Abstract
Background/Purpose
Concurrent chemoradiotherapy (CCRT) for squamous cell carcinoma of the head and neck (SCCHN) increases local tumor control, but at the expense of increased toxicity. We recently showed that several clinical/pre-treatment factors were associated with the occurrence of severe late toxicity. This study evaluates the potential relationship between radiation dose delivered to the pharyngeal wall and toxicity.
Methods
This was an analysis of long-term survivors from three previously reported RTOG trials of concurrent chemoradiotherapy for locally advanced SCCHN (RTOG 91–11; 97–03; and 99–14). Severe late toxicity was defined in this secondary analysis as chronic Grade 3–4 pharyngeal/laryngeal toxicity and/or requirement for a feeding tube ≥ 2 years after registration and/or potential treatment-related death (e.g. pneumonia) within 3 years. Radiation dosimetry (2-dimensional) analysis was performed centrally at RTOG Headquarters to estimate doses to four regions of interest along the pharyngeal wall (superior oropharynx, inferior oropharynx, superior hypopharynx, and inferior hypopharynx). Case-control analysis was performed, with a multivariate logistic regression model that included pre-treatment and treatment potential factors.
Results
A total of 154 patients were evaluable for this analysis, 71 cases (patients with severe late toxicities) and 83 controls; thus 46% of evaluable patients had a severe late toxicity. On multivariate analysis, significant variables correlated with the development of severe late toxicity; older age (odds ratio 1.062 per year; p = 0.0021) and radiation dose received by the inferior hypopharynx (odds ratio 1.023 per Gy; p=0.016). The subgroup of patients receiving < 60 Gy to the inferior hypopharynx had a 40% rate of severe late toxicity, compared with 56% for patients receiving > 60 Gy. Oropharyngeal dose was not associated with this outcome.
Conclusions
Severe late toxicity following CCRT is common in long term survivors. Age is the most significant factor, but hypopharyngeal dose also was associated.
Background/Introduction
For patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN), concurrent chemoradiotherapy (CCRT) improves 5-year survival by approximately 8% when CCRT is compared to radiotherapy alone.1 This increase in survivorship appears to be directly related to improvement in local-regional control.2,3,4,5,6 However, concurrent chemoradiotherapy significantly increases toxicity when compared to radiotherapy alone.7 Managing the delicate balance between tumor control and “acceptable” treatment related toxicity has become more difficult. Of particular concern is increased late toxicity, which is more difficult to manage than early/acute or subacute toxicity and is often permanent.8, 9 We recently published the results of a meta-analysis of late toxicity - specifically focusing on late, high grade pharyngeal/swallowing toxicity - based upon three prospective RTOG trials.10 This study showed that about 43% of long-term survivors had severe (RTOG Grade 3–5) late toxicity. This was found after excluding patients with significant pretreatment severe or life-threatening airway obstruction and/or dysphagia. Patients with active/recurrent cancer were also excluded. Factors associated with late toxicity included age, T-stage, primary tumor site, and post-treatment neck dissection.
Machtay et al., also showed that prescribed radiation dose (dose received by the tumor) was not associated with late toxicity; however, this study did not analyze the dose received by specific normal tissue structures within the head and neck.10 Eisbruch et al. showed that dysphagia and aspiration risk following chemoradiotherapy are strongly associated with radiotherapy dose to the pharyngeal constrictors, particularly the superior constrictors.11 This relationship has been further substantiated by several other authors.12, 13, 14, 15, 16, 17 Although these papers analyzed 3-dimensional dose-volume relationships, a criticism of these data is that they are often single-institution, retrospective studies, with small patient numbers and toxicity events. Thus, we retrospectively evaluated the (2-dimensional) dosimetric data available at RTOG Headquarters (HQ) from three prospective-multicenter trials, with large patient numbers and toxicity events, to determine if the radiation dose received by several critical normal tissue regions of interest would correlate with severe late toxicity.
Materials/Methods
The patients analyzed for this study were treated on one of three prospective trials that have been previously reported. All three studies enrolled patients with non-metastatic stage III/IV SCCHN, Karnofsky performance status of 60–100%; and adequate hematologic, renal, hepatic and cardiovascular function. These studies were performed in the 1990's and used conventional radiotherapy techniques, mostly 2-dimensional planning and delivery. No patient received intensity modulated radiation therapy (IMRT).
