Abstract
Background
In previous studies, the length of the glandulo-metaplastic esophageal mucosa (GMEM) at the gastroesophageal junction was assessed in a selected group of baboons. In this study, the length of the GMEM was measured in the entire esophagus in a cohort of unselected adult baboons.
Materials and Methods
In 15 female baboons, the entire esophagus was removed en bloc at autopsy, from the tongue to the angle of His. No part of the stomach was included. The length of GMEM was measured using a calibrated ocular microscale.
Results
GMEM was found in 11 out of the 15 esophagi. The total length of GMEM recorded in the 11 cases was 115 mm (mean 10.5 mm, range 1–45 mm). The mean age for animals with GMEM was 15.5 years (range 7–32 years) and for animals without GMEM was 14.0 years (range 7–20 years); the difference was non-significant (p<0.6). No significant association was found between the length of the GMEM and the age of the animals (p<0.6).
Conclusion
This study substantiates the notion that GMEM in baboons is a postnatal physiological adaptative process of the esophageal mucosa to daily regurgitation with rumination of gastric juices of low pH. The GMEM apparently progresses upwards, along the esophageal mucosa. The baboon might be an excellent animal model to study the series of histological events that take place in the distal esophagus under the influence of protracted gastroesophageal reflux.
Keywords: Baboons, esophagus, metaplasia, reflux
The esophagus of the baboon, a tubular, predominantly intrathoracic organ, is covered by stratified squamous epithelium displaying discrete papillae with one layer of basal cells and none to occasional intraepithelial lymphocytes (1). The distal portion near the gastric junction is usually thicker with deep papillae. Accessory mucus glands in the mucosa, the submucosa and even within muscle bundles are seen in the distal portion of the organ. These accessory glands consist of lobules built of acini containing both mucous-(chief) and serous (subsidiary)-secreting cells, connected to the lumen of the organ by a duct. Similar glands, accessory to the mucosa of the pharynx, are seen in the most proximal part of the organ. This organ is empirically divided into a proximal (cephalic), middle, and a distal third.
Under the influence of protracted gastric reflux, the normal squamous-lined mucosa becomes glandulo-metaplastic (2). The goal of this mucosal change is to buffer the low pH of the gastric juices entering the esophagus during regurgitation. The chewing of the regurgitated food, that is rumination, is a behavior linked to the physiological food processing machinery in baboons, as well as in other non-human primates (NHP) (2–4).
The glandulo-metaplastic esophageal mucosa (GMEM) the distal esophagus, is covered by columnar cells with mucous-producing glands located in the lamina propria; there are no glands in the submucosa or within muscle bundles (Figure 1).
Figure 1.
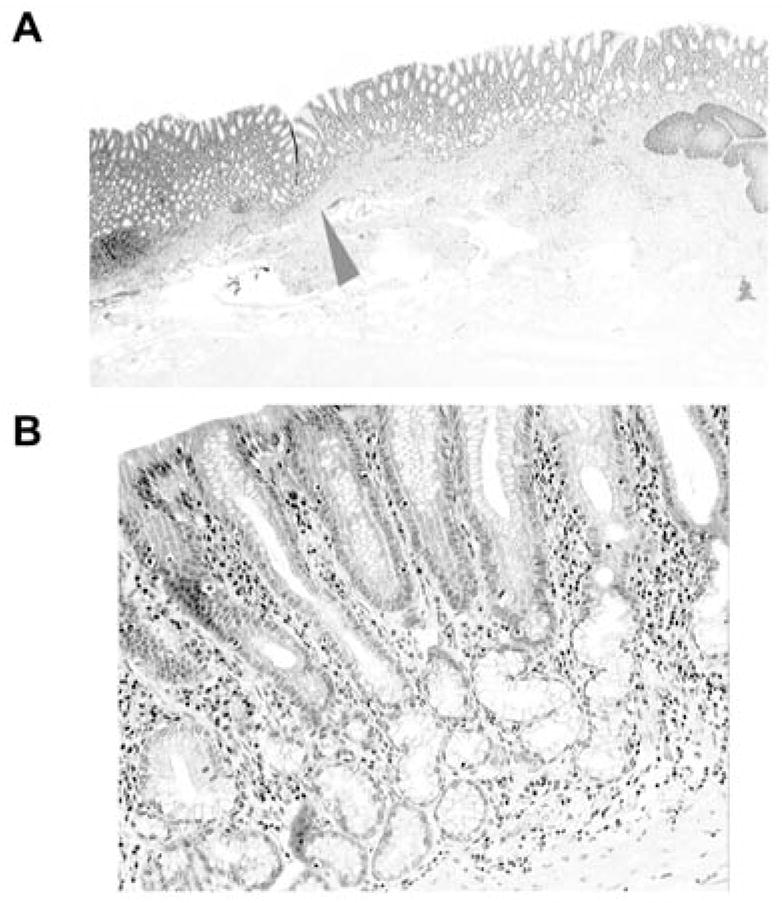
Glandulo-metaplastic esophageal mucosa (GMEM) in a baboon. A, Low power view: note the esophageal squamous epithelium on the right (H&E, ×2). B, Higher power view of Figure A (arrowhead), to demonstrate the mucous glands in the GMEM (H&E, ×10).
