Abstract
An attractive strategy to overcome multidrug resistance in cancer chemotherapy is to suppress P-glycoprotein (P-gp), which is a pump overproduced in cancer cells to remove cytotoxic drugs from cells. In the present study, a Ca2+-permeable channel TRPC5 was found to be overproduced together with P-gp in adriamycin-resistant breast cancer cell line MCF-7/ADM. Suppressing TRPC5 activity/expression reduced the P-gp induction and caused a remarkable reversal of adriamycin resistance in MCF-7/ADM. In an athymic nude mouse model of adriamycin-resistant human breast tumor, suppressing TRPC5 decreased the growth of tumor xenografts. Nuclear factor of activated T cells isoform c3 (NFATc3) was the transcriptional factor that links the TRPC5 activity to P-gp production. Together, we demonstrated an essential role of TRPC5–NFATc3–P-gp signaling cascade in P-gp induction in drug-resistant cancer cells.
A serious problem for cancer chemotherapy is the development of multidrug resistance in tumor cells. Many tumor cells overproduce a 170-kDa protein named P-glycoprotein (P-gp) during treatment. P-gp, coded by mdr1 gene, functions to pump different classes of cytotoxic drugs from tumor cells, rendering the tumor cells resistant to multiple chemotherapeutic drugs (1). P-gp overproduction has been reported in many tumors and in vitro-selected, drug-resistant cell lines (1). In clinical medicine, an attractive approach to overcome the multidrug resistance in cancer chemotherapy is to inhibit P-gp activity (1). In this regard, many P-gp antagonists (also named P-gp modulators) have been developed to inhibit P-gp activity. These modulators may be of clinical importance, because their coadministration with chemotherapeutic drugs has the potential to improve drug uptake into the P-gp–overproducing tumor cells, thereby reversing the multidrug resistance of tumor cells (1). Unfortunately, most of these drugs are either too toxic or induce intolerable pharmacokinetic interactions (1). Their clinical use is limited. It is highly desirable to find new agents that are more effective and less toxic.
Cytosolic Ca2+ ([Ca2+]i) is an important signal for transcriptional regulation of P-gp (2–4). Research has shown that chelation of Ca2+ by 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid abrogates the P-gp induction exerted by many drugs, whereas thapsigargin, which increases [Ca2+]i by inhibiting the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, enhances P-gp production (2, 3). Furthermore, a panel of different Ca2+ channel antagonists was found to reduce the P-gp expression (4).
TRP channels are a group of cation channels that play key functional roles in diverse physiological processes, including thermosensation, vascular tone regulation, and bone formation (5). Several TRP isoforms, including TRPM8, TRPV6, TRPV1, and TRPM1, have been shown to be involved in cancer pathogenesis (6). It has been suggested that TRPM8, TRPV6, and TRPV1 are oncogenic, whereas TRPM1 performs a tumor-suppressing function (6). These TRP channels are all Ca2+-permeable (5). The role of these TRP channels in cancer may involve changes in [Ca2+]i (6). However, to date there is still no report on TRP channel involvement in multidrug resistance and/or P-gp production.
In the present study we explored the role of TRPC channels in P-gp production and drug resistance. TRPC5 expression was found to be substantially up-regulated in the adriamycin-resistant breast cancer cell line MCF-7/ADM. Inhibition of TRPC5 caused a marked reduction in P-gp expression, leading to a reversal of adriamycin resistance in MCF-7/ADM cells. In animal models, suppressing TRPC5 activity/expression reversed the adriamycin resistance of solid tumors that were formed by MCF-7/ADM cell inoculation.
Results
Up-Regulation of P-gp and TRPC5 in Adriamycin-Resistant Human Breast Cancer Cells MCF-7/ADM.
Adriamycin is a frequently used chemotherapeutic drug in the treatment of breast cancer (7). Adriamycin binds to DNA and thereby blocks DNA replication and transcription, causing cytotoxicity to tumor cells (8). Adriamycin-resistant human breast cancer cells (MCF-7/ADM) were obtained by treating MCF-7/WT cells with stepwise increasing concentrations of adriamycin over 8 mo. Western blot analysis was performed to determine the P-gp protein expression level. A very high level of P-gp expression (molecular mass ∼170 kDa) was detected in MCF-7/ADM cells, whereas only a low level of P-gp expression was detected in its parental line MCF-7/WT (Fig. 1A).
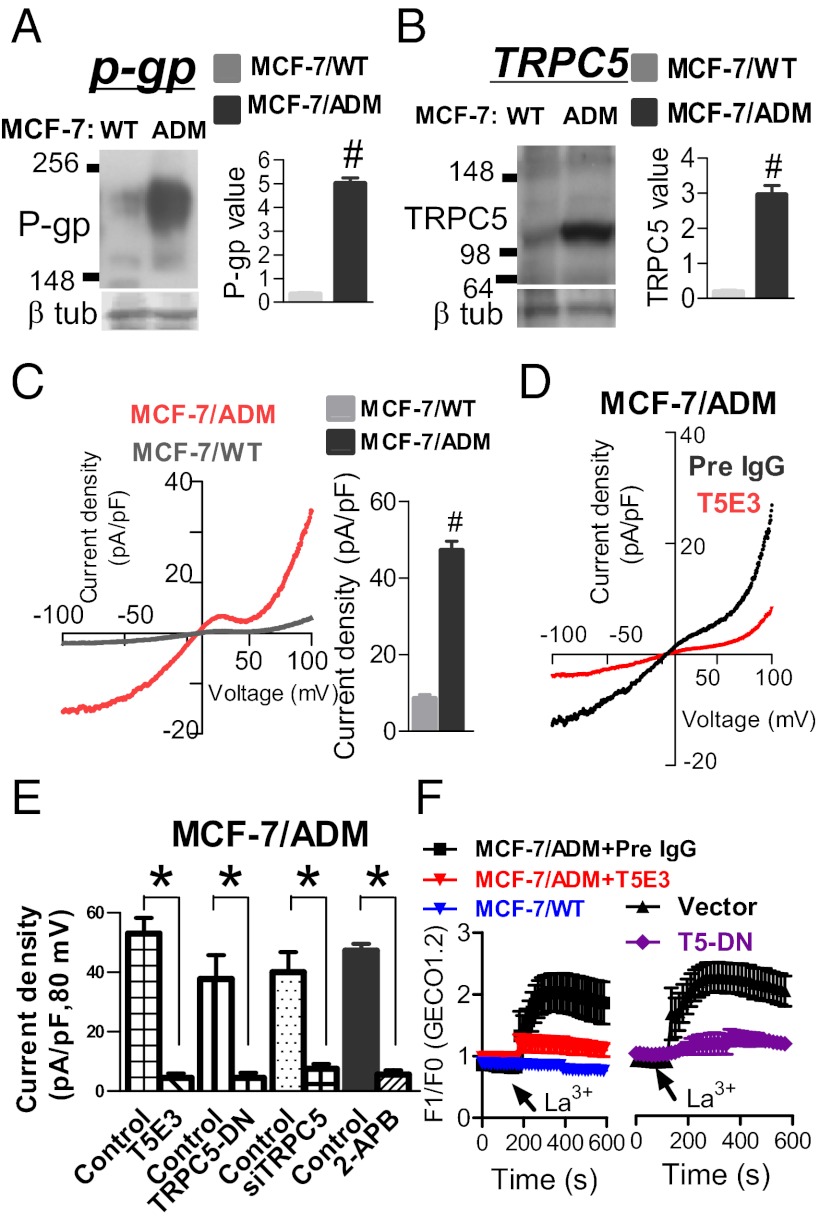
Fig. 1.
