SUMMARY
We have used Drosophila ovarian Follicle Stem Cells (FSCs) to study how stem cells are regulated by external signals and draw three main conclusions. First, the spatial definition of supportive niche positions for FSCs depends on gradients of Hh and JAK-STAT pathway ligands, which emanate from opposite, distant sites. FSC position may be further refined by a preference for low-level Wnt signaling. Second, hyperactivity of supportive signaling pathways can compensate for the absence of the otherwise essential adhesion molecule, DE-cadherin, suggesting a close regulatory connection between niche adhesion and niche signals. Third, FSC behavior is determined largely by summing the inputs of multiple signaling pathways of unequal potencies. Altogether our findings indicate that a stem cell niche need not be defined by short-range signals and invariant cell contacts; rather, for FSCs, the intersection of gradients of long-range niche signals regulates the longevity, position, number and competitive behavior of stem cells.
Keywords: Drosophila, Follicle Stem Cells, Signaling pathway, JAK-STAT, Hedgehog, Wnt
INTRODUCTION
Here we investigate Drosophila ovarian Follicle Stem Cells (FSCs), which serve as a model for stem cells that support epithelia requiring continuous regeneration (Margolis and Spradling, 1995; Nystul and Spradling, 2007, 2010). Importantly, more than one FSC is present within each insulated developmental unit. This arrangement allows ready replacement of one FSC lineage by another and competition for supportive niche positions, with two major consequences. First, a healthy FSC population can be maintained beyond the lifetime of a single stem cell. Second, somatic mutations that enhance FSC duplication or niche affinity might allow that FSC and its descendants to outcompete neighboring FSCs and thus take over the tissue, as in early steps in cancer. Both phenomena are likely relevant to human epithelial stem cells.
FSCs and Germline Stem Cells (GSCs) reside in the germarium (Fig. 1A), which is the most anterior structure of each of the roughly thirty ovarioles of an adult female. These stem cells support continuous egg production throughout the lifetime of well-fed flies. FSCs are maintained in their niche by cadherin- and integrin-mediated adhesive interactions while producing daughters that escape the niche and surround passing germline cysts (O'Reilly et al., 2008; Song and Xie, 2002). Most FSC daughters form an epithelium around the germline cells of growing egg chambers and divide roughly eight times before differentiating into a variety of specialized follicle cell types. A minority of FSC daughters arrest cell division earlier and either form polar cells at the anterior and posterior poles of developing egg chambers or stalk cells that separate egg chambers (Dobens and Raftery, 2000; Margolis and Spradling, 1995; Nystul and Spradling, 2010).
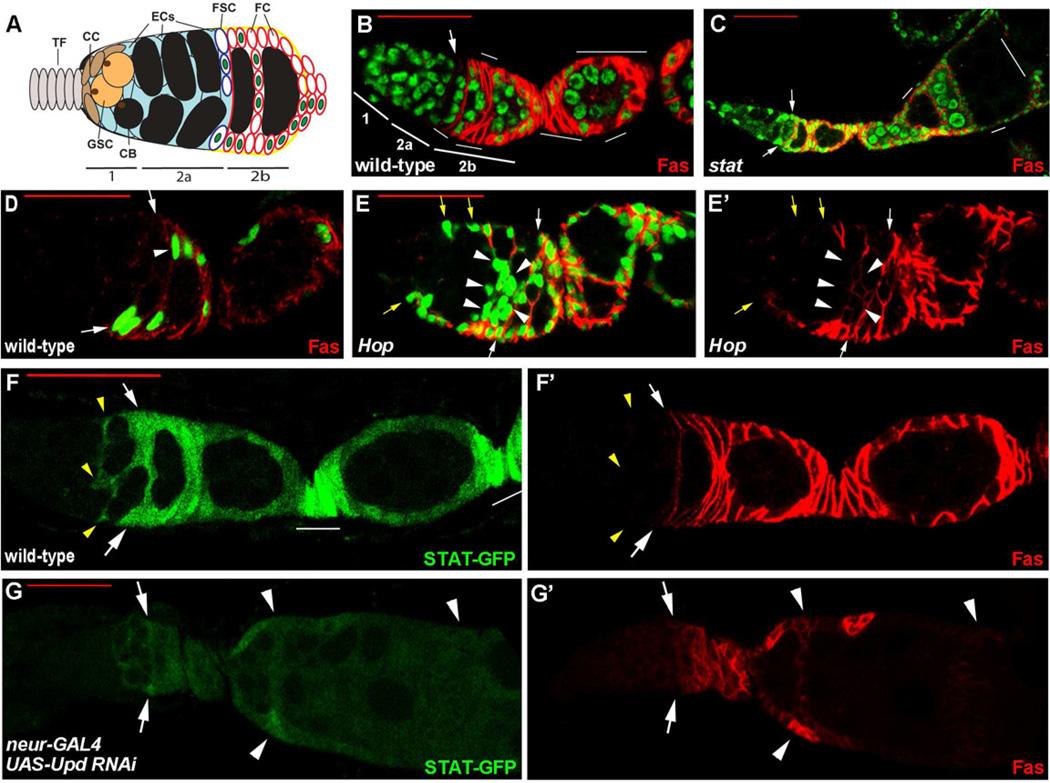
Figure 1. FSC responses to JAK-STAT pathway activity.
(A) Germarium cartoon showing differentiated Terminal Filament (TF) and Cap Cells (CC), which maintain Germline Stem Cells (GSCs) and express Hh and Wg strongly. Cystoblasts (CB) and subsequent, 2, 4, 8 and 16 cell germline cysts are in black, surrounded by somatic (blue) Escort Cells (ECs). A typical single marked Follicle Stem Cell (FSC) lineage is indicated by green nuclei, including FSC daughters that move laterally (up-down) or to the posterior (right). Most FSC daughters continue to proliferate as pre-follicle cells (FC) that express Fas3 (denoted by red outlines). (B, C) FSC clones negatively marked by the loss of GFP, showing (B) a wild-type FSC (arrow) and its derivatives (no green GFP; thin white lines) and (C) a Dstat92E mutant FSC clone where the mutant FSC has been lost and replaced by GFP-positive FSCs (arrows), while some GFP-negative FSC derivatives are still detected in later egg chambers (white lines). FSCs (arrows) are the most anterior (left) cells in their lineage, contact the germarial walls and do not express the surface protein Fas3 (red). In (C) there are some “fused” egg chambers, as expected from the requirement for JAK-STAT pathway activity to specify stalk cells. (D, E) FSC clones positively marked by GFP (green) and stained with antibody to Fas3 (red) for (D) a wild-type FSC and (E) an FSC expressing excess Hop (E’ shows Fas3 staining only). Excess JAK-STAT signaling produced extra marked cells anterior to normal FSC positions (white arrows). The most anterior ectopic GFP-positive cells did not express Fas3 (yellow arrows) but Fas3 was expressed in most ectopic anterior cells, whether lining the germarial walls (no arrows) or at internal positions (arrowheads). (F, G) JAK-STAT pathway activity reported by 10xSTAT-GFP (green) in ovarioles co-stained for (F’, G’) Fas3 (red). (F) In wild-type ovarioles the JAK-STAT pathway reporter was active in FSCs (arrows), their posterior derivatives (no arrows) and in posterior Escort Cells (yellow arrowheads). (G) In ovarioles where UAS-upd RNAi was driven by neur-GAL4 in polar cells JAK-STAT reporter activity was greatly reduced in the entire germarium, including FSCs (arrows) and in egg chambers, which were sometimes fused (arrowheads) with no intervening stalks. Red scale bars represent 25µm. See also Figure S1.
GSCs in the Drosophila ovary have been studied more thoroughly than FSCs and have substantially molded popular perceptions of archetypal stem cell biology. Physical constraints limit the number of GSCs that can contact a small population of differentiated niche cells known as Cap cells (Fig. 1A), which deliver a BMP signal locally to maintain GSCs in an undifferentiated state (Chen et al., 2011; Losick et al., 2011). FSC biology may be guided by quite different principles in several respects. First, FSC location is not simply defined as being adjacent to a single differentiated cell type (Nystul and Spradling, 2010). Second, FSCs respond to a far greater spectrum of extracellular signals than do GSCs (Kirilly et al., 2005; Song and Xie, 2003; Zhang and Kalderon, 2001). Third, FSC daughters only differentiate overtly after several rounds of proliferation.
FSCs are found at the beginning of germarial region 2b (Fig. 1A), where germline cysts first span the whole width of the germarium as an elongated disc (Nystul and Spradling, 2007, 2010). Hedgehog (Hh) and Wingless (Wg) ligands, which are required for FSC maintenance, derive from distant Terminal Filament and Cap cells at the extreme anterior of the germarium (Fig. 1A) (Forbes et al., 1996b; Song and Xie, 2003; Zhang and Kalderon, 2001). FSC maintenance also requires activity of DE-cadherin and integrin in the FSC (O'Reilly et al., 2008; Song and Xie, 2002). Escort cells are thought to be the critical FSC partners for homotypic cadherin-mediated interactions, and are present throughout the germarium anterior to the FSCs (Decotto and Spradling, 2005; Song and Xie, 2002). A basement membrane lines the entire germarial wall and is seeded with a critical integrin ligand by the FSC lineage itself (O'Reilly et al., 2008). From knowledge of these factors alone we might expect the entire anterior half of the germarium to anchor and support FSCs with a preference for the most extreme anterior positions close to Hh and Wg sources. Hence, a key question is whether additional signals or localized adhesive interactions define FSC position more precisely or control the number of FSCs supported in a germarium. Here we show that the JAK-STAT pathway is also a key regulator of FSCs and we examine how multiple signaling pathways collaborate to regulate FSC position and competitive behavior.