Briefly, RTOG 91–11 was a phase III larynx-preservation trial comparing induction chemo-radiation vs. concomitant chemoradiation, vs. radiation alone.18 For this analysis, only the CCRT arm was studied which consisted of 70 Gy (2 Gy once daily fractionation) plus three cycles of high-dose cisplatin (100 mg/m2, weeks 1, 4, and 7). Of 166 eligible patients on this arm from RTOG 91–11; 61 were assessable for late toxicity.
RTOG 97–03 was a 3-arm Phase IIR trial of several regimens of CCRT for stage III/IV SCCHN (excluding patients who were eligible for RTOG 91–11).19 Patients in arms 1 received 70 Gy with conventional fractionation plus daily cisplatin (10 mg/m2 per day) and 5-FU (400 mg/m2 per day via continuous infusion) for the last 2 weeks of RT. Patients in Arm 3 received weekly cisplatin (20 mg/m2 per week) and paclitaxel (30 mg/m2 per week) or once weekly. Arm 2 utilized an unconventional (week-on/week-off) RT technique, delivering concurrent chemoradiation (2 Gy plus daily 5-FU/hydoxyurea) over 13 weeks and was excluded from our analysis.. After excluding arm 2, 52 patients from RTOG 97–03 were assessable for late toxicity.
RTOG 99–14 was a single arm Phase II trial of concomitant boost radiotherapy with concurrent cisplatin for stage III/IV head and neck cancer.20 Radiation was delivered to 72 Gy over 6 weeks (as per RTOG 90–03) with 2 cycles of high-dose cisplatin in weeks 1 and 4. Of the 76 eligible patients in this trial, 41 were assessable for the purposes of this study.
As noted above, patients were excluded a priori from this analysis if they had severe pre-treatment dysfunction of their laryngopharynx (e.g. dependence upon a feeding tube prior to XRT). Patients also were excluded if they suffered early recurrence or cancer-related death. Our analysis thus focused specifically on long-term NED survivors of head and neck chemoradiotherapy. Patients with missing/inevaluable data on outcomes and/or missing radiotherapy simulation/portal films and/or dosimetry data were also excluded.
For this report, as defined in Machtay 2008, a severe late toxicity was defined as any or all of the following events:10
Grade 3 or greater toxicity present > 180 days after the start of XRT and attributable to dysfunction of the larynx and/or pharynx (e.g. dysphagia)
Requirement for a feeding tube/gastrostomy ≥ 2 years after the start of XRT.
Death with no evidence of disease (NED) of an unknown cause ≤ 3 years from study entry, in which laryngeal dysfunction was determined to be a contributing factor (e.g. aspiration pneumonia). A blinded review of these deaths was performed by one of the study authors (MM) without any knowledge of the patient's clinical pre-treatment and/or treatment related characteristics.
Patients who experienced ≥ 1 qualifying severe late toxicity event were considered to be a “case.” Patients were considered “controls” if they were alive NED > 2 years from study entry and did not experience any late toxicity event.
Dosimetric Analysis
As discussed elsewhere in this paper, the patients treated in these studies did not undergo 3-dimensional or IMRT treatment planning and delivery. Thus, the available data for estimation of received dose to organs at risk (OAR) were based upon analysis of simulation films, portal films, radiation prescription data, and hardcopy 2-dimensional dosimetry plots. These 2-dimensional plots generally provided estimated dose distribution in the region of the center of the primary tumor; data on dosimetry at “match lines” were not collected. For every patient, these data were reviewed by the senior author (MM) along with the lead RTOG physicist (JG) and RTOG dosimetrist (EO). These reviewers were `blinded' to the clinical outcomes (toxicity) of the patient.
