Abstract
Background
Attention-deficit/hyperactivity disorder (ADHD) is typically characterized by symptoms of inattention and hyperactivity/impulsivity, but there is increased recognition of a motivation deficit too. This neuropathology may reflect dysfunction of both attention and reward-motivation networks.
Methods
To test this hypothesis, we compared the functional connectivity density between 247 ADHD and 304 typically developing control children from a public magnetic resonance imaging database. We quantified short- and long-range functional connectivity density in the brain using an ultrafast data-driven approach.
Results
Children with ADHD had lower connectivity (short- and long-range) in regions of the dorsal attention (superior parietal cortex) and default-mode (precuneus) networks and in cerebellum and higher connectivity (short-range) in reward-motivation regions (ventral striatum and orbitofrontal cortex) than control subjects. In ADHD children, the orbitofrontal cortex (region involved in salience attribution) had higher connectivity with reward-motivation regions (striatum and anterior cingulate) and lower connectivity with superior parietal cortex (region involved in attention processing).
Conclusions
The enhanced connectivity within reward-motivation regions and their decreased connectivity with regions from the default-mode and dorsal attention networks suggest impaired interactions between control and reward pathways in ADHD that might underlie attention and motivation deficits in ADHD.
Keywords: ADHD, FCD mapping, impulsivity, inattention, networks, reward-motivation
Atention-deficiency/hyperactivity disorder (ADHD) is one of the most prevalent of all psychiatric disorders (1). ADHD is typically characterized by symptoms of inattention and hyperactivity/impulsivity (2), though there is increased recognition of reward and motivation deficits in this disorder (3). Impairments in brain dopamine (DA) neurotransmission have been implicated in the pathophysiology of ADHD (4–7). Indeed, studies using positron emission tomography in ADHD patients have revealed abnormalities in markers of DA neurotransmission (DA transporters, D2 receptors, synthesis, and release) in the reward-motivation pathway (midbrain, caudate, and ventral striatum), which were associated both with symptoms of inattention and with decreased scores in trait measures of motivation (8,9). In parallel, magnetic resonance imaging (MRI) studies have reported abnormal activation in prefrontal cortices (including inferior and dorsolateral regions and cingulate gyrus) and striatum, including caudate and ventral striatum (10), in individuals with ADHD compared with control subjects (11). Some of these changes are normalized by stimulant medications such as methylphenidate and amphetamine (12–14), supporting involvement of DA (possibly also noradrenergic [15]) neurotransmission in these functional changes (16).
More recently, MRI studies on resting-state functional connectivity (RSFC) have reported abnormal signal fluctuations in inferior frontal and superior parietal cortices (SPC), cingulum, and cerebellum (17–19); higher RSFC in anterior cingulum, pons, insula, cerebellum, and thalamus (20); and lower RSFC between putamen and posterior parietal cortex (21) and between SPC and cingulum in ADHD (22). Therefore, these studies implicate altered connectivity in parietal cortex (23) and anterior cingulum (24) in the neuropathology of ADHD, supporting the involvement of both executive-attention and reward-motivational networks in ADHD (25). However, until very recently, imaging studies were restricted to relatively small samples sizes (largest imaging study on ADHD included 25 ADHD participants), which limited their generalization. The recent release of an open access RSFC data set from 255 ADHD children (ADHD-200 Sample; http://fcon_1000.projects.nitrc.org/indi/adhd200/) now allows investigators to evaluate the functional brain connectivity patterns in a large sample of ADHD children.
Therefore, the purpose of this study was to compare the brain functional connectivity density (FCD) between ADHD children and typically developing children (TDC), taking advantage of this large RSFC data set, using a data-driven graph theory approach (26,27). Note, however, that the FCD has not been evaluated in ADHD, partially due to the overwhelming computational demands associated with graph theory computations. Recently, we proposed functional connectivity density mapping (28,29), an ultra-fast graph theory method for computing local and global FCD maps with high spatial resolution (3-mm isotropic), which allows identification of functional hubs (regions densely connected) with high sensitivity and discrimination among short-range FCD hubs and long-range FCD hubs (30). Based on previous neuroimaging findings, we hypothesized that ADHD will be associated with abnormal FCD in reward-motivational (ventral striatum and orbitofrontal cortex), attention (parietal cortex), and executive (dorsal cingulate) regions that would correlate with ADHD symptoms.
Methods and Materials
Subjects
Functional scans collected in resting conditions that corresponded to 255 children with ADHD and 316 typically developing control children (see Table 1 for additional demographic information) from the ADHD-200 Sample imaging database (http://fcon_1000.projects.nitrc.org/indi/adhd200/) were included in the study. To minimize variability across institutions, the study included data from research institutions that contributed to both study groups (ADHD and TDC). Thus, data sets from the Kennedy Krieger Institute (KKI), New York University Child Study Center (NYU), Oregon Health & Science University (OHSU), and Peking University (PU) were included in the study. Magnetic resonance imaging time series were collected in resting conditions using Siemens Magnetom Allegra and Trio (Siemens Medical Solutions, Erlangen, Germany) and Philips Gyroscan (Philips Medical Systems, Amsterdam, Netherlands) 3 Tesla MRI scanners and single-shot echo-planar imaging (Table S1 in Supplement 1).
Table 1.
Demographic Variables and ADHD Diagnoses
| ADHD Diagnosis | |||||||
|---|---|---|---|---|---|---|---|
| Data Set | TDC M/F | Age (Years) | ADHD M/F | Age (Years) | 1 | 2 | 3 |
| KKI | 34/27 | 10.3 ± 1.3 | 10/12 | 10.2 ± 1.6 | 16 | 1 | 5 |
| NYU | 46/51 | 12.2 ± 3.1 | 95/23 | 11.3 ± 2.7a | 73 | 2 | 43 |
| OHSU | 17/25 | 8.9 ± 1.2 | 26/11 | 8.8 ± 1.0 | 30 | 2 | 5 |
| PU | 71/45 | 11.7 ± 1.7 | 73/5 | 12.4 ± 2.0a | 29 | 0 | 49 |
ADHD, attention-deficit/hyperactivity disorder; F, female; KKI, Kennedy Krieger Institute; M, male; NYU, New York University Child Study Center; OHSU, Oregon Health & Science University; PU, Peking University; TDC, typically developing children.
