SUMMARY
The A2a adenosine receptor (AR) mediates several important physiological effects of adenosine, including vasodilation and inhibition of platelet aggregation. Until recently, no antagonist radioligand of sufficient selectivity or affinity was available. We describe the synthesis and characterization by radioligand binding of 125I-4-(2-[7-amino-2-{2-furyl}{1,2,4}triazolo{2,3-a}-{1,3,5}triazin-5-yl-amino]ethyl)phenol (125I-ZM241385) in membranes from two cell types that express A2a ARs. Membranes from Chinese hamster ovary (CHO) cells expressing a recombinant canine A2a AR bound 125I-ZM241385 with high affinity, and agonist competition experiments with 2-(p-carboxyethyl)-phenylamino-5′-N-carboxamidoadenosine, 5′-N-ethylcarboxamidoadenosine, and (−)-N6-[(R)-phenylisopropyl]adenosine revealed a potency order characteristic of an A2a AR binding site. Membranes from bovine striatum, which contain a native A2a AR, also bound 125I-ZM241385 with similarly high affinity and also displayed a pharmacological profile for displacement of radioligand binding that was consistent with that of an A2a AR. Also, under conditions in which 125I-ZM241385 bound with high affinity to a recombinant rat A2a AR expressed in CHO cells, no specific binding was detectable in membranes from CHO cells expressing functional rat A1, A2b, or A3 ARs, indicating that over the range of concentrations used in radioligand binding assays, 125I-ZM241385 is a highly selective antagonist radioligand for study of A2a ARs within a given species.
The multiple physiological effects of adenosine are mediated by its binding to specific cell-surface receptors. Biochemical and molecular cloning studies have identified four such receptors, termed A1, A2a, A2b, and A3. The A2 ARs are distinguished by their ability to interact with Gs and stimulate adenylyl cyclase activity (1, 2). However, just as characterization and purification of A1 ARs have been greatly facilitated by the development of specific antagonist ligands, characterization of the A2 ARs has been hindered by the dearth of similarly useful compounds. Only in the past 5 years have any selective agonist radioligands been developed for the study of A2a ARs (3–5) and, until recently, high-affinity selective antagonists did not exist. Some A2a-selective antagonists have been developed, such as the triazoloquinoxaline 4-amino-8-chloro-1-phenyl[1,2,4]triazolo[4,-3a]quinoxaline (6) and 8-styryl-substituted 1,3,7-alkylxanthines (7, 8). One of the latter class of compounds, KF17837, has been produced in a tritiated form and can bind with reasonable affinity to A2a ARs in rat striatum (9). However, this compound has been reported to be light sensitive, and the resulting product exhibits a reduced affinity for the A2a AR (8, 9). Also, for the study of A2a ARs in tissues expressing low levels of functional receptors, such as platelets or cells of myocardial origin (10), an iodinated radioligand with high specific activity would be desirable. Therefore, we used the recently developed, highly A2a AR-selective antagonist ZM241385 (11) to synthesize, purify, and characterize the first high affinity, iodinated antagonist radioligand selective for A2a ARs.
Experimental Procedures
Materials
NECA was the generous gift of Dr. Ray Olsson (University of South Florida, Tampa, Florida). (−)-N6-[(R)-phenylisopropyl]adenosine and adenosine deaminase were obtained from Boehringer-Mannheim. CGS21680 was purchased from Research Biochemicals International. Carrier-free Na125I (specific activity = 2200 Ci/mmol) was from Amersham International. Sources of other materials have been described previously (12, 13).
