SUMMARY
Uterine receptivity to embryo implantation is coordinately regulated by 17β-estradiol (E2) and progesterone (P4). Although increased E2 sensitivity causes infertility, the mechanisms underlying the modulation of E2 sensitivity are unknown. We show that nuclear receptor coactivator-6 (NCOA6), a reported coactivator for estrogen receptor α (ERα), actually attenuates E2 sensitivity to determine uterine receptivity to embryo implantation under normal physiological conditions. Specifically, conditional KO of Ncoa6 in uterine epithelial and stromal cells does not decrease, rather markedly increases E2 sensitivity, which disrupts embryo implantation and inhibits P4-regulated genes and decidual response. NCOA6 enhances ERα ubiquitination and accelerates its degradation, while loss of NCOA6 causes ERα accumulation in stromal cells during the pre-implantation period. At the same period, NCOA6 deficiency also caused a failure in downregulation of steroid receptor coactivator-3 (SRC-3), a potent ERα coactivator. Therefore, NCOA6 controls E2 sensitivity and uterine receptivity by regulating multiple E2-signaling components.
INTRODUCTION
Embryo implantation depends on molecular interactions between the hormone-primed uterus and the mature blastocyst (Wang and Dey, 2006). Implantation failure can be caused by defects in embryo or endometrium (Cakmak and Taylor, 2011; Valbuena et al., 1999). The endometrium is receptive to blastocyst implantation only in a restricted time “window” (Cakmak and Taylor, 2011; Wang and Dey, 2006), which is determined by the spatiotemporal regulation of the proliferation and differentiation status of uterine epithelial cells (ECs) and stromal cells (SCs) in response to P4 and E2 stimuli. Upon P4 priming during early mouse pregnancy, a small and temporal E2 increase at 3.5-day post-coitum (dpc) is essential for further induction of uterine SC proliferation and luminal EC differentiation to prepare the uterus for blastocyst attachment at 4.0 dpc (Matsumoto et al., 2002; Wang and Dey, 2006).
E2 action is mediated by ERα and ERβ. Studies using ERα and ERβ knockout (KO) mice have shown that ERα but not ERβ is essential for preparing endometrium for blastocyst attachment, while both receptors may be dispensable for P4-induced uterine decidualization (Wang and Dey, 2006). Furthermore, tightly regulated E2 concentrations determine the duration of receptive time window in the uterus. Lower E2 levels sustain but higher E2 levels shut down this time window (Ma et al., 2003). Although the exact role of E2 at the preimplantation stage is unclear in human uterus (Wang and Dey, 2006), high serum E2 levels or E2/P4 ratios induced by artificial ovulation induction may generate an adverse endometrial environment and reduce implantation rate (Valbuena et al., 1999). Failure of ERα downregulation at the implantation stage may also increase E2 sensitivity and reduce pregnant rate in women with polycystic ovary syndrome (PCOS) (Gregory et al., 2002).
NCOA6 (AIB3, ASC-2, RAP250, NRC, PRIP or TRBP) is a reported coactivator for multiple nuclear receptors and has many biological functions (Mahajan and Samuels, 2005). Ncoa6 KO in mice causes embryonic lethality due to developmental defects in placenta, heart and liver (Kuang et al., 2002; Mahajan and Samuels, 2005). In cultured cells, NCOA6 interacts with ERα and enhances its transcriptional function (Caira et al., 2000; Ko et al., 2000; Lee et al., 1999; Mahajan and Samuels, 2000). NCOA6 contains two LXXLL motifs and ERα mainly interacts with its N-terminal LXXLL motif (Mahajan and Samuels, 2005). Ncoa6 is expressed in many tissues, including uterine ECs and SCs (Zhang et al., 2003). The female heterozygous Ncoa6 KO mice exhibit a slightly reduced reproductive function (Mahajan and Samuels, 2005). However the physiological function and molecular mechanisms for NCOA6 during early pregnancy are unknown. In this study, we defined the function of Ncoa6 in early pregnancy by conditional KO of Ncoa6 in the progesterone receptor (PR)-positive cell lineages in mice, including both ECs and SCs in the uterus. We demonstrate the essential role of NCOA6 as a “coactivator” in uterine receptivity is actually to attenuate E2 sensitivity through accelerating ERα ubiquitination and degradation and downregulating SRC-3 in the endometrial epithelium at the preimplantation stage.
RESULTS
Conditional deletion of the Ncoa6 gene in the mouse uterus, corpus luteum and fallopian tube causes a failure of embryo attachment
Since Ncoa6-null mice display embryonic lethality, we generated the floxed Ncoa6 (Ncoa6f/f) mice, which were as normal as wild type (WT) mice (Fig. S1A–E). We next crossed these mice to PRCre/+ mice (Soyal et al., 2005) and generated Ncoa6f/f and PRCre/+;Ncoa6f/f (hereafter designated as Ncoa6d/d) mice. Total Ncoa6 mRNA and protein are abundant in Ncoa6f/f uteri, but are mostly diminished in Ncoa6d/d uteri (Fig. S1F–G). In Ncoa6f/f mice, Ncoa6 protein is detected in the uterine luminal and glandular ECs, endometrial SCs and myometrial cells. Low level Ncoa6 protein is also present in the corpus luteum and fallopian tube. In Ncoa6d/d mice, Ncoa6 is efficiently knocked out by the expression of PR-Cre transgene in the uterine ECs and SCs, corpus luteum, and fallopian tubule epithelium, while Ncoa6 protein is still expressed in the myometrium where little PR-Cre (Soyal et al., 2005) is expressed (Fig. 1A, Fig. S1H–J, and data not shown).