RESULTS
JAK-STAT pathway activity is critical for FSC function
JAK-STAT pathway activity is required directly in both germline and somatic “cyst” stem cells in the Drosophila testis for each type of stem cell to be maintained in its normal niche position (de Cuevas and Matunis, 2011). In Drosophila ovaries JAK-STAT signaling regulates GSC function indirectly by activating expression of a key GSC factor (Decapentaplegic; Dpp) in GSC-niche cells (Cap cells) (Losick et al., 2011). JAK-STAT requirements for FSC function have not been reported. The JAK-STAT pathway in Drosophila involves three Unpaired (Upd) family ligands, a receptor (Domeless), a single Janus Kinase, Hopscotch (Hop) and a single STAT (DStat92E), which is converted to a nuclear transcriptional activator by ligand-induced JAK phosphorylation (Arbouzova and Zeidler, 2006). We tested autonomous requirements for JAK and STAT components by creating mutant FSC clones marked by the loss of GFP (Fig. 1B) and scoring the persistence of these clones relative to controls. We found that both hop and D-Stat92E loss of function mutations produced severe cell autonomous loss of mutant FSC clones induced in young adults (Figs. 1C and 2A and Supplemental Table S1), implying an essential function for JAK-STAT signaling in maintaining FSCs.
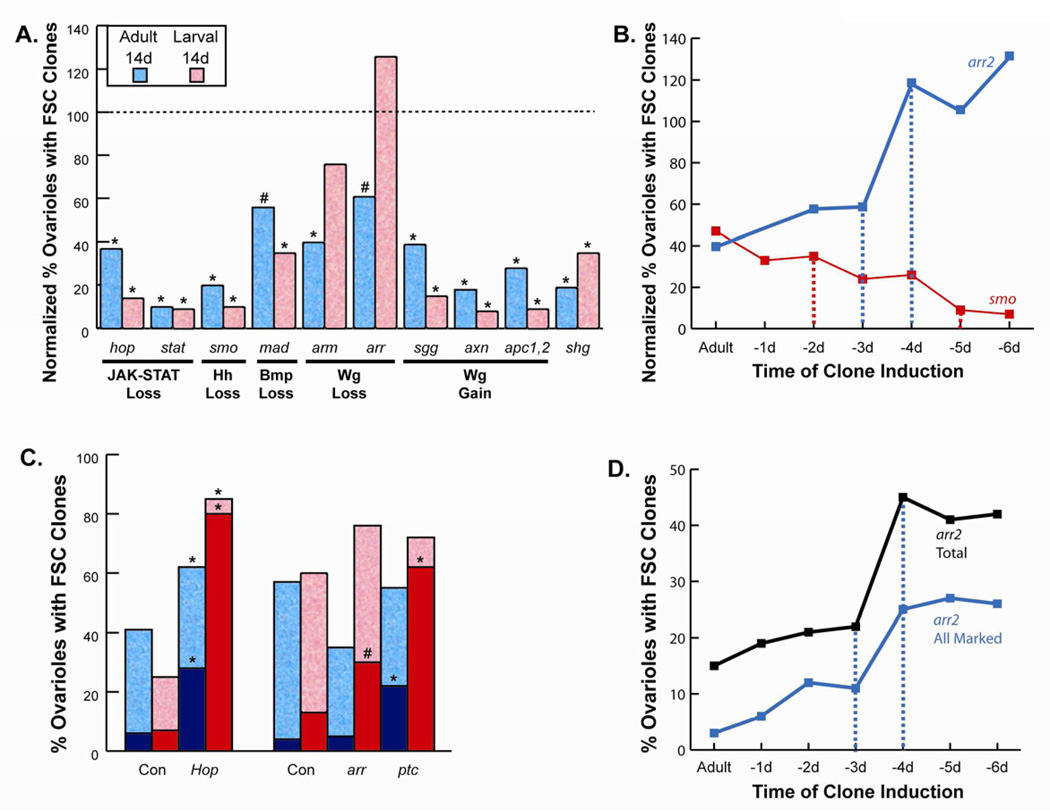
Figure 2. An FSC establishment phase during mid-pupation that is opposed by Wnt signaling.
(A) Negatively-marked FSC clones homozygous for the indicated mutations produced by heat-shock induced recombination in young adults (blue) or third instar larvae (red) were counted 14 days later and expressed as a percentage of control values (“normalized”) as a measure of FSC clone maintenance (raw data are in Table S1). Statistical differences from control values calculated by Fisher’s two-tailed test are indicated as p<0.0001 (*) or p<0.01 (#). (B) Normalized percentage of ovarioles retaining marked FSC clones was scored as in (A) for arr (blue) and smo (red) clones induced in adults and on each of the six days preceding eclosion. Broken lines de-limit major transition periods. (C) Absolute percentage of ovarioles retaining marked FSC clones 14 days after induction in adults (blue) or larvae (red) for FSCs expressing excess Hop or lacking function of arr or ptc, together with appropriate control (Con) wild-type genotypes tested strictly in parallel. Dark blue and red columns show the percentage of ovarioles where the entire germarium and subsequent egg chamber contained only marked FSC derivatives (“all marked”). Statistical differences from control values calculated by Fisher’s two-tailed test are indicated as p<0.0001 (*) or p<0.01 (#) (symbols are immediately above the relevant column). (D) Absolute percentage of ovarioles with any arr mutant FSC clone (black) or “all-marked” arr mutant FSC clones (blue) 14 days after induction in adults and on each of the six days preceding eclosion. Broken lines de-limit the major transition period.
In ovaries high level Upd expression in specialized “polar cells” at the anterior and posterior poles of developing egg chambers guides the differentiation of follicle cells according to their position along the AP axis and elicits strong expression of a JAK-STAT pathway reporter gene (10xSTAT-GFP) in nearby follicle cells (Bach et al., 2007; Xi et al., 2003) (Fig. 1F). We also saw strong 10xSTAT-GFP expression in the germarium. Expression was highest in posterior regions, including pre-follicle cells, FSCs and posterior Escort Cells near the FSCs, and much lower in anterior Escort Cells, with no detectable signal in the germline (Fig. 1F). GFP from the STAT reporter was greatly reduced in stat mutant FSC clones (Supplemental Fig. S1A), confirming its validity as a reporter of STAT activity in these experiments.
Polar cells first differentiate in the germarium (Nystul and Spradling, 2010). There, Upd produced in polar cells specifies adjacent stalk cells, which are required to separate egg chambers as they bud from the germarium (Assa-Kunik et al., 2007; McGregor et al., 2002). Upd expression has also been reported in terminal filament and cap cells at the extreme anterior of germaria (Lopez-Onieva et al., 2008). We therefore tested whether JAK-STAT pathway activity in FSCs depended on Upd ligand synthesized in either polar cells or anterior germarial cells. When upd RNAi was expressed in terminal filament and Cap cells (using bab-GAL4) or in Escort cells (using C587-GAL4) (Supplemental Fig. S1J, K) we did not see any reduction in 10XSTAT-GFP expression in FSCs (Supplemental Fig. S1E, F). However, expression of 10xSTAT-GFP was substantially reduced in all germarial cells, including FSCs, of most ovarioles when upd RNAi was expressed specifically in polar cells (using neur-GAL4) or in both polar cells and early stages of the FSC lineage (using 109-30 GAL4) (Fig. 1G and Supplemental Fig. S1C, D, H ,I).
Knockdown of Upd in polar cells produced fused egg chambers, indicating a defect in stalk cell specification (Fig. 1G), in only about 10% of ovarioles, suggesting that inhibition of Upd expression in the germarium was delayed or incomplete, as might be expected because strong neur-GAL4 expression is first seen at the posterior of the germarium (Supplemental Fig. S1H) after Upd protein has begun to specify adjacent stalk cells (Assa-Kunik et al., 2007; Nystul and Spradling, 2010). Incomplete inhibition of Upd expression in the germarium likely accounts for the incomplete penetrance of JAK-STAT pathway reporter inhibition we observed in FSCs (about 70% of ovarioles) because all ovarioles with fused egg chambers showed very strong loss of 10XSTAT-GFP expression in the germarium (Fig. 1G). We conclude that Upd produced in polar cells is the principal source of JAK-STAT activity in FSCs, consistent with our observation that STAT reporter activity in somatic cells declines substantially towards the anterior of the germarium (Fig. 1F).
We tested the response of FSCs to abnormally high JAK-STAT activity by expressing a UAS-Hop transgene in FSC clones using a modified MARCM method (Lee and Luo, 2001; Vied and Kalderon, 2009). Strong Hop expression induced a variety of dramatic phenotypes. Most notably, FSC lineages over-expressing Hop not only survived better than wild-type FSC clones (Fig. 2C) but also frequently produced germaria and subsequent egg chambers containing only derivatives of the marked Hop-expressing FSC (Fig. 1D,E), implying competitive displacement of unmarked (wild-type) FSCs. Such “all-marked” ovarioles are found only rarely for control wild-type clones (Fig. 2C) because the conditions for clone induction generally produce only one marked FSC in a germarium and FSC replacement among wild-type cells is relatively infrequent (Nystul and Spradling, 2010). Hence, most marked wild-type FSC clones are found in mosaic ovarioles together with unmarked FSC derivatives. Excess Hop also led to the cell autonomous accumulation of many extra marked cells anterior to the normal FSC position. Most of these cells expressed Fas3, like normal pre-follicle cells, but a few, like normal FSCs, did not (Fig. 1E). These phenotypes, indicating enhanced FSC activity, were markedly stronger when excess Hop expression in FSCs was initiated at late larval stages rather than in adults (Fig. 2C).