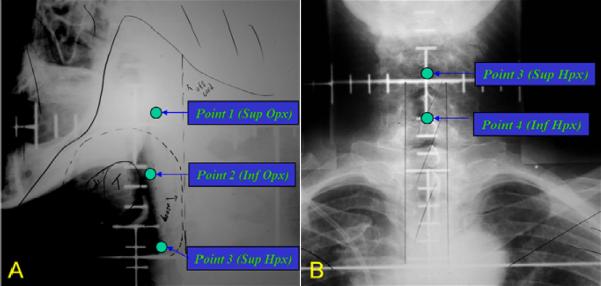
It was decided (prior to the initiation of this analysis) to focus on four regions of interest for dosimetric analysis. Specifically, the pharynx was divided into four regions of interest (ROI): Superior Oropharynx; Inferior Oropharynx; Superior Hypopharynx; and Inferior Hypopharynx (Figure 1). The 4 ROI's were identified on each simulation/portal film for each patient, and it was determined whether the ROI was clearly within the field, clearly outside of the field, or at the marginal edge of the field. In the latter scenario (marginal location of the OAR), it was estimated whether the OAR received 25% or 50% of the central-axis field dose for that treatment field. For each patient, the cumulative dose to each OAR, from all of the treatment fields, was summated. The summation doses were then used for the outcomes analysis for this paper.
Figure 1.
Simulation portals depicting anatomical regions of interest (ROI). These were selected as “dose points” and centrally reviewed by MM and RTOG QA HQ to determine if dose points were clearly within the field, clearly outside of the field, or at the marginal edge of the field. A) Lateral simulation film and ROI points: Point 1 – Superior Oropharynx (soft palate), Point 2 – Inferior Oropharynx (Inferior Tonsil), Point 3 Superior Hypopharynx (AE fold). B) AP simulation film and plotted ROI; Point 4 – Inferior Hypopharynx (approximate location of the cricopharyngeus).
Statistical Analysis
All analyses were performed using SAS/STAT® software, Version 9.2 for Windows, SAS Institute Inc., Cary, NC, USA. Chi-squared tests were used to compare pretreatment characteristics between the case and control groups and Z-tests were used to test for differences in binomial proportions. Univariate and multivariate logistic regression models were used to determine if there was any correlation between each OAR dose (continuous) and severe late toxicity. All models were stratified by treatment arm. Severe late RT toxicities > 180 days from XRT start and grade ≥ 3, were scored with the Radiation Therapy Oncology Group (RTOG) / European Organization for Research and Treatment of Cancer (EORTC) Late RT Morbidity Scoring Scheme21. [The multivariate analysis also included clinical factors, including age (continuous variable); gender; race (non-black vs. black); KPS (60–80 vs. 90–100); hemoglobin (continuous variable); pre-treatment weight (continuous variable); T-stage (T1/2 vs. T3/4); N Stage (Nx/N0/N1 vs. N2 vs. N3); tumor site (oral cavity/oropharynx vs. larynx/hypopharynx); radiotherapy dose received as assessed by late effects biologically equivalent dose (BED) model (total RT dose multiplied by (1+ [dose/fraction size] ÷ 3): continuous variable); chemotherapy dose received (<85% of planned dose vs. ≥ 85% of planned dose); and post-radiation neck dissection (yes vs. no). Variables' levels were grouped in order to avoid small cell counts. A stepwise selection procedure was used to build the multivariate logistic regression models using the above pretreatment/treatment variables. Entry criterion was set at p < 0.05. The odds ratios estimate how much more (less) likely it is to be in the case group versus the control group (reference group) among patients with the specific variable level's characteristic compared to those patients in the reference level (RL), after stratifying for treatment arm.
Results
The original, potential eligible patient population from these three studies was 397 (excluding patients from arm 2 of RTOG 9703); 134 patients were then excluded because of cancer progression, acute death due to cancer, or death with no evidence of disease; 80 patients were excluded because of severe pre-treatment laryngopharynx dysfunction due to tumor; and 29 patients were excluded due to missing data. Thus, the overall evaluable sample size for this report is 154 patients with a median follow-up of 4.5 years. Of these 154 patients, 71 had severe late toxicity (cases) and 83 patients had no severe late toxicity (controls). Thus, the crude rate of late toxicity in this study was 46%. If the original 397 patients are used as the `denominator,' the rate of severe late toxicity is 18%. This overtly illustrates the fact that crude rates of late toxicity are highly dependent upon the definition of the study sample at risk for late toxicity. Late toxicity events as seen by trial are outlined in Table 1. The pretreatment patient characteristics of the 154 evaluable cases and controls are presented in Table 2. On univariate analysis, KPS was significant (p = 0.049).
Table 1.