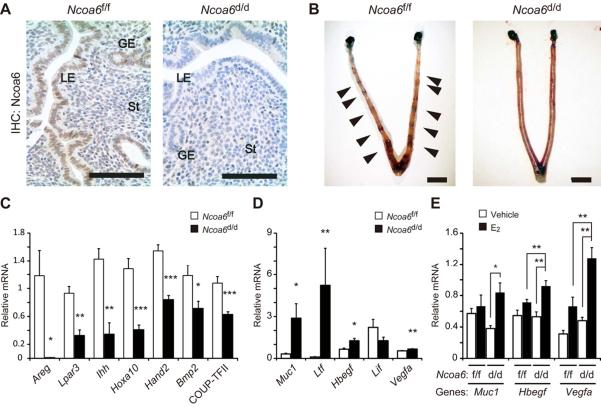
Fig. 1. Ncoa6d/d mice are infertile and exhibit altered expression patterns of P4 and E2−inducible genes in the uterus.
(A) Ncoa6 IHC (brown color) in Ncoa6f/f and Ncoa6d/d uterine sections. Scale bars represent 100 μm. LE, luminal epithelium; GE, glandular epithelium; St, stroma.
(B) Embryo ISs in Ncoa6f/f uterus (arrows) but not in Ncoa6d/d uterus at 4.5 dpc.
(C) Relative expression levels of P4−regulated genes in Ncoa6f/f (n=5) and Ncoa6d/d (n=5) uteri at 3.5 dpc. *, p<0.05; **, p < 0.01; ***, p< 0.0001.
(D) Relative expression levels of E2−regulated genes in Ncoa6f/f (n=5) and Ncoa6d/d (n=5) uteri at 3.5 dpc.
(E) Relative expression levels of Muc1, Hbegf and Vegfa in the uteri of Ncoa6f/f (n=5) and Ncoa6d/d (n=5) mice treated with sesame oil vehicle (V) or low dose E2 (6.7 ng/mouse).
The data in bargraph panels (C)–(E) are presented as Mean ± SEM.
During breeding with WT male mice for 6 months, 7 Ncoa6f/f females produced 21 litters with 6.4±2.6 pups per litter, but 9 Ncoa6d/d females did not produce any pups. We next assessed the effects of Ncoa6 loss on early pregnant events to pursue the cause of infertility. Ncoa6d/d (vs. Ncoa6f/f) mice showed no significant differences in ovulation (8.4±0.65 oocytes/mouse, n=8 vs. 7.5±0.4 oocytes/mouse, n=7), oocyte fertilization rate (46.4%, n=69 vs. 57.4%, n=54) at 1.5 dpc, and blastocyst formation rate at 3.5 dpc (53.6%, n=28 vs. 69.0%, n=29). At 3.5 dpc, the serum E2 (185±17 and 173±19 ng/ml) and P4 (39±4 and 35±1 ng/ml) levels in Ncoa6d/d (n=7) and Ncoa6f/f (n=6) mice are also similar, indicating a normal luteal function in Ncoa6d/d mice. At 4.5 dpc, we observed normal number of embryo implantation sites (ISs) (6.8±2.9 per uterus) in Ncoa6f/f uteri (n=10), but Ncoa6d/d uteri (n=10) had no IS although 3.3±1.4 unimplanted normal blastocysts per uterus could be recovered from these uteri (Fig. 1B). Furthermore, transfer of 101 WT blastocysts into the pseudo-pregnant Ncoa6f/f uteri (n=10) generated 41 embryo ISs, but transfer of 87 WT blastocysts into the pseudo-pregnant Ncoa6d/d uteri (n=8) only produced 1 embryo IS. These results demonstrate the failure of embryo attachment in Ncoa6d/d uteri. Therefore, Ncoa6 plays an essential role in the uterine receptivity for embryo attachment and implantation.
Ncoa6 deficiency results in abnormal expression of the implantation-related genes and increased E2 sensitivity in the uterus
To pursue the cause of implantation failure, we compared the expression levels of implantation-related genes that are regulated by P4 and E2 (Large and Demayo, 2011; Wang and Dey, 2006) between Ncoa6f/f and Ncoa6d/d uteri at 3.5 dpc. In Ncoa6d/d uteri, P4-regulated genes in the epithelium such as amphiregulin (Areg) (Ma et al., 2003), lysophosphatidic acid receptor-3 (Lpar3) (Hama et al., 2006) and Indian hedgehog (Ihh) (Lee et al., 2006), in both epithelium and stroma such as homeobox A10 (Hoxa10) (Cakmak and Taylor, 2011) and in the stroma such as heart and neural crest derivatives expressed 2 (Hand2) (Li et al., 2011) are significantly decreased. The Ihh-regulated downstream stromal genes such as bone morphogenetic protein-2 (Bmp2) and chicken ovalbumin upstream promoter transcription factor 2 (COUP-TFII) (Kurihara et al., 2007) are also significantly down-regulated in Ncoa6d/d uteri (Fig. 1C). On the other hand, E2-regulated genes including mucin-1 (Muc1), lactotransferrin (Ltf), heparin-binding EGF-like factor (Hbegf), vascular endothelial growth factor (Vegfa), fibroblast growth factors 1/2 (Fgf1/2) and insulin-like growth factor 1 (Igf1) in Ncoa6d/d uteri are expressed at higher levels than that in Ncoa6f/f uteri, while the expression of leukemia inhibitory factor (Lif), a glandular factor that regulates uterine receptivity, is slightly decreased in Ncoa6d/d uteri (Fig. 1D, and data now shown). These results suggest that Ncoa6 enhances P4-regulated genes but attenuates E2-regulated genes in the uterus during the preimplantation period under physiological conditions.
