Abstract
Lifetime breast cancer risk reflects an unresolved combination of early life factors including diet, body mass index, metabolic syndrome, obesity, and age at first menses. In parallel, the onset of allometric growth by the mammary glands around puberty is widely held to be estrogen (E)-dependent. Here we report that several physiological changes associated with metabolic syndrome in response to a diet supplemented with the trans-10, cis-12 isomer of conjugated linoleic acid lead to ovary-independent allometric growth of the mammary ducts. The E-independence of this diet-induced growth was highlighted by the fact that it occurred both in male mice and with pharmacological inhibition of either E receptor function or E biosynthesis. Reversal of the metabolic phenotype with the peroxisome proliferator-activated receptor-γ agonist rosiglitazone abrogated diet-induced mammary growth. A role for hyperinsulinemia and increased insulin-like growth factor-I receptor (IGF-IR) expression during mammary growth induced by the trans-10, cis-12 isomer of conjugated linoleic acid was confirmed by its reversal upon pharmacological inhibition of IGF-IR function. Diet-stimulated ductal growth also increased mammary tumorigenesis in ovariectomized polyomavirus middle T-antigen mice. Our data demonstrate that diet-induced metabolic dysregulation, independently of ovarian function, stimulates allometric growth within the mammary glands via an IGF-IR-dependent mechanism.
Epidemiological data consistently point to an early window of breast development as being sensitive to breast cancer risk factors associated with diet (1), body mass index (BMI) (2), obesity (3), and age at onset of puberty (4). This increased risk may reflect precocious breast development due to an earlier age at puberty (5) as a result of the obesity pandemic (6). However, lines of evidence indicate that this window is before the onset of puberty and first menses and is independent of ovarian function and increased BMI (7). Indeed, several studies point to a period of <10 y of age that confers the greatest risk to radiation-induced breast cancer (8), consistent with the fact that prepubescent female rats are most susceptible to chemical-induced mammary tumorigenesis (9). A role for diet during this period is highlighted by the fact that only girls who were 2–9 y old during the Dutch famine had an increased lifetime risk of developing breast cancer (10). Furthermore, data from a recent prospective study indicate that girls with a low BMI are at a greater risk for developing benign breast disease (11). Combined, lines of evidence such as these point to an early window of breast development before the onset of puberty that could be sensitive to one or more dietary components or diet-induced metabolic change.
The majority of mammary gland (MG) development occurs during postnatal life and primarily initiates with a phase of allometric growth around the onset of puberty that results in extension of the ductal network from the nipple into the surrounding adipose stroma (12). This allometry is widely held to commence in response to the synthesis and secretion of estrogen (E) by the ovaries (13), where ovariectomy ablates ductal elongation (12) whereas E copotentiates the actions of growth hormone (GH) and its stroma-derived paracrine mediator, insulin-like growth factor-I (IGF-I) (14). Much of this regulation is conferred by the surrounding adipose microenvironment of the mammary fat pad, which itself is modified by diet and developmental state (15).
Major clinical signs of metabolic syndrome include impaired insulin signaling associated with visceral adiposity, dyslipidemia, hypertension, and inflammation (16), where hepatic steatosis separately from obesity may more accurately correlate with impaired insulin signaling (17). A diet-dependent model that recapitulates many aspects of the metabolic syndrome involves feeding rodents the trans-10, cis-12 isomer of conjugated linoleic acid (10,12 CLA), an octadecadienoic fatty acid that is present in foodstuffs either due to the hydrogenation of vegetable oils (18) or at low levels due to biohydrogenation in ruminants (19). When 10,12 CLA is fed to mice, it dysregulates metabolic function coincident with lipoatrophy, transient hypertriglyceridemia, adipose tissue inflammation, hepatic steatosis, and hyperinsulinemia (20–22). The lipoatrophic effect of 10,12 CLA has led to its widespread adoption as a weight-loss supplement (23) that gives rise to elevated plasma triacylglycerol and LDL:HDL cholesterol levels (24). This collection of phenotypes induced by dietary 10,12 CLA therefore provides a useful and defined model of metabolic disruption for studying its effects on MG growth.
Here we report that early allometric growth in the MG of mice occurs independently of estrogenic stimulation following diet-induced metabolic changes resulting from ingestion of 10,12 CLA. These data highlight not only that E-independent allometric growth of the mammary ducts is induced by a dietary component, but also that aspects of the metabolic syndrome elicit this growth, which may increase E-independent breast cancer risk.
Results
Dietary 10,12 CLA Increases Mammary Ductal Growth.
Morphology of the MG from ovary-intact female Balb/cJ mice fed a diet containing 1% 10,12 CLA after weaning at 21 d was first analyzed at 35 d. Mice fed 10,12 CLA for 14 d had greater ductal elongation compared with mice fed the control diet (P < 0.05; Fig. 1). The MG of mice fed 10,12 CLA accumulated 10,12 CLA (P < 0.05; Table S1) coincident with reduced adiposity measured as wet MG mass (P < 0.05; Table S2). We also investigated whether dietary 10,12 CLA altered ductal elongation in ovariectomized (OVX) mice supplemented with E for 14 d. Similar to ovary-intact mice, OVX mice treated with E tended to have increased ductal growth in response to 10,12 CLA (P = 0.09; Fig. S1).
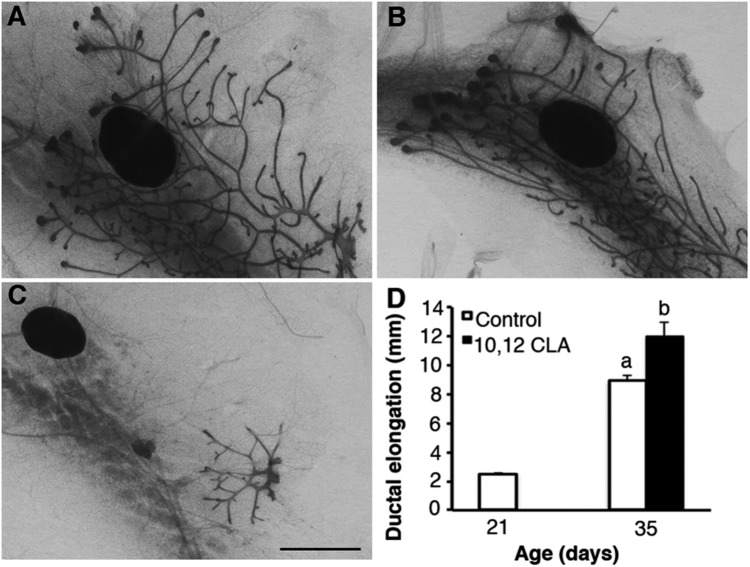
Fig. 1.