Receptor cDNAs and expression
Recombinant ARs were expressed in CHO cells; these cells have since been demonstrated to be devoid of ARs as determined by radioligand binding and adenylyl cyclase assays (12–14). CHO cells stably expressing rat A3 ARs under the control of a cytomegaloviral promoter have been described and characterized previously (12,13). The rat A2a. AR cDNA (15) was subcloned as a HindIII/XbaI fragment into similarly digested pCMV5, and the resulting construct was used to transiently transfect CHO cells using a DEAE-Dextran procedure. For expression of the rat A1 and A2b ARs, CHO cells were transiently transfected with pcDNA/rat A2b AR and pCMV5/rat A1 AR constructs that have been previously described (12,16). For expression of the canine A2a AR, a HindIII/XbaI fragment encoding the cDNA was subcloned into the polylinker region of similarly digested pM2N expression vector (17), which was generously donated by Dr. Simon Cook, Onyx Pharmaceuticals, Richmond, California. This vector contains both a neo gene, to facilitate selection of stable clones, and a modified murine metallothionin promoter with several additional metal-responsive elements, to provide optimal heavy metal inducibility of any introduced gene. Generation of cell lines with stably incorporated pM2N/ A2a AR was achieved by transfection of CHO cells using a modified calcium phosphate precipitation-glycerol shock procedure followed by selection in G418-containing media. Resistant clones were isolated, expanded, and assayed for heavy metal induction of [3H]CGS21680 binding in isolated membrane preparations. One cell line (CHOΔA2a) was expanded and used for further experiments. A control cell line (CHOΔneo) containing the pM2N cDNA with no insert was also generated. Optimal induction of A2a AR expression was achieved by incubating transfected CHO cells at ~80% confluence with 100 μM ZnCl2 and 2 μM CdCl2 for 48 hr, with replacement of the media ~ 16 hr before cell harvest.
Synthesis and radioiodination of ZM241385
The characterization of ZM241385 as an A2a AR-selective antagonist has been described previously (11). ZM241385 was synthesized by the addition of 2.74 g of 4-(aminoethyl)phenol to a stirred suspension of 1.4 g of 7-amino-2-(2-furyl)-5-methylsulfonyl-[1,2,4]triazolo-[1,5-a] [1,3,5]triazine in acetonitrile and being mixed overnight. After evaporation of the solvent, the residue was purified by chromatography over silica (100 g), eluting with dichloromethane containing 50% (v/v) methanol. The resulting solid (1.23 g) was crystallized from ethyl acetate to give the pure final product (m.p. 225–227°; elemental analysis for C16H15N7O2: formula weight, 337; calculated, C, 57.0; H, 4.5; N, 29.1%; found, C, 56.7; H, 4.6; N, 29.4%). Nuclear magnetic resonance spectra of ZM241385 solutions were consistent with the predicted structure (Fig. 1).
Fig. 1.

Chemical structure of 125I-ZM241385. The iodination of the parent compound and purification of the radioligand are described in Experimental Procedures.
For iodination, 0.1 mg of ZM241385 was dissolved in 1 ml of methanol and 10μl was taken to dryness under nitrogen. After resuspension in 40μl of 0.3 M NaH2PO4, pH 7.5, 1.5 mCi Na125I was added, followed by 10 μl of 1 mg/ml chloramine T. After incubation at room temperature for 2 min, the reaction was stopped by the addition of 25 μl of 2 mg/ml sodium metabisulfite. Separation of 125I-ZM241385 from the parent compound was achieved by application of the iodination mixture to a Waters 501 HPLC system. Resolution was achieved by reverse-phase HPLC using a 60% (v/v) methanol/ 40% (v/v) 20 mM ammonium formate, pH 8.0, mobile phase, and a C18 μBondapak column at a flow rate of 0.75 ml/min. The 125I-ZM241385 peak was defined by measurement of UV absorbance and γ radiation. Under these conditions, 125I-ZM241385 (elution time = 14.8 min) was completely resolved from the starting material (elution time = 8.9 min). Therefore, the specific activity of 125I-ZM241385 was assumed to be 2200 Ci/mmol.