To assess the specific role of Ncoa6 in P4 and E2 signaling pathways, we treated ovariectomized Ncoa6f/f and Ncoa6d/d mice with P4 or low dose E2 (6.7 ng/mouse). P4 treatment equally induced the expression of P4-regulated genes including Areg, Ihh, Hoxa10, Bmp2, and COUP-TFII in Ncoa6f/f and Ncoa6d/d uteri (Fig. S1K). Furthermore, at 2.5 dpc when P4 is dominant and E2 is not secreted, the expression of Ihh mRNA in Ncoa6f/f and Ncoa6d/d uteri is also similar (Fig. S1L). Low dose E2 treatment was unable to induce significant expression of E2-regulated genes such as Muc1, Hbegf, Vegfa, Myc and Igf1 in Ncoa6f/f uteri and also incapable to stimulate the growth of these uteri. On the contrary, the same treatment resulted in remarkable induction of these E2-regulated genes in Ncoa6d/d uteri and uterine weight gain (Fig. 1E, Fig. S1M–N). Moreover, at 3.5 dpc the uteri of Ncoa6d/d mice were grossly heavier than the uteri of Ncoa6f/f mice in response to hormonal stimulation (Fig. S1O). These results demonstrate that Ncoa6 does not directly modulate P4 sensitivity, but directly suppresses E2 sensitivity and that the E2 supersensitivity resulted from Ncoa6 KO may inhibit uterine receptivity, as high dose E2 treatment makes uterus refractory to embryo attachment (Ma et al., 2003).
Ncoa6 deficiency increases uterine epithelial proliferation, stromal ERα protein and epithelial E2 response during the preimplantation period
In normal pregnant uteri, SC growth and cessation of EC proliferation assemble a closed linear uterine lumen for blastocyst attachment. In agreement with this, Ncoa6f/f uteri have closed lumens at 3.5 dpc. However, Ncoa6d/d uteri display intricately extended luminal and glandular epithelia that surround the curving lumens with many branches on the uterine cross sections (Fig. 2A), suggesting an epithelial overgrowth. Cell proliferation markers Ki-67 and phospho-histone H3 (pHH3) are undetectable in the epithelium of Ncoa6f/f uteri at 3.5 dpc. However, Ncoa6d/d uterine epithelium exhibits robust Ki-67 and pHH3 expression, indicating abnormally sustained EC proliferation during the preimplantation period (Fig. 2B, Fig. S2A). Since the EC proliferation is promoted by E2, the proliferative epithelium also indicates the excess E2 signaling caused by Ncoa6 KO.
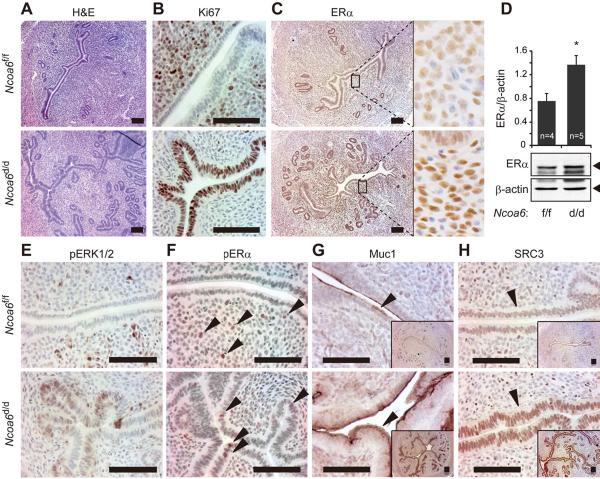
Fig. 2. Ncoa6 deficiency causes epithelial over proliferation and elevation of ERα, phosphorylated ERα, Muc1 and SRC-3 proteins in the uterus at 3.5 dpc.
(A) H&E-stained Ncoa6f/f and Ncoa6d/d uterine sections.
(B)–(C) Ki67 IHC for Ki67 and ERα in Ncoa6f/f and Ncoa6d/d uterine sections. The boxed areas in the left panels of (C) are enlarged in the right panels, which show a stronger ERα immunoreactivity in the stroma of Ncoa6d/d uterus vs. Ncoaf/f uterus.
(D) Representative Western blot results for ERα in tissue lysates prepared from Ncoa6f/f and Ncoa6d/d uteri. Band intensities were determined by densitometry and normalized to β-actin. The data are presented as Mean ± SEM. *, p=0.02.
(E)–(H) IHC for pERK1/2, S118-phosphorylated ERα (pERα), Muc1 and SRC-3 in Ncoa6f/f and Ncoa6d/d uterine sections. Arrows indicate cell nuclei with pERα immunoreactivity (F), Muc1 immunoreactivity in the apical membrane of luminal epithelium (G) and SRC-3 immunoreactivity in the nuclei of luminal ECs (H). Scale bars in all panels indicate 100 μm.
We further examined ERα in the uterus. In comparison with Ncoa6f/f uteri, Ncoa6d/d uteri exhibit much stronger stromal ERα immunostaining at 3.5 dpc (Fig. 2C). Western blot analysis further confirmed an overall two-fold ERα increase in Ncoa6d/d vs. Ncoa6f/f uteri (Fig. 2D). Because stromal ERα is responsible for E2-induced EC proliferation in normal uterus (Cooke et al., 1997; Winuthayanon et al., 2010), the increased ERα may be involved in epithelial overgrowth of Ncoa6d/d uteri.