Dietary 10,12 CLA stimulates ductal elongation in the mammary glands of ovary-intact peripubertal mice. Representative mammary gland whole mounts of female Balb/cJ mice weaned onto either the (A) control or the (B) 10, 12 CLA diet at 21 d and then euthanized at 35 d. C is a representative whole mount at 21 d. (D) Ductal elongation was measured as the farthest distance that the duct termini extended from the nipple. (Scale bar, 2 mm.) Data are means ± SEM (n = 4–5/group). a,bMeans with different superscripts are different (P < 0.05).
Dietary 10,12 CLA Induces Ovary- and E-Independent Mammary Ductal Growth.
Surprisingly, we found that mice ovariectomized at 21 d and then fed 10,12 CLA for another 21 d had significant elongation of the ductal network (P < 0.05; Fig. 2) that extended to the supramammary lymph node, comparable to that typical in ovary-intact females around 33 d of age (25). The ductal morphology was characterized by enlarged, bifurcated, and proliferative terminal end buds (TEB) (Fig. 2E) that contrasted to the blunted, quiescent ductal termini in control-fed females (Fig. 2A). The 10,12 CLA-induced TEB development was evident just 7 d after commencement of the diet (Fig. 2A). Ductal elongation in response to 10,12 CLA in OVX mice (Fig. 2B) and the overall ductal area (Fig. 2C) increased over time to reach a maximum by 35 d (P < 0.05; Fig. 2), which was sustained at 42 and 63 d. The MG of mice fed 10,12 CLA also had a lower mass and adiposity after they ingested 10,12 CLA for only 7 d and increased liver mass by 35 d of age (P < 0.05; Fig. S2). Given that allometric growth in the MG was originally defined as “a growth coefficient relative to metabolic size” (13), we used this approach to establish whether 10,12 CLA-stimulated ductal growth in OVX females was allometric. Indeed, OVX mice fed 10,12 CLA had a growth coefficient of 3.95 compared with 0.60 for control-fed mice (Fig. 2D). These values are similar to those reported for allometric growth in ovary-intact mice and for isometric growth in OVX mice, respectively (13).
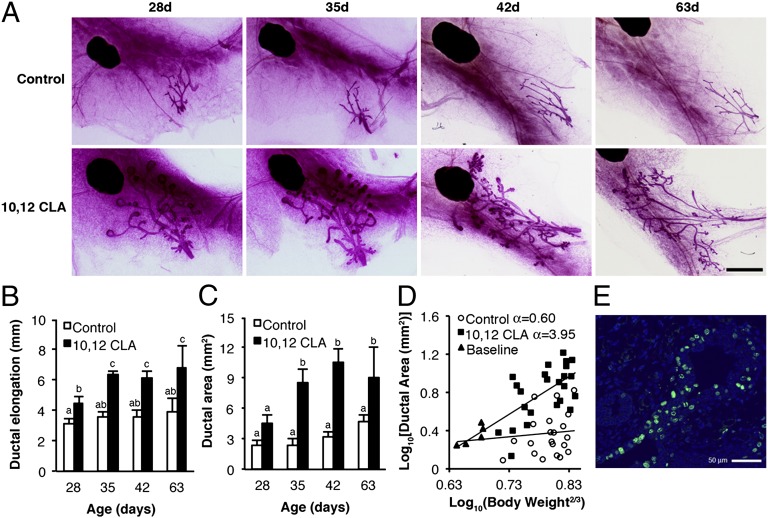
Fig. 2.
Dietary 10,12 CLA stimulates mammary gland growth in OVX peripubertal mice. (A) Representative mammary gland whole mounts from OVX Balb/cJ mice fed either the control or the 10, 12 CLA diet from 22 d and euthanized at 28, 35, 42, or 63 d. (Scale bar, 2 mm.) (B) Ductal elongation measured per Fig. 1. (C) Ductal area measured as the polygon area surrounding the ductal network. (D) Linear regression of log10 (Ductal area) and log10 (Body weight2/3) from OVX mice fed either the control or the 10,12 CLA diet from 22 d until euthanized at 28, 35, or 42 d of age. A subset of mice was euthanized at 21 d (Baseline). Ninety-five percent confidence limits for control and 10,12 CLA groups were −0.85–2.04 and 2.62–5.28, respectively. (E) Representative terminal end bud from a 42-d-old OVX mouse fed 10,12 CLA for 21 d. Proliferating cells were detected by 5-ethynyl-2′-deoxyuridine histochemistry (green), overlaid with DAPI (blue). Data are means ± SEM (n = 4–10/group). a,b,cMeans with different superscripts are different (P < 0.05).
We also determined whether this effect of 10,12 CLA was strain-specific by feeding 10,12 CLA to 129SVE, C57BL/6, and FVB mice for 21 d post ovariectomy. The 10,12 CLA-induced MG phenotype differed subtly between strains. Ductal elongation increased approximately twofold in Balb/cJ mice (P < 0.05; Fig. S3I) whereas ductal area nearly doubled in 129SVE mice (P < 0.05; Fig. S3J). Similarly, epithelial area increased in 10,12 CLA-fed FVB mice (P < 0.05; Fig. S3M). There was no change in ductal elongation or area in C57BL/6 mice fed 10,12 CLA. Mass of the MG was reduced by 10,12 CLA in all strains whereas liver mass increased consistently (P < 0.05; Fig. S3 K and L).
Given the ovary independence of this growth stimulation, we also assessed whether dietary 10,12 CLA stimulated ductal elongation in the MG of peripubertal male 129SVE mice. Males of this strain, unlike Balb/cJ, maintain a ductal rudiment about the nipple. Indeed, ductal area was increased after 21 d of dietary 10,12 CLA versus the control diet (P < 0.05; Fig. 3). The amount of outgrowth induced by 10,12 CLA was similar to that recorded in OVX females.