Membrane preparation and radioligand binding
Membranes were prepared from bovine striatum and stored at −80° in 1-ml aliquots as previously described (4). For use in radioligand binding, an aliquot of membranes was thawed and added to 9 ml of binding buffer (50 mM HEPES, pH 6.8,10 mM MgCl2) containing 0.3 unit/ml adenosine deaminase and incubated at 37° for 15 min. After centrifugation, membranes were resuspended in 9 ml of binding buffer containing 0.1 unit/ml adenosine deaminase with a motor-driven Teflon pestle in a Potter-Elvehjem homogenizer for use in radioligand binding assays. Membranes were prepared from CHO cells by scraping of the cells into 5 ml of lysis buffer (10 mM HEPES, pH 7.5, 5 mM EDTA) after cell monolayers were washed several times with ice-cold buffer. After Dounce homogenization on ice (20 strokes), membranes were pelleted by centrifugation and similarly resuspended in binding buffer supplemented with 0.3 unit/ml adenosine deaminase for immediate use in radioligand binding assays.
Binding studies were performed in a 250-μl reaction volume containing 150 μl of membrane suspension, 50 μl of radioligand, and 50 μl of water or competing ligand. Incubations were carried out for 1 hr at 37° with agitation and were terminated by vacuum filtration over 0.3% (v/v) polyethylimine-treated glass-fiber filters and rapid washing with ice-cold binding buffer containing 0.03% (w/v) 3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonate using a Brandel cell harvester. For saturation analysis of 125I-ZM241385 binding, radioligand concentrations ranging from 0.25 nM to 4–8 nM were used. Nonspecific binding was defined by the inclusion of 50 μM NECA. Saturation and competition curves were analyzed by a previously validated computer-assisted curve-fitting program (18). IC50 values obtained from competition curves were converted to Ki values using the Cheng-Prusoff equation (19). Data are presented as mean ± standard error for the number of experiments indicated.
Adenylyl cyclase assays were performed on isolated membranes as previously described (12).
Results and Discussion
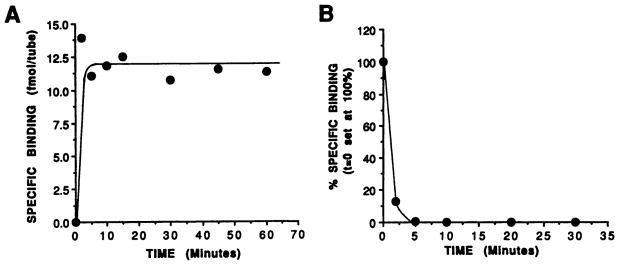
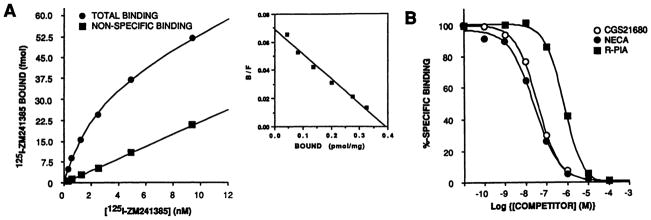
ZM241385 has been demonstrated previously to be a highly selective antagonist ligand for blockade of A2a ARs(11). Specifically, it exhibits 80-fold selectivity for A2a ARs versus A2b ARs in functional assays, as well as 500–1000-fold and 500,000-fold selectivities for A2a ARs versus A1 and A3 ARs, respectively, in radioligand binding assays (11). Inspection of the chemical structure also revealed that it was amenable to radioiodination, as shown in Fig. 1, due to the presence of a phenolic functional group. Such a radioligand would potentially be of great use for the study of A2a ARs. Therefore, 125I-ZM241385 was synthesized, purified by HPLC (see Experimental Procedures), and characterized with respect to its ability to bind to recombinant AR subtypes. Fig. 2A demonstrates that 125I-ZM241385 bound very rapidly to membranes from CHO cells expressing a recombinant canine A2a AR. Steady state appeared to be reached as early as the first time point examined (2 min) and was sustained for at least 60 min (Fig. 2A). Binding was rapidly reversed on the addition of NECA to a concentration of 50 μM, with >80% dissociation being evident as early as a 2-min incubation (Fig. 2B). Fig. 3A demonstrates that 125I-ZM241385 bound to a single saturable high affinity site in membranes from CHO cells expressing a recombinant canine A2a AR, exhibiting Kd and Bmax values of 1.62 ± 0.49 nM and 0.47 ± 0.07 pmol/mg protein, respectively (three experiments). In addition, competition experiments demonstrated that 125I-ZM241385 binding was displaced by agonist ligands with a pharmacological profile consistent with that of an A2a AR, i.e., CGS21680 and NECA were significantly more potent than (−)-N6-[(R)-phenylisopropyl]adenosine, and that the receptor exhibited a lower affinity for xanthine antagonists compared with A1 ARs (1, 2) (Fig. 3B and Table 1). No specific binding was observed in membranes from the negative control CHOΔneo cell line (data not shown).