E2-induced EC proliferation is originated from stroma and mediated by the FGFs-FGFR-ERK paracrine signaling pathway (Li et al., 2011). Thus, we investigated the downstream signaling components of ERK in the epithelium. At 3.5 dpc, numerous phospho-ERK1/2-positive cells were detected in the epithelium of Ncoa6d/d uteri, but undetectable in the epithelium of Ncoa6f/f uteri (Fig. 2E). Accordingly, the phosphorylated ERα on Ser118, a target site of phospho-ERK1/2, was not detected in ECs of Ncoa6f/f uteri, but evidently detected in ECs of Ncoa6d/d uteri at 3.5 dpc (Fig. 2F). Since the Ser118 phosphorylation promotes transcriptional activity of ERα, we analyzed the expression of an ERα target, Muc1, in the uterine epithelium. Muc1 expression was markedly increased in Ncoa6d/d uteri vs. Ncoa6f/f uteri (Fig. 2G). Since Muc1 is a barrier on the surface of luminal ECs that prevents embryo attachment, the increased Muc1 may cause blastocyst attachment failure in Ncoa6d/d uteri.
Ncoa6-deleted uteri fail to downregulate SRC-3 in the luminal epithelium
Since the SRC family coactivators enhance ERα transcriptional activity (Xu et al., 2009), we compared their expressions in Ncoa6f/f and Ncoa6d/d uteri. In non-pregnant mice, SRC-3 protein is equally expressed in ECs of both Ncoa6f/f and Ncoa6d/d uteri throughout the estrous cycle (Fig. S2B). At 3.5 dpc, SRC-3 is significantly reduced in the epithelium of Ncoa6f/f uteri, as reported previously in normal uteri (Jeong et al., 2007). However, high level SRC-3 is still retained in ECs of Ncoa6d/d uteri (Fig. 2H). SRC-3 mRNA is also significantly increased in Ncoa6d/d uteri (Fig. S2C). The expression levels of SRC-1 and SRC-2 are comparable between Ncoa6f/f and Ncoa6d/d uteri at 3.5 dpc (IHC data not shown). Since SRC-3 is a strong coactivator for nuclear receptors including ERα and other transcription factors such as E2F1, AP-1 and PEA3 (Xu et al., 2009), these results suggest that SRC-3 overexpression may also contribute to the increased E2 sensitivity and cell proliferation in the epithelium of Ncoa6d/d uteri.
Failure of decidual response in Ncoa6d/d uteri due to reduced Bmp2 expression
Excess E2 stimulation suppresses SC differentiation and decidualization in human and rat uteri (Basir et al., 2001; Kennedy, 1986). To determine the functional impact of E2 supersensitivity and its accompanied downregulation of P4 signaling in Ncoa6d/d uteri, we performed decidual response assay as described in Supplemental Information. The decidual response in Ncoa6d/d uteri was diminished and accordingly, the decidual response markers, including alkaline phosphatase activity and mRNA expression of Bmp2 and follistatin (Fst) were not induced (Fig. S3A–D). Interestingly, this failure of decidual response could be partially restored by administration of exogenous BMP2 protein (Fig. S3E–F). Therefore, Ncoa6 KO-caused downregulation of Bmp2 expression is partially responsible for the failure of decidualization in Ncoa6d/d uteri.
Inhibition of ER function rescues embryo implantation in Ncoa6d/d uteri
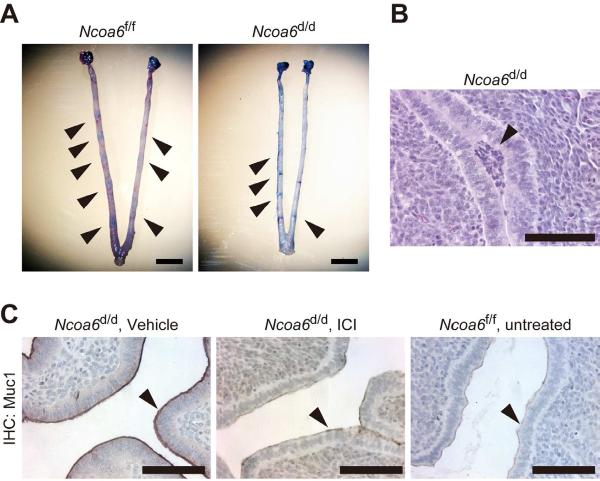
To test could suppression of E2 supersensitivity rescue implantation failure in Ncoa6d/d uteri, we treated Ncoa6f/f and Ncoa6d/d mice with a low dose of ER antagonist ICI-182780 (ICI) at 3.0 dpc, the time just before the temporal E2 secretion occurred. At 4.5 dpc, we observed all Ncoa6f/f uteri (n=6) had ISs (6.2±1.0 per uterus), so did 5 out of 6 Ncoa6d/d uteri (3.2±2.1 per uterus) (Fig. 3A–B). ICI treatment also strikingly reduced Muc1 expression in the luminal ECs of Ncoa6d/d uteri at 4.5 dpc, making its expression similar to that in the untreated Ncoa6f/f uteri (Fig. 3C). These results demonstrate that the Ncoa6 deficiency-caused E2 supersensitivity is responsible for the implantation failure in Ncoa6d/d uteri.
Fig. 3. Inhibition of E2 supersensitivity with ICI-182780 treatment rescues embryo implantation in Ncoa6d/d uteri.
(A) ISs (arrows) in Ncoa6f/f and Ncoa6d/d uteri at 4.5 dpc after ICI-182780 treatment.
(B) A H&E-stained embryo (arrow) attached to the epithelium of Ncoa6d/d uterus at 4.5 dpc in a mouse treated with ICI-182780.