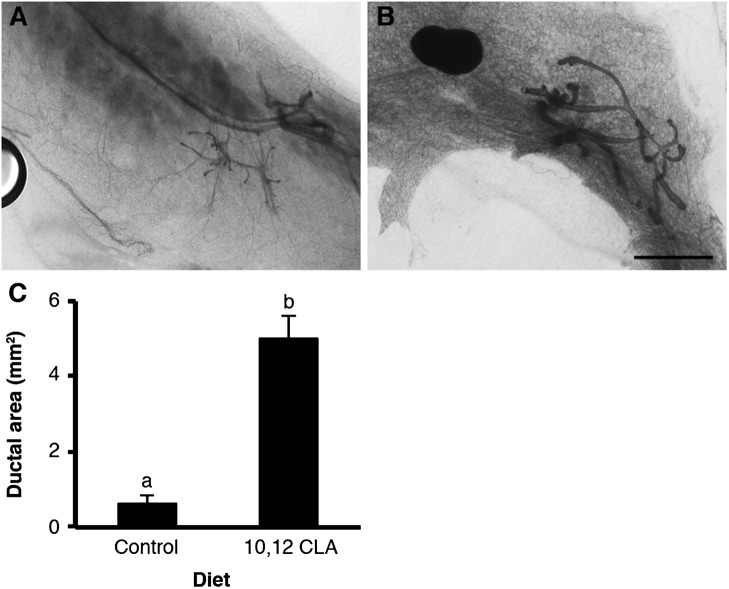
Fig. 3.
Dietary 10,12 CLA stimulates the mammary glands of peripubertal male mice. Representative mammary gland whole mounts from 129SVE male mice weaned onto either the (A) control or the (B) 10,12 CLA diet at 21 d and euthanized at 43 d. (Scale bar, 2 mm.) (C) Ductal area determined per Fig. 2. Data are means ± SEM (n = 7/group). a,bMeans with different superscripts are different (P < 0.05).
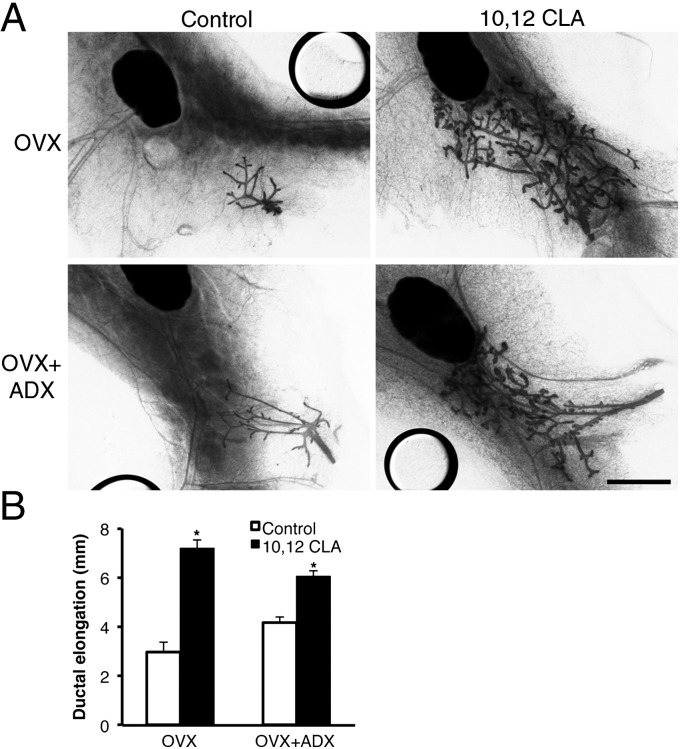
To further confirm the E independence of this diet-induced phenotype, we either blocked estrogen receptor (ER) activity in 10,12 CLA-fed OVX mice by coadministering ICI 182,780 or inhibited aromatization by coadministering letrozole. Neither compound affected 10,12 CLA-induced ductal elongation (P < 0.05; Fig. 4). The efficacy of ICI 182,780 was confirmed by its ability to suppress E-induced uterotrophy (P < 0.05; Fig. S4). Any potential for adrenal-stimulated growth was excluded when similar responses to 10,12 CLA were recorded in adrenalectomized, OVX female mice (P < 0.05; Fig. 5), despite lower corticosterone levels (P < 0.05; Fig. S4). Combined, our findings clearly establish that dietary 10,12 CLA induces E-independent allometric mammary growth.
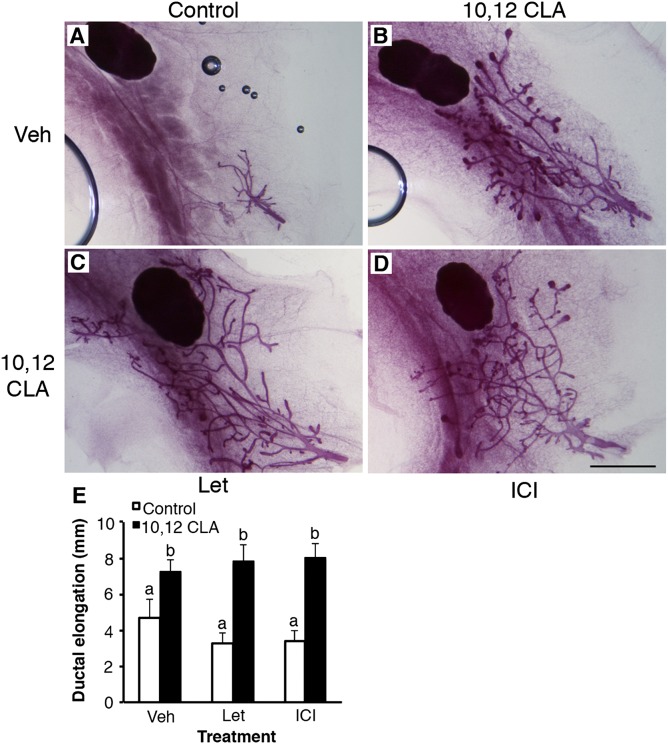
Fig. 4.
Ovary-independent mammary growth induced by dietary 10,12 CLA is not mediated by the E receptor or endogenous E biosynthesis. Representative mammary gland whole mounts from ovariectomized Balb/cJ mice fed either the (A) control or (B) 10,12 CLA diet from 22 d and euthanized at 42 d. Mice were coadministered daily injections of (A and B) sesame oil vehicle (Veh), (C) letrozole (Let), or (D) ICI 182,780 (ICI). (Scale bar is 2 mm.) (E) Ductal elongation measured per Fig. 1. Data are means ± SEM (n = 4–6/group). a,bMeans with different superscripts are different (P < 0.05).
Fig. 5.
Dietary 10,12 CLA stimulates mammary growth in peripubertal mice independently of both the ovaries and the adrenal glands. (A) Representative mammary gland whole mounts from Balb/cJ mice either ovariectomized (OVX) or ovariectomized and adrenalectomized (OVX+ADX), at 21 d. Mice were then fed either the control or the 10,12 CLA diet for a further 21 d. (Scale bar, 2 mm.) (B) Ductal elongation measured per Fig. 1. Data are means ± SEM (n = 3–5/group). *Within a surgical treatment group control-fed and 10,12 CLA-fed mice are different (P < 0.05).