Summary of Patients used in this Analysis
| Radiation Therapy Oncology Group Study |
||||
|---|---|---|---|---|
| Characteristic | 91-11 (Arm 1) | 97-03 (Arms 1 & 3) | 99-14 (Arm 1) | Total |
| Original No. of eligible patients | 166 | 155 | 16 | 397 |
| Reason for exclusion | ||||
| Pretreatment swallowing disability1 | 20 | 42 | 18 | 80 |
| Acute death2 | 10 | 8 | 3 | 21 |
| Missing data/inevaluable for RT review | 21 | 6 | 2 | 29 |
| Dead NED3 (known cause and not RX related) | 9 | 4 | 1 | 14 |
| Cancer progression4 | 45 | 43 | 11 | 99 |
| Total excluded | 105 | 103 | 35 | 243 |
|
| ||||
| Total analyzable for this study | 61 | 52 | 41 | 154 |
| Late Toxicity Events 5 | ||||
| Feeding tube dependence ≥ 2 years post-RT | --6 | 16 | 7 | 23 |
| RTOG late toxicity criteria, grade 3+ | ||||
| Pharyngeal Dysfunction (dysphagia) | 12 | 11 | 9 | 32 |
| Laryngeal dysfunction | 16 | 3 | 0 | 19 |
| Mucositis | 1 | 0 | 5 | 6 |
| Other (e.g. infection, fistula, wt. loss) | 4 | 0 | 3 | 7 |
| Dead NED [unknown cause and could be RX Related] ≤ 3 years after study entry | 3 | 2 | 2 | 7 |
|
| ||||
| Any late toxicity events (cases) | 28 | 24 | 19 | 71 |
| No late toxicity events (controls) | 33 | 28 | 22 | 83 |
Abbreviation: NED, no evidence of disease; RX, treatment; RT, radiation therapy
Pretreatment swallowing disability: airway obstruction, dysphagia, odynophagia, feeding tube, or gastrostomy
Acute: 180 days from the start of RT for grade 5 toxicity or 180 days from the date of randomization for non-toxicity death
Patients are NED if they do not have any of the following events/failures: persistence, local/regional recurrence, distant mets, second primary, death by study cancer, or death by second primary
Cancer progression is having at least one of the previous events/failures.
Numbers do not add up along columns due to some patients having more than one toxicity event.
Feeding tube data were not collected in RTOG 91-11
Table 2.
Characteristics of Patients with Severe Late Toxicities (Cases) and Controls
| Cases (n = 71) |
Controls (n = 83) |
||||
|---|---|---|---|---|---|
| Characteristic | No. | % | No. | % | P-value |
| Age, years | |||||
| Median | 59 | 54 | |||
| Min-Max | 33–77 | 26–78 | |||
| ≤ 70 | 63 | 89 | 77 | 93 | 0.38 |
| > 70 | 8 | 11 | 6 | 7 | |
| Sex | |||||
| Male | 53 | 75 | 63 | 76 | 0.86 |
| Female | 18 | 25 | 20 | 24 | |
| Race | |||||
| Non-Black | 63 | 89 | 78 | 94 | 0.24 |
| Black | 8 | 11 | 5 | 6 | |
| KPS | |||||
| 70–80 | 16 | 23 | 9 | 11 | 0.049 |
| 90–100 | 55 | 77 | 74 | 89 | |
| Hemoglobin, g/dL | |||||
| Median | 14.3 | 14.1 | |||
| Min-Max | 10.1–17.2 | 9.9–18.2 | |||
| ≤ 13.5 | 18 | 25 | 31 | 37 | 0.11 |
| > 13.5 | 53 | 75 | 52 | 63 | |
| Weight loss in previous 6 months, kg | |||||
| ≤ 5 | 54 | 76 | 69 | 83 | |
| > 5 | 17 | 24 | 14 | 17 | |
| T stage | |||||
| T1/T2 | 16 | 23 | 28 | 34 | 0.28 |
| T3/T4 | 55 | 77 | 55 | 66 | |
| N stage | |||||
| NX/N0/N1 | 32 | 45 | 35 | 42 | 0.71 |
| N2 | 34 | 48 | 39 | 47 | |
| N3 | 5 | 7 | 9 | 11 | |
| Tumor site | |||||
| Oral cavity/oropharynx | 31 | 44 | 43 | 52 | 0.31 |
| Oral Cavity | 5 | 7 | 1 | 1 | |
| Oropharynx | 26 | 37 | 42 | 51 | |
| Larynx/hypopharynx | 40 | 56 | 40 | 48 | |
| Larynx | 30 | 42 | 35 | 42 | |
| Hypopharynx | 10 | 14 | 5 | 6 | |
| Radiotherapy dose-intensity delivered, BED1 Gy | |||||
| Mean | 115.7 | 115.6 | 0.74 | ||
| Median | 116.7 | 116.7 | |||
| Min-Max | 108.7–116.7 | 108.6–120.0 | |||
| Neck dissection after RT | |||||
| Yes | 19 | 27 | 21 | 25 | 0.84 |
| No | 52 | 73 | 62 | 75 | |
| Chemotherapy dose delivered2 % | |||||
| < 85 | 10 | 14 | 10 | 12 | 0.71 |
| ≥ 85 | 61 | 86 | 73 | 88 | |
Abbreviations: No., number; KPS, Karnofsky performance status; BED, biologically equivalent dose; RT, radiation therapy.