ADHD-TDC→.01 < p < .05 (two-sample t test).
Psychiatric Diagnosis
Psychiatric diagnoses (Table 2) were established at each institution through psychiatric interviews with experienced child psychiatrists using the Schedule of Affective Disorders and Schizophrenia for Children–Present and Lifetime Version administered to parents and children (NYU and PU); the Diagnostic Interview for Children and Adolescents, Fourth Edition (KKI); the Conners’ Parent Rating Scale–Revised, Long version (KKI, NYU); the ADHD Rating Scale IV (KKI and PU); the Kiddie Schedule for Affective Disorders and Schizophrenia administered to a parent (OHSU); the parent and teacher Conners’ Rating Scale, Third Edition (OHSU); and the Computerized Diagnostic Interview Schedule for Children-IV and the ADHD Rating Scale IV (PU). Intelligence was evaluated with the Wechsler Abbreviated Scale of Intelligence (NYU); the Wechsler Intelligence Scale for Chinese Children–Revised (PU); and the Wechsler Intelligence Scale for Children, Fourth Edition (KKI and OHSU). Inattention, hyperactivity/impulsivity, and ADHD index, an overall measure of symptom severity, were rated by parents. Subjects were diagnosed as TDC, ADHD-hyperactive/impulsive, ADHD-inattentive, or ADHD-combined (Table 1).
Table 2.
Diagnosis Variables
| Variable | KKI | NYU | OHSU | PU | Total Sample |
|---|---|---|---|---|---|
| ADHD Index | TDC: 45.2 ± 4.3 | TDC: 45.1 ± 6.0 | TDC: NA | TDC: 28.1 ± 6.0 | TDC: 38.4 ± 10.0 |
| ADHD: 73.5 ± 9.8a | ADHD: 71.1 ± 8.5a | ADHD: NA | ADHD: 51.0 ± 8.9a | ADHD: 64.6 ± 13.2a | |
| Inattention | TDC: 45.7 ± 4.9 | TDC: 45.1 ± 5.7 | TDC: 47.0 ± 6.2 | TDC: 15.1 ± 3.7 | TDC: 35.3 ± 15.4 |
| ADHD: 73.4 ± 10.6a | ADHD: 70.2 ± 9.0a | ADHD: 72.9 ± 7.9a | ADHD: 28.8 ± 5.9a | ADHD: 58.9 ± 21.1a | |
| Impulsivity | TDC: 46.6 ± 4.5 | TDC: 46.1 ± 5.4 | TDC: 45.9 ± 6.6 | TDC: 13.1 ± 3.4 | TDC: 34.9 ± 16.5 |
| ADHD: 72.7 ± 10.8a | ADHD: 67.9 ± 12a | ADHD: 70.4 ± 13a | ADHD: 23.3 ± 7.8a | ADHD: 55.7 ± 23.6a | |
| IQ | TDC: 111 ± 10 | TDC: 110 ± 14 | TDC: 118 ± 13 | TDC: 118 ± 13 | TDC: 115 ± 13 |
| ADHD: 106 ± 15a | ADHD: 106 ± 14a | ADHD: 108 ± 14a | ADHD: 105 ± 13a | ADHD: 106 ± 14a |
ADHD, attention-deficit/hyperactivity disorder; IQ, intelligence quotient; KKI, Kennedy Krieger Institute; NA, not applicable; NYU, New York University Child Study Center; OHSU, Oregon Health & Science University; PU, Peking University; TDC, typically developing children.
ADHD < TDC → p < 10−10 (two-sample t test).
Image Preprocessing
Image realignment and spatial normalization to the stereotactic space of the Montreal Neurological Institute (MNI) with voxel size of 3 × 3 × 3mm3 were carried out using SPM2 (Wellcome Trust Centre for Neuroimaging, London, United Kingdom). Physiological noise was minimized using band-pass temporal filtering and removal of motion-related signal fluctuations (28). Voxels located at white matter and cerebrospinal fluid regions were excluded using a gray matter mask.
Functional Connectivity Density Mapping
Functional connectivity density mapping (28,29) was used to compute the strength of the local functional connectivity density (lFCD) and the global functional connectivity density (gFCD). The number of functional connections, k(x0), was determined through Pearson correlations between time-varying signals at x0 and those in other voxels using an arbitrary threshold R > .6; this correlation threshold was selected in our previous work because r < .4 increased false-positive rate and central processing unit (CPU) time and r > .7 led to lFCD maps with reduced dynamic range and lower sensitivity; thus, we fixed r = .6 for all calculations (28). See Supplement 1 for further details on the computation of lFCD and gFCD.
Short- and Long-Range FCD
The lFCD included all voxels that belonged to the local cluster of functionally connected voxels and was equated to short-range FCD (intraregional connectivity). The strength of the long-range FCD (interregional connectivity) was equated to gFCD – lFCD to remove all connected voxels that belonged to the local cluster (30). Short- and long-range FCD maps were spatially smoothed with an 8-mm Gaussian kernel in SPM2 to minimize the differences in the functional anatomy of the brain across subjects.
Subject Motion
To control for the effect of subject’s motion on functional connectivity measures, we computed the mean absolute displacement of the brain from every time frame to the next (31). The eight ADHD children (seven boys and one girl) with the largest mean displacements (>.3 mm) were excluded from the analysis to match the mean displacement across ADHD and TDC participants (mean displacement: .0794 ± .002 [ADHD] and .0725 ± .003 [TDC]; p = .09, t test).