Diet Induces Mammary Growth via Metabolic Dysregulation and the Insulin/IGF-I Axis.
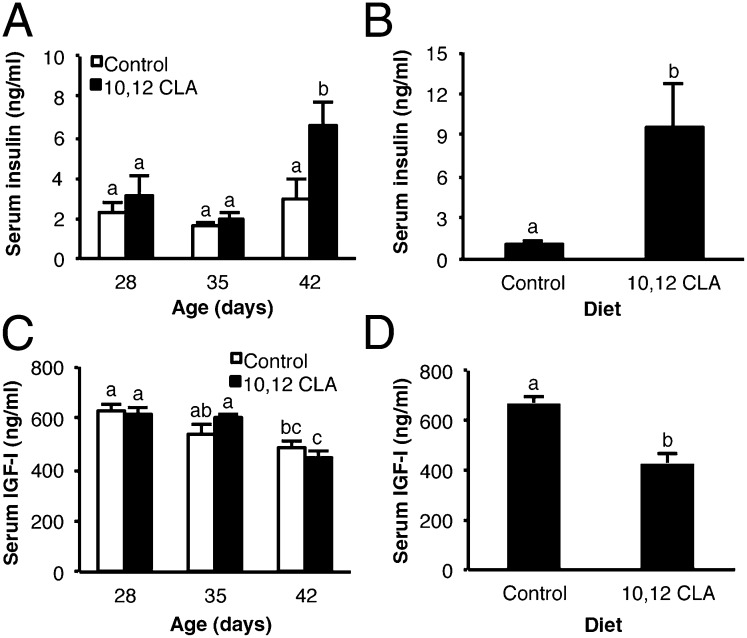
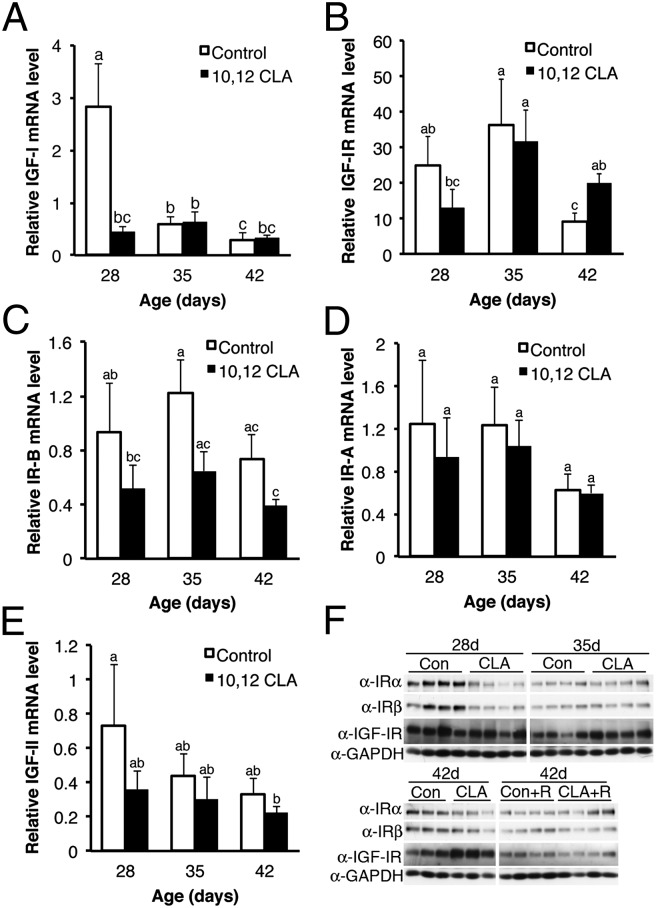
We considered which aspects of diet-induced metabolic dysregulation could induce allometric mammary growth where insulin-responsive IGF-I signaling regulates ductal growth (14). Intake of 10,12 CLA increased serum insulin in OVX female Balb/cJ and male 129SVE mice by 42 or 43 d of age, respectively (P < 0.05; Fig. 6 A and B). By contrast, 10,12 CLA fed to OVX mice did not alter serum IGF-I levels that independently declined with age (P < 0.05; Fig. 6C). Interestingly, serum IGF-I levels in male 129SVE mice were lower after the 10,12 CLA diet (P < 0.05; Fig. 6D). Given key roles for local IGF axis components in the MG (14), we also examined their expression following dietary 10,12 CLA. Although there was no main effect of diet on IGF-I mRNA levels in the MG (P = 0.08), 10,12 CLA suppressed its expression at 28 d (P < 0.05; Fig. 7A). The level of insulin-like growth factor-I receptor (IGF-IR) mRNA in the MG of 10,12 CLA-fed females was elevated at 42 d, but not at 28 or 35 d of age (P < 0.05; Fig. 7B). There was a significant negative main effect of 10,12 CLA on insulin receptor (IR)-B mRNA levels (P < 0.05; Fig. 7C), whereas there was no main effect of diet on IR-A mRNA levels, although they decreased with age (P < 0.05; Fig. 7D). There was a trend toward a suppressive main effect of 10,12 CLA on IGF-II mRNA levels (Fig. 7E; P = 0.06).
Fig. 6.
Dietary 10,12 CLA elevates serum insulin in OVX peripubertal mice. Serum insulin (A and B) and IGF-I (C and D) were measured by ELISA. (A and C) OVX Balb/cJ mice were fed either the control or the 10,12 CLA diet from 22 d to 28, 35, or 42 d. (B and D) Male 129SVE mice were fed either the control or the 10,12 CLA diet from 21 to 43 d. Data are means ± SEM (n = 4–5/group). a,b,cMeans with different superscripts are different (P < 0.05).
Fig. 7.
Dietary 10,12 CLA alters gene and protein expression profiles for components of the insulin/IGF axis in mammary glands of OVX mice. Total RNA from the mammary glands of OVX Balb/cJ mice fed either the control or the 10,12 CLA diet from 22 d to 28, 35, or 42 d was analyzed for mRNA expression of (A) IGF-I, (B) IGF-IR, (C) IR-B, (D) IR-A, and (E) IGF-II. Data are means ± SEM (n = 3–7/group). a,b,cMeans with different superscripts are different (P < 0.05). (F) Western blot analysis of total proteins from the mammary glands of OVX Balb/cJ mice fed either the control or the 10,12 CLA diet from 22 d to 28, 35, or 42 d for IRα, IRβ, IGF-IR, and GAPDH. A subset of OVX mice was administered rosiglitazone (R) concurrent with the diets.
