Abstract
Drylands occupy large portions of the Earth, and are a key terrestrial biome from the socio-ecological point of view. In spite of their extent and importance, the impacts of global environmental change on them remain poorly understood. In this introduction, we review some of the main expected impacts of global change in drylands, quantify research efforts on the topic, and highlight how the articles included in this theme issue contribute to fill current gaps in our knowledge. Our literature analyses identify key under-studied areas that need more research (e.g. countries such as Mauritania, Mali, Burkina Faso, Chad and Somalia, and deserts such as the Thar, Kavir and Taklamakan), and indicate that most global change research carried out to date in drylands has been done on a unidisciplinary basis. The contributions included here use a wide array of organisms (from micro-organisms to humans), spatial scales (from local to global) and topics (from plant demography to poverty alleviation) to examine key issues to the socio-ecological impacts of global change in drylands. These papers highlight the complexities and difficulties associated with the prediction of such impacts. They also identify the increased use of long-term experiments and multidisciplinary approaches as priority areas for future dryland research. Major advances in our ability to predict and understand global change impacts on drylands can be achieved by explicitly considering how the responses of individuals, populations and communities will in turn affect ecosystem services. Future research should explore linkages between these responses and their effects on water and climate, as well as the provisioning of services for human development and well-being.
Keywords: climate change, desertification, drylands, ecosystem services, human livelihood, poverty alleviation
1. Dryland ecosystems: definition and importance
Drylands occupy large portions of the Earth's surface characterized by low and highly variable precipitation that does not compensate for the evaporative demands imposed by the intense solar radiation and extreme temperatures [1]. Different criteria have been used over the years to define aridity and to set the climatic boundaries of drylands [2]. The classification proposed by the United Nations Environmental Programme (UNEP), based on the aridity index (AI, the ratio of mean annual precipitation to mean annual potential evapotranspiration), is widely used nowadays [3]. According to this criterion, drylands are defined as regions with an AI < 0.65, and are subdivided in four categories: hyper-arid (AI < 0.05), arid (0.05 < AI < 0.20), semi-arid (0.20 < AI < 0.50) and dry–subhumid (0.50 < AI < 0.65). Overall, these areas cover 5.1 × 107 ha, totalling 41 per cent of the land surface (see [1] for a detailed account of the area occupied by each dryland subtype).
The climatic characteristics of drylands, coupled with the relatively low fertility of their soils [4], impose important limitations on their biota [5]. As a consequence of these constraints, dryland vegetation is typically sparse and forms a ‘two-phase’ mosaic (figure 1) where discrete vegetation patches, mostly grasses and shrubs, are separated by a matrix of bare ground and/or biological soil crusts (BSCs hereafter) dominated by lichens, mosses and cyanobacteria [6]. The extensive areas devoid of vascular vegetation characterizing dryland landscapes, their harsh climate and some environmental problems caused by the mismanagement of their natural resources (e.g. dust bowls and desertification; see [7,8]), have contributed to the general public's impression that drylands are unproductive and ‘useless’ ecosystems, both from the ecological and socio-economical points of view [9].
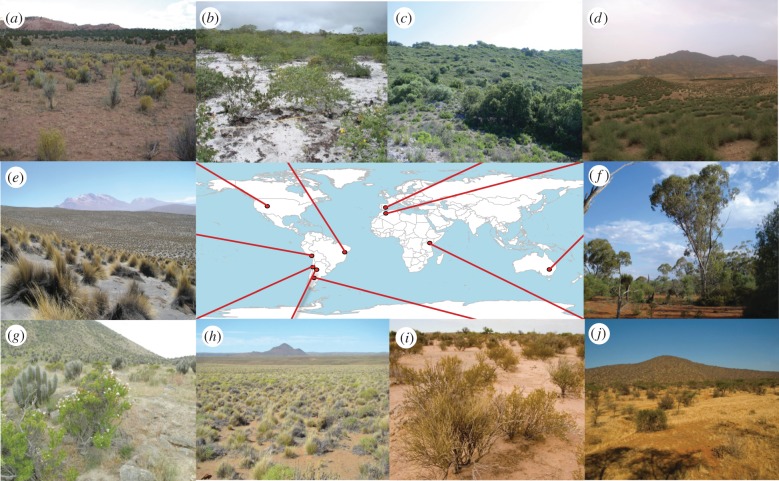
Figure 1.