Statistical Analyses
One-way analysis of variance (ANOVA) with three zero-mean covariates (age, gender, and mean displacement) was implemented in SPM to map group differences, independently for short- and long-range FCD, and also for RSFC. Statistical significance was based on pFWE < .05, corrected for multiple comparisons at the cluster level with a family-wise error (FWE) correction.
Results
Demographic Variables and Clinical Measures
There were some differences in the clinical characteristics of participants from the various institutions (Table 1). For the whole sample, the age of ADHD children did not differ from that of the TDC, but the proportion of boys was higher in the ADHD sample (204 boys vs. 51 girls) than in the TDC sample (168 boys vs. 148 girls) (p < .0001). Twelve children were removed from the TDC group, 9 who were receiving psychotropic medications (4 boys and 5 girls) and 3 who had no information on medication status (2 boys and 1 girl); thus, the final TDC sample included 304 participants. In the ADHD group, 67 children were receiving psychotropic medication; the medication status was missing for 73 ADHD children; and the remaining 115 ADHD children were medication naïve.
ADHD index scores were not reported for the OHSU data set and parent-rated scores were missing for 8 TDC girls, 12 TDC boys, 2 ADHD girls, and 7 ADHD boys from the remaining institutions (KKI, NYU, and PU). The available scores of inattention, hyperactivity/impulsivity, and ADHD index were highly intercorrelated (r > .84) and showed significant correlation with IQ (r < −.2; p < .0001). Scores of inattention, hyperactivity/impulsivity, and ADHD index were significantly lower for PU than for KKI, NYU, and OHSU (p < 10−32; t test), which likely reflects differences between the ADHD Rating Scale IV (PU) and the Conners’ Parent Rating Scale–Revised, Long version and the parent and teacher Conners’ Rating Scale, Third Edition (KKI, NYU, and OHSU). Therefore, separate group comparisons of clinical measures (and the regression analysis with the clinical measures, see below) were carried out for the PU data set and for the rest of the data sets (KKI, NYU, and OHSU), which were combined.
For the PU data set, scores of inattention, hyperactivity/impulsivity, and ADHD index were higher for ADHD children than for TDC (separated analyses for boys and girls; p < 10−5; t test); IQ was significantly lower for ADHD boys than for TDC boys (p < 10−6), but this group difference was not observed in girls, likely due to the reduced number of ADHD girls in the PU data set. Gender differences in scores of inattention, hyperactivity/impulsivity, ADHD index, and IQ were not observed for ADHD children or TDC in the PU data set (p > .08). For the KKI-NYU-OHSU combined sample, scores of inattention, hyperactivity/impulsivity, and ADHD index were also higher for ADHD children than for TDC (separated analyses for boys and girls; p < 10−30; t test); the lower IQ for ADHD was more pronounced in ADHD boys (p < 10−7) than in girls (p < 10−2), but this gender × group interaction effect did not reach statistical significance. Intelligence quotient was higher for TDC boys than for TDC girls (p < .0003). Scores of inattention and ADHD index were higher for ADHD girls than for ADHD boys (p < 10−7), but these gender differences were not observed in TDC (p > .22) and the gender × group interaction effect was statistically significant (p < .0001; one-way ANOVA).
FCD
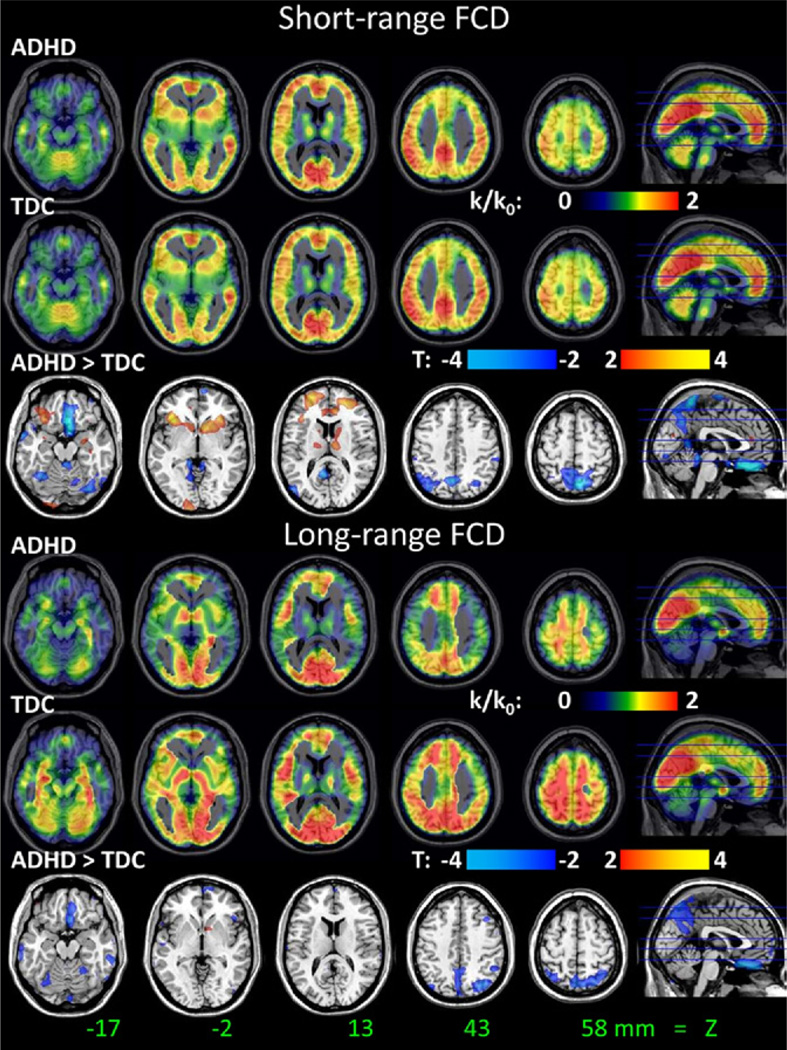
For TDC and ADHD children, the short-range FCD patterns were highly bilateral and maximal in posterior cingulate/ventral precuneus, occipital, inferior, superior and lateral parietal, ventral, and dorsolateral prefrontal cortices (Figure 1). The long-range FCD patterns were also highly bilateral for TDC and ADHD children and were maximal in ventral posterior occipital, ventral prefrontal, parietal, and dorsolateral prefrontal cortices. Group comparisons showed that short-range FCD was lower in SPC (Brodmann area [BA] 7) and precuneus and higher in inferior orbitofrontal cortex (OFC)/insula, ventral striatum, and superior frontal cortex (BA 10) for ADHD children than for TDC (pFWE < .05; Table 3, Figure 1). ADHD children also had lower long-range FCD in SPC and cerebellum than TDC (pFWE < .05; Table 3, Figure 1).