In previous work, we assessed the length of the GMEM in sections from the gastroesophageal junction of a selected group of adult NHP mainly baboons (1, 4).
The purpose of the present study was to audit the frequency and length of the GMEM in the entire esophagus in a consecutive cohort of adult baboons.
Materials and Methods
Fifteen adult female baboons (Papio spp), members of a colony at the Southwest National Primate Research Center, Southwest Foundation for Biomedical Research were investigated.
The conditions of animal housing were reported elsewhere (3). Briefly, the NHP were housed in metal and concrete indoor-outdoor cages and fed with commercial monkey diets, occasionally supplemented with a variety of fruit and vegetables. Water was available ad libitum. The animal management was carried out in accordance with the Institutional Animal Care and Use Committee guidelines.
The baboons were euthanized for management purposes. The entire esophagus was removed en bloc at autopsy, from the tongue (not included) to the angle of His (where the esophagus meets the stomach). No part of the stomach was included. The esophageal specimens were cut into 4 cm-long blocks, starting from the most proximal (oral) resection border. Blocks were sequentially numbered from 1 and each portion was opened wide, adhered to cardboard, cut into longitudinal sections. The sections were then fixed in 10% neutral buffered formalin, embedded in paraffin, cut at 5 μm, stained with hematoxylin and eosin (H&E) and evaluated under light microscopy. The length of the GMEM was assessed using a calibrated ocular micro scale under light microscopy.
Statistical analysis
The length of the GMEM (in mm) in relation to the age of the animals was tested using the nonparametric Wilcoxon matched pairs test. Statistical significance was defined as p<0.05.
Results
The results are presented in Table I. A total of 283 sections were reviewed. The Table shows that GMEM was found in 11 out of the 15 baboons. The total length of GMEM recorded in the 11 cases was 115 mm (mean 10.5 mm, range 1–45 mm).
Table I.
The length of the glandulo-metaplastic esophageal mucosa (GMEM), as measured in sections from the entire esophagus (from the tongue to the angle of His) in 15 adult baboons. The number of sections reviewed/case and the age of the animals are also given.
| Baboon | No. sections examined | Length (mm) of the GMEM in 11 baboons* | Age (in years) |
|---|---|---|---|
| Total | 283 | 115 | 232 |
| Mean | 18.9 | 10.5 | 15.5 |
GMEM was found in 11 out of the 15 baboons.
The mean age of animals with GMEM was 15.5 years (range 7–32 years), while for animals without GMEM, it was 14.0 years (range 7–20 years). The difference was non-significant (p<0.6).
No significant association was found between the length of the GMEM and the age of the animals (p<0.6).
Discussion
Sections from the entire esophagus, from the base of the tongue to the angle of His, were histological scrutinized in a cohort of consecutive baboons. No gastric mucosa was included in the preparations, thus contrasting with previous studies on a selected group of baboons (1, 2), in which histological sections from the gastroesophageal junction having GMEM were investigated.
The present results demonstrated that the GMEM occurred in the majority (n=11) of the 15 consecutive esophagi investigated. The length of the GMEM was apparently not influenced by the postnatal age of the animals. The GMEM seems to have progressed upwards along the esophageal mucosa.
The origin of the GMEM remains controversial. It has been postulated that the mucosal defects occurring concomitantly with reflux can be repaired with columnar-lined epithelium originating from a cephalic creeping substitution of the junctional glandular epithelium (cardiac mucosa) (5), from esophageal glands (6), or from squamous stem cells (7). However, we found no indication that the GMEM originated from normal esophageal glands, as the GMEM in baboons lacks the serous-secreting cells normally present in the esophageal glands proper. The GMEM in baboons appears to be a genuine glandular transformation of the normal esophageal squamous mucosa. One plausible explanation for this mucosal transformation is that these animals chew the regurgitated food (3, 4, 8–12), and chew it again (that is ruminate). Apparently, gastroesophageal reflux (GER) in baboons is a life-long physiological food processing mechanism (3, 4). The daily reflux of gastric juices of low pH eventually leads to GMEM of the distal esophagus.