The production of “all marked” ovarioles is also induced by excess Hh pathway activity in FSCs lacking patched (ptc) activity (Vied and Kalderon, 2009; Zhang and Kalderon, 2001). That phenotype was also stronger when initiated at larval stages (Fig. 2C). Thus, JAK-STAT activity, like Hh pathway activity, is stringently required in FSCs for normal self-renewal. Moreover, excessive activity in each pathway produces dramatically enhanced FSC activity, implying that the level of activity in these two pathways is a major determinant of FSC behavior. The Hh signal derives from Cap cells anterior to the FSC (Forbes et al., 1996a; Hartman et al., 2011), whereas the JAK-STAT ligand, Upd, derives principally from polar cells posterior to FSCs. These observations suggest the simple possibility that FSCs must reside at the intersection of the two ligand distributions where both Hh and JAK-STAT pathway activities are adequately high.
FSC establishment and maintenance; opposing Wnt pathway roles
It has previously been reported that normal FSC maintenance requires Wnt and BMP pathway activities and is impaired, to a lesser degree, by excessive Wnt pathway activity (Kirilly et al., 2005; Song and Xie, 2003). For FSC clones induced in adults we found excessive Wnt pathway activity (due to apc1 apc2, axn or sgg mutations) to be more deleterious than loss of Wnt signaling (due to arr or arm mutations), contrary to the earlier report (Song and Xie, 2003). Also, by comparing all pathway manipulations side by side in analogous tests we could see that FSC maintenance in adults over 14 days was reduced more by disruption of Hh (smo: 20% of control) and JAK-STAT pathways (hop & stat average: 23% of control) than by the loss of Wnt (arm and arr average: 43% of control) and BMP contributions (mad: 56% of control) (Fig. 2A and Supplemental Table S1).
We also induced mutant FSC clones during development. Almost all pathway manipulations tested here (Fig. 2A, C), or other critical FSC gene mutations tested previously (Wang et al., 2012), produced stronger effects when induced prior to adulthood, suggesting that FSC establishment depends on, but is more sensitive to, many of the same factors that regulate adult FSC maintenance. Although Wnt pathway hyperactivation (due to sgg, axn or apc1 apc2 mutations) followed this general trend, both arm and arr mutant FSC clones (which lack Wnt pathway activity) survived much better if induced prior to adulthood (Fig. 2A). A time course revealed that the critical transition time for the dramatic change in the response of FSCs to Wnt pathway manipulation was 3–4 days before eclosion (Fig. 2B). The opposite response for smo mutant FSC clones (FSC loss) was centered over a similar developmental stage, spanning 2-5 days prior to adulthood (Fig. 2B).
Strikingly, arr mutant FSCs induced in larvae actually exhibited a phenotype indicating enhanced FSC function, producing many “all-marked” ovarioles (Fig. 2C). The critical developmental stage for the production of frequent all-marked clones was the same as for enhanced FSC clone survival (Fig. 2B,D), suggesting that the two phenomena are causally linked. If a marked FSC ceases to function it can be replaced by the progeny of another, unmarked, FSC in a mosaic germarium, leading to loss of the marked FSC clone (Nystul and Spradling, 2007; Zhang and Kalderon, 2001). However, once a germarium contains only marked FSCs and their progeny, a marked FSC can only be replaced by another marked cell. Hence, we conclude that arr mutant FSCs survive better in adults if first induced at larval stages because they are stabilized by prior homogenization of the stem cell population. These observations not only inform us about the effects of Wnt signaling on FSCs but they also illustrate a general principle that may be very important in the development of cancer: namely, full colonization of niches within a developmental unit can provide an important mechanism for stabilizing a specific mutant stem cell genotype.
In summary, we found that normal levels of Wnt signaling contribute only weakly to FSC maintenance in adults. More importantly, normal levels of Wnt signaling oppose FSC establishment and excess Wnt signaling strongly impairs both FSC establishment and maintenance. The strong negative responses of FSCs to excess Wnt pathway activity may be important in ensuring that FSCs are not established and maintained too close to Cap cells, which are located at the anterior end of the germarium and express high levels of Wg (Forbes et al., 1996b). Conversely, it is possible that FSCs are also weakly constrained from residing too far from Cap cells because of the weak positive influence of Wg on FSC maintenance. These effects of Wg likely supplement the positional constraints imposed by Hh and JAK-STAT signals.
How does the level of Hh signaling influence FSC function?
If gradients of Hh and Wg emanating from cells anterior to FSCs and Upd emanating from cells posterior to FSCs define supportive positions for FSCs we would expect FSCs to be sensitive to the quantitative activity in each pathway. Our results with mutations producing complete loss of pathway activity or extremely high pathway activities are consistent with this idea, but what about intermediate activities close to those in normal FSCs? We were able to pursue that question further for Hh signaling.
Hh expression in the germarium is high only in Terminal Filament and Cap cells (Forbes et al., 1996a) and functional tests suggested that these are the key sites of Hh expression to support FSCs (Hartman et al., 2011; King et al., 2001). It might therefore be expected that only low levels of Hh reach the rather distant FSCs, forming only a shallow gradient at that position. However, Hh release from the anterior of the germarium involves an unusual specialized mechanism guided by expression of the Hh-sequestering protein Boi (Brother of Ihog) in Hh-expressing cells (Hartman et al., 2011). Moreover, the Hh pathway reporter ptc-lacZ is in fact expressed at high levels throughout the anterior of the germarium up to and including the FSCs; FSC daughters generally have slightly lower ptc-lacZ levels, which decline further towards the posterior (Vied and Kalderon, 2009) (Fig. 3C). The decline in Hh pathway activity posterior to FSCs supports the possibility that Hh may indeed have a critical role as a graded instructive stem cell factor. We addressed two concepts within that hypothesis by testing FSC function when we attenuated or blocked responses to Hh (to see if a natural ligand gradient was essential) and simultaneously imposed various levels of constitutive pathway activity (to see if a specific level of pathway activity in the FSC was essential).
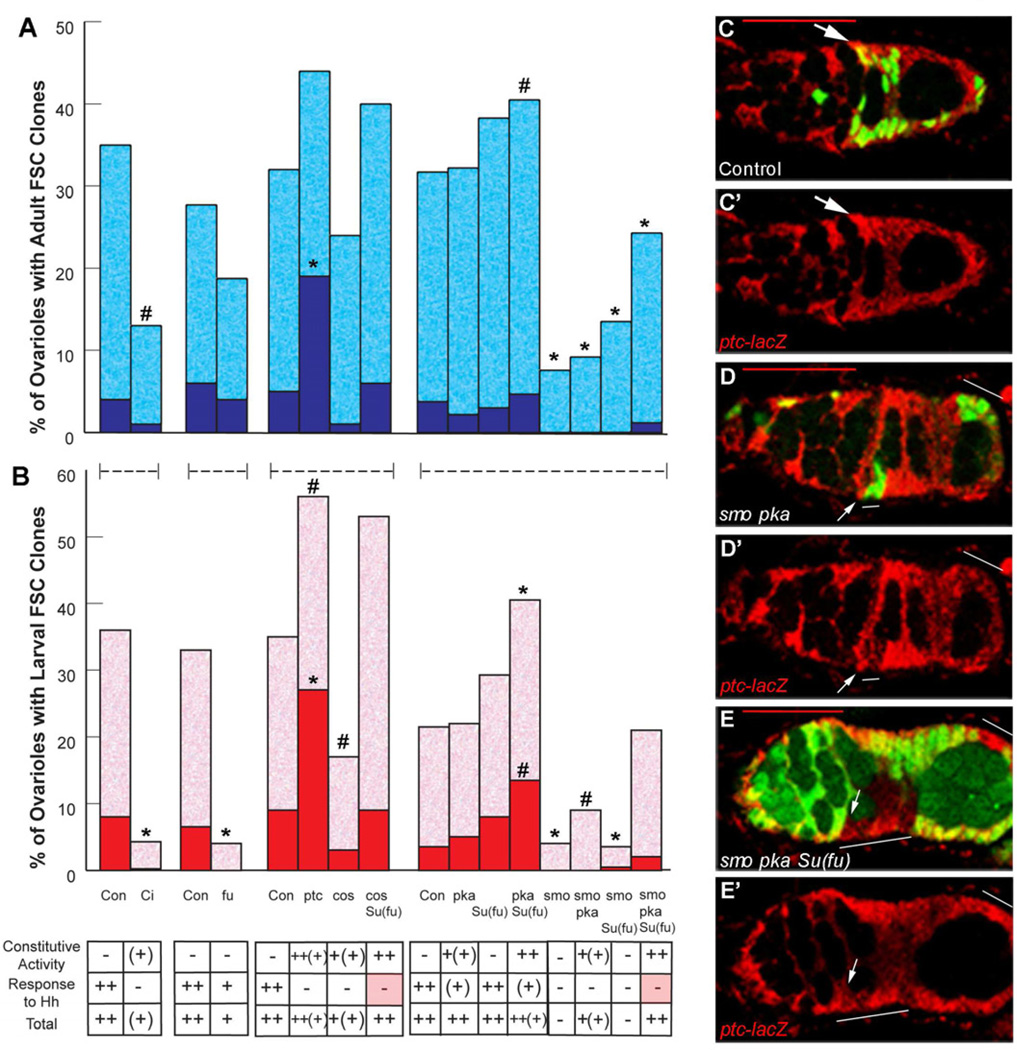
Figure 3. Hh pathway activity in FSCs must be high and precise but need not be higher than in daughters.
(A, B) Percentage of ovarioles retaining marked FSC clones 14 days after induction in (A) adults (blue) or (B) larvae (red), with dark colors indicating “all-marked” clones for FSCs lacking function of the indicated Hh signaling pathway components and controls (Con). At the bottom, the resulting strength of constitutive pathway activity, response to Hh and the sum of both are indicated based on extensive studies in wing discs. Statistical differences from control values calculated by Fisher’s two-tailed test are indicated as p<0.0001 (*) or p<0.01 (#). (C-E) Hh pathway activity is reported here by staining β-galactosidase (red) from a ptc-lacZ transgene, shown alone (C’–E’) or together with FSC clone markers (green in C–E). Hh pathway activity is (C, C’) slightly higher in a wild-type FSC (arrow) than in more posterior daughters, (D, D’) reduced in the FSC (arrow) of a positively-marked smo pka clone (green; white line) and (E, E’) high and uniform among the FSC and daughters of a negatively-marked (no green; white line) smo pka clone in Su(fu) mutant animals. Red scale bars represent 25µm.