Figure 1.
Distribution of short-range (top panel) and long-range (bottom panel) functional connectivity density (FCD) in the human brain for 247 attention-deficit/hyperactivity disorder children and 304 typically developing children and the statistical differences (t score) between the groups. Functional connectivity density mapping threshold used to compute short- and long-range FCD: r > .6. One-way analysis of variance with three covariates (age, gender, and mean motion) was used to contrast short- and long-range FCD maps across groups. ADHD, attention-deficit/hyperactivity disorder; TDC, typically developing children.
Table 3.
Statistical Significance (t Scores; One-Way ANOVA) of FCD Differences Between ADHD Patients (n = 247) and Matched Healthy Control Subjects (n = 304), as well as the Corresponding Effects of Gender and Age on FCD
| Region | BA | x (mm) | y (mm) | z (mm) | TDC (t) | ADHD (t) | ADHD > TDC (t) | F > M (t) | Age (t) |
|---|---|---|---|---|---|---|---|---|---|
| Short-Range FCD = lFCD: ADHD > Control | |||||||||
| OFC | 47 | −30 | 27 | −3 | 34.5 | 35.1 | 3.0 | 2.7 | ns |
| OFC | 47 | 33 | 24 | −6 | 38.7 | 40.2 | 4.0 | ns | ns |
| OFC | 47 | 36 | 27 | −12 | 38.4 | 39.0 | 3.2 | ns | 2.9 |
| Ventral Striatum | −12 | 12 | −6 | 30.3 | 30.8 | 2.6 | ns | ns | |
| Ventral Striatum | 9 | 12 | −6 | 29.9 | 30.2 | 2.4 | 2.6 | −1.8 | |
| Superior Frontal | 10 | 24 | 60 | 18 | 28.1 | 30.5 | 3.8 | ns | ns |
| Short-Range FCD = lFCD: Control > ADHD | |||||||||
| Precuneus | 30 | 6 | −54 | 9 | 33.5 | 25.4 | −3.6 | 2.7 | ns |
| Precuneus | 7 | −6 | −66 | 60 | 39.3 | 30.3 | −3.8 | ns | 2.8 |
| Precuneus | 5 | 3 | −48 | 69 | 28.9 | 21.1 | −3.6 | ns | ns |
| Long-Range FCD = lFCD − gFCD: Control > ADHD | |||||||||
| Cerebellum | Crus | −54 | −66 | −27 | 10.1 | 5.3 | −2.8 | ns | ns |
| SPC | 7 | −24 | −66 | 63 | 7.2 | 4.0 | −1.8 | ns | ns |
| SPC | 7 | −27 | −75 | 48 | 16.3 | 10.1 | −3.4 | ns | ns |
Statistical values averaged in 9-mm isotropic (cubic; 27 imaging voxels) regions of interest centered at the Montreal Neurological Institute coordinates (x, y, z) of the local maxima.
ADHD, attention-deficit/hyperactivity disorder; ANOVA, analysis of variance; BA, Brodmann area; F, female; FCD, functional connectivity density; gFCD, global functional connectivity density; lFCD, local functional connectivity density; M, male; ns, nonsignificant; OFC, orbitofrontal cortex; SPC, superior parietal cortex, TDC, typically developing children.
Region of Interest Analyses
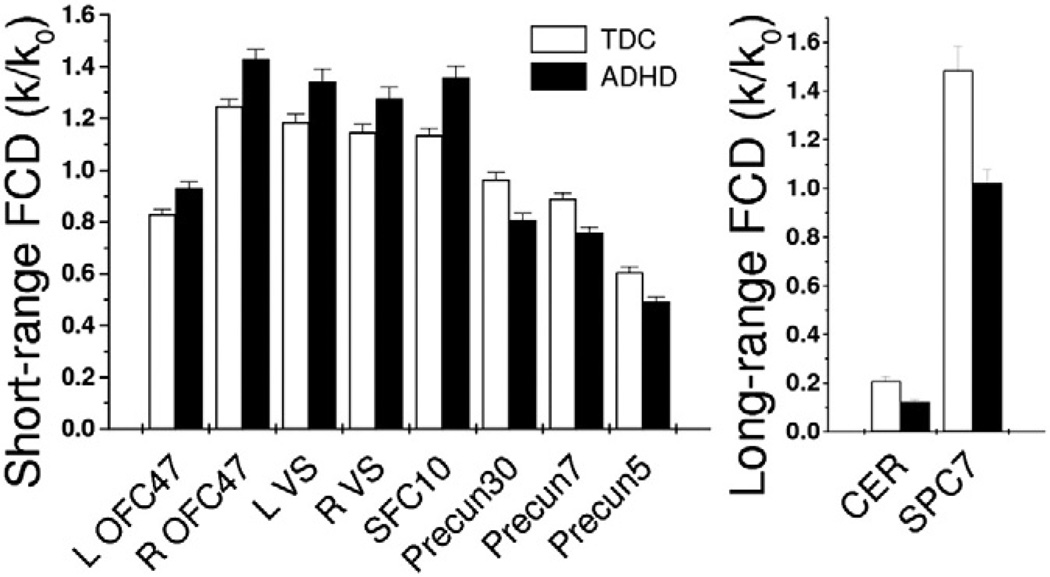
Average FCD measures in regions of interest (ROIs) that differed between the groups showed that the strength of short- and long-range FCD varied between regions and differed between TDC and ADHD children (Figure 2, Table 3). The average strength of the short-range FCD in ROIs located in left and right OFC/insula, ventral striatum, and caudate (Table 3) was 15 ± 2% higher for ADHD children than for TDC. Conversely, the short- and long-range FCD were lower in ADHD children than TDC in parietal cortex (16 ± 2% and 33 ± 4%, respectively) and cerebellum (15 ± 3%and 40 ± 10%, respectively). Short-range FCD in OFC/insula and ventral striatum was positively correlated with long-range FCD in SPC, independently for ADHD children and TDC (r > .16; p < .01).