Despite the pH of the gastric acid being similar in baboons (13) and humans (14) at the time of pH testing, the daily reflux of that acid into the esophagus might be more continuous in baboons than in humans. In contrast to its human counterpart, the baboon receives no medication for this natural gastric reflux, a treatment often administered to humans with symptoms for remittent waves of GER.
During the gastric phase of digestion, humans usually adopt an upright (orthostatic) position while baboons adopt, by nature, an oblique position. This oblique position might exacerbate the reflux of the gastric contents into the esophagus in these animals.
It has been recognized that a high fat intake in humans relaxes the lower esophageal sphincter (LES) (15, 16), thus encouraging GER. This situation seems not to apply to baboons inasmuch as the regular daily diet in monkeys is only 4% fat, whereas the limiting dietary fat recommended by The American Heart Association in humans is 30%. Hence, the daily fat intake does not seem to be a factor explaining the high frequency of GMEM in baboons.
Higher-ranking baboons usually have a greater access to food than lower-ranking baboons (3, 4), a behavior also found in numerous captive species of baboons. It is well known that stress both causes and increases the severity of symptoms in the gastrointestinal tract (17, 18) by changes in the motility and in the function of the LES. Corticotropin-releasing factor (CRF) is the prime mediator of the stress response (19). A response of CRF receptors to stress is an impaired or delayed emptying of the stomach contents, a situation that may encourage the development of GER. As low-ranking baboons housed in a group setting usually have much lower access to food than high-ranking baboons, it is not inconceivable that low-ranking baboons are subjected to daily stress (20, 21). Hence, the stress to which low-ranking baboons are subjected to might encourage the development of GMEM.
The chemical component of the refluxate bathing the mucosal microenvironment may also contribute to the development of the GMEM. In this respect, Mahadeva et al. (22) found that GER symptoms in humans were more common and correlated with an increased columnar-lined esophagus in British patients than in South-East Asian patients. The authors suggested that the different mucosal microenvironments were responsible for these differences. Consequently, possible differences in the chemical components of the refluxate bathing the esophageal mucosal microenvironment might help to explain the differences in frequency and length of the GMEM mucosa in baboons.
One possibility to explore the effect of the diet on the evolution of GMEM in the distal esophagus could be to assess the frequency and the length of the columnar-lined metaplastic mucosa in baboons at other facilities engaged in primate research. Different food regimens and behavioral attitudes at other facilities would identify whether these parameters have any bearing in the triggering of the glandulo-metaplastic transformation of the esophageal squamous epithelium in baboons.
In conclusion, the present investigation in baboons substantiates the notion that GMEM is a postnatal physiological process of adaptation of the esophageal mucosa to protracted regurgitation of low pH gastric acid, into the esophagus. Based on these considerations, the baboon might be an excellent animal model to study the series of histological events that takes place in the distal esophagus under the influence of protracted GER.