Up-regulation of TRPC5 and P-gp in MCF-7/ADM cells. (A and B) Representative immunoblots (Left) and data summary (Right) of P-gp (A) and TRPC5 expression (B). Left lanes in immunoblots are samples from MCF-7/WT cells (WT), whereas right lanes are samples from MCF-7/ADM cells (ADM). Lower in immunoblots is β-tubulin control. In data summary, relative expression is shown, in which intensity of bands for TRPC5 and P-gp was divided by corresponding internal control (β-tubulin). (C–E) Whole-cell patch clamp studies comparing TRPC5 activity under hypotonic conditions between MCF-7/WT and MCF-7/ADM cells (C), and effect of T5E3, TRPC5-DN, TRPC5-siRNA, and 2-APB in MCF-7/ADM cells under hypotonicity (D and E). Shown are representative whole-cell current–voltage relationships (Left in C and D) and summary of data at +80 mV under different conditions (Right in C and E). 2-APB, 60 μM; T5E3 or preimmune IgG, 20 μg/mL; TRPC5-DN or empty plasmid, 8 μg per well in six-well plates; TRPC5-siRNA or scrambled siRNA, 200 nM. Values are means ± SE of three to five experiments. #P < 0.05 vs. MCF-7/WT; *P < 0.05 vs. control. (F) [Ca2+]i measurement using GECO1.2. Shown are time courses of [Ca2+]i changes in response to La3+ (100 μM) in MCF-7/WT and MCF-7/ADM cells treated with or without T5E3 or TRPC5-DN.
Expression of TRPC proteins was examined. TRPC5 expression (molecular mass ∼110–120 kDa) was substantially higher in MCF-7/ADM cells than in MCF-7/WT cells (Fig. 1B). Immunostaining showed that TRPC5 was mostly localized at cell surface and in some puncta or vesicular structures near the cell surface (Fig. S1). The expression of several other TRPC isoforms, including TRPC1, TRPC3, TRPC4, and TRPC6, showed no difference between MCF-7/ADM and MCF-7/WT cells (Fig. S2). TRPC2 is a pseudogene in human, therefore it was not studied. In Western blots, β-tubulin was used as an internal control to ensure that a similar amount of proteins was loaded onto different gel lanes (Fig. 1 A and B).
Time course study showed that up-regulation of P-gp and TRPC5 could be found as early as 6 wk after adriamycin treatment (Fig. S3). The up-regulation became higher by week 9 and even higher at 8 mo (Fig. 1 A and B and Fig. S3). We also established a paclitaxel-resistant MCF-7 cell line (MCF-7/PTX) by treating MCF-7/WT cells with stepwise increasing concentrations of paclitaxel over 10 mo. The expression of P-gp and TRPC5 was also found to be markedly higher in MCF-7/PTX than in MCF-7/WT cells (Fig. S4).
Functional Presence of TRPC5 in MCF-7/ADM Cells.
Functional existence of TRPC5 was determined by patch clamp and [Ca2+]i measurement. TRPC5 is activated by hypotonicity (9). In patch clamp recording, the whole-cell current under hypotonicity was much larger in MCF-7/ADM than in MCF-7/WT cells (Fig. 1C). This current displayed a double-rectifying current–voltage relationship, which is typical of TRPC5 (10). The whole-cell current diminished in MCF-7/ADM cells that were pretreated with a TRPC5-specific blocking antibody, T5E3 (11) (Fig. 1 D and E). Application of 2-aminoethoxydiphenylborate (2-APB), a commonly used TRPC5 pharmacological antagonist, also reduced the whole-cell current (Fig. 1E). A dominant-negative TRPC5 construct (TRPC5-DN) was used to disrupt the function of endogenous TRPC5 (12), whereas a TRPC5-specific siRNA was used to suppress the expression of TRPC5. Transfection of MCF-7/ADM cells with TRPC5-DN or TRPC5-siRNA diminished the whole-cell current (Fig. 1E). As controls, pretreatment of the cells with preimmune IgG or transfection with an empty plasmid or a scrambled siRNA had little effect on the whole-cell current (Fig. 1E). Immunoblots confirmed that the TRPC5-siRNA could effectively knock down the expression of TRPC5 proteins by 73% ± 4% (Fig. S5).
Because P-gp could pump Ca2+-sensitive fluorescence dyes out of the cells (13), we could not use conventional dye Fura-2 or Fluo-4 to study TRPC5-mediated Ca2+ entry. Instead, a Ca2+-sensitive molecular construct was used (14). It is known that La3+ potentiates TRPC5 activity but inhibits many other Ca2+-permeable channels (15). Application of La3+ (100 μM) elicited a rise in [Ca2+]i in MCF-7/ADM but not in MCF-7/WT cells (Fig. 1F). The La3+-elicited [Ca2+]i rise in MCF-7/ADM cells was inhibited by T5E3 and TRPC5-DN, indicating the involvement of TRPC5 (Fig. 1F).
Effect of TRPC5 Suppression on P-gp Expression.