BED for toxicity = Total dose × (1 + Actual dose/Fx /3)
Chemotherapy dose delivered = (total dose patient [pt] received) / (BSA × total dose specified in the study protocol). If the pt refused chemo, received the wrong chemo agent(s), or there was no chemo documentation than these pts were treated as receiving none of their specified chemo dose(= 0%).
Univariate analyses of dosimetric data (mean dose to ROI's) are shown in Table 3. The case group was associated with higher mean dose to the superior hypopharynx (63.3 Gy vs. 57.0 Gy; p=0.015) and inferior hypopharynx (49.7 Gy vs. 41.4 Gy, p=0.025) compare to the controls. Mean dose to the superior oropharynx (69.6 Gy vs. 69.5 Gy; p=0.87) and inferior oropharynx (63.8 Gy vs. 63.3 Gy; p=0.77) were not associated with severe late toxicity.
Table 3.
Doses to Organs at Risk (OAR) for Patients with Severe Late Toxicities (Cases) and Controls
| Cases (n = 71) |
Controls (n = 83) |
||||
|---|---|---|---|---|---|
| OAR | No. | % | No. | % | P-value |
| Superior Oropharynx, Gy Epiglottis | |||||
| Mean | 69.6 | 69.5 | 0.87 | ||
| Median | 70 | 70 | |||
| Min-Max | 50.6–72.0 | 55.0–72.3 | |||
| Inferior Oropharynx, Gy Superior Constrictor | |||||
| Mean | 63.8 | 63.3 | 0.77 | ||
| Median | 67.5 | 67.1 | |||
| Min-Max | 25.6–72.0 | 2.1–72.3 | |||
| Superior Hypopharynx, Gy Cricoarytenoid | |||||
| Mean | 63.3 | 57.0 | 0.015 | ||
| Median | 70 | 70 | |||
| Min-Max | 31.5–72.0 | 2.1–72.5 | |||
| Inferior Hypopharynx, Gy | |||||
| Mean | 49.7 | 41.4 | 0.025 | ||
| Median | 58.0 | 50.6 | |||
| Min-Max | 2.1–72.0 | 2.1–72.0 | |||
Univariate and multivariate logistic regression models are shown in Table 4. In the univariate analysis, older age was statistically significantly associated with severe late toxicity (p = 0.0012), as we found in our previous analysis10.
Table 4.