Figure 2.
Bar plot showing the average values for short-range (left) and long-range (right) functional connectivity density (FCD) across subjects and regions of interest (ROIs) for attention-deficit/hyperactivity disorder (ADHD) children (n = 247) and typically developing children (TDC) (n = 304). Error bars are standard errors of the mean. Statistical significance between ADHD and TDC for all ROIs: p < .01. Cubic ROI volume: .73 cc (27 imaging voxels); ROI center coordinates in Table 3. Numbers in region labels are Brodmann areas. CER, cerebellum; L, left hemisphere; OFC, orbitofrontal cortex; Precun, precuneus; R, right hemisphere; SFC, superior frontal cortex; SPC, superior parietal cortex; VS, ventral striatum.
The short-range FCD in ADHD boys from the PU data set showed negative correlations with hyperactivity/impulsivity and the ADHD index in cerebellum, SPC, and precuneus (r < −.24; p < .05; Figure 3) and for the TDC boys positive correlations were observed in ventral striatum with scores of inattention and the ADHD index, in left OFC/insula with hyperactivity/impulsivity and the ADHD index, and in superior frontal gyrus (BA 9) with hyperactivity/impulsivity scores (r > .25; p < .05; Figure 3). The long-range FCD in ADHD boys from the PU data set showed negative correlations in SPC with inattention, hyperactivity/impulsivity, and the ADHD index scores (r < −.32; p = .01; Figure 3).
Figure 3.
Scatter plots showing correlations between rating scores and average values of short- and long-range functional connectivity density (FCD) in parietal (top panels) and reward-motivational (bottom panels) regions of interest (ROIs). Sample: 67 attention-deficit/hyperactivity disorder (ADHD) boys (full circles) and 63 typically developing children (TDC) boys (open circles) from Peking University. Cubic ROI volume: .73 cc (27 imaging voxels); ROI center coordinates in Table 3. OFC, orbitofrontal cortex.
For the KKI-NYU-OHSU combined sample, rating scores did not show significant correlations with FCD in the ROIs (separate analyses for TDC and ADHD boys and girls; |r| < .2). Correlations with scores of IQ were not significant for any ROIs.
RSFC Networks
We mapped the networks functionally connected to the left SPC (cubic ROI seed; ROI volume = 125 voxels; center coordinates: x, y, z = [−24, −66, 63] mm), an attention region that showed lower short- and long-range FCD for ADHD than for TDC, and that of the ventral striatum (ROI coordinates: x, y, z = [9, 15, −3] mm), a reward-motivation region that showed higher short-range FCD for ADHD than for TDC.
For both ADHD and TDC, the positive SPC-RSFC mapped into a bilateral network that included other SPC regions (BAs 2, 5, 7, and 40); superior, middle, and inferior occipital gyri (BA 19); inferior temporal cortex (BA 37); and the superior frontal gyrus (frontal eye field, BA 6) (pFWE < .001; Figure 4); the negative RSFC mapped into anterior and posterior insula; anterior cingulum (BAs 24 and 32); superior frontal gyrus (BA 9) and OFC (BAs 10 and 11); inferior frontal (BAs 45 and 47), supramarginal gyrus (BA 40), angular gyrus (BA 39), precuneus (BA 39), superior (BA 22), middle (BA 21), and inferior (BA 20) temporal gyri; temporal pole (BA 21); parahippocampal gyrus (BAs 30 and 36); midbrain; dorsal striatum (caudate, putamen, and pallidum); thalamus; and vermis. The positive strength of the RSFC was lower for ADHD children than for TDC in precuneus and SPC, and the negative RSFC strength was lower in medial OFC and temporal cortices (pFWE < .05; Figure 4). Thus, the lower SPC-RSFC for ADHD mapped into the attention network regions that showed lower FCD and the reward-cin TDC.
Figure 4.
Statistical significance (color-coded t score) of resting-state functional connectivity patterns for the superior parietal cortex (SPC) seed (cubic region of interest [ROI] centered at x, y, z = (−24, −66, 63) mm; ROI volume = 125 imaging voxels) for 304 typically developing children (TDC) and 247 attention-deficit/hyperactivity disorder (ADHD) children and their differences. One-way analysis of variance with three covariates (age, gender, and mean motion). RSFC, resting-state functional connectivity.
For both ADHD and TDC, the positive RSFC of the ventral striatum mapped into a bilateral network that included middle OFC (BA 11), anterior cingulum (BA 32), superior frontal gyrus (BA 10), OFC/insula, middle temporal gyrus (BA 22), temporal pole (BA 38), anterior thalamus, hypothalamus, caudate, hippocampus, precuneus, temporal pole, and parahippocampal gyrus (pFWE < .001). The negative RSFC of the ventral striatum mapped into the primary visual cortex (BA 17). The strength of the RSFC with ventral striatum was lower for ADHD children than for TDC in precuneus, temporal pole, and parahippocampal gyrus (pFWE < .05).