Examples of dryland vegetation. (a) Mixed shrubland dominated by Ericameria nauseosum in Utah, USA (photo by M. A. Bowker); (b) shrubland dominated by Chamaecrista cytisoides in Brazil (photo by R. Romao); (c) shrubland dominated by Quercus coccifera and Rosmarinus officinalis in Spain (photo by J. L. Quero); (d) Stipa tenacissima grassland in Morocco (photo by F. T. Maestre); (e) Festuca orthophylla grassland in Peru (photo by J. Monerris); (f) open woodland dominated by Eucalyptus populnea and Acacia aneura in Australia (photo by S. Soliveres); (g) shrubland dominated by Eulychnia acida in Chile (photo by C. Barraza); (h) grassland dominated by various Stipa and Festuca species in Argentina (photo by J. Gaitán); (i) Larrea cuneifolia shrubland in Argentina (photo by E. Pucheta); (j) savannah of Acacia totalis in Kenya (photo by V. Polo).
The vision of drylands as areas of little value does not match reality. Not only are these ecosystems very diverse, but they also provide a fascinating natural laboratory to study evolution and species adaptation to extreme conditions, as well as offering ecosystem services that are essential for the maintenance of life [2,10]. Drylands include some of the most diverse biomes in terms of animal diversity, such as deserts and xeric shrublands [11], and host about 20 per cent of the major centres of plant diversity worldwide [12]. In addition to their high plant diversity, which are in some cases higher than those found in more productive biomes [13,14], dryland ecosystems also harbour highly diverse microbial and soil communities [15,16]. Many dryland species also show high levels of genetic differentiation among populations [17]. Such a biodiversity is crucial for maintaining ecosystem multifunctionality (i.e. the ability of ecosystems to maintain multiple functions and services simultaneously, such as carbon storage, productivity and the build-up of nutrient pools [18]), as suggested by recent studies carried out with vascular plants and BSCs worldwide [19–22]. Moreover, drylands also display a wide array of vegetation (figure 1) and soil types. As an example of the latter, a global survey of 224 dryland ecosystems in 16 countries identified 26 different soil types [19].
Drylands are also of paramount importance for humans for multiple reasons. First of all, over 38 per cent of the global human population lives in them [11]. Second, drylands possess a massive amount of key resources, including most of the world's oil reserves [23], as well as large deposits of valuable minerals such as gold, copper and silver [12]. Third, these environments are crucial for achieving global sustainability, and for the well-being of human populations worldwide [8,24]. Over 90 per cent of the dryland human settlements are located in developing countries, and a substantial part of them lag far behind the rest of the world with regards to development indicators [10]. Some features of these settlements, coupled to their biophysical features, underlie their socio-economical situation in the so-called drylands syndrome [7]. These characteristics include the sparse distribution of human populations over the territory, and their remoteness from markets and political centres. These limitations impose serious difficulties on delivering services efficiently, and on deploying effective communication, healthcare and educational systems [8]. Furthermore, severe land degradation and desertification is also present in 10–20% of drylands, and their consequences are estimated to directly affect approximately 250 million people in the developing world [7]. These numbers are likely to expand substantially as a consequence of both climatic changes and the projected exponential human population growth [25].
2. Global environmental change effects on drylands
Increases in temperature, nutrient availability and atmospheric carbon dioxide (CO2) concentration, as well as changes in precipitation patterns and land use are key drivers of ongoing global environmental change (hereafter global change) faced by terrestrial ecosystems worldwide [25]. With the aim to set the context for this theme issue, in this section we highlight some of the most important socio-ecological consequences of global change in drylands. However, we do not intend to provide a comprehensive overview on how such changes will affect dryland ecosystems and the people living in them, as such a thorough review is beyond the scope of this introduction.