Fig. 2.
Association and dissociation kinetics of 125I-ZM241385 binding to membranes from CHO cells expressing the canine A2a AR. A, Membranes from CHO cells expressing the canine A2a AR were incubated with 1 nM 125I-ZM241385 at 37° for the indicated times before separation of bound radioligand from free by vacuum filtration over glass-fiber filters as described in Experimental Procedures. Nonspecific binding was determined using 50 μM NECA. This represents one of two experiments that produced quantitatively similar results. B, Membranes from CHO cells expressing the canine A2a AR were incubated with 1 nM 125I-ZM241385 at 37° for 30 min, at which time NECA was added to a concentration of 50 μM. Samples were then processed at the indicated times after the addition by vacuum filtration as described in A. Data are expressed as a percentage of the specific binding observed before the addition of NECA at t = 0. This represents one of two experiments that produced quantitatively similar results.
Fig. 3.
Binding of 125I-ZM241385 to membranes from CHO cells expressing the canine A2a AR. Membranes were prepared from CHOΔA2a cells after induction with ZnCl2/CdCl2 for use in radioligand binding assays as described in Experimental Procedures. A, Saturation isotherm for 125I-ZM241385 binding. Inset, Scatchard transformation of the specific binding data from the same experiment. This represents one of three experiments, data from which are given in Results and Discussion. B, Agonist competition for 125I-ZM241385 binding. 125I-ZM241385 was present at a concentration of 0.5 nM. Specific binding of radioligand at this concentration accounted for 85% of the total binding. The amount of specific binding has been normalized and expressed as a percentage. Pooled data from three such experiments are given in Table 1.
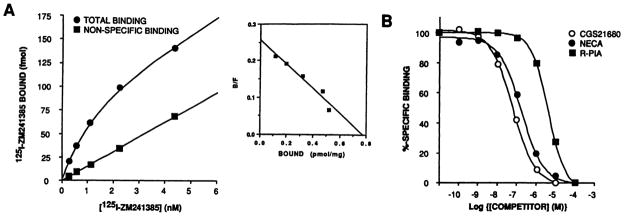
TABLE 1. Competition for 125I-ZM241385 binding.
Competition binding was performed as described in Experimental Procedures on membranes prepared from bovine striatum and from CHOΔA2a cells after induction of receptor expression by incubation with 100 μM ZnCl2 and 2 μM CdCl2 for 48 hr. Data are presented from three separate experiments.
| Competing ligand | Bovine striatum
|
CHOΔA2a
|
|||
|---|---|---|---|---|---|
| Ki | nM | Ki | nM | ||
| nM | nM | ||||
| Agonist | |||||
| CGS21680 | 41.5 ± 10.9 | 0.73 ± 0.05 | 33.0 ± 6.2 | 0.82 ± 0.04 | |
| NECA | 116.1 ± 18.0 | 0.79 ± 0.04 | 28.7 ± 7.2 | 0.83 ± 0.10 | |
| R-PIA | 2244 ± 889 | 0.97 ± 0.27 | 725 ± 135 | 1.01 ±0.02 | |
| Antagonist | |||||
| XAC | 153.5 ± 63.9 | 1.16 ± 0.04 | 53.0 ± 22.2 | 0.99 ± 0.01 | |
| BW1433 | 86.3 ± 8.2 | 0.88 ± 0.07 | 79.9 ± 25.0 | 1.02 ±0.05 | |
BW1433, 1,3-dipropyl-8-(4-acrylate)phenylxanthine; XAC, xanthine amine congener.