Functionality of the insulin/IGF axis also occurs at the posttranscriptional level (26). Western blot analysis revealed that IGF-IR protein abundance was increased in the MG of 35- and 42-d-old OVX mice fed 10,12 CLA (P < 0.05; Fig. 7F and Fig. S5A). This increase was manifest in the epithelial compartment, given that IGF-IR abundance was not altered in the contralateral epithelium-free mammary fat pad (P > 0.05; Fig. S5B). Concordant with the mRNA expression results, both IRα and IRβ protein levels were reduced in the intact MG of 10,12 CLA-fed mice at 42 d of age (P < 0.05; Fig. 7 and Fig. S5 C–F).
Insulin Sensitization and IGF-IR Blockade Reverses Diet-Induced Mammary Growth.
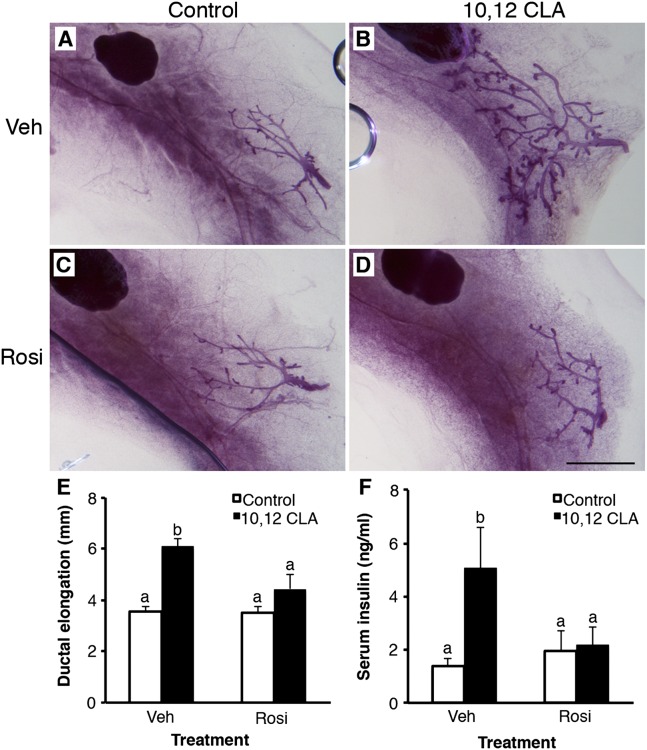
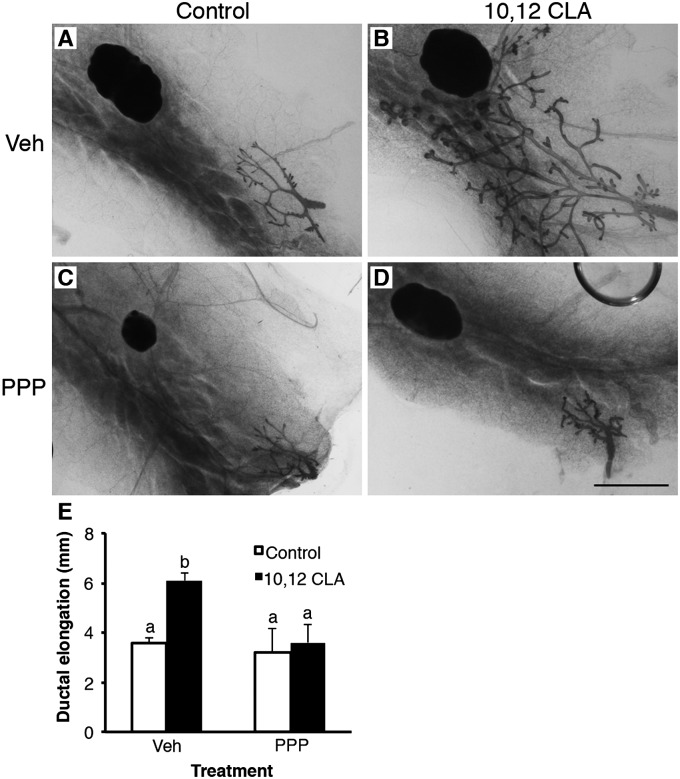
Given that many aspects of the metabolic syndrome can be reversed by therapeutic sensitization to insulin, we coadministered the peroxisome proliferator-activated receptor-γ (PPARγ) agonist rosiglitazone (Rosi) to OVX mice fed the 10,12 CLA diet until 42 d of age. Ductal growth induced by 10,12 CLA was abolished by Rosi (P < 0.05; Fig. 8 A–E). In parallel, Rosi ameliorated the effect of 10,12 CLA on MG mass (P < 0.05; Fig. S6), negated the 10,12 CLA-stimulated hyperinsulinemia (P < 0.05; Fig. 8F), and abrogated the elevated IGF-IR protein levels in the MG (P < 0.05; Fig. 7F and Fig. S5A). Given these data and the aforementioned changes in components of the insulin/IGF-I axis including increased IGF-IR abundance, we next coadministered the IGF-IR inhibitor picropodophyllotoxin (PPP) to OVX mice fed the control or 10,12 CLA diet. Treatment with PPP completely blocked 10,12 CLA-induced ductal elongation (P < 0.05; Fig. 9), which was similar to the effect of Rosi. These data indicate that E-independent growth stimulation occurs alongside altered IGF-IR function and dysregulated insulin action.
Fig. 8.
PPARγ agonist rosiglitazone inhibits ovary-independent 10,12 CLA-induced mammary gland growth. (A–D) Representative mammary whole mounts from ovariectomized Balb/cJ mice fed either the control (A, C) or the 10,12 CLA diet (B and D) from 22 d and coadministered either DMSO (Veh; A and B) or rosiglitazone (Rosi; C and D) for a further 21 d. (Scale bar, 2 mm.) (E) Ductal elongation measured per Fig. 1. (F) Serum insulin concentrations. Data are means ± SEM (n = 3–7/group). a,bMeans with different superscripts are different (P < 0.05).
Fig. 9.
Blockade of IGF-IR abrogates ovary-independent 10,12 CLA-induced mammary growth. (A–D) Representative mammary whole mounts from ovariectomized Balb/cJ mice fed either the control (A, C) or the 10,12 CLA diet (B and D) from 22 d and coadministered either DMSO (Veh; A and B) or PPP (C and D) for a further 21 d. (Scale bar, 2 mm.) (E) Ductal elongation measured per Fig. 1. Data are means ± SEM (n = 3–6/group). a,bMeans with different superscripts are different (P < 0.05).