Univariate and Multivariate Logistic Regression Models for Severe Late Toxicity
| Variable | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Univariate | |||
|
| |||
| Age, continuous | 1.065 | 1.025–1.107 | 0.0012 |
|
| |||
| KPS | |||
| 60–80 | 2.40 | 0.98–5.85 | 0.054 |
| 90–100 | 1.00 | ||
|
| |||
| Hemoglobin (g/dl), continuous | 1.19 | 0.95–1.50 | 0.13 |
|
| |||
| T Stage | |||
| T1/T2 | 1.00 | ||
| T3/T4 | 1.85 | 0.87–3.93 | 0.11 |
|
| |||
| Tumor Site | |||
| Oral cavity/oropharynx | 1.00 | ||
| Larynx/hypopharynx | 2.32 | 0.82–6.58 | 0.12 |
|
| |||
| Superior Hypopharynx Dose (Gy), continuous | 1.034 | 1.008–1.062 | 0.011 |
|
| |||
| Inferior Hypopharynx Dose (Gy), continuous | 1.025 | 1.006–1.044 | 0.0079 |
|
| |||
| Multivariate | |||
|
| |||
| Age, continuous | 1.062 | 1.022–1.104 | 0.0021 |
|
| |||
| Inferior Hypopharynx Dose (Gy), Continuous | 1.023† | 1.004–1.043 | 0.016 |
Abbreviations: RL=reference level
Interpretation: An increase in dose of 1 Gy means an increase in the odds of being in the case group by 2%; an increase in dose of 5 Gy means an increase in the odds of being in the case group by 12%; an increase in dose of 10 Gy means an increase in the odds of being in the case group by 26%.
T-stage and tumor site were notsignificant in this study. Increased dose to the superior and inferior hypopharynx were also associated with increased odds of being in the case group (p=0.011 and p=0.0079, respectively). All other pre-treatment/treatment variables examined did not approach statistical significance with p-values greater than 0.20 (data not shown). In the multivariate analysis, only age and dose to the inferior hypopharynx remained statistically significant. Older patients and patients receiving higher doses to their inferior hypopharynx were more likely to have a severe late toxicity event (OR, 1.062 per Gy; CI [1.022 – 1.104], p = 0.0021 and OR, 1.023 per Gy; CI [1.004 – 1.043], p = 0.016, respectively).
To further characterize the dose dependent toxicity associated with hypopharyngeal dose, rates of toxicity were analyzed in all 154 patients based on dose to hypopharyngeal ROI's. As demonstrated in Table 5a, patients who received ≤ 50 Gy to both the superior and inferior hypopharynx demonstrated a significantly lower rate of severe late toxicity (8/28= 29%) than patients who received > 60 Gy to both subregions (33/59 = 56%; p = 0.011). Tables 5b and 5c display further breakdowns of crude complication rates by tumor site, T-stage and age. Of note, 7/7 of patients with oral cavity/oropharynx cancer who received hypopharynx dose > 60 Gyhad severe late toxicity.
Table 5a.
Univariate Analysis of Dose Characteristics
| Inferior Hypopharynx Dose (Gy) |
||||
|---|---|---|---|---|
| ≤ ;50 | 51–60 | > 60 | ||
| Superior Hypopharynx Dose (Gy)† | ≤ 50 | 8/28 (29%) | --- | --- |
| 50–60 | 10/20 (50%) | 3/6 (50%) | --- | |
| > 60 | 9/20 (45%) | 8/21 (38%) | 33/59 (56%) | |
| Age (yrs)* | ≤ 60 | 14/46 (30%) | 5/16 (31%) | 21/40 (53%) |
| > 60 | 13/22 (59%) | 6/11 (55%) | 12/19 (63%) | |
p-value = 0.011 [8/28 (29%) vs. 33/59 (56%)]
p-value = 0.012 [14/46 (30%) vs. 12/19 (63%)]
Table 5b.
Univariate Analysis of Dose Characteristics by Tumor Site and T Stage
| Inferior Hypopharynx Dose (Gy) |
||||
|---|---|---|---|---|
| ≤ 50 | 51–60 | > 60 | ||
| Tumor Site | Oral cavity/oropharynx | 20/57 (35%) | 4/10 (40%) | 7/7 (100%) |
| Larynx/hypopharynx | 7/11 (64%) | 7/17 (41%) | 26/52 (50%) | |
| T Stage | T1/T2 | 9/26 (35%) | 2/7 (29%) | 5/11 (45%) |
| T3/T4 | 18/42 (43%) | 9/20 (45%) | 28/48 (58%) | |
Table 5c.