Cells respond to Hh by blocking proteolytic processing of the transcription factor Ci-155 to a shorter repressor form, Ci-75, and also by increasing the activity of Ci-155 activator in a dose-dependent manner (Jiang and Hui, 2008; Smelkinson et al., 2007). FSCs lacking Ci altogether were rapidly lost (Fig. 3A,B) (Zhang and Kalderon, 2001), as were FSC clones lacking Fused (Fu) kinase activity (Fig. 3A, B), which is required for full activation of Ci-155 (Zhou and Kalderon, 2011). Thus, neither inhibition of Ci-75 repressor production nor low level Ci-155 activity sufficed to maintain FSCs.
To evaluate whether normal FSC behavior depends on responding to the natural Hh ligand gradient we tested two FSC genotypes that abrogate responses to ligand (which depend substantially on Costal 2 (Cos2) and absolutely on Smoothened (Smo)) but substitute high-level constitutive pathway activity (by inactivating Suppressor of fused (Su(fu)) and either Cos2 or Protein Kinase A (PKA)) (Jiang and Hui, 2008; Zhou et al., 2006; Zhou and Kalderon, 2011). We found that both cos2 and smo pka mutant FSC clones in Su(fu) mutant animals were maintained very well; they also produced no significant enhancement of FSC function, which was scored by the production of “all-marked” ovarioles (Fig. 3A,B). Since the cos2 Su(fu) and smo pka Su(fu) genotypes impose the same Hh pathway activity on FSCs and their daughters we conclude that FSCs need not have higher Hh pathway activity than their daughters.
Now that we have established that only the absolute level of Hh pathway activity in FSCs is important we can assess how that level influences FSC behavior. smo pka mutant FSCs are highly defective (Fig. 3A,B) and exhibited reduced ptc-lacZ staining in FSCs, down to a level normally seen at the posterior of the germarium (Fig. 3D). Thus, the lower end of the range of Hh pathway activity observed in normal germaria is clearly insufficient to support an FSC. Even cos2 mutant FSCs, which have no detectable loss of ptc-lacZ expression (data not shown), have a significant functional deficit (Fig. 3B), indicating that even a small reduction below normal Hh pathway activity is detrimental.
At the high end of the spectrum, loss of ptc reproduces maximal pathway stimulation by Hh in wing discs. That condition is also reproduced almost precisely in wing discs by cos2 Su(fu) and smo pka Su(fu) mutant cells. Nevertheless, of the three genotypes only ptc produces a high proportion of all-marked mutant FSC clones. Thus, enhanced FSC activity can be triggered by a very small increase in Hh pathway activity.
In summary, both the ptc-lacZ reporter and functional studies suggest that normal Hh pathway activity is very high in FSCs and that small deviations in either direction can produce substantial FSC loss or FSC duplication. In other words, normal FSC function requires Hh pathway activity to fall within a very precise range.
Distinct morphological responses to different pathways in FSCs and their daughters
Within a normal FSC lineage the most anterior cell does not detectably express Fas3 and is situated adjacent to the germarial wall, whereas the majority of FSC pre-follicle cell progeny occupy more posterior positions and express high levels of Fas3 (Nystul and Spradling, 2007; Zhang and Kalderon, 2001). Immediate FSC pre-follicle cell daughters occasionally have not yet accumulated detectable levels of Fas3 and may be very close to an FSC as they begin to move to the posterior or migrate laterally across the interior of the germarium towards an FSC on the opposite germarial wall (Fig. 1A,D) (Nystul and Spradling, 2007, 2010). When ptc mutant FSC clones were marked with GFP and observed 7–21 days later we saw additional GFP-positive, Fas3-negative cells close to the normal FSC position and adjacent to the germarial wall (Fig. 4A), as described previously (Vied and Kalderon, 2009; Zhang and Kalderon, 2001). At least some of those ectopic cells behave like extra FSCs because they were previously shown to contribute more FSC lineages than were normally seen in a single ovariole (Zhang and Kalderon, 2001). When ptc FSC clones were induced in larvae rather than adults there were generally greater numbers of FSC-like cells and these were frequently accompanied by supernumerary GFP-positive, Fas3-negative cells away from the germarial walls, possibly daughters of the supernumerary FSC-like cells (Supplemental Fig. S2C).
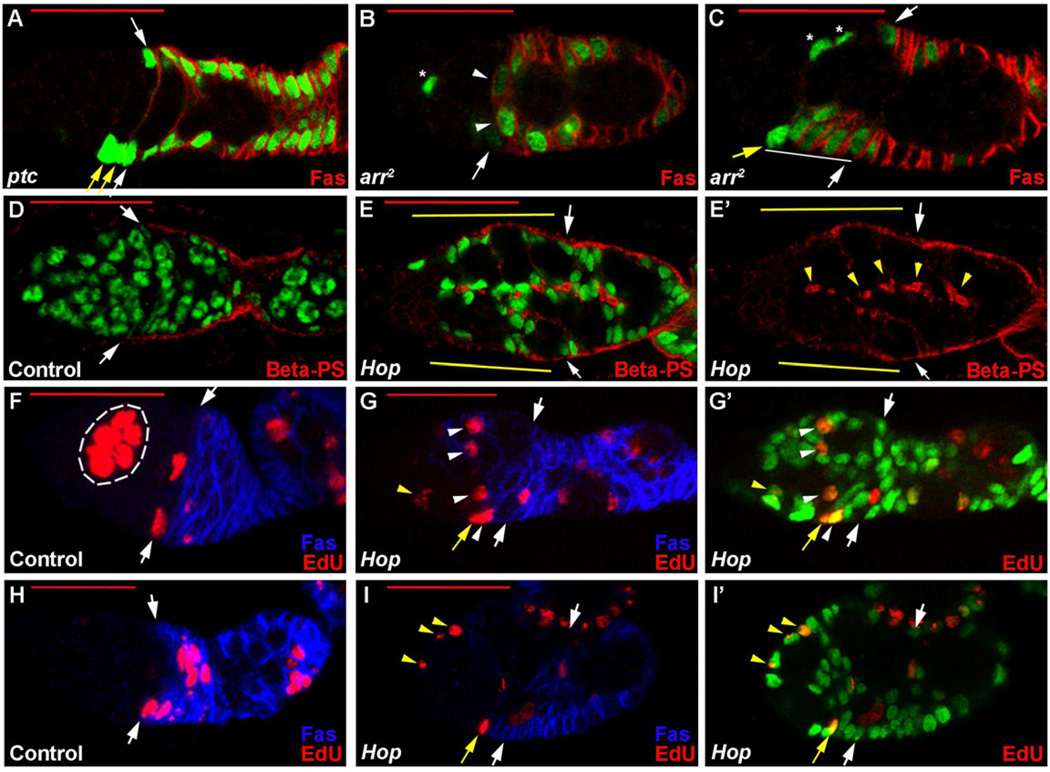
Figure 4. FSC responses to signaling pathways are diverse and include accumulation of cells anterior to normal FSC positions.
(A–E) FSC clones homozygous for the designated mutations or expressing excess Hop were marked positively with GFP (green) and stained for (A–C) Fas3 (red) or (D–E) β-PS integrin (red). Clones were induced in larvae (B, C, E) or adults (A, D). White arrows show normal FSC positions. (A) Yellow arrows mark extra FSC-like ptc mutant cells (against the germarial wall and lacking Fas3 staining) anterior to the normal FSC position. (B) White arrowheads indicate ectopic Fas3-positive arr FSC derivatives. (C) Ectopic Fas3-positive arr FSC derivatives (white line) extend anterior to the normal FSC position and, in this example, include an FSC-like Fas3-negative cell at the extreme anterior (yellow arrow). Marked Escort cells (asterisks) are commonly seen in ovarioles with positively marked clones of various genotypes, including wild-type clones. In (E, E’) yellow lines indicate anterior extension of β-PS integrin staining along the basement membrane interface of ectopically anterior FSC derivatives; ectopic staining at the interfaces of internal Hop-expressing cells is indicated by yellow arrowheads. (F–I) EdU labeling (red) of ovarioles containing positively-marked (green) control clones (F, H) or FSC clones expressing Hop (G, I), and stained for Fas3 (blue), showing just red and blue channels (F–I) or just red and green channels (G’, I’). In most control germaria (F, H) only germline cysts (outlined in white in F) incorporate EdU anterior to FSCs, but several Hop-expressing cells anterior to FSCs are labeled by EdU, including Fas3-positive cells (white arrowheads in G), Fas3-negative FSC-like cells (yellow arrows in G and I) and far anterior cells (yellow arrowheads in G and I). Red scale bars represent 25µm. See also Figure S2.
By contrast to the ptc phenotype, we did not see frequent additional Fas3-negative FSC-like cells close to normal FSC positions for FSC clones lacking arr function or expressing activated TKV (Fig. 4B,C and Supplemental Fig. 2B). However, for arr mutant clones induced in larvae we did see some abnormal phenotypes. First, we frequently saw a row of Fas3-expressing cells straddling the germarium at the region 2a/b border where we normally see only thin Fas3-positive processes or single Fas3-positive cells (Fig. 4B). Second, we occasionally observed Fas3-expressing cells extending further anterior than the normal FSC position along a germarial wall (Fig. 4C). This suggests that an FSC may be in an abnormally anterior position but FSC identification was often ambiguous, either because the most anterior of these ectopic cells sometimes expressed Fas3 or because Fas3 expression was weak in all of the ectopically anterior cells. We conclude that arr mutant FSC derivatives often occupy abnormal positions in the germarium, which in some instances suggest that the FSC is ectopically anterior.