Medication
ADHD children that were not using psychotropic medications had higher short-range FCD than TDC in OFC/insula and ventral striatum (p < .05; ROI analyses), but this effect was not observed in medicated ADHD children (Figure 5), suggesting that ADHD medications could normalize the short-range FCD in reward-motivation regions. However, the short-range FCD differences between medicated and unmedicated ADHD children were not statistically significant (p > .1). On the other hand, the medicated ADHD group exhibited lower long-range FCD than TDC in cerebellum and SPC (p < .05), but this effect was not observed in ADHD children that were not using psychotropic medications (Figure 5).
Figure 5.
Effect of medications on short-range functional connectivity density (FCD) in reward-motivational regions of interest (ROIs) (right orbitofrontal cortex [OFC]/insula and ventral striatum [VS]) and of long-range FCD in cerebellum and superior parietal cortex (SPC) ROIs. Statistical significance: *p < .05. Sample: 119 unmedicated and 68 medicated attention-deficit/hyperactivity disorder (ADHD) children and 304 unmedicated typically developing children (TDC). Cubic ROI volume: .73 cc (27 imaging voxels); ROI center coordinates in Table 3. Cereb, cerebellum; med, medication.
Gender
Both genders demonstrated higher short-range FCD in ventral striatum for the ADHD group compared with TDC (p < .05; ROI analyses), but the higher short-range FCD in OFC/insula for ADHD was pronounced for boys but not for girls (p < .001; Figure S3 in Supplement 1). The long-range FCD in SPC and cerebellum was lower for ADHD than for TDC for boys (p < .001) but not for girls and showed a gender × diagnosis interaction effect (p < .05; one-way ANOVA with gender as a covariate).
Discussion
Here, we used graph theory to map changes in brain functional connectivity (both short- and long-range FCD) associated with ADHD in a large data set (247 ADHD patients and 304 TDC). ADHD children demonstrated 15% higher short-range connectivity in regions classically associated with reward and motivation and 33% lower long-range connectivity in regions classically associated with cognitive processing (parietal cortex). Furthermore, we document lower RSFC strength between reward-motivation and attention networks for ADHD children than for TDC that might help understand impairments in cognitive (attention/executive [32]) and reward (motivation [33]) in ADHD.
Hyper Short-Range Connectivity
Ventral striatum, caudate, and OFC, brain regions that are implicated in motivation and reward (34), demonstrated higher strength of short-range connectivity for ADHD children than for TDC, and there was a positive association between rating scores and short-range connectivity in some of these regions (ventral striatum, OFC/insula) for TDC boys from PU. The higher proportion of short-range FCD in regions involved with motivation/reward in ADHD children is consistent with our previous studies showing lower density of DA transporters and D2/D3 receptors in ventral striatum and caudate in ADHD adults than in control subjects that were associated with inattention and with reduced scores in trait measures of motivation (9). Dopamine neurons in the ventral tegmental area project to ventral striatum caudate and OFC, modulating the neuronal activity in these brain regions (35). Dopamine changes the efficacy of other neurotransmitter signals in the brain (36), apparently by reducing spontaneous background activity (37), and thus, the lower dopaminergic function in ADHD might cause larger higher spontaneous activity and increased short-range FCD. This hypothesis is consistent with the higher amplitude of low-frequency fluctuations in lateral OFC (17) and the higher RSFC of the anterior cingulate with OFC/insula and inferior frontal cortices previously reported for ADHD children than for TDC (20). However, it contrasts with the lower functional connectivity of the OFC/insula reported in ADHD adults when compared with control subjects while performing a working memory task (38). These differences could reflect not just the conditions of the studies (resting versus cognitive task) but also the developmental stages of the subjects (children versus adults).
The weaker functional connectivity of the OFC/insula and ventral striatum with posterior parietal regions for ADHD children than for TDC is also consistent with the dopaminergic modulation of parietal activity (39) and with prior reports of decreased functional connectivity between striatal and posterior cortical regions in ADHD (23,40).
Hypo Long-Range Connectivity
Superior parietal cortices and cerebellum demonstrated lower long-range FCD (as well as short-range FCD) for ADHD children than for TDC and decreased FCD in these regions showed significant correlation with inattention and impulsivity/hyperactivity for ADHD children from the PU cohort. We previously showed that brain activity in SPC was correlated with the availability of DA transporters in caudate (39). Thus, lower dopaminergic function in ADHD patients (8) could impair brain activity and the long-range connectivity in SPC, a region that includes prominent long-range functional connectivity hubs (29). This is consistent with the impairments in parietal lobe function of ADHD patients (32). The connectivity of SPC with dorsal attention network regions (dorsal precuneus and SPC; positive RSFC) and that with OFC and temporal networks (middle and inferior temporal cortex; negative RSFC) were weaker for ADHD children than for TDC.
Hypoconnectivity
Seed-voxel correlation analysis revealed that the RSFC network of the SPC (BA 7) and ventral striatum showed decreased positive connectivity for ADHD children than for control subjects in other SPC regions that are part of the dorsal attention network (41) and with the precuneus, which is part of the default mode network (DMN), and decreased negative connectivity with OFC/insula and ventral striatum. The DMN is routinely deactivated during goal-directed cognitive tasks (42), and impaired suppression of the DMN is associated with attentional lapses during performance of a cognitive task (43). Impaired connectivity between the dorsal attention network and DMN has been proposed to contribute to inattention in ADHD (44). Our findings support reduced connectivity between these two networks in children with ADHD. The reduced connectivity with the lateral OFC/insula is consistent with findings in children and young adults with ADHD showing executive function deficits that include the lateral OFC (45).
Our findings are consistent with the dual pathway model of ADHD that identifies dysregulation of two independent components: a cognitive component (poor inhibitory control) that implicates the mescortical dopamine pathway and a motivational component (delayed aversion) that implicates the mesolimbic dopamine pathway (46). Future studies that assess the intersubject variability in the connectivity of these two pathways in individuals with ADHD and their relationship to clinical symptoms and outcomes will allow investigators to evaluate the potential of RSFC as a clinical biomarker in ADHD.