Diet-Induced Mammary Growth Facilitates the Growth of Transformed Epithelium.
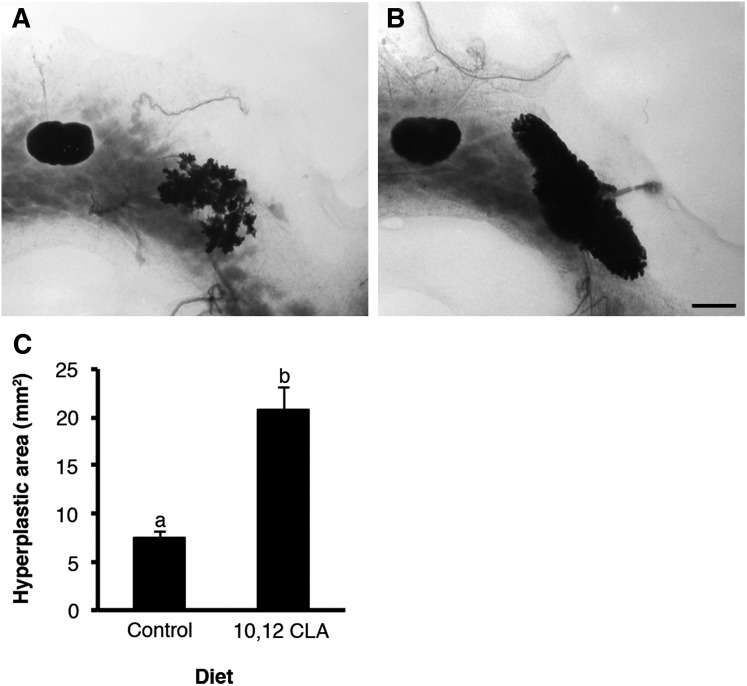
We sought to establish whether 10,12 CLA-induced growth of the normal MG would also stimulate growth of genetically transformed mammary epithelium in polyomavirus middle T-antigen (PyMT) transgenic mice that are predisposed to early onset mammary tumors during allometric growth (27). Heterozygous PyMT mice were ovariectomized at 21 d and fed the control or 10,12 CLA diets for 21 d. Whole-mount analysis highlighted that the 10,12 CLA diet markedly increased the area of epithelial hyperplasia (P < 0.05; Fig. 10) to an extent that was proportional to that recorded in the MG of OVX females (Fig. 2). There was no difference in the histopathology of these hyperplasias.
Fig. 10.
Dietary 10,12 CLA accelerates tumorigenesis in transgenic mice expressing the PyMT antigen. (A and B) Representative mammary whole mounts from ovariectomized mice heterozygous for PyMT fed either the (A) control or (B) 10,12 CLA diet from 22 to 42 d. (Scale bar, 2 mm.) (C) Average total hyperplastic area. Data are means ± SEM (n = 5/group). a,bMeans with different superscripts are different (P < 0.05).
Discussion
Dogma asserts that the mammary ducts undergo early allometric growth around the onset of puberty in response to an increase in ovarian E synthesis (12, 14), consistent with the widespread demonstration that ovariectomy abolishes mammary growth. Decades of research have established that ductal growth reflects the concerted actions of E, progesterone (P), and GH (12). Specifically, GH stimulates the local synthesis of IGF-I by the mammary stroma (28) whereas E enhances this effect of GH (29) by activating GH-inducible stromal ER (30). In turn, IGF-I stimulates TEB formation (14) that is enhanced by E (29). Maximal growth of the mammary ducts in response to IGF-I ultimately depends on facilitation by either E (29) or P, while the combination of E, P, and IGF-I is essential for alveolar development (31).
Here we reveal that allometric ductal growth in OVX peripubertal mice initiates in response to a dietary component, namely 10,12 CLA. Most notably, this growth is independent of ovarian stimulation, any action of E via its receptor, or systemic E biosynthesis. Identification of this E-independent mechanism is a major extension of studies showing that ovary-intact mice fed 10,12 CLA had increased TEB number (32) or premature lobuloalveolar development (33).
Epidemiological studies have consistently highlighted an as-yet-unclear relationship between early life diet (34, 35), obesity (3), BMI (2), and breast cancer risk. Dietary fat intake has generally failed to account for breast cancer risk across numerous epidemiological analyses (36), although consumption of hydrogenated fats was recently associated with a greater risk (37). We found that dietary 10,12 CLA induced allometric growth coincident with aspects of metabolic dysregulation including lipoatrophy, hyperinsulinemia, and hepatic steatosis, whereas others have also described adipose inflammation (22) in response to this diet. We posit that dietary 10,12 CLA confers its effects on the MG by inducing aspects of the metabolic syndrome that have been repeatedly linked to increased breast cancer risk (38) concurrent with the obesity epidemic, particularly via its effects in young girls (6). Our finding that the MG of various strains of mice differed in their response to 10,12 CLA despite having similar metabolic changes supports a potential genetic basis for how diet and/or diet-induced metabolic dysregulation affects breast cancer risk in different populations (39).
A candidate role for the insulin/IGF-I axis in facilitating this diet-induced phenotype was manifest at several levels. We suggest that hyperinsulinemia in response to dietary 10,12 CLA may have contributed to increased epithelial growth, given that systemic and local IGF-I levels were unaffected. Such a mechanism would coincide with the ability of hyperinsulinemia to stimulate growth of the mammary ducts and mammary tumors in a nonobese model of type 2 diabetes (40). Furthermore, postmenopausal women with elevated insulin levels are at a higher risk for developing breast cancer independently of any effect of circulating E (41) whereas breast cancer patients with type 2 diabetes have a poorer outcome (42). Our finding that the insulin-sensitizing actions of Rosi completely reversed the effects of 10,12 CLA on mammary growth lends support to the recent proposal that metformin may confer benefits as an adjuvant breast cancer therapy (38). Despite these lines of evidence, any potential for insulin to promote normal mammary development remains unclear given that IGF-I–deficient mice fail to undergo mammary growth (14).