To determine the use of 125I-ZM241385 in identifying A2a ARs in cells that may express multiple AR subtypes, radioligand binding was performed in membranes from CHO cells expressing rat AR subtypes. Similar to the results obtained with the expressed canine A2a AR, CHO cells transiently transfected with a rat A2a AR cDNA bound 125I-ZM241385 with high affinity (Kd = 0.66 ± 0.03 nM, Bmax = 2.67 ± 0.10 pmol/mg protein; three experiments). However, over the range of 125I-ZM241385 concentrations capable of labeling A2a ARs (0.25–8 nM), no specific binding to rat A1 or A3 ARs could be detected despite the ability to detect these receptors by [3H]1,3-dipropyl-8-cyclopentylxanthine and 125I-4-amino-benzyl-5′-N-methylcarboxamidoadenosine binding, respectively (data not shown). In addition, we failed to detect any specific binding of a similar range of concentrations of 125I-ZM241385 to membranes from CHO cells transiently transfected with a rat A2b AR cDNA (data not shown). This was not due to the lack of expression of functional receptor as parallel adenylyl cyclase assays on the same membranes preparations demonstrated that 50 μM NECA could produce a 2.6 ± 0.1-fold stimulation of activity above basal (three experiments). Therefore, under our assay conditions, no specific binding of 125I-ZM241385 could be detected to A1 A2b, or A3 ARs, indicating that this radioligand is highly selective for identification of A2a ARs within a given species.
To be useful as a radioligand, 125I-ZM241385 should be capable of labeling A2a ARs expressed endogenously by a given cell type as well as recombinant proteins. Therefore, 125I-ZM241385 binding was performed on membranes from bovine striatum, the A2a AR of which has previously been well characterized by 125I-2-(4-[2-{(4-aminophenyl)methyl-carbonyl)ethyl]phenyl)ethylamino-5′-N-ethylcarboxamidoadenosine and [3H]CGS21680 binding (4, 20). Saturation analysis revealed that 125I-ZM241385 bound to a single saturable high affinity site, with Kd and Bmax values of 1.39 ± 0.39 nM and 0.72 ± 0.08 pmol/mg protein, respectively (three experiments) (Fig. 4A). Therefore, the Kd values exhibited by the native bovine A2a AR and the recombinant canine receptor are essentially identical. Also, agonist competition for 125I-ZM241385 binding exhibited the characteristic profile of an A2a AR (Fig. 4B and Table 1) and was very similar to that of the recombinant canine A2a AR (Fig. 3B and Table 1). The slight differences in the Ki values for some of the competing ligands between the bovine and canine receptors probably reflect species-dependent differences that have also been observed for displacement of agonist radioligand binding at A2a ARs (21).
Fig. 4.
Binding of 125I-ZM241385 to membranes from bovine striatum. Membranes were prepared from bovine striatum for use in radioligand binding assays as described in Experimental Procedures. A, Saturation isotherm for 125I-ZM241385 binding. Inset, Scatchard transformation of the specific binding data from the same experiment. This represents one of three experiments, data from which are given in Results and Discussion. B, Agonist competition for 125I-ZM241385 binding. 125I-ZM241385 was present at a concentration of 0.5 nM. Specific binding of radioligand at this concentration accounted for 70% of the total binding. The amount of specific binding has been normalized and expressed as a percentage. Pooled data from three such experiments are given in Table 1.