Univariate Analysis of Age by Tumor Site and T Stage
| Age |
|||
|---|---|---|---|
| ≤ 60 | > 60 | ||
| Tumor Site | Oral cavity/oropharynx | 15/48 (31%) | 16/26 (62%) |
| Larynx/hypopharynx | 25/54 (46%) | 15/26 (58%) | |
| T Stage | T1/T2 | 10/35 (29%) | 6/9 (67%) |
| T3/T4 | 30/67 (45%) | 25/43 (58%) | |
Discussion
This retrospective analysis of a series of prospective randomized trials demonstrates a high risk of severe late toxicity following CCRT for SCCHN, approximately 46% in the cohort studied. This high rate reflects the strict definition of patients eligible for analysis – patients with significant pre-treatment adverse function and patients with early recurrence of cancer were excluded, because they are not really evaluable for late effects. Wehypothesize that some studies that report lower rates of late toxicity included patients in their denominator that are not truly evaluable for late toxicity.
In our previous analysis of factors associated with severe late toxicity after CCRT for locally advanced SCCHN, we found no significant association between cumulative radiation therapy dose to the tumor and late toxicity10. We postulated that this was due to the narrow dose range prescribed and the generally excellent compliance seen in these rigorous protocol driven studies. For our current study, doses (point doses) were estimated to the superior and inferior aspects of the oropharynx and hypopharynx, as representative ROI's. This is one of the largest studies ever performed on the correlation between doses to normal tissues and late complications in head and neck cancer.
Upon reevaluation of the data, age remained the most significant risk factor. However, in addition, higher point dose estimates to the hypopharynx (superior and/or inferior) are associated with increased risk of severe late toxicity. Point doses to the oropharynx, however, were not associated with an increased late toxicity risk. Not surprisingly, patients with larynx/hypopharynx cancer tended to have higher point doses to the inferior hypopharynx, as shown in Table 5b. However, patients with oral cavity/oropharynx cancer who had high hypopharyngeal dose had a high rate of severe late toxicity as well.
Utilizing modern treatment modalities to decrease doses to structures of the hypopharynx may decrease the overall risk of late toxicity for patients receiving CCRT for SCCHN.
Our study has several limitations. First, and most importantly, we used 2-dimensional treatment plans and crude methods of estimating doses to normal anatomic structures. RTOG trials in the 1990's used treatment planning and delivery techniques appropriate for that time era, and thus 3-D dose-volume data are not available. However, despite not having detailed 3-D data, we nonetheless identified an association between point doses (hypopharynx) and risk of toxicity. We very well recognize that more complex analyses of dose-volume relationships may further quantify the nature of this association. In fact, several series have been published reporting these complex relationships, and demonstrating associations between dose-volume parameters and late toxicity.17, 21, 22, 23, 24 However, these series are relatively small and since they are from single “centers of excellence,” they may not necessarily reflect the broader experience with chemoradiotherapy as compared with our series. We do plan to study 3-D/IMRT within the more recent data sets from 3-D/IMRT studies from the 2000's, including RTOG 0522 (a randomized trial comparing chemoradiotherapy with versus without cetuximab).
A second potential limitation of our report is that it is a retrospective meta-analysis of three separate clinical trials, all with slightly different eligibility criteria, CCRT regimen, and year of activation. However, this analysis does represent one of the largest multi-institutional centrally reviewed, prospectively obtained data sets analyzing late toxicity in the CCRT era. Each patient received quality treatment, appropriate standard of care for their time period, that was carefully vetted by field RTOG investigators and RTOG Headquarters..
Third, the exclusion of patients with pre-existing severe dysfunction of the laryngopharyngeal structures from this type of analysis is controversial. Determination of treatment related severe toxicity is subjective, and it can be difficult to determine if severe dysfunction after treatment is the result of treatment or the result of damage caused by pre-existing cancer. RTOG on-study data collection forms very clearly allowed us to identify those patients who had significant pre-existing laryngopharyngeal dysfunction prior to enrollment and treatment. We felt that it was important to include only those patients who had clear-cut late radiation induced complications and no signs of local recurrence as “cases” in this case-control series, and to maintain maintain consistency with our previous published report on late toxicity.10
We urge other investigators in the field of late radiation toxicity to similarly account for confounding factors.