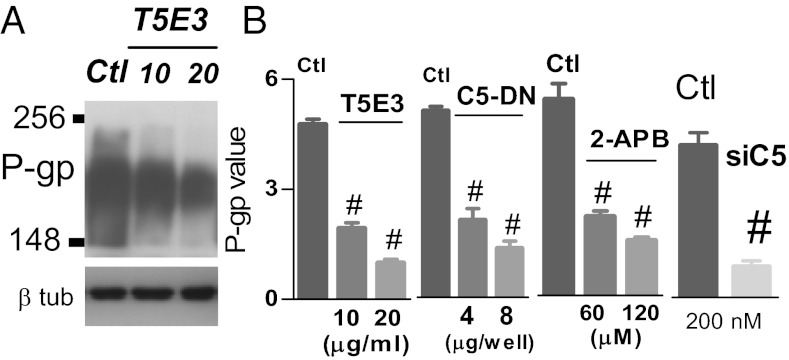
A possible role of TRPC5 in controlling P-gp expression was explored. Inhibition of TRPC5 by T5E3 (11) or 2-APB (16) suppressed the P-gp expression in a concentration-dependent manner in MCF-7/ADM cells (Fig. 2). TRPC5-DN and TRPC5-siRNA also caused a similar reduction in P-gp expression in MCF-7/ADM cells (Fig. 2). In contrast, knocking down P-gp had no effect on TRPC5 expression (Fig. S6). As a control for T5E3, preimmune IgG treatment had no effect on P-gp expression (Fig. 2). Mock transfection with an empty plasmid or a scrambled siRNA also had no effect on P-gp expression (Fig. 2). siRNAs targeted against TRPC1, TRPC3, TRPC4, TRPC6, stromal interaction molecule 1 (STIM1), and Orail were also developed (Fig. S7). Most of these siRNAs had no effect on P-gp expression (Fig. S7). The only exception was Orail-siRNA, which was able to cause a moderate reduction in P-gp expression (∼25%) (Fig. S7). In comparison, suppressing/inhibiting TRPC5 with T5E3, TRPC5-DN, or TRPC5-siRNA had a much larger effect on P-gp expression (∼75–80% reduction) (Fig. 2) than that of Orai1-siRNA.
Fig. 2.
TRPC5 is essential for P-gp production in MCF-7/ADM cells. MCF-7/ADM cells were treated overnight with T5E3 [or preimmune IgG as control (Ctl) in A and B], TRPC5-DN (or vector as control in B), TRPC5-siRNA (or scrambled siRNA as control in B), or 2-APB (or 0.1% DMSO as control in B) at the indicated concentration. A, Upper: P-gp expression; A, Lower: β-tubulin control. In data summary (B), relative expression is shown, in which the intensity of bands for P-gp was divided by corresponding β-tubulin band. Values are means ± SE of four experiments. #P < 0.05 vs. control.
Presumably, the role of TRPC5 in stimulating/maintaining P-gp overproduction may be related to Ca2+ entry through TRPC5. Normal culture medium contains growth factors, which are known to stimulate TRP channel activity (17). Application of human fibroblast growth factor (hFGF) was found to induce a [Ca2+]i rise in MCF-7/ADM cells (Fig. S8). TRPC5-siRNA greatly suppressed the hFGF-induced [Ca2+]i rise, supporting a key role of TRPC5 (Fig. S8). Orai1-siRNA and TRPC1-siRNA also slightly reduced the hFGF-induced [Ca2+]i rise, whereas TRPC3-siRNA, TRPC4-siRNA, TRPC6-siRNA, and STIM1-siRNA had no effect (Fig. S8). Involvement of Orail in hFGF-induced [Ca2+]i rise agrees with its role in P-gp production (Fig. S7), but TRPC1-mediated Ca2+ influx might not be important for P-gp production (Fig. S7). Because TRPC5 played a much more important role than Orail in determining P-gp expression, we only focused on TRPC5 in latter study.
Effect of TRPC5 Suppression on Subcellular Distribution of Adriamycin and Adriamycin Resistance.
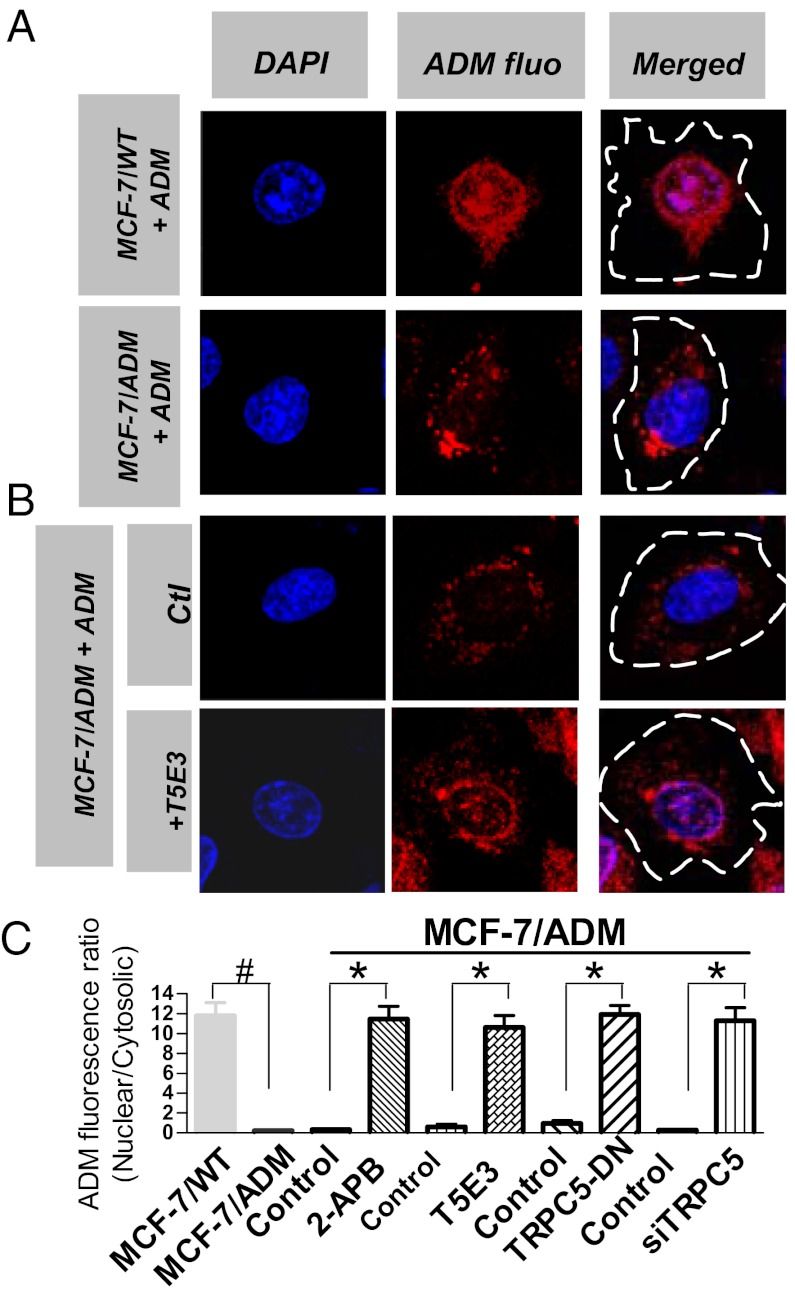
Adriamycin has natural red fluorescence (7). Subcellular distribution of adriamycin was monitored by a confocal laser-scanning microscope as previously reported (7). As expected, adriamycin was found to be mostly accumulated inside the nucleus in MCF-7/WT cells (Fig. 3A), similar to the results reported by others (7). In MCF-7/ADM cells, adriamycin accumulation was much lower, with most of residual adriamycin located near cell peripheral regions but not in nucleus (Fig. 3 A and B). This was expected, because P-gp would pump adriamycin out of cells in adriamycin-resistant cells (7). Interestingly, inhibiting TRPC5 activity or suppressing TRPC5 expression using T5E3, TRPC5-DN, TRPC5-siRNA, or 2-APB resulted in adriamycin reaccumulation in the nucleus of MCF-7/ADM cells (Fig. 3 B and C). These data agree with the notion that TRPC5 is important for P-gp function.