The phenotypes from over-expressing Hop in FSCs were readily distinguished from those of ptc, arr and TKV*-expressing FSCs by three criteria. First, a very large number of ectopic cells could accumulate, typically extending very far anterior and leading to changes in the shape of germline cysts (Fig. 1E and Fig. 4E). Second, the majority of ectopic anterior cells expressed Fas3 either strongly or in a weak and patchy pattern (Fig. 1E). When uniformly strong Fas3 staining was observed one or more of the most anterior cells, generally adjacent to the germarial walls, had no Fas3 staining; that pattern recapitulates the normal progression of Fas3 staining in an FSC lineage but from an ectopically anterior starting point (Fig. 1E). Third, the laminin A ligand and the PS integrin beta subunit (βPS) normally accumulate only along the basement membrane lining the germarial walls in regions adjacent and posterior to the FSCs, presumably because only FSCs and pre-follicle cells secrete the critical ligand, laminin A, required to engage and organize surface integrin (Fig. 4D) (O'Reilly et al., 2008). However, Hop-expressing cells anterior to the normal FSC lineage also stained strongly for βPS along the germarial walls (Fig. 4E). In fact, when large numbers of anterior Hop-expressing cells were present some ectopic βPS staining was also seen at the interfaces between clustered ectopic Hop-expressing cells that were far from the germarial wall (Fig. 4E).
We also used EdU labeling to see if ectopic Hop-expressing cells were proliferating. In wild-type germaria Escort cells anterior to the region 2a/b border only very rarely incorporate EdU (Kirilly et al., 2011) (Fig. 4F,H). By contrast, we saw extensive EdU incorporation into somatic cells in the anterior half of germaria harboring Hop-expressing FSC clones (Fig. 4G,I). EdU labeling was seen exclusively in GFP-marked cells, derived from the Hop-expressing FSC, and was observed both in Fas3-expressing cells and extreme anterior Fas3-negative cells, consistent with the latter cells acting like FSCs. Thus, ectopic cells with elevated JAK-STAT signaling were extremely numerous and exhibited characteristics of excess FSCs and their pre-follicle cell derivatives in abnormally anterior locations. By comparison, supernumerary ptc FSC-like cells accumulated predominantly close to normal FSC positions, arr mutant FSCs and their derivatives were occasionally more anterior than normal, and activated TKV did not produce any distinctive FSC phenotypes.
FSC signaling pathways and adhesive niche interactions
The aberrant positioning of FSC derivatives with deficient Wnt pathway or hyperactive JAK-STAT pathway activities suggested that a significant outcome of these signals may be to regulate migration or adhesion of FSCs. We therefore investigated the relationship between signaling pathways and a known contributor to FSC-niche adhesion, DE-cadherin (Shotgun; Shg) (Song and Xie, 2002).
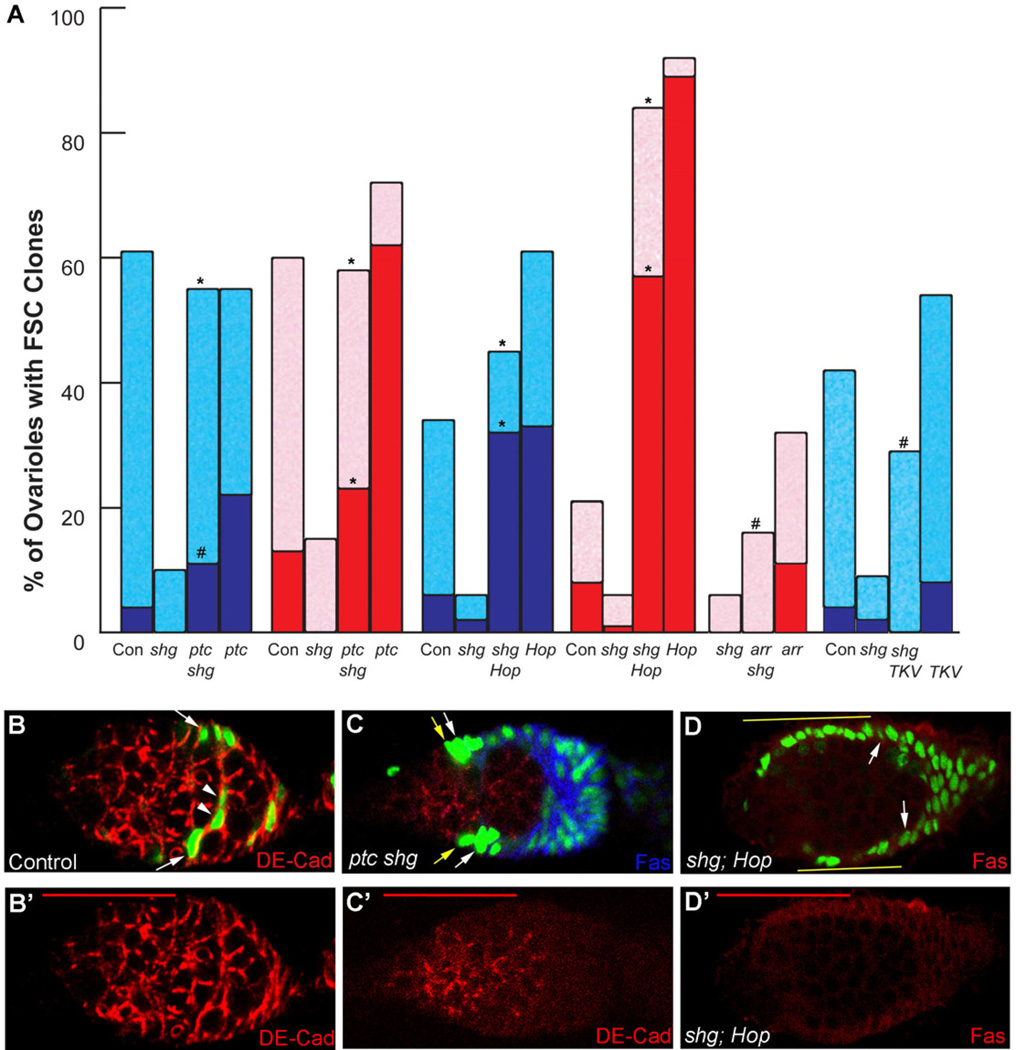
Remarkably, we found that both ptc mutations and excess Hop expression in FSC clones fully rescued the FSC maintenance defect of null shg mutant FSCs and even produced frequent “all-marked” germaria and other phenotypes characteristic of enhanced FSC activity (Fig. 5A–D). The ability of Hh and JAK-STAT pathways to override the lack of DE-cadherin emphasizes their potent effects on FSCs, either rendering them adhesion-independent or driving a distinct mode of adhesion.
Figure 5. Signaling pathways can compensate for loss of Cadherin function in FSCs.
(A) Percentage of ovarioles retaining marked FSC clones 14 days after induction in adults (blue) or larvae (red), with dark colors indicating “all-marked” clones; experiments test rescue of shg (cadherin) loss of function by excess Hh (ptc) or JAK-STAT (Hop) pathway activity, loss of Wnt pathway activity (arr) or activated BMP receptor (TKV). Statistical differences for shg mutant FSCs with and without potential rescue were calculated by Fisher’s two-tailed test and indicated as p<0.0001 (*) or p<0.01 (#) (symbols directly above relevant potential rescue column). (B, C) Positively-marked clones (green) stained for DE-cadherin (red) (shown alone in B’, C’) and Fas3 (blue in C) to show loss of Cadherin in (C) ptc shg compared to (B) wild-type FSC derivatives. Yellow arrows indicate ectopic FSC-like cells in (C). (D) Ectopic anterior shg Hop FSC derivatives (green; yellow lines) associate closely with the germarial walls and express Fas3 (red). Red scale bars represent 25µm.
Loss of DE-cadherin in FSC clones expressing excess Hop accentuated the accumulation of ectopically anterior marked cells along the germarial walls, rather than internally (Fig. 5D). That response is consistent with reduced interaction of FSCs and their derivatives with Escort Cells or germline cysts in the absence of DE-cadherin. The strong association of FSC derivatives expressing excess Hop along anterior germarial walls in the presence or absence of DE-cadherin (Fig. 1E, Fig. 5D and Supplemental Fig. S2A), together with the associated anterior extension of βPS integrin staining (Fig. 4E) suggests that the JAK-STAT pathway might drive anterior migration of FSCs through enhanced integrin interactions with the basement membrane.
The loss of shg mutant FSCs was also substantially rescued by activated TKV expression in adult clones and was partially rescued by loss of arr in larval-induced clones (Fig. 5A). However, loss of DE-cadherin did reduce the survival of arr and TKV-expressing FSC clones and the generation of “all-marked” ovarioles by arr mutant FSCs (Fig. 5A). These observations suggest two possible interpretations. One possibility is that BMP or Wnt pathways might act in part by modulating adhesion through DE-cadherin. The alternative, which is consistent with our earlier conclusions, is that compensatory changes in FSCs induced by the BMP and Wnt pathways are simply weaker than for Hh and JAK-STAT pathways; hence FSCs still retain some dependence on an adhesive contribution from DE-cadherin.