(C) IHC for Muc1 in uterine sections prepared from vehicle (sesame oil)- or ICI-182780-treated Ncoa6d/d mice and untreated Ncoa6f/f mice at 4.5 dpc. Arrows indicate Muc1 immunoreactivity located at the surface of luminal ECs. Scale bars in all panels indicate 100 μm.
Ncoa6 accelerates ERα ubiquitination and degradation
In pursuing how ERα is increased in Ncoa6d/d uteri, we measured its mRNA levels. ERα mRNA is comparably expressed in Ncoa6f/f and Ncoa6d/d uteri at 3.5 dpc (Fig. S4A). We then assessed how Ncoa6 deficiency affected ERα protein stability in uterine SCs. SCs isolated from Ncoa6d/d uteri at the preimplantation stage displayed higher levels of ERα protein than those from Ncoa6f/f uteri (Fig. 4A–B, Fig. S4B–C). When protein synthesis was blocked by cyclohexamide (CHX), ERα degraded slightly faster in Ncoa6f/f SCs versus Ncoa6d/d SCs in the absence of E2 (Fig. 4A, Fig. S4B). More interestingly, E2 treatment induced a rapid ERα degradation in Ncoa6f/f SCs, but only slightly accelerated ERα degradation in Ncoa6d/d SCs (Fig. 4B, Fig. S4C). Moreover, Ncoa6 knockdown and overexpression in human MCF-7 cells increased and decreased ERα protein, respectively (Fig. S4D and data not shown). These results suggest that Ncoa6 levels are inversely correlated with ERα levels.
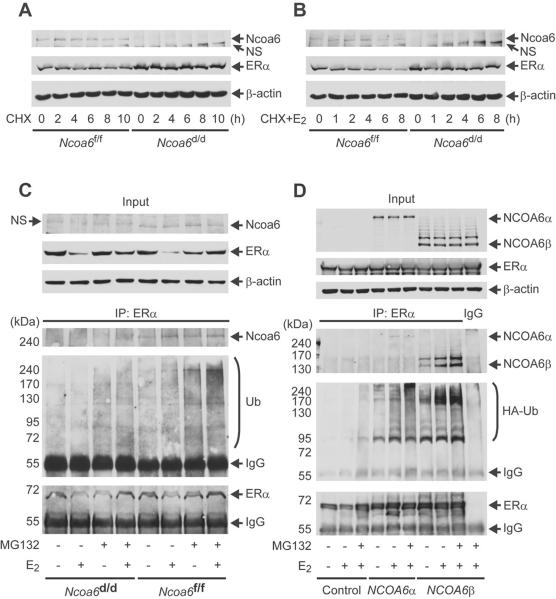
Fig. 4. Ncoa6 enhances ER4α ubiquitination and degradation.
(A) The isolated Ncoa6f/f and Ncoa6d/d uterine SCs were cultured in the absence of estrogen and treated with CHX. Cells were collected at different time points for Western blot.
(B) The above cells were treated with E2 and CHX as indicated and assayed by Western blot.
(C) Ncoa6f/f and Ncoa6d/d uterine SCs were treated with MG132 and/or E2 as indicated. A small portion of cell lysate was assayed by Western blot (upper panel). The larger portion of the cell lysate was subjected to co-immunoprecipitation (Co-IP) using ERα antibody, followed by Western blot analysis of the eluates using antibodies against Ncoa6, ubiquitin and ERα (lower panels). The amount of sample loaded was adjusted to comparable input of precipitated ERα. The IgG bands were from the antibody for Co-IP.
(D) HeLa cells were co-transfected with hERa-expressing plasmid and a plasmid expressing hNCOA6α, hNCOA6β (a splicing isoform without exon 10) or no protein (control). The transfected cells were treated with MG132 and/or E2 as indicated. A small portion of the cell lysate was assayed by Western blot (upper panel). The remaining cell lysate was subjected to Co-IP using ERa antibody or control IgG. The eluted precipitates were analyzed by Western blot for Ncoa6, HA-ubiquitin and ERα.
Next, we examined whether Ncoa6 is involved in ERα ubiquitination-dependent degradation. In Ncoa6f/f uterine SCs, Ncoa6 is associated with ERα in both presence and absence of E2 as evidenced by co-immunoprecipitation (Fig. 4C). ERα ubiquitination is detected at low levels in untreated cells, but it is significantly induced in E2-treated Ncoa6f/f cells. Ubiquitinated ERα further increases after these cells are treated with proteasome inhibitor MG132, and reaches the peak level upon combined E2 and MG132 treatment (Fig. 4C). However, ubiquitinated ERα is barely detectable in un-treated or E2-treated Ncoa6d/d SCs. Ubiquitinated ERα is also significantly lower in MG132-treated and MG132+E2-treated Ncoa6d/d SCs vs. the same reagents-treated Ncoa6f/f SCs (Fig. 4C). Furthermore, both human NcoA6α and NcoA6β isoforms (Li and Xu, 2011) ectopically expressed in HeLa cells form complexes with ERα and robustly enhance ERα ubiquitination (Fig. 4D). Finally, amino acid sequence analysis suggests that Ncoa6 does not contain any conserved homologous domain to any ubiquitin ligase, and in vitro ERα ubiquitination and proteasome-mediated degradation assays using purified human Ncoa6 proteins failed to support it as an E3 ligase (data not shown). Taken together, these results suggest that Ncoa6 may serve as a molecular modulator to promote ERα ubiquitination and degradation. This function of Ncoa6 may suppress E2 sensitivity through downregulation of ERα protein in the uterine stroma to allow embryo implantation.