Fig. 3.
TRPC5 affects subcellular distribution of adriamycin (ADM) in MCF-7/ADM cells. Representative fluorescent images of nuclear DAPI staining (Left, blue), adriamycin autofluorescence (Center, red), and merged images (Right) in MCF-7/WT (A) and MCF-7/ADM (A and B) cells. (B) Representative images showing the effect of TRPC5 suppression by T5E3 (20 μg/mL, overnight) on adriamycin redistribution at the subcellular level. Dashed lines in merged images outline the cell boundary, which could be visualized at higher magnification in differential interference contrast mode. (C) Summary data of adriamycin subcellular distribution expressed as nuclear/cytosolic ratio. TRPC5-DN (8 μg per well in six-well plates, 48 h); TRPC5-siRNA (200 nM, 48 h); 2-APB (100 μM, overnight). Adriamycin (10 μg/mL, overnight) was added in the culture medium 1 d before fluorescence measurement. n = 4–6 experiments. #P < 0.05 vs. MCF-7/WT; *P < 0.05 vs. respective control.
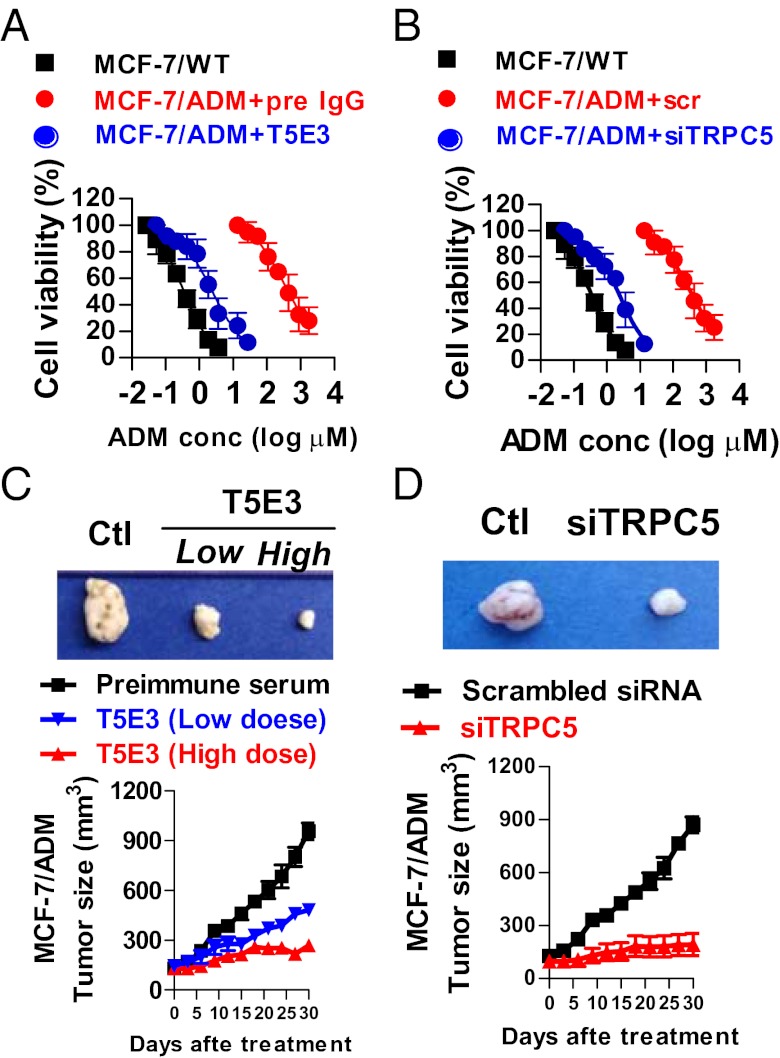
We next explored the possibility of whether inhibiting TRPC5 could reverse the drug resistance to adriamycin in MCF-7/ADM cells. Results from MTT assays showed that MCF-7/ADM cells were much more resistant to adriamycin-induced cell death (Fig. 4) than MCF-7/WT. MCF-7/ADM cells displayed a 630-fold higher resistance to adriamycin than MCF-7/WT, as determined by MTT cytotoxicity assay. MCF-7/ADM cells also displayed resistance to many other chemotherapeutic drugs, including paclitaxel, epirubicin, vincristine, and mitoxantrone (Table S1). Administration of T5E3, TRPC5-DN, TRPC5-siRNA, or 2-APB caused a remarkable reversal of adriamycin resistance in MCF-7/ADM cells, with respective adriamycin IC50 values decreased by 172-fold, 106-fold, 92-fold, and 207-fold, respectively (Fig. 4 A and B and Fig. S9 A and B). T5E3 or TRPC5-DN also reversed the resistance to paclitaxel in MTT assay (Fig. S10).
Fig. 4.
Inhibition of TRPC5 reverses adriamycin resistance in MCF-7/ADM cells by MTT assay and reduces the growth of human breast tumor xenografts in athymic nude mice. (A and B) MTT assay. MCF-7/ADM cells were treated with T5E3 (20 μg/mL, overnight; A) or TRPC5-siRNA (600 nM, 48 h; B), followed by adriamycin incubation at different concentration for 48 h. Preimmune IgG and scrambled siRNA were used as respective controls. In MTT assay, cell viability was measured and expressed as percentage of no-adriamycin control. (C and D) Representative photographs (Upper) of harvested tumors 30 d after T5E3 (C) or TRPC5-siRNA (D) and corresponding tumor growth curves (Lower) measured at indicated time points. Female nude mice bearing xenograft tumors derived from MCF-7/ADM were injected at the tumor sites with T5E3 [2 μg (low dose) or 4 μg (high dose) or preimmune IgG as control], or TRPC5-siRNA (40 pmole, scrambled siRNA as control). Values are means ± SE of three to eight experiments.