The biophysical characteristics of drylands make them highly vulnerable to global change drivers, and to climate change in particular [8,26–28]. Two components of climate change are of particular interest for drylands: the expected increase in temperature and the predicted changes in precipitation patterns. There is much ongoing discussion on the range of temperature increase, which depends on the greenhouse gases emission scenario and the geographical region considered, and this may have been overestimated in dryland regions owing to model deficiencies [29]. However, there is generalized agreement by most models that a warming of over 3°C and a 100 per cent increase in the frequency of extremely warm years is expected for drylands worldwide by the late twenty-first century [25]. Projections of the changes in precipitation amounts and patterns are subject to a greater degree of uncertainty. A multi-model analysis projects variations in annual precipitation from −30 per cent to +25 per cent in drylands, depending on the geographical region considered [30]. Specifically, most climatic models project that drylands located in China are expected to see an increase in total rainfall amounts by 10–20%, but precipitation will be reduced by 5–30% in large portions of the drylands in the Americas, Africa, Australia and the Mediterranean Basin [30]. However, regional models predict important local-scale variations superimposed upon these overall trends [31–33]. Even in areas where precipitation will increase, the expected rise in temperature will increase evapotranspiration rates, which may cancel out the expected positive effects of enhanced precipitation on soil moisture and ecosystem productivity. As a consequence of expected climatic changes, soil moisture is projected to decrease by 25 per cent in a substantial portion of drylands worldwide [30]. However, these overall trends may need to be interpreted with caution when translated to concrete situations in the light of expected changes in rainfall variability. Climate models project an increase in precipitation variability in drylands, including more extreme rainfall events and intense droughts [34]. Large rainfall pulses infiltrate deeper and last longer than smaller rain events [35], and thus even areas undergoing decreases in annual precipitation could experience increased soil moisture if precipitation becomes more variable but is characterized by larger pulses [36,37]. Nevertheless, there is evidence that climate change will exacerbate the aridity of most drylands worldwide, such as the southwestern US [38], the Mediterranean Basin [39], southern Africa [40], Australia [41], South America [42] and China [33].
Predicting effects of global change on dryland ecosystems is not straightforward. This is because of the complex interactions and contrasting effects of predicted changes in different global change drivers. For instance, while the expected reductions in water availability will likely exacerbate water stress and reduce productivity of dryland vegetation [43–45], increases in [CO2] may improve the water use efficiency (WUE) of plants, and thus ameliorate and potentially counterbalance negative effects of reduced soil moisture [46–48]. However, improvements in WUE may not suffice to compensate negative effects on soil moisture of increased evapotranspiration and reduced rainfall scenarios, particularly when feedbacks between elevated CO2, water availability and vegetation are taken into account [49]. Indeed, reductions in above-ground biomass with increased warming and drought in drylands have been widely documented [50–53]. The size and frequency of rainfall events modulate processes such as soil and ecosystem respiration [54,55], microbial activity [56] and plant physiology and primary productivity [57]. Thus, modifications in precipitation patterns with climate change will largely affect ecosystem functioning in drylands [58], although some of these changes may not be necessarily negative (see [59] for a review). For example, increases in above-ground net primary productivity (ANPP) with increases in rainfall variability (less but more intense rainfall events) have been observed in semi-arid steppes from North America [36].
Global change will have important effects on dryland organisms other than vascular plants. The dryland biota in general is known to be well-adapted to infrequent, intense and unpredictable pulses of precipitation [4]. However, increasing temperatures and prolonged drought events associated with global change will pose physiological water balance challenges to a wide suite of organisms, including birds [60], reptiles [61] and insects [62], as transpiration increases. Expected changes in temperature and rainfall frequency may even promote extreme mortality events, as recently recorded in organisms as disparate as small birds [63] and mosses [64]. Recent experimental studies have also shown that warming by 2–3°C will reduce the cover and diversity of BSC-forming lichens [65], and will promote declines in both bacterial and fungal activity and biomass, overall bacterial diversity and the bacteria:fungi ratio [64,66,67] in drylands.
Experiments and syntheses conducted in recent years have projected modifications and disruptions in species and multi-trophic interactions in drylands [61,68]. The complexities in the responses of organisms to global change challenge the establishment of general predictions. However, the expected overall decrease in water availability under global change is likely to promote higher competition among vascular plants, stronger herbivory effects on plants and more intense predator–prey interactions (see [61] for a review). Such responses may cascade throughout the whole ecosystem, ultimately promoting shifts in the structure and composition of dryland communities [69].