DISCUSSION
We have demonstrated that Ncoa6 KO in the uterine SCs and ECs results in embryo implantation failure, indicating that Ncoa6 is absolutely required for appropriate development of uterine endometrial receptivity. Furthermore, Ncoa6 deficiency robustly increases uterine E2 sensitivity as evidenced by the increased expression of ERα target genes under physiological conditions, the abnormally sustained EC proliferation during the preimplantation period and the supersensitive response to low dose E2-induced gene expression and uterine growth. Moreover, suppression of ERα function rescues embryo implantation, proving that E2 supersensitivity is responsible for the failure of embryo implantation caused by Ncoa6 deficiency. These discoveries clearly indicate that Ncoa6 actually attenuates E2/ERα physiological function to permit embryo implantation in the uterus, although it was previously reported as an ERα coactivator based on experiments performed using cultured cells (Mahajan and Samuels, 2005). These findings also qualify Ncoa6 as an essential negative modulator of uterine E2 sensitivity during the preimplantation period.
Since E2-induced uterine EC proliferation is indirectly stimulated by the ERα-mediated FGFs and IGF1 expression in the stroma and the subsequent paracrine activation of the receptor tyrosine kinases and downstream protein kinase signaling pathways in ECs (Hewitt et al., 2010; Li et al., 2011), the Ncoa6 deficiency-induced uterine E2 supersensitivity should be initiated from the increased ERα protein in the stroma. Our data, when combined with the existing knowledge, suggest that the increase in stromal ERα in Ncoa6d/d uteri promotes the expression of growth factors such as IGF1 and FGFs, which in turn activate their receptors and downstream protein kinase pathways such as ERK. ERK-mediated phosphorylation of S118 enhances ERα activity in ECs. The enhanced ERK pathway may also upregulate SRC-3 through activating the E2F1/SP1–SRC-3 positive feedback regulatory loop (Mussi et al., 2006). Since SRC-3 is a potent ERα coactivator (Xu et al., 2009), the persistent expression of high level SRC-3 may work synergistically with the activated ERα in ECs of Ncoa6d/d uteri to support the sustained Muc1 expression at 3.5 dpc, preventing embryo attachment. In addition, since ERK activation also leads to activation of multiple transcription factors such as E2F1, AP-1 and PEA3 that use SRC-3 as a coactivator to promote cell proliferation (Xu et al., 2009), the upregulated SRC-3 in Ncoa6d/d uteri may be partially responsible for the sustained EC proliferation at 3.5 dpc, causing a blockage of EC differentiation.
In early pregnancy, counterbalanced E2 and P4 signaling pathways coordinately regulate uterine receptivity. Overstimulation with E2 dosages that inhibit uterine receptivity has been shown to inhibit P4/PR signaling (Tibbetts et al., 1998) and downregulate PR target genes such as Ihh (Matsumoto et al., 2002), Lpar3 (Hama et al., 2006) and Areg (Ma et al., 2003) in the uterine ECs. Thus, the decreased expression of PR target genes including Ihh, Areg and Lpar3 in ECs of Ncoa6d/d uteri may be a result of inhibition from Ncoa6 deficiency-triggered E2 supersensitivity. The reduced Ihh expression should explain the decreased expression of Ihh-inducible genes Bmp2 and COUP-TFII in the stroma of Ncoa6d/d uteri (Kurihara et al., 2007; Lee et al., 2010). Since both Bmp2 and COUP-TFII are essential for uterine decidual response (Kurihara et al., 2007; Lee et al., 2010), the Ncoa6 deficiency-impaired decidual response should be caused by the decreased expression of these genes, as evidenced by the partial rescue of decidual response in Ncoa6d/d uteri treated with BMP2 protein. However, the regulatory mechanisms for the decreased expression of Hoxa10 and Hand2 in the stroma of Ncoa6d/d uteri are currently unclear, which could be a consequence of impaired uterine receptivity caused by E2 supersensitivity.
In yeast, the 19S components of the 26S proteasome interact with transcriptional elongation factors to enhance transcription elongation in an independent manner to their proteolytic roles (Ferdous et al., 2001). In cultured mammalian cells, E2-bound ERα recruits coactivators and proteasome components to the target gene promoters to form a transcriptional activation complex, in which the proteasome components accelerate transcription by degrading ERα that has finished its transcriptional activation task, which may be required to re-load the next run of transcriptional initiation (Lonard et al., 2000; Reid et al., 2003). Interestingly, the current study demonstrates that Ncoa6 interacts with ERα to accelerate ERα ubiquitination and proteasome-mediated degradation regardless of E2-binding status. More importantly, this Ncoa6-associated ERα degradation plays an essential role to avoid abnormal increase in ERα protein and thereby prevent the uterus from acquiring E2 supersensitivity for appropriate embryo implantation. Therefore, Ncoa6-accelerated ERα ubiquitination and degradation do not functionally couple with transcriptional activation, but actually attenuate the expression of ERα target genes in the uterus under natural conditions.
Increased levels of ERα and SRC-3 expression have been found in the endometrium of women with PCOS associated with E2 supersensitivity and poor reproductive performance (Gregory et al., 2002). Similarly, in Ncoa6d/d mouse uteri Ncoa6 deficiency also increases ERα in the stroma and SRC-3 in the epithelium during the pre-implantation period and resulted in E2 supersensitivity and implantation failure. This suggests that loss of Ncoa6 expression or function might be involved in ERα and SRC-3 upregulation and E2 supersensitivity observed in the uteri of women with PCOS.