Effect of TRPC5 Antagonists on the Growth of MCF-7/ADM Tumor Xenografts.
An animal model of human breast tumor was established by inoculating MCF-7/ADM cells in athymic nude mice (BALB/cAnNCr-nu/nu) using the method developed by others (18). The tumor continued to grow in size under adriamycin treatment, indicating adriamycin resistance (Fig. 4 C and D and Fig. S9 C and D). Immunostaining showed that TRPC5 and P-gp were abundantly coexpressed in the tumor, as determined by serial sections of the same tumor (Fig. S11E). Injection of T5E3, a lentivirus carrying TRPC5-DN (lenti-TRPC5-DN), TRPC5-siRNA, or 2-APB at tumor sites substantially reduced the tumor growth (Fig. 4 C and D and Fig. S9 C and D). Immunohistochemistry also confirmed that the P-gp expression was substantially reduced in T5E3-, lenti-TRPC5-DN-, TRPC5-siRNA-, or 2-APB-treated tumor xenografts (Fig. S11 A–D).
The growth of tumor xenografts was also resistant to paclitaxel, another common cancer chemotherapeutic drug structurally unrelated to adriamycin (Fig. S10). Similarly, paclitaxel-resistant tumor growth was markedly reduced by tumor site injection of T5E3, lenti-TRPC5-DN, TRPC5-siRNA, or 2-APB (Fig. S10).
Involvement of NFATc3 in P-gp Induction.
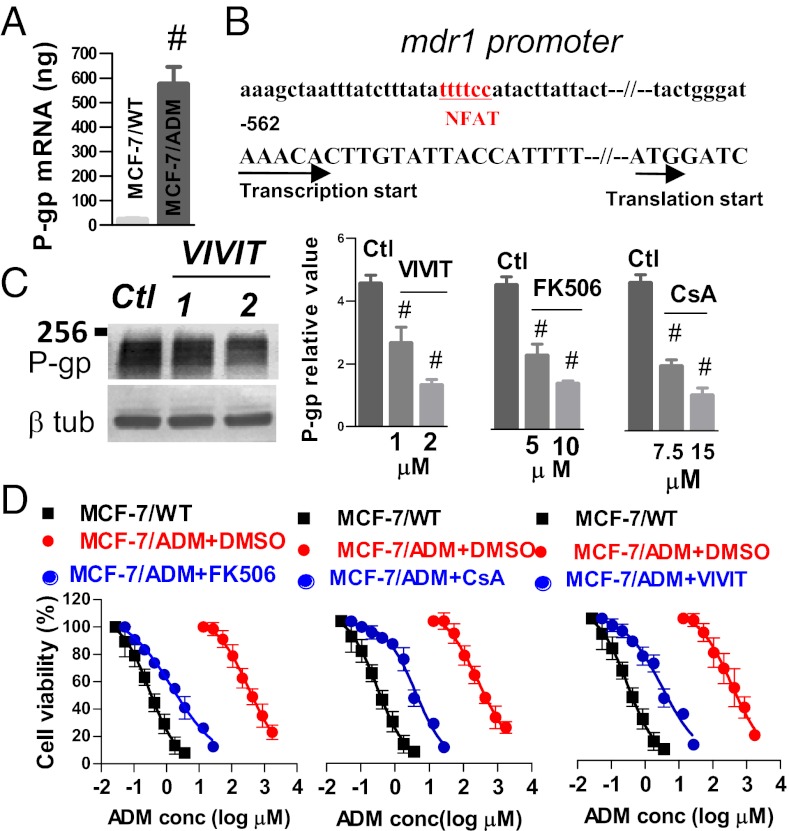
Real-time PCR was used to compare P-gp expression at the mRNA level between MCF-7/ADM and MCF-7/WT cells. The results demonstrated a much higher expression level of P-gp mRNA in MCF-7/ADM than in MCF-7/WT cells (Fig. 5A). Search in the primary nucleotide sequence using the TFSEARCH database identified a potential binding site for a Ca2+-dependent transcription factor NFAT (nuclear factor of activated T cells) (Fig. 5B). The binding site is located in the 5′ upstream region of mdr1 transcription initiation site at the position −537 to −542 nt (Fig. 5B). VIVIT, FK506, and cyclosporin A (19, 20) were used to inhibit NFAT activity. The results show that all three inhibitors dose-dependently reduced P-gp protein expression (Fig. 5C). In the absence of NFAT inhibitors, cellular adriamycin accumulation was lower and located near cell peripheral regions but not in nucleus in MCF-7/ADM cells (Fig. S12). NFAT inhibitors induced a reaccumulation of adriamycin in the nucleus (Fig. S12). In MTT assays, these inhibitors caused a remarkable reversal of adriamycin resistance, making MCF-7/ADM cells sensitive to adriamycin-induced cell death again (Fig. 5D).
Fig. 5.
NFAT regulates P-gp expression and alters adriamycin resistance in MCF-7/ADM cells. (A) Comparison of P-gp mRNA level between MCF-7/WT and MCF-7/ADM cells using real-time PCR. A total of 1 × 105 cells were used. (B) Sketch map of 5′ upstream flanking sequence of mdr1 gene. A putative NFAT binding site is shown. Transcription start point is labeled as +1. (C and D) Effect of FK506, cyclosporin A, or VIVIT treatment on P-gp expression (C) and on cell viability by MTT assay (D). In C, MCF-7/ADM cells were treated overnight with these agents at the indicated concentration. Shown are representative immunoblots (Left) and data summary (Right). In data summary, shown are relative expression, in which the intensity of bands for P-gp was divided by corresponding β-tubulin band. Vehicle (labeled as Ctl) contained 0.1% DMSO. Values are means ± SE of four experiments. #P < 0.05 vs. Ctl. In D, MCF-7/ADM cells were treated overnight with FK506 (10 μM, Left), cyclosporin A (15 μM, Middle), or VIVIT (2 μM, Right), followed by adriamycin incubation at different concentrations for 48 h. Data are expressed as percentage of no-adriamycin control. Values are means ± SE of four experiments.