Our data are also consistent with a potential role for cross-talk between insulin and IGF-I receptors during MG development (43). Interestingly, levels of the IGF-IR protein, more so than expression of its mRNA, were increased in the MG of 10,12 CLA-fed mice. A similar situation was recorded during pancreatic neuroendocrine carcinogenesis whereby levels of IR and IGF-IR mRNA were increased only slightly versus a pronounced induction at the translational level (26). Furthermore, functionality of the IGF-IR is required for the diet-induced mammary growth recorded here as highlighted by the fact that it was reversed by PPP, consistent with the essential role of the IGF-IR in mediating normal mammary ductal development (44). These data align with recent findings from a kinome scan pointing to an insulin/IGF-I receptor-dependent pathway for E-independent growth of breast cancer cells (45), reinforcing key roles for the IGF-IR in breast cancer and its therapeutic targeting that likely converges with its many candidate roles during the metabolic syndrome (46, 47). Whether certain dietary constituents such as 10,12 CLA or metabolic dysregulation impinge on mammary growth during puberty via the synergistic relationships that exist between IGF-I and E (29) or P (31) remains to be established.
In conclusion, our findings highlight a striking link between diet, metabolic dysregulation, and allometric MG growth that is independent of estrogenic stimulation. These results lend support to increasing evidence suggesting a relationship between breast cancer risk and early life events that clearly include dietary components and their effects on aspects of metabolic dysregulation.
Materials and Methods
All details regarding mice, strains, diets, surgery, treatments, and housing conditions are outlined in SI Materials and Methods. Diets were isocaloric and based on a modified AIN93G diet that contained 15% fat by weight, with 10,12 CLA replacing 1% fat by weight. The 10,12 CLA content in the experimental diet was 6.81% of total fatty acids, whereas it was undetectable in the control diet (Table S3). Mice were fed the control diet for 1 d following ovariectomy and then randomly assigned to either the control diet or 10,12 CLA. Daily injections started concurrently with diet assignments. All details of the analysis of mammary gland development, circulating hormones, fatty acids, gene expression (Table S4), proteins, and statistics are outlined in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Trish Berger for letrozole, Dr. Alexander Borowsky for PyMT mice and the University of California, Davis Animal Science Vivarium staff and Dr. Agnese Mariotti (Centre Pluridisciplinaire d’Oncologie, Lausanne, Switzerland) for their contributions. This research was supported in part by a grant from Dairy Management Inc. (to R.C.H. and A.L.L), by the University of California, Davis Cancer Center (R.C.H.), by United States Department of Defense Postdoctoral Award W81XWH-09-1-0657 (to L.A.), and by a University of California, Davis Jastro Graduate Research Award (to G.E.B).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210527109/-/DCSupplemental.
References
- 1.Michels KB, Rosner BA, Chumlea WC, Colditz GA, Willett WC. Preschool diet and adult risk of breast cancer. Int J Cancer. 2006;118:749–754. doi: 10.1002/ijc.21407. [DOI] [PubMed] [Google Scholar]
- 2.Ruder EH, Dorgan JF, Kranz S, Kris-Etherton PM, Hartman TJ. Examining breast cancer growth and lifestyle risk factors: Early life, childhood, and adolescence. Clin Breast Cancer. 2008;8:334–342. doi: 10.3816/CBC.2008.n.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baer HJ, et al. Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: A prospective cohort study. Breast Cancer Res. 2005;7:R314–R325. doi: 10.1186/bcr998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkey CS, Frazier AL, Gardner JD, Colditz GA. Adolescence and breast carcinoma risk. Cancer. 1999;85:2400–2409. doi: 10.1002/(sici)1097-0142(19990601)85:11<2400::aid-cncr15>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Himes JH. Examining the evidence for recent secular changes in the timing of puberty in US children in light of increases in the prevalence of obesity. Mol Cell Endocrinol. 2006;254–255:13–21. doi: 10.1016/j.mce.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Lee JM, et al. Weight status in young girls and the onset of puberty. Pediatrics. 2007;119:e624–e630. doi: 10.1542/peds.2006-2188. [DOI] [PubMed] [Google Scholar]
- 7.Aksglaede L, Sørensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: The Copenhagen Puberty Study. Pediatrics. 2009;123:e932–e939. doi: 10.1542/peds.2008-2491. [DOI] [PubMed] [Google Scholar]
- 8.Ronckers CM, Erdmann CA, Land CE. Radiation and breast cancer: A review of current evidence. Breast Cancer Res. 2005;7:21–32. doi: 10.1186/bcr970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haslam SZ. Age as a modifying factor of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in the Lewis rat. Int J Cancer. 1979;23:374–379. doi: 10.1002/ijc.2910230316. [DOI] [PubMed] [Google Scholar]
- 10.Elias SG, Peeters PH, Grobbee DE, van Noord PA. Breast cancer risk after caloric restriction during the 1944–1945 Dutch famine. J Natl Cancer Inst. 2004;96:539–546. doi: 10.1093/jnci/djh087. [DOI] [PubMed] [Google Scholar]
- 11.Berkey CS, et al. Prospective study of growth and development in older girls and risk of benign breast disease in young women. Cancer. 2011;117:1612–1620. doi: 10.1002/cncr.25692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hovey RC, Trott JF, Vonderhaar BK. Establishing a framework for the functional mammary gland: From endocrinology to morphology. J Mammary Gland Biol Neoplasia. 2002;7:17–38. doi: 10.1023/a:1015766322258. [DOI] [PubMed] [Google Scholar]
- 13.Flux DS. Growth of the mammary duct system in intact and ovariectomized mice of the CHI strain. J Endocrinol. 1954;11:223–237. doi: 10.1677/joe.0.0110223. [DOI] [PubMed] [Google Scholar]
- 14.Ruan W, Kleinberg DL. Insulin-like growth factor I is essential for terminal end bud formation and ductal morphogenesis during mammary development. Endocrinology. 1999;140:5075–5081. doi: 10.1210/endo.140.11.7095. [DOI] [PubMed] [Google Scholar]
- 15.Hovey RC, Aimo L. Diverse and active roles for adipocytes during mammary gland growth and function. J Mammary Gland Biol Neoplasia. 2010;15:279–290. doi: 10.1007/s10911-010-9187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. American Heart Association National Heart, Lung, and Blood Institute Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 17.Bremer AA, Mietus-Snyder M, Lustig RH. Toward a unifying hypothesis of metabolic syndrome. Pediatrics. 2012;129:557–570. doi: 10.1542/peds.2011-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung MO, Yoon SH, Jung MY. Effects of temperature and agitation rate on the formation of conjugated linoleic acids in soybean oil during hydrogenation process. J Agric Food Chem. 2001;49:3010–3016. doi: 10.1021/jf001296v. [DOI] [PubMed] [Google Scholar]