Combinatorial effects of changes in signaling pathway activities
It was initially surprising to us that at least four signaling pathways have a significant impact on FSC function. The signals presumably convey information about the suitability of the extracellular environment for supporting stem cell function, with at least Hh, JAK-STAT and Wnt pathways dictating stem cell positions or numbers, but how do these signaling pathways converge within the stem cell? Favorable pathway changes can alter FSC numbers, position and adhesion-dependence, suggesting regulation of FSC proliferation or adhesion, but they produce distinctive phenotypes, indicating diverse initial targets. One possibility is that each pathway contributes to a specific mode of cell adhesion or a single facet of the FSC’s proliferation machinery, each of which is absolutely required for an FSC to function effectively as a stem cell. Alternatively, the inputs of each pathway might act through different biochemical paths but contribute to a common functionally important end-point, such as retention in the germarium or proliferation, which are undeniably essential FSC properties. In the latter scenario, the inputs of different pathways are effectively summed and hence excess activity in one pathway may sometimes compensate for reduced activity in another pathway. We tested that possibility for a variety of combinations of pathway perturbations.
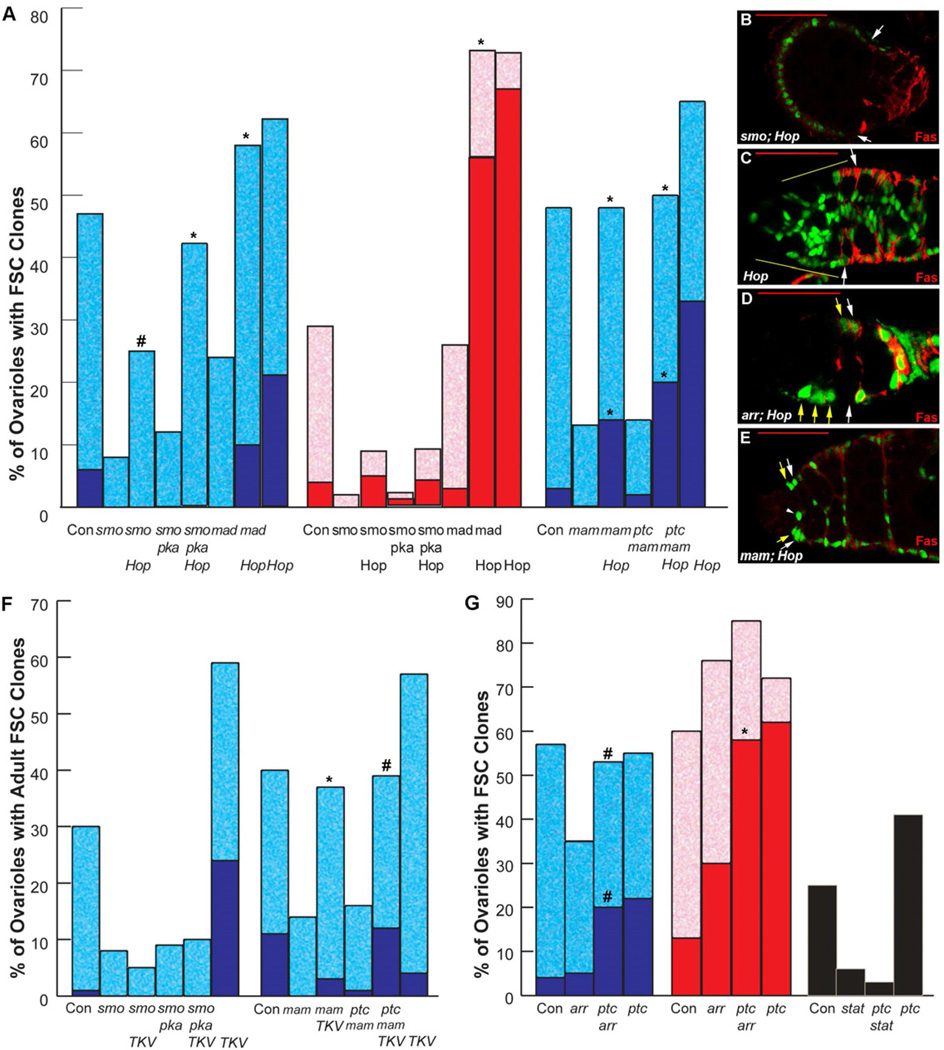
According to the strength of phenotypes elicited by both pathway inhibition and hyperactivity, Hh and JAK-STAT signaling have emerged as the major influences on FSCs (Fig. 2A,C). First we examined the possibility that excess JAK-STAT activity might compensate for loss of Hh signaling. Excess Hop did not significantly rescue the loss of smo mutant FSC clones induced in larvae but did substantially restore such lineages for mutant clones induced in adults, for which smo FSC phenotypes are less severe (Fig. 6A). A similar pattern was seen for attempted rescue of smo pka FSC clones. In rescued smo or smo pka FSC lineages over-expressing Hop in adults there was no displacement of wild-type FSC lineages to produce all-marked clones (Fig. 6A). In fact, those genotypes produced FSC clones with an approximately normal phenotype (Supplemental Fig. S3A), suggesting that excess JAK-STAT pathway activity can indeed compensate for the deficits in niche adhesion or proliferation that curtail the maintenance of FSCs in the absence of Hh signaling. However, since rescue is incomplete for smo FSC clones and slightly better for smo pka clones (Fig. 6A), it appears that excess JAK-STAT activity cannot quite compensate quantitatively for complete loss of Hh signaling in adult FSCs.
Figure 6. Epistasis tests indicate convergence and hierarchy of signaling pathways in FSCs.
(A, F, G) Percentage of ovarioles retaining marked FSC clones 14 days after induction in adults (blue or black) or larvae (red), with dark colors indicating “all-marked” clones; experiments test rescue of indicated loss of function FSC genotypes by expression of excess Hop (A) or activated TKV (F), or loss of ptc (G) in the mutant FSC. Statistical differences for mutant FSC genotypes with and without potential rescue were calculated by Fisher’s two-tailed test and indicated by * p<0.0001 or # p<0.01 (symbols directly above relevant potential rescue column). In (G) stat rescue tests, ovarioles in which the other (unscored) FSC lineage was ptc were not counted to avoid any influence of ptc mutant FSCs out-competing other genotypes; hence, there is no “all-marked” category. (B–E) Positively-marked clones (green) stained for Fas3 (red) showing (B) smo mutant Hop-expressing cells accumulating in an abnormal anterior structure instead of populating posterior egg chambers, (C, D) reduced anterior accumulation of Hop-expressing cells in the absence of arr function (larval-induced clone), and (E) excess Hop phenotype in the absence of mam function (which prevents polar and stalk cell specification and hence failure of normal egg chamber budding). White arrows indicate normal FSC positions; yellow arrows or lines indicate ectopically anterior marked cells (in C–E). Red scale bars represent 25µm. See also Figure S3.
Interestingly, among the small proportion of smo or smo pka larval-induced FSC clones rescued by Hop overexpression (Fig. 6B), roughly half had a remarkable phenotype, in which a structure resembling an egg chamber, containing only marked Fas3-positive FSC derivatives, appeared to be budding from the anterior end of the germarium while no marked mutant FSC derivatives extended posteriorly to contribute to normal follicle cell lineages (Supplemental Fig. S3B-D). These strikingly abnormal phenotypes suggest that graded Hh or JAK-STAT ligand signals ordinarily contribute to ensuring the normal posteriorly directed path of FSC pre-follicle cell daughters.
To test whether excess Hh pathway activity can compensate for JAK-STAT pathway deficiency we generated ptc and stat clones simultaneously (on 2R and 3R chromosome arms). We observed a similar loss of stat mutant FSC clones relative to control clones whether these FSCs also were homozygous for ptc (2% vs 23%) or not (3% vs 18%), showing that excess Hh pathway activity provided no rescue for FSCs lacking JAK-STAT activity (Fig. 6G). This test was only possible for clones induced in adults because clones induced in larvae resulted in severe reductions in eclosion of adults of the appropriate genotype. Thus, at least for FSC maintenance, excess Hh pathway activity cannot compensate for the loss of JAK-STAT signaling. This result is perhaps to be expected given our finding that excess Hh pathway activity engendered by the ptc mutation is only marginally higher than the normal Hh pathway activity experienced by FSCs.
We also tested the interaction of Hh and JAK-STAT signaling with other pathways. Excess JAK-STAT activity fully rescued survival of mad mutant FSCs deficient for BMP signaling (Fig. 6A). Indeed, the resulting phenotype was very similar to that induced by Hop over-expression alone. Potential rescue of BMP pathway deficient FSCs by excess Hh signaling could not be tested conveniently. In the reciprocal test, excess BMP pathway activity due to activated TKV expression did not rescue the survival of smo mutant FSC clones at all (Fig. 6F), as reported previously (Kirilly et al., 2005). Loss of Wnt pathway activity in adults produces only a weak loss of FSCs. Survival of arr mutant FSC clones induced in adults was restored by simultaneous loss of ptc (activating the Hh pathway) to produce a phenotype very similar to that of ptc alone (Fig. 6G). This set of positive rescue results supports the idea that the positive contributions of Wnt and BMP pathways to FSC maintenance can be substituted by increasing Hh or JAK-STAT pathways. They also support a clear hierarchy of rescue potential that mirrors the severity of phenotypes for each pathway. We did, however, find two exceptions to these general conclusions, as described below.
First, loss of arr function reduced the severity of phenotypes induced by excess Hop. The resultant phenotypes were notably weaker than for Hop over-expression alone or for rescue of mam mutant FSCs (discussed below) by Hop, even for clones induced in larvae, where loss of Wnt signaling alone normally enhances FSC function (Fig. 6A,C-E and Supplemental Fig. S3E). Thus, a positive contribution of Wnt signaling is required to elicit the strongest phenotypes due to increased JAK-STAT pathway activity. This contrasts with the lack of requirement for the BMP pathway for JAK-STAT pathway hyperactivity phenotypes and the lack of requirement for Wnt pathway activity in FSCs responding to excess Hh pathway activity. We infer that there is some mechanistic inter-dependence between the positive actions of Wnt signaling and JAK-STAT signaling in FSCs.