Dryland biogeochemistry is largely driven by rainfall and the associated effects on net primary productivity [35,70,71]. Thus, changes in precipitation frequency and amount, as well as in evapotranspiration rates projected by climatic models, are expected to result in important changes in processes such as soil C and nutrient cycles. These alterations may be mediated by changes in land use (see Thomas [72] for a discussion), and by modifications in the richness and composition of biotic components, such as plants and BSCs, induced by global change [65,73–75]. Experimental reductions in overall precipitation have been found to lower litter decomposition rates in the Argentinean Patagonia [76] and the Chihuahuan Desert [77]. However, similar results have not been found in observational studies [78]. Contradictory responses of nitrogen (N) mineralization and availability to changes in water availability have also been reported [76,79,80]. Maestre et al. [20] found that a 2.5°C experimental warming enhanced soil CO2 efflux, particularly in areas dominated by BSCs, in a semi-arid environment from Spain. However, Lellei-Kovàcs et al. [81] did not find a significant effect of warming on this variable in a semi-arid forest–steppe ecosystem from Hungary. Studies conducted in the Sonoran Desert suggest that soil respiration may be less related to soil moisture and more to available carbon [82]. These results contrast with many other studies conducted in drylands worldwide suggesting that C fluxes are either tied to photosynthesis, and therefore fluxes will change as plants respond to variation in soil moisture availability [54], or that variations in seasonal rainfall, coupled to associated changes in temperature, largely regulate C fluxes [73,83]. In this direction, reductions in approximately 30 per cent in annual precipitation have been reported to lower daily soil CO2 efflux by 50 per cent in Mediterranean semi-arid shrublands [51]. Moreover, less frequent, more intense rainfall pulses have been found to increase this variable up to 30 per cent in the Chihuahuan Desert [37]. Modelling studies suggest that expected changes in precipitation and temperature, coupled with increases in [CO2], will increase soil respiration in a nonlinear fashion in this desert [84]. Other studies have found that soil organic matter can be negatively affected by expected changes in climate and [CO2] in drylands [74]. However, this response does not seem to be universal, as global change may promote C storage in soil through increased biomass production and reduced C turnover [85]. These results emphasize the nonlinear dependency between biogeochemical cycles in drylands and global change, and the difficulties faced by dryland researchers when projecting the ecological effects of such change.
The effects of other global change drivers, such as enhanced N availability, on drylands are also difficult to predict. Studies have reported both positive and negative effects of increased N fertilization on these ecosystems (see [86] for a review). These effects depend both on the amount of N considered and on factors such as overall water availability and the relative concentrations of other nutrients, such as phosphorus [87]. In addition, there is evidence that the frequencies of wildland fires in many dryland regions (e.g. the Mediterranean Basin) have increased during the last century [88]. Expected increases in temperature and decreases in ambient moisture are likely to further increase fire recurrence intervals [89]. More fires may act synergistically with N deposition and the invasion by exotics to alter vegetation structure [86]. For example, many drylands are being invaded by exotic species, a process that reduces ecosystem functioning [90] and is expected to be further worsened with global change [91]. Moreover, the productivity and cover of these exotics has also been reported to be enhanced with N deposition [92]. This process may favour the accumulation of flammable biomass and enhance the connectivity of otherwise isolated plant patches, further altering fire regime and severity [93].