- 19.Lock AL, Bauman DE. Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids. 2004;39:1197–1206. doi: 10.1007/s11745-004-1348-6. [DOI] [PubMed] [Google Scholar]
- 20.Halade GV, Rahman MM, Fernandes G. Differential effects of conjugated linoleic acid isomers in insulin-resistant female C57Bl/6J mice. J Nutr Biochem. 2010;21:332–337. doi: 10.1016/j.jnutbio.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Clément L, et al. Dietary trans-10,cis-12 conjugated linoleic acid induces hyperinsulinemia and fatty liver in the mouse. J Lipid Res. 2002;43:1400–1409. doi: 10.1194/jlr.m20008-jlr200. [DOI] [PubMed] [Google Scholar]
- 22.Poirier H, Shapiro JS, Kim RJ, Lazar MA. Nutritional supplementation with trans-10, cis-12-conjugated linoleic acid induces inflammation of white adipose tissue. Diabetes. 2006;55:1634–1641. doi: 10.2337/db06-0036. [DOI] [PubMed] [Google Scholar]
- 23.Larsen TM, Toubro S, Astrup A. Efficacy and safety of dietary supplements containing CLA for the treatment of obesity: Evidence from animal and human studies. J Lipid Res. 2003;44:2234–2241. doi: 10.1194/jlr.R300011-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Tricon S, et al. Opposing effects of cis-9,trans-11 and trans-10,cis-12 conjugated linoleic acid on blood lipids in healthy humans. Am J Clin Nutr. 2004;80:614–620. doi: 10.1093/ajcn/80.3.614. [DOI] [PubMed] [Google Scholar]
- 25.Hovey RC, et al. Effects of neonatal exposure to diethylstilbestrol, tamoxifen, and toremifene on the BALB/c mouse mammary gland. Biol Reprod. 2005;72:423–435. doi: 10.1095/biolreprod.104.029769. [DOI] [PubMed] [Google Scholar]
- 26.Ulanet DB, Ludwig DL, Kahn CR, Hanahan D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc Natl Acad Sci USA. 2010;107:10791–10798. doi: 10.1073/pnas.0914076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: A transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walden PD, Ruan W, Feldman M, Kleinberg DL. Evidence that the mammary fat pad mediates the action of growth hormone in mammary gland development. Endocrinology. 1998;139:659–662. doi: 10.1210/endo.139.2.5718. [DOI] [PubMed] [Google Scholar]
- 29.Ruan W, Catanese V, Wieczorek R, Feldman M, Kleinberg DL. Estradiol enhances the stimulatory effect of insulin-like growth factor-I (IGF-I) on mammary development and growth hormone-induced IGF-I messenger ribonucleic acid. Endocrinology. 1995;136:1296–1302. doi: 10.1210/endo.136.3.7867584. [DOI] [PubMed] [Google Scholar]
- 30.Feldman M, Ruan W, Tappin I, Wieczorek R, Kleinberg DL. The effect of GH on estrogen receptor expression in the rat mammary gland. J Endocrinol. 1999;163:515–522. doi: 10.1677/joe.0.1630515. [DOI] [PubMed] [Google Scholar]
- 31.Ruan W, Monaco ME, Kleinberg DL. Progesterone stimulates mammary gland ductal morphogenesis by synergizing with and enhancing insulin-like growth factor-I action. Endocrinology. 2005;146:1170–1178. doi: 10.1210/en.2004-1360. [DOI] [PubMed] [Google Scholar]
- 32.Ip MM, et al. The t10,c12 isomer of conjugated linoleic acid stimulates mammary tumorigenesis in transgenic mice over-expressing erbB2 in the mammary epithelium. Carcinogenesis. 2007;28:1269–1276. doi: 10.1093/carcin/bgm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foote MR, Giesy SL, Bernal-Santos G, Bauman DE, Boisclair YR. t10,c12-CLA decreases adiposity in peripubertal mice without dose-related detrimental effects on mammary development, inflammation status, and metabolism. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1521–R1528. doi: 10.1152/ajpregu.00445.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers IS, et al. Diet throughout childhood and age at menarche in a contemporary cohort of British girls. Public Health Nutr. 2010;13:2052–2063. doi: 10.1017/S1368980010001461. [DOI] [PubMed] [Google Scholar]
- 35.Fuemmeler BF, Pendzich MK, Tercyak KP. Weight, dietary behavior, and physical activity in childhood and adolescence: Implications for adult cancer risk. Obes Facts. 2009;2:179–186. doi: 10.1159/000220605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyd NF, et al. Dietary fat and breast cancer risk revisited: A meta-analysis of the published literature. Br J Cancer. 2003;89:1672–1685. doi: 10.1038/sj.bjc.6601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, John EM, Horn-Ross PL, Ingles SA. Dietary fat, cooking fat, and breast cancer risk in a multiethnic population. Nutr Cancer. 2008;60:492–504. doi: 10.1080/01635580801956485. [DOI] [PubMed] [Google Scholar]
- 38.Ibarra-Drendall C, Dietze EC, Seewaldt VL. Metabolic syndrome and breast cancer risk: Is there a role for metformin? Curr Breast Cancer Rep. 2011;3:142–150. doi: 10.1007/s12609-011-0050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rose DP, Haffner SM, Baillargeon J. Adiposity, the metabolic syndrome, and breast cancer in African-American and white American women. Endocr Rev. 2007;28:763–777. doi: 10.1210/er.2006-0019. [DOI] [PubMed] [Google Scholar]
- 40.Novosyadlyy R, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010;70:741–751. doi: 10.1158/0008-5472.CAN-09-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gunter MJ, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Currie CJ, et al. Mortality after incident cancer in people with and without type 2 diabetes: Impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowzee AM, Ludwig DL, Wood TL. Insulin-like growth factor type 1 receptor and insulin receptor isoform expression and signaling in mammary epithelial cells. Endocrinology. 2009;150:3611–3619. doi: 10.1210/en.2008-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnette SG, Hadsell DL. Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology. 2001;142:4937–4945. doi: 10.1210/endo.142.11.8500. [DOI] [PubMed] [Google Scholar]
- 45.Fox EM, et al. A kinome-wide screen identifies the insulin/IGF-I receptor pathway as a mechanism of escape from hormone dependence in breast cancer. Cancer Res. 2011;71:6773–6784. doi: 10.1158/0008-5472.CAN-11-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: An update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 47.Gallagher EJ, LeRoith D. Minireview: IGF, insulin, and cancer. Endocrinology. 2011;152:2546–2551. doi: 10.1210/en.2011-0231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.