Conclusion
We were able to identify a significant association between hypopharyngeal dose points and toxicity based on 2D analysis. When this region of interest is not involved with tumor, efforts should be made to limit dose as best as possible. Future RTOG analyses on 3-D/IMRT data sets will be performed once data from modern studies mature with respect to late toxicity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pignon JP, LeMaitre A, Maillard E, et al. Meta-analysis in head and neck cancer (MACH-NC) : an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009 Jul;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Brizel DM, Albers MA, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;328:1798–1804. doi: 10.1056/NEJM199806183382503. [DOI] [PubMed] [Google Scholar]
- 3.Wendt TG, Grabenbauer CG, Rodel CM, et al. Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: A randomized multicenter study. J Clin Oncol. 1998;16:1318–1324. doi: 10.1200/JCO.1998.16.4.1318. [DOI] [PubMed] [Google Scholar]
- 4.Olmi P, Crispino S, Fallai C, et al. Locoregionally advanced carcinoma of the oropharynx: Conventional radiotherapy vs. accelerated hyperfractionated radiotherapy vs. concomitant radiotherapy and chemotherapy – a multicenter randomized trial. Int J Radiat Oncol Biol Phys. 2003;55:78–92. doi: 10.1016/s0360-3016(02)03792-6. [DOI] [PubMed] [Google Scholar]
- 5.Staar S, Rudat V, Stuetzer H, et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy: Results of a multicentric randomized German trial in advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:1161–1171. doi: 10.1016/s0360-3016(01)01544-9. [DOI] [PubMed] [Google Scholar]
- 6.Bensadoun RJ, Benezery K, Dassonville O, et al. French multicenter phase III randomized study testing concurrent twice-a-day radiotherapy and cisplatin/5-fluorouracil chemotherapy (BiRCF) in unresectable pharyngeal carcinoma: Results at 2 years (FNCLCC-GORTEC) Int J Radiat Oncol Biol Phys. 2006;64:983–994. doi: 10.1016/j.ijrobp.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 7.Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother Oncol. 2003;66:253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 8.Denis F, Garaud P, Bardet E, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2003;22:69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53(1):23–28. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 10.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head and neck cancer: Which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 12.Dirix P, Abbeel S, Vanstraelen B, et al. Dysphagia after chemoradiotherapy for head-and-neck squamous cell carcinoma: dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2009;75(2):385–392. doi: 10.1016/j.ijrobp.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 13.Caudell JJ, Schaner PE, Desmond RA, et al. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2009;76(2):403–409. doi: 10.1016/j.ijrobp.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Teguh D, Levendag P, Sewnaik A, et al. Results of fiberoptic endoscopic evaluation of swallowing vs radiation dose in the swallowing muscles after radiotherapy of cancer in the oropharynx. Radiother Oncol. 2008;89(1):57–63. doi: 10.1016/j.radonc.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Lavendag P, Teguh D, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by radiation therapy dose to the superior and middle constrictor muscle: A dose-effect relationship. Radiother Oncol. 2007;85(1):64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Feng FY, Kim HM, Lyden TH, et al. Intensity modulated chemoradiotherapy aiming to reduce dysphagia in patienst with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010 Jun 1;28(16):2732–8. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010 Dec 1;78(5):1356–65. doi: 10.1016/j.ijrobp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 19.Garden AS, Harris J, Vokes E, et al. Preliminary results of RTOG 9703: A phase II randomized trial of concurrent radiation (RT) and chemotherapy for advanced squamous cell carcinomas (SCC) of the head. J Clin Oncol. 2004 Jul 15;22(14):2856–64. doi: 10.1200/JCO.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Ang KK, Harris J, Garden A, et al. Concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: Radiation Therapy Oncology Group phase II trial 99-14. J Clin Oncol. 2005;23:3008–3015. doi: 10.1200/JCO.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 21.Cox J, Stetz J, T P. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC) Int Journal of Rad Oncol Biol Phys. 1995;31(5):1341–6. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 22.Teguh D, Levendag P, Sewnaik A, et al. Results of fiberoptic endoscopic evaluation of swallowing vs radiation dose in the swallowing muscles after radiotherapy of cancer in the oropharynx. Radiother Oncol. 2008;89(1):57–63. doi: 10.1016/j.radonc.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Feng F, Kim H, Lyden T, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: Early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68(5):1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 24.Dirix P, Abbeel S, Vanstraelen B, et al. Dysphagia after chemoradiotherapy for head-and-neck squamous cell carcinoma: dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2009;75(2):385–392. doi: 10.1016/j.ijrobp.2008.11.041. [DOI] [PubMed] [Google Scholar]