Medication
Medicated ADHD children showed less enhancement of the connectivity in reward/motivation regions, which could reflect the decreases in background activity associated with enhanced dopaminergic and noradrenergic signaling (37). Indeed, these findings are consistent with studies in ADHD children showing that methylphenidate normalized the hypersensitive OFC activation to reward (25). On the other hand, the reduced connectivity in cerebellum and SPC was more accentuated in ADHD treated children than in those without medication. Because ADHD treated children also tended to show lower FCD in these regions than control subjects, it is unlikely that the finding is driven by medication. Moreover, studies on effects of medication tend to support an amelioration of cerebellar changes in ADHD with stimulant medications (47), as well as an improvement on parietotemporal activation (25). Thus, we would have expected the connectivity to be lower in unmedicated than in medicated ADHD children, but this apparent discrepancy could reflect a more severe phenotype in medicated that in unmedicated ADHD children.
Study Limitations
The small number of ADHD girls in this study (n = 50) limited the statistical power of our gender analyses, and thus the findings predominately reflect the data from ADHD boys (n = 197). The phenotypic characterization of subjects in the ADHD-200 Sample database did not provide standardized parent-rated scores (PU used the ADHD Rating Scale IV; the remaining institutions used the Conners’ Parent Rating Scale–Revised, Long version) and missed ADHD index for one of data sets (OHSU), as well as scores of inattention and hyperactivity/impulsivity for some of the subjects. Since the symptoms varied significantly across institutions, we were unable to analyze functional connectivity differences as a function of ADHD subtypes. Thus, we were unable to assess the correlation with ADHD symptoms for the whole sample, and the restricted statistical power might have limited our findings to the PU data set. Furthermore, medication status was missing for a large number of subjects; the doses and types of medications and whether the subjects were or were not under the effects of the medication during the functional magnetic resonance imaging scan were not documented. Since stimulant medications are very effective in suppressing ADHD symptoms (32), it was surprising that there were no differences in symptom scores between medicated and unmedicated ADHD children. This could indicate that either the scores were obtained when they were not under the effects of medications or if they were scored while under the effects of the medication that their symptoms were more severe than those of the unmedicated ADHD children. Since age affects the connectivity pattern, it would have been desirable to have a measure of developmental stage (hormonal status) in addition to that provided by age alone. Some of the variability across research institutions is likely to reflect differences in resting conditions (e.g., eyes opened/closed, awake/sleep, etc.), which could affect group comparisons (48,49). Micro movement (31) and respiration (50) can also increase the variability of the functional connectivity patterns. The mean displacement covariate partially controlled for micro movements; the relative fast acquisition (repetition time < 2.5 seconds) and the .01 to .10 Hz band-pass filtering partially controlled for the effect of respiration. We could not control additional physiologic effects because heart rate and respiration data were not available. Resting-state functional connectivity studies do not interrogate the functional roles of the brain regions, which are known only by analogy with other studies, and explore brain network properties in a limited way (51). Therefore, caution must be exercised when interpreting the functional significance of RSFC results.
In conclusion, here, we document higher short-range FCD in reward-motivation network regions encompassing ventral striatum and orbitofrontal cortex and lower long-range FCD in attention (SPC) and default-mode (precuneus) network regions for ADHD children than for control subjects. We also document a decreased connectivity between corticostriatal and parietal networks that might underlie the impairment in attention and the decreased motivation to sustain attention, as well as the impaired inhibition of the default mode networks in ADHD (25,52).
Supplementary Material
Acknowledgments
This work was accomplished with support from the National Institute on Alcohol Abuse and Alcoholism (2RO1AA09481).
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.National Institutes of Health. National Institutes of Health Consensus Development Conference Statement: Diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD) J Am Acad Child Adolesc Psychiatry. 2000;39:182–193. doi: 10.1097/00004583-200002000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Biederman J. Attention-deficit/hyperactivity disorder: A life-span perspective. J Clin Psychiatry. 1998;59(suppl 7):4–16. [PubMed] [Google Scholar]
- 3.Haenlein M, Caul W. Attention deficit disorder with hyperactivity: A specific hypothesis of reward dysfunction. J Am Acad Child Adolesc Psychiatry. 1987;26:356–362. doi: 10.1097/00004583-198705000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Volkow N, Wang G, Newcorn J, Telang F, Solanto M, Fowler J, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- 5.Prince J. Catecholamine dysfunction in attention-deficit/hyperactivity disorder: An update. J Clin Psychopharmacol. 2008;28:S39–S45. doi: 10.1097/JCP.0b013e318174f92a. [DOI] [PubMed] [Google Scholar]
- 6.Ernst M, Zametkin A, Matochik J, Jons P, Cohen R. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci. 1998;18:5901–5907. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst M, Zametkin A, Matochik J, Pascualvaca D, Jons P, Cohen R. High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1209–1215. doi: 10.1176/ajp.156.8.1209. [DOI] [PubMed] [Google Scholar]
- 8.Volkow N, Wang G, Kollins S, Wigal T, Newcorn J, Telang F, et al. Evaluating dopamine reward pathway in ADHD: Clinical implications. JAMA. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volkow N, Wang G, Newcorn J, Kollins S, Wigal T, Telang F, et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2011;16:1147–1154. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrmann M, Biehl S, Jacob C, Deckert J. Neurobiological and psychophysiological correlates of emotional dysregulation in ADHD patients. Atten Defic Hyperact Disord. 2010;2:233–239. doi: 10.1007/s12402-010-0047-6. [DOI] [PubMed] [Google Scholar]