References
- 1.Rubio CA, Dick EJ, Jr, Hubbard GB. The columnar-lined mucosa in the distal esophagus. A preliminary study in baboons. In Vivo. 2009;23:273–275. [PMC free article] [PubMed] [Google Scholar]
- 2.Rubio CA, Dick EJ, Schlabritz-Loutsevitch NE, Orrego A, Hubbard GB. The columnar-lined mucosa at the gastroesophageal junction in non-human primates. Int J Clin Exp Pathol. 2009;2:481–488. [PMC free article] [PubMed] [Google Scholar]
- 3.Glover EJ, Leland MM, Dick EJ, Jr, Hubbard GB. Gastroesophageal reflux disease in baboons (Papio sp.): a new animal model. J Med Primatol. 2008;37:18–24. doi: 10.1111/j.1600-0684.2007.00217.x. [DOI] [PubMed] [Google Scholar]
- 4.Glover E, Leland M, Hubbard G. An association between gastric regurgitation and disease in nonhuman primates. Am J Primatol. 2005;66:174–179. [Google Scholar]
- 5.Gillen P, Keeling P, Byrne PJ. Experimental columnar metaplasia in the canine oesophagus. Br J Surg. 1988;75:113–115. doi: 10.1002/bjs.1800750208. [DOI] [PubMed] [Google Scholar]
- 6.Bremner CG, Lynch VP, Ellis FH., Jr Barrett’s esophagus: congenital or acquired? An experimental study of esophageal mucosal regeneration in the dog. Surgery. 1970;68:209–216. [PubMed] [Google Scholar]
- 7.Miyashita T, Ohta T, Fujimura T, Ninomiya I, Fushida S, Hattori T, Miwa K. Duodenal juice stimulates oesophageal stem cells to induce Barrett’s oesophagus and oesophageal adenocarcinoma in rats. Oncol Rep. 2006;15:1469–1475. [PubMed] [Google Scholar]
- 8.Baker K, Easley S. An analysis of regurgitation and reingestion in captive chimpanzees. Appl Anim Behav Sci. 1996;49:403–415. [Google Scholar]
- 9.Gould E, Bres M. Regurgitation and reingestion in captive gorillas: description and intervention. Zoo Biol. 1986;5:241–250. [Google Scholar]
- 10.Howell S, Fritz J, Downing S, Bunuel M. Treating chronic regurgitation behaviour: a case study. Lab Anim. 1997;26:30–33. [Google Scholar]
- 11.Gould E, Bres M. Regurgitation in gorillas: possible model for human eating disorders (rumiation/bulimia) J Dev Behav Pediatr. 1986;7:314–319. doi: 10.1097/00004703-198610000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Morgan L, Howell S, Fritz J. Regurgitation and reingestion in a captive chimpanzee (Pan troglodytes) Lab Anim. 2003;22:42–45. [Google Scholar]
- 13.Lakhoo K, Parekh D, Lawson HH, Rogers G, Van der Walt LA, Hunter S. Gastric acid secretion and gastrin release in the baboon. Dig Dis Sci. 1992;37:1313–1318. doi: 10.1007/BF01295997. [DOI] [PubMed] [Google Scholar]
- 14.Wenner J, Johnsson F, Johansson J, Oberg S. Acid reflux immediately above the squamocolumnar junction and in the distal esophagus: simultaneous pH monitoring using the wireless capsule pH system. Am J Gastroenterol. 2006;101:1734–1741. doi: 10.1111/j.1572-0241.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi M, Oka H, Hashimoto T, Asakuma Y, Takao M, Gon G, Yamamoto M, Tsuji Y, Yamamoto N, Shimada M, Lee K, Ashida K. Obesity as a risk factor for GERD in Japan. J Gastroenterol. 2008;43:57–62. doi: 10.1007/s00535-007-2128-7. [DOI] [PubMed] [Google Scholar]
- 16.Castell DO. Obesity and gastroesophageal reflux. Is there any relationship? Eur J Gastroenterol Hepatol. 1996;8:625–626. [PubMed] [Google Scholar]
- 17.Kamolz T, Melanovich T. Psychological and emotional aspects of gastroesophageal reflux in man. Gastroenterology. 1970;58:199–203. doi: 10.1046/j.1442-2050.2002.00261.x. [DOI] [PubMed] [Google Scholar]
- 18.Bhathia V, Tandon R. Stress and the gastrointestinal tract. J Gastroenterol Hepatolo. 2005;20:332–339. doi: 10.1111/j.1440-1746.2004.03508.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu SV, Yuan PQ, Wang L, Peng YL, Chen CY, Taché Y. Identification and characterization of multiple corticotropin-releasing factor type 2 receptor isoforms in the rat esophagus. Endocrinology. 2007;148:1675–1687. doi: 10.1210/en.2006-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naliboff BD, Mayer M, Fass R, Fitzgerald LZ, Chang L, Bolus R, Mayer EA. The effect of life stress on symptoms of heartburn. Psychosom Med. 2004;66:426–434. doi: 10.1097/01.psy.0000124756.37520.84. [DOI] [PubMed] [Google Scholar]
- 21.Rubio CA, Sveander M, Lagergren J. Re-adaptation of the esophageal mucosa of rats to protracted stress. In Vivo. 2001;15:413–16. [PubMed] [Google Scholar]
- 22.Mahadeva S, Raman MC, Ford AC, Follows M, Axon AT, Goh KL, Moayyedi P. Gastro-oesophageal reflux is more prevalent in Western dyspeptics: a prospective comparison of British and South-East Asian patients with dyspepsia. Aliment Pharmacol Ther. 2005;21:1483–1490. doi: 10.1111/j.1365-2036.2005.02455.x. [DOI] [PubMed] [Google Scholar]