The second example concerns the interaction of signaling pathways with another important FSC factor, Mastermind (Mam). Mam is normally a co-activator for the Notch pathway but Mam appears to have an additional, non-canonical function in FSCs because Mam is required for FSC maintenance whereas Notch activity is not (Vied and Kalderon, 2009). It was previously shown that mam mutant FSCs are not rescued at all by loss of ptc (Vied and Kalderon, 2009). By contrast, high level Hop expression allowed both mam and ptc mam FSC clones to survive as well as control clones, additionally producing ectopic cells in the anterior of the germarium and displacing wild-type FSC lineages to produce "all-marked" ovarioles to a degree only slightly lower than observed for Hop over-expression alone (Fig. 6A,E). Thus, excess Hop, in contrast to excess Hh pathway activity, rescued FSCs lacking Mam activity. More surprisingly, activated TKV also rescued both mam and ptc mam mutant FSC maintenance extremely well (Fig. 6F). The selective requirement for Mam function in order to respond to Hh signaling is consistent with earlier evidence that Mam may take on a non-canonical co-activator function in Hh signaling in FSCs (Vied and Kalderon, 2009). In summary, epistasis analyses revealed evidence of some molecular collusions (between Mam and Hh signaling, and between Wnt and JAK-STAT pathways) but mostly showed that one signaling pathway can compensate for another within a consistent hierarchy of influence, implying that the FSC effectively sums the inputs of signaling pathways to regulate critical functions, such as niche adhesion and proliferation.
DISCUSSION
Stem cells are generally maintained in appropriate numbers at defined locations. It is therefore expected that a specific extracellular environment defines a supportive niche and regulates stem cell numbers. However, the mechanisms for supporting and regulating stem cells may vary widely (Losick et al., 2011). In the Drosophila germarium, GSCs are principally regulated directly by a single (BMP) pathway that is activated by signals from immediately adjacent Cap cells and acts within GSCs to prevent differentiation (Chen et al., 2011; Losick et al., 2011). Here we have shown that in the same tissue, FSCs are regulated by the activity of at least four major signaling pathways, that the ligands for at least three of these pathways (Wnt, Hh and JAK-STAT) derive from distant cells and that these pathways appear to collaborate in order to define supportive niche positions for FSCs and the number of FSCs that are supported. Most crucially, FSCs provide a particularly interesting paradigm where the intersection of gradients of long-range niche signals regulates stem cell maintenance, position, number and competitive behavior.
Intersecting gradients of long-range signals define FSC niches
We examined how the strength of a signaling pathway specifies FSC numbers and supportive niche positions by manipulating the Hh pathway. Normally, Hh pathway activity is marginally higher in FSCs than in their daughters and is progressively lower in more posterior cells, consistent with Hh emanating from Cap and Terminal Filament cells at the anterior tip of the germarium (Forbes et al., 1996a;Hartman et al., 2011; King et al., 2001; Vied and Kalderon, 2009). We found that small reductions in Hh pathway activity led to FSC loss while small increases caused FSCs to outcompete their neighbors. FSCs must therefore reside reasonably close to the anterior of the germarium in order to receive sufficient stimulation by Hh, but what prevents FSCs from moving progressively further anterior and enjoying even stronger Hh stimulation? Wg is expressed in anterior Cap cells along with Hh (Forbes et al., 1996b; Song and Xie, 2003). Here we found that excess Wnt pathway activity strongly impairs FSC maintenance and that loss of Wnt pathway activity during FSC establishment can lead to enhanced FSC function and to a modest accumulation of Wg-insensitive FSC derivatives in ectopically anterior positions. These observations suggest that anterior Wg expression contributes to limiting the anterior spread of FSCs. However, Wg-insensitive cells do not spread to the extreme anterior of germaria, suggesting that additional factors control the position of FSCs along the anterior-posterior axis of the germarium.
In fact, we saw apparent FSC duplication and anterior movement of FSC derivatives, including Fas3-negative FSC-like cells, very dramatically in response to elevated JAK-STAT pathway activity. Furthermore, the pattern of expression of a reporter of JAK-STAT pathway activity and its response to localized inhibition of ligand production showed that the JAK-STAT pathway in FSCs is activated primarily by ligand emanating from more posterior, polar cells. Hence, we suggest that normal FSCs are unable to function in significantly more anterior positions because they would receive inadequate stimulation of the JAK-STAT pathway.
Thus, the combination of graded distributions of Hh, Wnt and JAK-STAT pathway ligands appears to be instrumental in setting the anterior-posterior position of FSCs and how many FSCs may be supported in each germarium. Neither the Hh nor the JAK-STAT pathway activity gradients appear to be classical smooth gradients but both are high in the central 2a/b region of the germarium (where FSCs are located) and considerably lower in either the anterior (JAK-STAT) or posterior (Hh) third of the germarium. Although FSCs are normally supported by both Hh and JAK-STAT pathways, JAK-STAT pathway hyperactivity could substantially compensate for loss of Hh pathway activity to support FSCs that are neither rapidly lost nor displace wild-type FSCs. We therefore conclude that the sum of quantitative inputs of these two pathways is a key parameter for supporting normal FSC function.
How do multiple signaling pathways collaborate to influence stem cells?
We first considered that Hh, Wnt and JAK-STAT pathways might have a major effect on the migratory or adhesive properties of FSCs partly because favorable pathway manipulations led to ectopically positioned FSC-like cells in the germarium and displacement of wild-type FSCs. It is possible that enhanced proliferation could also contribute significantly to these phenotypes. Indeed, FSC proliferation is likely modulated by several signaling pathways and has been shown to be important for FSC retention in the niche (O'Reilly et al., 2008; Wang and Kalderon, 2009). However, to date, manipulation of cell proliferation alone in an FSC has not produced the displacement of wild-type FSCs that we observed in response to altered Hh, JAK-STAT and Wnt pathways (Wang and Kalderon, 2009).
Further evidence for FSC signals regulating adhesion comes from our observation that favorable mutations in all four signaling pathways studied here obviated, to a remarkable degree for Hh and JAK-STAT pathways, the normal requirement of FSCs for DE-cadherin function. Again, it is possible that enhanced FSC proliferation may compensate for defective niche adhesion. In fact, partial restoration of FSC maintenance has previously been seen in response to excess Cyclin E or E2F activity for FSCs lacking a regulator of the actin cytoskeleton likely to contribute to adhesion (Wang et al., 2012). Nevertheless, the continued retention of FSCs in the germarium despite the absence of DE-cadherin is most simply explained if Hh and JAK-STAT pathways alter FSC adhesive properties to favor FSC retention.
The cellular interactions guiding the location of FSCs are likely quite complex, involving pre-follicle cells, Escort Cells, germline cysts and the basement membrane. Some of our observations suggest that JAK-STAT signaling might act, in part, by promoting integrin interactions with the basement membrane. Normally, laminin A ligand and strong integrin staining along the basement membrane do not extend further anterior than the FSCs (O'Reilly et al., 2008). Perhaps excess JAK-STAT signaling facilitates increasingly anterior deposition of laminin A and organization of adhesive integrin complexes, promoting simultaneous anterior migration and basement membrane adhesion of cells of the FSC lineage. In support of this hypothesis we saw anterior extension of integrin staining and apparent anterior migration of Hop-expressing cells, principally along germarial walls. However, the requirement or sufficiency of these changes in integrin organization remains to be tested.
For excessive Hh signaling, ectopic cells also often associated with germarial walls but these cells did not accumulate in far anterior positions or change the pattern of integrin staining, so enhanced integrin-mediated associations seem unlikely to explain the phenotype. The Hh hyperactivity phenotype is very strong in the absence of DE-cadherin function in FSCs, so what other adhesive function might be altered by Hh signaling? Partial restoration of smo mutant FSC maintenance by increased DE-cadherin expression (Wang et al., 2012) provides some further support that adhesive changes are an important component of the FSC response to Hh. As noted previously (Zhang and Kalderon, 2000), ptc mutant follicle cells rarely contact germline cells in mosaic egg chambers, preferentially occupying positions between egg chambers or surrounding the follicle cell epithelium (Supplemental Fig. S2D), suggesting that excess Hh pathway activity in cells of the FSC lineage may reduce their affinity for germline cells or their propensity to integrate into an epithelium. Adhesion to posteriorly moving germline cysts and a nascent follicular epithelium would seem a priori to be the major influences tending to pull FSCs and their daughters away from a stable germarial association. A reduction in FSC or FSC daughter interactions with germline cysts or with pre-follicle cells might therefore lead to increased retention of FSCs in the neighborhood of the normal FSC niche, facilitating accumulation of extra FSCs or allowing FSC retention even in the absence of DE-cadherin.
Potential relevance to cancer development
Most cancers involve signaling pathway mutations and several such mutations likely originate in stem cells, where selective pressures may eliminate or amplify mutant cell lineages (Visvader, 2011). It is therefore important to understand how signaling pathways regulate stem cells. Our studies on FSCs highlight some significant principles that may be widely relevant to human epithelial cell cancers. First, activating mutations in signaling pathways normally required for maintenance of the stem cell in question can amplify the number of stem or stem-like cells in a local environment. This produces an increased number of identical but independent genetic lineages, greatly facilitating the acquisition and selection of secondary mutations that push a mutant stem cell lineage towards a cancerous phenotype. Second, signaling pathway mutations can enhance a stem cell’s ability to compete for niche positions, promoting occupation of all available niches in an insulated developmental compartment. These stem cells are now no longer vulnerable to competition from wild-type stem cells and are effectively immortalized if, as for FSCs, daughter cells readily replace lost stem cells. Third, signaling pathway alterations can compensate for deficits in other pathways or other contributors to normal stem cell function. Hence, stem cell self-renewal can now tolerate significant further mutations and changes in their environment that accompany cancer progression. Loss of epithelial cadherin function provides a specific example of a significant mutation that would be expected often to contribute to cancer development by spurring an epithelial to mesenchymal cell transition, but which can (in FSCs) only be propagated in stem cells after mutational hyperactivation of a key signaling pathway. Finally, our studies emphasize that it is possible for many pathways to exert strong influences on a single stem cell type; in FSCs, Hh, JAK-STAT and PI3K (Wang et al., 2012) pathway hyperactivity phenotypes are extremely strong, while Wnt and BMP pathways can also play significant roles.