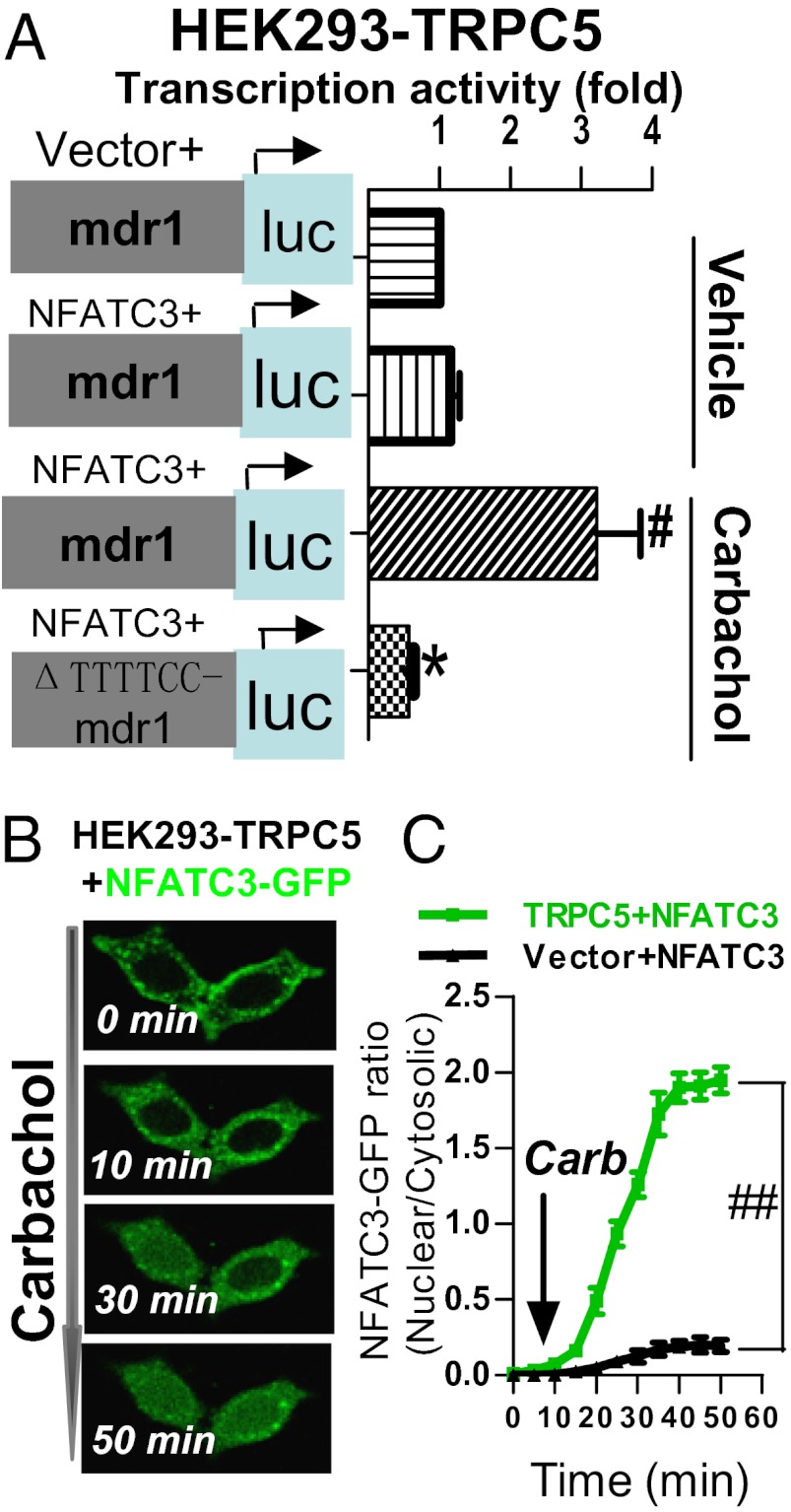
There are four isoforms of NFAT, NFATc1–4. Luciferase reporter assay was used to determine the specific NFAT isoform(s) involved. The 5′ flanking 800-bp fragment, which is located upstream of mdr1 transcriptional initiation site and contains mdr1 promoter and enhancers, was cloned into the luciferase reporter vector to “report” the transcriptional activity of mdr1 promoter. We used an HEK293 cell line that was stably expressing TRPC5, and the cells were incubated with carbachol to stimulate TRPC5 activity. The cells were further transfected with a specific NFAT isoform together with the luciferase reporter vector carrying the 5′ flanking 800-bp sequence of mdr1 gene. The results demonstrated that NFATc3 stimulated the transcriptional activity of mdr1 promoter in these cells, whereas other NFAT isoforms failed to so do (Fig. 6A and Fig. S13). Importantly, deletion of a putative NFAT binding site at −537 to −542 nt (ΔTTTTCC) in the 5′ flanking region of mdr1 gene abrogated the NFATc3-mediated activation of P-gp transcription (Fig. 6A). NFAT is a Ca2+-dependent transcription factor. A rise in [Ca2+]i is expected to induce NFAT translocation from cytosol to nucleus, where it stimulates gene transcription (21). Indeed, in HEK293 cells that were cotransfected with TRPC5 and GFP-tagged NFATc3, stimulation of TRPC5 with carbachol induced a time-dependent NFATc3 translocation from cytoplasm to nucleus (Fig. 6 B and C). In control cells that were transfected with empty plasmid without TRPC5, carbachol application failed to induce NFAT nuclear translocation (Fig. 6C). Together these results established a signaling cascade involving TRPC5, NFATc3 nuclear translocation, and P-gp production.
Fig. 6.
NFATc3 links TRPC5 activity to P-gp production. (A) NFATc3 stimulates the transcriptional activity of mdr1 gene. HEK293 cells stably expressing TRPC5 were further cotransfected with NFATc3 (or empty vector) and the luciferase reporter plasmid carrying 5′ flanking 800-bp sequence of mdr1 gene (or its ΔTTTTCC version). The cells were incubated with or without 100 μM carbachol, as indicated. Horizontal axes indicate relative transcriptional activity in luciferase assay in HEK293 cells overexpressing indicated constructs. Activity in the cells carrying vector + mdr1-luc + TRPC5 was normalized to 1. Values are means ± SE of six experiments. #P < 0.05 vs. vehicle; *P < 0.05 vs. mdr1. (B and C) Representative images (B) and summary data (C) of time-dependent migration of GFP-tagged NFATc3 from cytosol to nucleus after 100 μM carbachol challenge. Transfected constructs are indicated. Values are means ± SE of four experiments. #P < 0.05 vs. vector + NFATc3.