In conclusion, we synthesized and characterized a selective antagonist radioligand for the study of the A2a AR. Although another antagonist radioligand, [3H]KF17837S, has been described for this receptor (9), its usefulness in detecting the low levels of A2a ARs expressed in many tissues may be limited due to its lower specific activity and light sensitivity. Because of its excellent selectivity and high affinity and the facile nature of the synthesis, which results in its high specific activity, 125I-ZM241385 would be more suitable for these purposes. In addition, the availability of an antagonist radioligand can facilitate a more detailed investigation of the atypical coupling of the A2a AR to Gs that we and others have noted (5, 22, 23).
Acknowledgments
We thank Drs. Scott Rivkees and Steven Reppert for rat A1 A2a, and A2b adenosine receptor cDNAs; Dr. Simon Cook for providing pM2N; Mary Pound for technical assistance; and Linda Scherich for preparation of the manuscript.
ABBREVIATIONS
- AR
adenosine receptor
- CHO
Chinese hamster ovary
- ZM241385
4-(2-[7-amino-2-{2-furyl}{1,2,4}triazolo{2,3-a}{1,3,5}triazin-5-yl-amino]ethyl)phenol
- CGS21680
2-(p-carboxyethyl)phenylamino-5′-N-carboxamidoadenosine
- KF17837
1,3-dipropyl-7-methyl-(3,4-dimethoxystyryl)xanthine
- HPLC
high performance liquid chromatography
- NECA
5′-N-ethylcarboxamidoadenosine
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
References
- 1.Olah ME, Stiles GL. Adenosine receptor subtypes: characterization and therapeutic regulation. Annu Rev Pharmacol Toxicol. 1995;35:581–606. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- 2.Tucker AL, Linden J. Cloned receptors and cardiovascular responses to adenosine. Cardiovasc Res. 1993;27:62–67. doi: 10.1093/cvr/27.1.62. [DOI] [PubMed] [Google Scholar]
- 3.Jarvis MF, Schulz R, Hutchison AJ, Do UH, Sills MA, Williams M. [3H]CGS21680, a selective A2 adenosine receptor agonist, directly labels A2 receptors in rat brain. J Pharmacol Exp Ther. 1989;251:888–893. [PubMed] [Google Scholar]
- 4.Barrington WW, Jacobson KA, Hutchison AJ, Williams M, Stiles GL. Identification of the A2 adenosine receptor binding subunit by photoaffinity crosslinking. Proc Natl Acad Sci USA. 1989;86:6572–6576. doi: 10.1073/pnas.86.17.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luthin DR, Olsson RA, Thompson RD, Sawmiller DR, Linden J. Characterization of two affinity states of adenosine A2a receptors with a new radioligand, 2-[2-(4-amino-3-[125I]iodophenyl)ethylamino]adenosine. Mol Pharmacol. 1995;47:307–313. [PubMed] [Google Scholar]
- 6.Sarges R, Howard HR, Browne RG, Lebel LA, Seymour PA, Koe BK. 4-Amino[11,2,4]triazolo[4,3-α]-quinoxalines: a novel class of potent adenosine receptor antagonists and potential rapid-onset antidepressants. J Med Chem. 1990;33:2240–2254. doi: 10.1021/jm00170a031. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson KA, Nikodijevic O, Padgett WL, Gallo-Rodriguez C, Maillard M, Daly JW. 8-(3-Chlorostyryl)caffeine (CSC) is a selective A2-adenosine receptor antagonist in vitro andin vivo. FEBS Lett. 1993;323:141–144. doi: 10.1016/0014-5793(93)81466-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nonaka H, Ichimura M, Takeda M, Nonaka Y, Shimada J, Suzuki F, Yamaguchi K, Kase H. KF17837 [(E)-8-(3, 4-dimethoxystyryl)-1,3-dipropyl-7-methylxanthine], a potent and selective adenosine A2 receptor antagonist. Eur J Pharmacol. 1994;267:335–341. doi: 10.1016/0922-4106(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 9.Nonaka H, Mori A, Ichimura M, Shindou T, Yanagawa K, Shimada J, Kase H. Binding of [3H]KF17837S, a selective adenosine A2 adenosine receptor antagonist, to rat brain membranes. Mol Pharmacol. 1995;46:817–822. [PubMed] [Google Scholar]