EXPERIMENTAL PROCEDURES
Clonal Analysis and Stem Cell Counts
Adult flies or larvae of the appropriate genotype were heat shocked twice approximately 8 hours apart for 1 hour at 37°C. FRT101 flies were given an additional 1 hour heat shock at 37°C between two and four hours prior to dissection in order to induce hsp70-GFP expression. For positive marking, flies were incubated at 29°C for one to two days prior to dissection in order to increase the expression of UAS-GFP. At least 50 ovarioles were evaluated for stem cell counts and in most cases over 100 ovarioles were counted. In only one case were fewer than 50 ovarioles evaluated for stem cell clones in the data presented due to insufficient flies (14 day larval ptcS2;UAS-Hop).
For time course experiments (Figure 2) parents were transferred daily and all animals, starting at 6 days prior to eclosion up to 1 day old females, were heat-shocked at the same time. The flies were dissected, fixed and stained 14 days after heat shock and stem cell counts were performed for 100 to 150 ovarioles for each time point. Details of fly stocks can be found in Supplementary Materials.
Immunohistochemistry
Ovaries were dissected in PBS and fixed in 4% Paraformaldehyde in PBS for 20 minutes at room temperature. The tissue was blocked in 1% BSA for 1 hour and stained with the appropriate primary antibodies: anti-Fasciclin III (1:250), anti-DE-Cadherin (1:5) and anti-βPS (1:100) (University of Iowa Developmental Studies Hybridoma Bank (DSHB) under the auspices of the NICHD), anti-β-Galactosidase (Cappel) at 1:2000 and anti-GFP (A6455, Molecular Probes) at 1:2000 for 1 hour or overnight at 4°C. Secondary antibodies were Alexa-488, Alexa-594 or Alexa 647 from Molecular Probes used at 1:1000 for 1 to 2 hours at room temperature. DNA was stained with Hoechst 33258 (Molecular Probes) (DNA not shown but used for stem cell counts).
EdU labeling protocol
Cells in S phase were detected using the Click-iT EdU Imaging Kit C10339 from Invitrogen. Ovaries dissected into PBS were rinsed twice in 3% BSA in PBS, incubated 30 min in 15 mM EdU in PBS and fixed in 4% paraformaldehyde for 10 min. Ovaries were rinsed 10 min with 3% BSA, permeabilized 20 min with PBST (PBS containing 0.1% Triton X-100, 0.05% Tween-20) and incubated in primary antibody for 1 h. After rinsing once in PBST and twice in 3% BSA, the Click-iT reaction was performed. Ovaries were then rinsed and then incubated in secondary antibody for 1 h. Ovaries were rinsed three times in PBST, once in PBS, and mounted in Dapi-Fluoromount-G (SouthernBiotech).
Supplementary Material
Highlights.
The JAK-STAT pathway is a major regulator of Drosophila Follicle Stem Cells
Wnt signaling in Follicle Stem Cells (FSCs) opposes FSC establishment
A hierarchy of several signaling pathways combine quantitatively to regulate FSCs
FSC position is determined principally by graded long-range signals
ACKNOWLEDGMENTS
We thank Christina Atiya, Pui-Leng Ip, Steve Marks, Zhu Wang and Qianhe Zhou for assistance with experiments and discussions, Erika Bach, Nick Brown, Doug Harrison and Gary Struhl for generously contributing important fly stocks, the Bloomington Drosophila stock center, and the Developmental Studies Hybridoma Bank. We thank Jamila Horabin for providing laboratory support to C.V. to complete experiments. Supported by NIH grant GM079351 to DK and a NYSTEM summer undergraduate program in stem cell science award C026076 to NSF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:2605–2616. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- Assa-Kunik E, Torres IL, Schejter ED, Johnston DS, Shilo BZ. Drosophila follicle cells are patterned by multiple levels of Notch signaling and antagonism between the Notch and JAK/STAT pathways. Development. 2007;134:1161–1169. doi: 10.1242/dev.02800. [DOI] [PubMed] [Google Scholar]
- Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, Baeg GH. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. doi: 10.1016/j.modgep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang S, Xie T. Restricting self-renewal signals within the stem cell niche: multiple levels of control. Curr Opin Genet Dev. 2011 doi: 10.1016/j.gde.2011.07.008. [DOI] [PubMed] [Google Scholar]
- de Cuevas M, Matunis EL. The stem cell niche: lessons from the Drosophila testis. Development. 2011;138:2861–2869. doi: 10.1242/dev.056242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Developmental Cell. 2005;9:501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Dobens LL, Raftery LA. Integration of epithelial patterning and morphogenesis in Drosophila ovarian follicle cells. Dev Dyn. 2000;218:80–93. doi: 10.1002/(SICI)1097-0177(200005)218:1<80::AID-DVDY7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Forbes AJ, Lin H, Ingham PW, Spradling AC. hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development. 1996a;122:1125–1135. doi: 10.1242/dev.122.4.1125. [DOI] [PubMed] [Google Scholar]
- Forbes AJ, Spradling AC, Ingham PW, Lin H. The role of segment polarity genes during early oogenesis in Drosophila. Development. 1996b;122:3283–3294. doi: 10.1242/dev.122.10.3283. [DOI] [PubMed] [Google Scholar]
- Hartman TR, Zinshteyn D, Schofield HK, Nicolas E, Okada A, O'Reilly AM. Drosophila Boi limits Hedgehog levels to suppress follicle stem cell proliferation. J Cell Biol. 2011;191:943–952. doi: 10.1083/jcb.201007142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King FJ, Szakmary A, Cox DN, Lin H. Yb modulates the divisions of both germline and somatic stem cells through piwi- and hh-mediated mechanisms in the Drosophila ovary. Molecular Cell. 2001;7:497–508. doi: 10.1016/s1097-2765(01)00197-6. [DOI] [PubMed] [Google Scholar]
- Kirilly D, Spana EP, Perrimon N, Padgett RW, Xie T. BMP signaling is required for controlling somatic stem cell self-renewal in the Drosophila ovary. Developmental Cell. 2005;9:651–662. doi: 10.1016/j.devcel.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Kirilly D, Wang S, Xie T. Self-maintained escort cells form a germline stem cell differentiation niche. Development. 2011;138:5087–5097. doi: 10.1242/dev.067850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. [erratum appears in Trends Neurosci 2001 Jul;24(7):385] Trends in Neurosciences. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Lopez-Onieva L, Fernandez-Minan A, Gonzalez-Reyes A. Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development. 2008;135:533–540. doi: 10.1242/dev.016121. [DOI] [PubMed] [Google Scholar]
- Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- McGregor JR, Xi R, Harrison DA. JAK signaling is somatically required for follicle cell differentiation in Drosophila. Development. 2002;129:705–717. doi: 10.1242/dev.129.3.705. [DOI] [PubMed] [Google Scholar]
- Nystul T, Spradling A. An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell. 2007;1:277–285. doi: 10.1016/j.stem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Nystul T, Spradling A. Regulation of epithelial stem cell replacement and follicle formation in the Drosophila ovary. Genetics. 2010;184:503–515. doi: 10.1534/genetics.109.109538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly AM, Lee HH, Simon MA. Integrins control the positioning and proliferation of follicle stem cells in the Drosophila ovary. J Cell Biol. 2008;182:801–815. doi: 10.1083/jcb.200710141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelkinson MG, Zhou Q, Kalderon D. Regulation of Ci-SCFSlimb binding, Ci proteolysis, and hedgehog pathway activity by Ci phosphorylation. Dev Cell. 2007;13:481–495. doi: 10.1016/j.devcel.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Xie T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14813–14818. doi: 10.1073/pnas.232389399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Xie T. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development. 2003;130:3259–3268. doi: 10.1242/dev.00524. [DOI] [PubMed] [Google Scholar]
- Vied C, Kalderon D. Hedgehog-stimulated stem cells depend on non-canonical activity of the Notch co-activator Mastermind. Development. 2009;136:2177–2186. doi: 10.1242/dev.035329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- Wang ZA, Huang J, Kalderon D. Drosophila follicle stem cells are regulated by proliferation and niche adhesion as well as mitochondria and ROS. Nature communications. 2012;3:769. doi: 10.1038/ncomms1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZA, Kalderon D. Cyclin E-dependent protein kinase activity regulates niche retention of Drosophila ovarian follicle stem cells. Proc Natl Acad Sci U S A. 2009;106:21701–21706. doi: 10.1073/pnas.0909272106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi R, McGregor JR, Harrison DA. A gradient of JAK pathway activity patterns the anteriorposterior axis of the follicular epithelium. Developmental Cell. 2003;4:167–177. doi: 10.1016/s1534-5807(02)00412-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kalderon D. Regulation of cell proliferation and patterning in Drosophila oogenesis by Hedgehog signaling. Development. 2000;127:2165–2176. doi: 10.1242/dev.127.10.2165. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kalderon D. Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature. 2001;410:599–604. doi: 10.1038/35069099. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Apionishev S, Kalderon D. The contributions of protein kinase A and smoothened phosphorylation to hedgehog signal transduction in Drosophila melanogaster. Genetics. 2006;173:2049–2062. doi: 10.1534/genetics.106.061036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Kalderon D. Hedgehog Activates Fused through Phosphorylation to Elicit a Full Spectrum of Pathway Responses. Dev Cell. 2011;20:802–814. doi: 10.1016/j.devcel.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.