Discussion
The major findings of this study are as follows: (i) TRPC5 and P-gp expression were substantially up-regulated in the adriamycin-resistant breast cancer cell line MCF-7/ADM. (ii) Suppressing TRPC5 activity/expression with T5E3, TRPC5-DN, TRPC5-siRNA, or 2-APB caused a marked reduction in P-gp protein expression in MCF-7/ADM cells. These treatments also altered adriamycin distribution at the subcellular level, resulting in adriamycin reaccumulation in nucleus. In MTT assay, these treatments, to a great extent, reversed the adriamycin resistance of MCF-7/ADM cells. (iii) Injection of T5E3, lenti-TRPC5-DN, TRPC5-siRNA, or 2-APB at the tumor site reversed the adriamycin resistance of tumor xenografts that were formed by inoculating MCF-7/ADM cells in athymic nude mice, causing significant reduction in tumor size. (iv) Inhibiting NFAT in MCF-7/ADM cells also reduced P-gp protein expression, induced a reaccumulation of adriamycin in nucleus, and reversed the adriamycin resistance in MTT cell death assay. (v) In luciferase reporter assay, NFATc3 stimulated the transcriptional activity of mdr1 promoter, the effect of which was abrogated by deleting an NFAT binding site at the 5′ flanking region of mdr1 gene. Together, these results highlight a key functional role of the TRPC5–NFATc3–P-gp signaling pathway in the development of multidrug resistance in cancer cells. A schematic illustration is shown in Fig. S14.
It has been well documented that a rise in [Ca2+]i stimulates P-gp production in cancer cells (3, 4). However, the molecular identity of Ca2+-permeable channels that were involved in the process is unknown. TRPC5 is a Ca2+-permeable nonselective cation channel. The channel plays an important role in neuronal growth cone extension (12, 22), vascular smooth muscle migration (22), and animal fear behavior (23). The expression level of TRPC5 was reported to vary in different tissues/cells (22). Excessive expression of TRPC5 channels has been shown to be associated with cardiovascular disorders (24). In the present study we uncovered another function of TRPC5: its control of P-gp expression and multidrug resistance in drug-resistant cancer cells. Three different indexes for multidrug resistance were monitored, including P-gp expression level, adriamycin expulsion from cells, and adriamycin-induced cancer cell death. In adriamycin-resistant MCF-7/ADM cells, P-gp expression level was high, adriamycin accumulation was low and outside of the nucleus, and the cells were resistant to adriamycin-induced cell death. Inhibition of TRPC5 by T5E3, TRPC5-DN, TRPC5-siRNA, or 2-APB reversed all three of these multidrug resistance indexes. These data demonstrated that TRPC5 is a key protein that can control/regulate P-gp expression and multidrug resistance in the breast cancer cell MCF-7/ADM. TRPC5 may not be the only Ca2+-permeable channel that could affect P-gp expression. Orail-siRNA was also found to reduce the expression of P-gp, although the effect of Orai1-siRNA on P-gp expression was much smaller than that of TRPC5-siRNA.
We further examined the effect of TRPC5 inhibition on growth characteristics of human breast tumor xenografts in an athymic nude mouse model. Treatment of mice bearing human breast tumor xenografts with TRPC5 inhibitor T5E3, lenti-TRPC5-DN, TRPC5-siRNA, or 2-APB substantially inhibited adriamycin- and paclitaxel-resistant tumor growth. These results suggest that inhibiting/suppressing TRPC5 could be a means to reverse multidrug resistance in the animal model of human breast tumor. Among the four methods that were used to inhibit/suppress TRPC5, T5E3, lenti-TRPC5-DN, and TRPC5-siRNA are highly specific to TRPC5, whereas 2-APB is less specific. 2-APB has been commonly used to inhibit TRPC5 (16), but it can also inhibit other TRPCs, such as TRPC1. In addition, it activates TRPV1-3 (25). T5E3 is a blocking antibody that was designed to target the permeation pore of TRPC5 (11) and has been shown to selectively and effectively block TRPC5-mediated Ca2+ entry and cation currents (11). TRPC5-DN carries mutations within the TRPC5 pore region, selectively disrupting the function of endogenous TRPC5 (12). TRPC5-siRNA could effectively suppress the expression of TRPC5 (Fig. S5). Therefore, the results from T5E3, lenti-TRPC5-DN, and TRPC5-siRNA were more reliable than those from 2-APB. Whether T5E3 and/or lenti-TRPC5-DN and/or TRPC5-siRNA can be developed into potential therapeutic tools to overcome the multidrug resistance in cancer chemotherapy still needs further investigation.
NFAT are Ca2+-dependent transcription factors. Their activation by Ca2+ is mediated through the Ca2+-binding proteins calmodulin and calcineurin (21). Previously, Ca2+ entry through several other TRPC channels, including TRPC1, TRPC3, and TRPC6, has been shown to stimulate NFAT-dependent gene transcription (26, 27). A putative NFAT binding site can be found in the 5′ flanking sequence of mdr1 gene. Thus, we explored the possible involvement of NFAT in TRPC5-mediated P-gp production. We found that treatment of MCF-7/ADM cells with NFAT inhibitor—VIVIT, FK506, or cyclosporin A—substantially reduced P-gp expression and caused adriamycin reaccumulation in nucleus. These NFAT inhibitors also reversed adriamycin resistance in MTT cell death assay. Several lines of evidence indicate that NFATc3 is particularly important. (i) Overexpression of NFATc3 stimulated the transcriptional activity of mdr1 gene in luciferase reporter assay; (ii) carbachol stimulation of TRPC5 induced a nuclear translocation of NFATc3; and (iii) deletion of a putative NFAT binding site at the 5′ flanking region (containing promoter and enhancer) of mdr1 gene abrogated the NFATc3-mediated activation of mdr1 gene transcription. These data strongly suggest that Ca2+ entry through TRPC5 stimulates NFATc3 to enhance P-gp production.
In conclusion, our data demonstrated that TRPC5-mediated Ca2+ entry, via NFATc3, stimulates P-gp overproduction in adriamycin-resistant MCF-7/ADM breast cancer cells. Inhibiting/suppressing TRPC5 could reverse the adriamycin resistance in these cancer cells and reduce the growth of tumor xenografts formed by these cancer cells.
Materials and Methods
Additional methods are described in SI Materials and Methods.
Luciferase Activity Assays.
HEK293 cells stably expressing TRPC5 were transiently cotransfected with 0.2 μg reporter plasmid (mdr1-luc or ΔTTTTCC-mdr1-luc), 0.2 μg expression plasmid (NFATc3), and 0.04 μg pRL-CMV, using Lipofectamine 2000 reagent. After 48 h, cells were harvested for luciferase reporter assay.
Whole-Cell Patch Clamp.
Whole-cell current was measured with an EPC-9 patch clamp amplifier as described elsewhere (28). The holding potential was 0 mV, and current–voltage relationship was obtained using a ramp protocol from +100 mV to −100 mV, with 500-ms duration.
[Ca2+]i Measurement.
A Ca2+-sensitive molecular construct, GECO1.2, was transfected into MCF-7/WT and MCF-7/ADM cells (14). GECO1.2 fluorescence signals were measured at room temperature (∼23 °C) using an Olympus fluorescence imaging system.