EXPERIMENTAL PROCEDURES
Mouse experiments
Animal protocols were approved by Baylor College of Medicine Animal Care and Use Committee. PR-Cre mice were described previously (Soyal et al., 2005). Ncoa6f/f and Ncoa6d/d mice were generated as illustrated in Fig. S1 and Supplemental Information. For collecting uterine specimens from pregnant or pseudo-pregnant mice, 8–10 week-old females were paired with intact or vasectomized WT males. Copulatory plugs were checked in the morning, and the morning when vaginal plug was observed was designated as 0.5 dpc. Ovulation and fertilization were examined at 1.5 dpc. The early blastocyst development was examined at 3.5 dpc. ISs were examined at 4.5 or 5.5 dpc. The serum concentrations of E2 and P4 were measured using blood samples collected at 3.5 dpc. Detail method is described in the Supplemental Information. For E2 and P4 treatments, 6-week-old female mice were ovariectomized, allowed to rest for 2 weeks, and then subcutaneously injected with vehicle (sesame oil), 6.7 ng of E2 or 1 mg of P4 in sesame oil per mouse. Six hours later, uteri were collected, frozen in liquid nitrogen, and stored at −80°C for analysis. For ICI treatment, a previous protocol (Lee et al., 2010) was slightly modified. Each mouse was intraperitoneally injected with 5 ng of ICI in 200 μl of sesame oil at 11 pm on 3.0 dpc. ISs were analyzed at 4.5 dpc.
RT-PCR
RNA was isolated from uteri using Trizol reagent and converted to cDNA using the Reverse Transcriptase Core kit (Eurogentec). Relative mRNA levels were measured using matched Universal TaqMan real-time PCR probes (Roche) and gene-specific primers. Ncoa6 mRNA was measured using qPCR Core kit for SYBR Green I (Eurogentec) with matched primers. All probe and primer sets for qPCR are listed in Supplemental Information. 18S RNA was measured using the TaqMan Ribosomal RNA Control Reagents (Applied Biosystems) and used to normalize relative mRNA levels in each sample.
Primary culture of uterine stromal cells
Uterine SCs were isolated from pseudo-pregnant mice at 3.5 dpc as described in Supplemental Information. For analysis of ERα degradation and ubiquitination, cells were cultured in DMEM/F12 medium with 10% charcoal-stripped FBS for 48 hours, followed by treatment with reagents as indicated.
IHC
Five-μm thick uterine sections were prepared from formalin-fixed and paraffin-embedded tissues and processed for H&E staining and IHC as described (Lee et al., 2006). Antibodies for IHC are listed in Supplemental Information.
Transfection
Plasmid constructs are described in Supplemental Information. HeLa cells were transfected with plasmids using lipofectamine 2000 (Invitrogen). MCF-7 cells were transfected with plasmids using lipofectamine LTX (Invitrogen). Total amount of DNA used in each transfection assay was balanced by adding the pcDNA3 parent plasmid.
Western blot and CHW chase
Protein was extracted from whole uterus or cultured cells using RIPA buffer with proteinase inhibitors and phosphatase inhibitors. Western blotting was performed with 30 mg of protein in each lysate and antibodies listed in Supplemental Information. To assay ERα stability, mouse uterine SCs were treated with 10 μg/ml of CHX. Thirty minutes after adding CHX, cells were treated with vehicle (ethanol) or 10 nM of E2. Band intensities of Western blots were quantified by densitometry using Image J software.
ERα ubiquitination assay
Mouse uterine SCs were pre-treated with vehicle (DMSO) or 10 μM of MG132 for 30 minutes, then treated with vehicle (ethanol) or 10 nM of E2 for 6 hours. HeLa cells were transfected with mock or expression vectors for hERα, hNCOA6α, hNCOA6β and HA-ubiquitin. The transfected cells were treated with vehicle or 10 μM of MG132 for 6.5 hours and with vehicle or 10 nM of E2 for 6 hour (added 0.5 hours later after MG132 addition). The treated cells were lysed in TNE buffer (10 mM Tris-HCl, pH 7.8, 1% NP40, 0.15 M NaCl, 1 mM EDTA) containing proteinase inhibitors and phosphatase inhibitors. ERα was immunoprecipitated from cell lysate with 500 mg protein using ERα antibody. The precipitates were assayed by Western blots using ERα, ubiquitin and Ncoa6 antibodies.
Statistical analysis
Data are presented as Mean ± SEM. Statistical analyses were performed using two-sided, unpaired Student-t test, Mann-Whiney's U test, One-Way ANOVA or analysis of covariance (ANCOVA). Multiplicity adjustment methods (Bonferroni method) were used when multiple pairwise comparisons were performed. Chi-square tests were used to evaluate differences of data in percentages.
