43.Chiang CW, et al. The adenosine transporter of Toxoplasma gondii. Identification by insertional mutagenesis, cloning, and recombinant expression. J Biol Chem. 1999;274:35255–35261. doi: 10.1074/jbc.274.49.35255. [DOI] [PubMed] [Google Scholar]
- 44.De Koning HP, Al-Salabi MI, Cohen AM, Coombs GH, Wastling JM. Identification and characterisation of high affinity nucleoside and nucleobase transporters in Toxoplasma gondii. Int J Parasitol. 2003;33:821–831. doi: 10.1016/s0020-7519(03)00091-2. [DOI] [PubMed] [Google Scholar]
- 45.Aloisi F, Agresti C, Levi G. Establishment, characterization, and evolution of cultures enriched in type-2 astrocytes. J Neurosci Res. 1988;21:188–198. doi: 10.1002/jnr.490210211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.