- 10.Behnke N, Muller W, Neumann J, Schmitz W, Scholz H, Stein B. Differential antagonism by 1,3-dipropylxanthine-8-cyclopentylxanthine and 9-chloro-2-(2-furanyl)-5,6-dihydro-1,2,4-triazolo(1,5-c)quinazolin-5-imine of the effects of adenosine derivatives in the presence of isoprenaline on contractile response and cyclic AMP content in cardiomyocytes: evidence for the co-existence of A1- and A2-adenosine receptors on cardiomyocytes. J Pharmacol Exp Ther. 1990;254:1017–1023. [PubMed] [Google Scholar]
- 11.Poucher SM, Keddie JR, Singh P, Stoggall SM, Caulkett PWR, Jones G, Collis MG. The in vitro pharmacology of ZM241385, a potent, non-xanthine, A2a selective adenosine receptor antagonist. Br J Pharmacol. 1995;115:1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olah ME, Gallo-Rodriguez C, Jacobson KA, Stiles GL. 125I-4-Aminobenzyl-5′-N-methylcarboxamidoadenosine, a high affinity radioligand for the rat A3 adenosine receptor. Mol Pharmacol. 1994;45:978–982. [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer TM, Gettys TW, Stiles GL. Interaction with and regulation of multiple G-proteins by the rat A3 adenosine receptor. J Biol Chem. 1995;270:16895–16902. doi: 10.1074/jbc.270.28.16895. [DOI] [PubMed] [Google Scholar]
- 14.Rivkees SA, Reppert SM. RFL9 encodes an A2b-adenosine receptor. Mol Endocrinol. 1992;6:1598–1604. doi: 10.1210/mend.6.10.1333049. [DOI] [PubMed] [Google Scholar]
- 15.Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack A, Adler E, Reppert SM. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 16.Stehle JH, Rivkees SA, Lee JJ, Weaver DR, Deeds JD, Reppert SM. Molecular cloning and expression of the cDNA for a novel A2-adenosine receptor subtype. Mol Endocrinol. 1992;6:384–393. doi: 10.1210/mend.6.3.1584214. [DOI] [PubMed] [Google Scholar]
- 17.Cook SJ, Rubinfeld B, Albert I, McCormick F. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 1993;12:3475–3485. doi: 10.1002/j.1460-2075.1993.tb06022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLean A, Hancock AA, Lefkowitz RJ. Validation and statistical analysis of a computer modeling method for quantitative analysis of radioligand binding data for mixtures of pharmacological receptor subtypes. Mol Pharmacol. 1982;22:3099–3108. [PubMed] [Google Scholar]
- 19.Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of an inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 20.Nanoff C, Stiles GL. Solubilization and characterization of the A2 adenosine receptor. J Recept Res. 1993;13:961–973. doi: 10.3109/10799899309073703. [DOI] [PubMed] [Google Scholar]
- 21.Stone GA, Jarvis MF, Sills MA, Weeks B, Snowhill EW, Williams M. Species differences in high-affinity adenosine A2 binding sites in striatal membranes from mammalian brain. Drug Dev Res. 1988;15:31–46. [Google Scholar]
- 22.Nanoff C, Jacobson KA, Stiles GL. The A2 adenosine receptor: guanine nucleotide modulation of agonist binding is enhanced by proteolysis. Mol Pharmacol. 1991;39:130–135. [PMC free article] [PubMed] [Google Scholar]
- 23.Gross W, Lohse MJ. Mechanism of activation of A2 adenosine receptors. II. A restricted collision-coupling model of receptor-effector interaction. Mol Pharmacol. 1991;39:24–530. [PubMed] [Google Scholar]