Adriamycin Accumulation and NFATc3-GFP Translocation.
Subcellular distribution of adriamycin and NFATc3-GFP was determined using a laser scanning confocal microscope.
In Vivo Tumorigenicity Assay.
MCF-7/ADM cells were s.c. injected into the flanks of female nude mice (5 × 106 cells per mouse). When the tumors reached ∼200 mm3, nude mice bearing xenograft tumors were injected with adriamycin (3 mg/kg, i.p., once every 3 d). Animals were also injected once every 3 d at the tumor sites with TRPC5 antagonists; the injection was scheduled 1 d before adriamycin injection.
Supplementary Material
Acknowledgments
This work was supported by Hong Kong Research Grants Council Grants CUHK479109, CUHK478710, and CUHK478011 (to X.Y.); Strategic Investment Scheme C (to X.Y.); and a Group Research Grant from the Chinese University of Hong Kong (to X.Y.); “Strategic Priority Research Program” of the Chinese Academy of Sciences Grant XDA01040000 and Ministry of Science and Technology of China 2011CB966200 (to J.J.); China National Natural Science Foundation Grant 81100185 (to X.M.) and 81130057 (to J.J.); and China National Natural Science Foundation Grant 81101667 and Jiangsu Province Natural Science Foundation Grant BK2009071 (to R.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202989109/-/DCSupplemental.
References
- 1.Goda K, Bacsó Z, Szabó G. Multidrug resistance through the spectacle of P-glycoprotein. Curr Cancer Drug Targets. 2009;9:281–297. doi: 10.2174/156800909788166493. [DOI] [PubMed] [Google Scholar]
- 2.Shtil AA, Azare J. Redundancy of biological regulation as the basis of emergence of multidrug resistance. Int Rev Cytol. 2005;246:1–29. doi: 10.1016/S0074-7696(05)46001-5. [DOI] [PubMed] [Google Scholar]
- 3.Riganti C, et al. Digoxin and ouabain induce P-glycoprotein by activating calmodulin kinase II and hypoxia-inducible factor-1alpha in human colon cancer cells. Toxicol Appl Pharmacol. 2009;240:385–392. doi: 10.1016/j.taap.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 4.Komoto C, et al. Reversal effects of Ca2+ antagonists on multidrug resistance viadown-regulation of MDR1 mRNA. Kobe J Med Sci. 2007;53:355–363. [PubMed] [Google Scholar]
- 5.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehen’kyi V, Prevarskaya N. Oncogenic TRP channels. Adv Exp Med Biol. 2011;704:929–945. doi: 10.1007/978-94-007-0265-3_48. [DOI] [PubMed] [Google Scholar]
- 7.Altan N, Chen Y, Schindler M, Simon SM. Tamoxifen inhibits acidification in cells independent of the estrogen receptor. Proc Natl Acad Sci USA. 1999;96:4432–4437. doi: 10.1073/pnas.96.8.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zunino F, et al. The interaction of adriamycin and its beta anomer with DNA. Biochim Biophys Acta. 1977;476:38–46. doi: 10.1016/0005-2787(77)90283-0. [DOI] [PubMed] [Google Scholar]
- 9.Gomis A, Soriano S, Belmonte C, Viana F. Hypoosmotic- and pressure-induced membrane stretch activate TRPC5 channels. J Physiol. 2008;586:5633–5649. doi: 10.1113/jphysiol.2008.161257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beech DJ. Canonical transient receptor potential 5. Handb Exp Pharmacol. 2007;179:109–123. doi: 10.1007/978-3-540-34891-7_6. [DOI] [PubMed] [Google Scholar]
- 11.Xu SZ, et al. Generation of functional ion-channel tools by E3 targeting. Nat Biotechnol. 2005a;23:1289–1293. doi: 10.1038/nbt1148. [DOI] [PubMed] [Google Scholar]
- 12.Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- 13.Homolyat L, et al. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J Biol Chem. 1993;68:21493–21496. [PubMed] [Google Scholar]
- 14.Zhao Y, et al. An expanded palette of genetically encoded Ca2+ indicators. Science. 2011;333:1888–1891. doi: 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung S, Mühle A, Schaefer M, Strotmann R, Schultz G, Plant TD. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem. 2003;278:3562–3571. doi: 10.1074/jbc.M211484200. [DOI] [PubMed] [Google Scholar]
- 16.Xu SZ, et al. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: A differential, extracellular and voltage-dependent effect. Br J Pharmacol. 2005b;145:405–414. doi: 10.1038/sj.bjp.0706197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiorio Pla A, et al. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J Neurosci. 2005;25:2687–2701. doi: 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue W, Zhou D, Chen S, Brodie A. A new nude mouse model for postmenopausal breast cancer using MCF-7 cells transfected with the human aromatase gene. Cancer Res. 1994;54:5092–5095. [PubMed] [Google Scholar]
- 19.Pu WT, Ma Q, Izumo S. NFAT transcription factors are critical survival factors that inhibit cardiomyocyte apoptosis during phenylephrine stimulation in vitro. Circ Res. 2003;92:725–731. doi: 10.1161/01.RES.0000069211.82346.46. [DOI] [PubMed] [Google Scholar]
- 20.Crabtree GR, Schreiber SL. SnapShot: Ca2+-calcineurin-NFAT signaling. Cell. 2009;138:210–221, 210, e1. doi: 10.1016/j.cell.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 22.Jiang LH, Gamper N, Beech DJ. Properties and therapeutic potential of transient receptor potential channels with putative roles in adversity: focus on TRPC5, TRPM2 and TRPA1. Curr Drug Targets. 2011;12:724–736. doi: 10.2174/138945011795378568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riccio A, et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137:761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bush EW, et al. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- 25.Hu H, Grandl J, Bandell M, Petrus M, Patapoutian A. Two amino acid residues determine 2-APB sensitivity of the ion channels TRPV3 and TRPV4. Proc Natl Acad Sci USA. 2009;106:1626–1631. doi: 10.1073/pnas.0812209106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohba T, et al. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol. 2007;42:498–507. doi: 10.1016/j.yjmcc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 27.Poteser M, et al. PKC-dependent coupling of calcium permeation through transient receptor potential canonical 3 (TRPC3) to calcineurin signaling in HL-1 myocytes. Proc Natl Acad Sci USA. 2011;108:10556–10561. doi: 10.1073/pnas.1106183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen B, et al. Epinephrine-induced Ca2+ influx in vascular endothelial cells is mediated by CNGA2 channels. J Mol Cell Cardiol. 2008;45:437–445. doi: 10.1016/j.yjmcc.2008.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.