Supplementary Material
HIGHLIGHTS
Ncoa6 loss in the uterus disrupts embryo implantation by increasing E2 sensitivity
Ncoa6 loss upregulates stromal ERα and epithelial SRC-3 in early pregnant uteri
Ncoa6 promotes ERα ubiquitination and degradation
The “coactivator” Ncoa6 actually attenuates ERα function in the uterus
ACKNOWLEDGEMENTS
We thank Dr. T. Takahashi for technical advice. This work is partially supported by NIH grants CA119689, DK058242 and CA112403.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Basir GS, O WS, Ng EH, Ho PC. Morphometric analysis of periimplantation endometrium in patients having excessively high oestradiol concentrations after ovarian stimulation. Hum Reprod. 2001;16:435–440. doi: 10.1093/humrep/16.3.435. [DOI] [PubMed] [Google Scholar]
- Caira F, Antonson P, Pelto-Huikko M, Treuter E, Gustafsson JA. Cloning and characterization of RAP250, a novel nuclear receptor coactivator. J Biol Chem. 2000;275:5308–5317. doi: 10.1074/jbc.275.8.5308. [DOI] [PubMed] [Google Scholar]
- Cakmak H, Taylor HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update. 2011;17:242–253. doi: 10.1093/humupd/dmq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdous A, Gonzalez F, Sun L, Kodadek T, Johnston SA. The 19S regulatory particle of the proteasome is required for efficient transcription elongation by RNA polymerase II. Mol Cell. 2001;7:981–991. doi: 10.1016/s1097-2765(01)00250-7. [DOI] [PubMed] [Google Scholar]
- Gregory CW, Wilson EM, Apparao KB, Lininger RA, Meyer WR, Kowalik A, Fritz MA, Lessey BA. Steroid receptor coactivator expression throughout the menstrual cycle in normal and abnormal endometrium. J Clin Endocrinol Metab. 2002;87:2960–2966. doi: 10.1210/jcem.87.6.8572. [DOI] [PubMed] [Google Scholar]
- Hama K, Aoki J, Bandoh K, Inoue A, Endo T, Amano T, Suzuki H, Arai H. Lysophosphatidic receptor, LPA3, is positively and negatively regulated by progesterone and estrogen in the mouse uterus. Life Sci. 2006;79:1736–1740. doi: 10.1016/j.lfs.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Li Y, Li L, Korach KS. Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. J Biol Chem. 2010;285:2676–2685. doi: 10.1074/jbc.M109.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Han SJ, Aronow BJ, Lydon JP, O'Malley BW, DeMayo FJ. The p160 steroid receptor coactivator 2, SRC-2, regulates murine endometrial function and regulates progesterone-independent and -dependent gene expression. Endocrinology. 2007;148:4238–4250. doi: 10.1210/en.2007-0122. [DOI] [PubMed] [Google Scholar]
- Kennedy TG. Intrauterine infusion of prostaglandins and decidualization in rats with uteri differentially sensitized for the decidual cell reaction. Biol Reprod. 1986;34:327–335. doi: 10.1095/biolreprod34.2.327. [DOI] [PubMed] [Google Scholar]
- Ko L, Cardona GR, Chin WW. Thyroid hormone receptor-binding protein, an LXXLL motif-containing protein, functions as a general coactivator. Proc Natl Acad Sci U S A. 2000;97:6212–6217. doi: 10.1073/pnas.97.11.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang SQ, Liao L, Zhang H, Pereira FA, Yuan Y, DeMayo FJ, Ko L, Xu J. Deletion of the cancer-amplified coactivator AIB3 results in defective placentation and embryonic lethality. J Biol Chem. 2002;277:45356–45360. doi: 10.1074/jbc.C200509200. [DOI] [PubMed] [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3:e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large MJ, Demayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol. 2011 doi: 10.1016/j.mce.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Kurihara I, Jeong JW, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of ERalpha activity by COUP-TFII is essential for successful implantation and decidualization. Mol Endocrinol. 2010;24:930–940. doi: 10.1210/me.2009-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38:1204–1209. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- Lee SK, Anzick SL, Choi JE, Bubendorf L, Guan XY, Jung YK, Kallioniemi OP, Kononen J, Trent JM, Azorsa D, et al. A nuclear factor, ASC-2, as a cancer-amplified transcriptional coactivator essential for ligand-dependent transactivation by nuclear receptors in vivo. J Biol Chem. 1999;274:34283–34293. doi: 10.1074/jbc.274.48.34283. [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331:912–916. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Xu J. Identification and characterization of the alternatively spliced nuclear receptor coactivator-6 isoforms. Int J Biol Sci. 2011;7:505–516. doi: 10.7150/ijbs.7.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard DM, Nawaz Z, Smith CL, O'Malley BW. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell. 2000;5:939–948. doi: 10.1016/s1097-2765(00)80259-2. [DOI] [PubMed] [Google Scholar]
- Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100:2963–2968. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan MA, Samuels HH. A new family of nuclear receptor coregulators that integrate nuclear receptor signaling through CREB-binding protein. Mol Cell Biol. 2000;20:5048–5063. doi: 10.1128/mcb.20.14.5048-5063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan MA, Samuels HH. Nuclear hormone receptor coregulator: role in hormone action, metabolism, growth, and development. Endocr Rev. 2005;26:583–597. doi: 10.1210/er.2004-0012. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol. 2002;245:280–290. doi: 10.1006/dbio.2002.0645. [DOI] [PubMed] [Google Scholar]
- Mussi P, Yu C, O'Malley BW, Xu J. Stimulation of steroid receptor coactivator-3 (SRC-3) gene overexpression by a positive regulatory loop of E2F1 and SRC-3. Mol Endocrinol. 2006;20:3105–3119. doi: 10.1210/me.2005-0522. [DOI] [PubMed] [Google Scholar]
- Reid G, Hubner MR, Metivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell. 2003;11:695–707. doi: 10.1016/s1097-2765(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- Tibbetts TA, Mendoza-Meneses M, O'Malley BW, Conneely OM. Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol Reprod. 1998;59:1143–1152. doi: 10.1095/biolreprod59.5.1143. [DOI] [PubMed] [Google Scholar]
- Valbuena D, Jasper M, Remohi J, Pellicer A, Simon C. Ovarian stimulation and endometrial receptivity. Hum Reprod. 1999;14(Suppl 2):107–111. doi: 10.1093/humrep/14.suppl_2.107. [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 2010;107:19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wu RC, O'Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615–630. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liao L, Kuang SQ, Xu J. Spatial distribution of the messenger ribonucleic acid and protein of the nuclear receptor coactivator, amplified in breast cancer-3, in mice. Endocrinology. 2003;144:1435–1443. doi: 10.1210/en.2002-0018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.