- 11.Rubia K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: A review. Biol Psychiatry. 2011;69:e69–e87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Rubia K, Halari R, Cubillo A, Smith A, Mohammad A, Brammer M, et al. Methylphenidate normalizes fronto-striatal underactivation during interference inhibition in medication-naïve boys with attention-deficit hyperactivity disorder. Neuropsychopharmacology. 2011;36:1575–1586. doi: 10.1038/npp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bidwell L, McClernon F, Kollins S. Cognitive enhancers for the treatment of ADHD. Pharmacol Biochem Behav. 2011;99:262–274. doi: 10.1016/j.pbb.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Posner J, Maia T, Fair D, Peterson B, Sonuga-Barke E, Nagel B. The attenuation of dysfunctional emotional processing with stimulant medication: An fMRI study of adolescents with ADHD. Psychiatry Res. 2011;193:151–160. doi: 10.1016/j.pscychresns.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins T, Arnsten A. The neuropsychopharmacology of fronto-executive function: Monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuczenski R, Segal D. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: Comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- 17.Zang Y, He Y, Zhu C, Cao Q, Sui M, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, Wu Q, Guo L, Li Q, Long X, Huang X, et al. Abnormal spontaneous brain activity in medication-naïve ADHD children: A resting state fMRI study. Neurosci Lett. 2011;502:89–93. doi: 10.1016/j.neulet.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 19.Liston C, Cohen M, Teslovich T, Levenson D, Casey B. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: Pathway to disease or pathological end point? Biol Psychiatry. 2011;69:1168–1177. doi: 10.1016/j.biopsych.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, et al. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett. 2006;400:39–43. doi: 10.1016/j.neulet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Cao X, Cao Q, Long X, Sun L, Sui M, Zhu C, et al. Abnormal resting-state functional connectivity patterns of the putamen in medication-naïve children with attention deficit hyperactivity disorder. Brain Res. 2009;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Castellanos F, Margulies D, Kelly C, Uddin L, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity Disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uddin L, Kelly A, Biswal B, Margulies D, Shehzad Z, Shaw D, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 2008;169:249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Fair D, Posner J, Nagel B, Bathula D, Dias T, Mills K, et al. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubia K, Halari R, Cubillo A, Mohammad A, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Buckner R, Sepulcre J, Talukdar T, Krienen F, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomasi D, Volkow N. Functional connectivity density mapping. Proc Natl Acad Sci U S A. 2010;107:9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomasi D, Volkow N. Functional connectivity hubs in the human brain. Neuroimage. 2011;57:908–917. doi: 10.1016/j.neuroimage.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomasi D, Volkow N. Aging and functional brain networks [published online ahead of print July 5] Mol Psychiatry. 2011 doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Dijk K, Sabuncu M, Buckner R. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swanson J, Baler R, Volkow N. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: A decade of progress. Neuropsychopharmacology. 2011;36:207–226. doi: 10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripp G, Wickens J. Research review: Dopamine transfer deficit: A neurobiological theory of altered reinforcement mechanisms in ADHD. J Child Psychol Psychiatry. 2008;49:691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 34.Wise R. Brain reward circuitry: Insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 35.Haber S, Fudge J. The primate substantia nigra and VTA: Integrative circuitry and function. Crit Rev Neurobiol. 1997;11:323–342. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- 36.Kiyatkin E, Rebec G. Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol. 1996;75:142–153. doi: 10.1152/jn.1996.75.1.142. [DOI] [PubMed] [Google Scholar]
- 37.Rolls E, Thorpe S, Boytim M, Szabo I, Perrett D. Responses of striatal neurons in the behaving monkey. 3. Effects of iontophoretically applied dopamine on normal responsiveness. Neuroscience. 1984;12:1201–1212. doi: 10.1016/0306-4522(84)90014-9. [DOI] [PubMed] [Google Scholar]
- 38.Wolf R, Plichta M, Sambataro F, Fallgatter A, Jacob C, Lesch K, et al. Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2009;30:2252–2266. doi: 10.1002/hbm.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomasi D, Volkow N, Wang R, Telang F, Wang G, Chang L, et al. Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One. 2009;4:e6102. doi: 10.1371/journal.pone.0006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonuga-Barke E, Castellanos F. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Tomasi D, Volkow N. Association between functional connectivity hubs and brain networks. Cereb Cortex. 2011;21:2003–2013. doi: 10.1093/cercor/bhq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weissman D, Roberts K, Visscher K, Woldorff M. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 44.Kelly A, Uddin L, Biswal B, Castellanos F, Milham M. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of frontostriatal and fronto-cortical brain abnormalities in children and adults with attention deficit hyperactivity disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention [published online ahead of print April 27] Cortex. 2011 doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Sonuga-Barke E. Psychological heterogeneity in AD/HD—a dual pathway model of behaviour and cognition. Behav Brain Res. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- 47.Bledsoe J, Semrud-Clikeman M, Pliszka S. A magnetic resonance imaging study of the cerebellar vermis in chronically treated and treatment-naïve children with attention-deficit/hyperactivity disorder combined type. Biol Psychiatry. 2009;65:620–624. doi: 10.1016/j.biopsych.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fornito A, Bullmore E. What can spontaneous fluctuations of the blood oxygenation-level-dependent signal tell us about psychiatric disorders? Curr Opin Psychiatry. 2010;23:239–249. doi: 10.1097/YCO.0b013e328337d78d. [DOI] [PubMed] [Google Scholar]
- 49.Morcom A, Fletcher P. Does the brain have a baseline? Why we should be resisting a rest. Neuroimage. 2007;37:1073–1082. doi: 10.1016/j.neuroimage.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 50.Birn R, Murphy K, Bandettini P. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Hum Brain Mapp. 2008;29:740–750. doi: 10.1002/hbm.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wig G, Schlaggar B, Petersen S. Concepts and principles in the analysis of brain networks. Ann N Y Acad Sci. 2011;1224:126–146. doi: 10.1111/j.1749-6632.2010.05947.x. [DOI] [PubMed] [Google Scholar]
- 52.Castellanos F, Sonuga-Barke E, Milham M, Tannock R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends Cogn Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.