Ongoing global change is also promoting important shifts in species composition, and reductions in species richness in drylands worldwide [94–96]. These processes will likely have cascading effects on other biota and on ecosystem functioning [93,97,98]. As an example, a recent survey of global drylands has shown that plant species richness is positively linked to ecosystem multifunctionality, which is also negatively related to annual mean temperature [19]. These results suggest that changes in climate and biodiversity expected under global change will negatively impact the provision of ecosystem functions and services in drylands. The phenomenon of shrub encroachment (i.e. the expansion of woody vegetation into former grasslands [99]), a key land-cover change affecting drylands worldwide, has multiple effects on ecosystem structure and functioning [100]. Although this phenomenon has not been found to significantly affect ecosystem attributes such as biodiversity at the global scale [100], it may either enhance or reduce plant species richness at local and regional scales [97,101]. Some studies have suggested that different global change drivers, such as increases in [CO2] and in the frequency of large precipitation events, may favour shrubs at the expenses of grasses in drylands [49,102,103]. Thus, it is likely that shrub encroachment will be augmented in the future [35], even if other factors known to promote this land-cover change (e.g. grazing [99]) are reduced. Many dryland regions are experiencing other land-use changes, including: (i) large-scale growth of urban and industrial areas (e.g. the case of Phoenix in the USA [104]), (ii) the afforestation of former grassland areas (e.g. large-scale plantations of fast-growing tree species in Australia, Argentina, Paraguay and Uruguay [105]), (iii) the replacement of traditional agricultural uses by modern, irrigation-based, agriculture (e.g. Almería province in southeast Spain [106]), and (iv) the extensive deforestation of dry forests (e.g. Cerrado, Caatinga, Chaco of Brazil, Bolivia, Paraguay and Argentina) to expand the area devoted to the production of intensive crops such as soy [107–109]. These processes are substantially affecting the hydrology [110–112] and biogeochemistry [113] of dryland ecosystems worldwide. Land-use change is also a major threat to their biodiversity [28], and the effects on ecosystem processes and services may act synergistically with those of other global change drivers [114].
Global change will also have dramatic impacts on the human populations in drylands. Such impacts are mostly linked to water availability, food security and socio-political conflicts in developing regions, as well to reductions and/or alterations in other ecosystem services important for human development and well-being. The intrinsic variability in precipitation characterizing drylands, which is inversely proportional to the total amount received [115], together with recurrent droughts promoted by phenomena such as El Niño, have a direct impact on millions of people who practise subsistence farming in drylands and whose crop yields are largely dependent on precipitation [116,117]. Most climatic projections agree with the fact that many drylands, such as the Mediterranean Basin, western USA, southern Africa and northeastern Brazil, will suffer a decrease of water resources with ongoing climate change because of increases in the variability of rainfall and in the frequency and duration of droughts [10,30]. These climatic changes will exacerbate food production and security issues. Moreover, they are likely to worsen already existing socio-political conflicts and limit the development of agricultural programmes, particularly in regions where water conflicts are already arising (e.g. Egypt, Sudan and Israel [118,119]).
Expected changes in water availability will likely increase the frequency and magnitude of humanitarian crisis in many dryland areas around the world [120]. Health conditions and diseases are likely to be magnified under climate change because of the expansion of illness vectors (e.g. malaria and dengue [121,122]), and the reductions in the availability and reliability of freshwater supply. Both factors will increase the incidence of gastrointestinal diseases [123]. Human populations living in drylands have a great variety of mechanisms to adapt to slow climatic changes and extreme climatic events, which strengthen their ability to cope with projected water shortages [124,125]. However, there is increasing agreement that global change will increase human migration in drylands [126]. Not surprisingly, the term climatic refugees, persons who migrate because of direct and indirect effects of global change, is being increasingly used [127]. Recent studies have also highlighted the tight connections between drought and warming and the rise of armed conflicts in drylands worldwide [66,128,129]. Thus, accumulated evidence suggests that the expected changes in precipitation and temperature will further worsen the disastrous humanitarian consequences resulting from armed conflicts and food shortages, as well as migrations, in many developing dryland regions.
Human populations living in drylands will also be affected by the alterations induced by global change in ecosystem services other than food production and freshwater supply. For example, primary production is a key supporting service that in drylands is negatively correlated with temperature and positively with precipitation [130]. Expected changes in these climatic attributes in most dryland regions will likely reduce the productivity of both crops and natural vegetation. This will not only affect food production and security, but also will have key implications for soil conservation and climate regulation, given the strong impacts of vegetation and its development on processes such as soil erosion, nutrient cycling, carbon sequestration and water run-off and infiltration [5,95,111,112]. Such impacts may act synergistically with global change-induced biodiversity losses, as biodiversity is crucial for maintaining ecosystem services in drylands [19,131]. Climate-induced migrations and land-use changes are also expected to negatively impact on important cultural services, such as social identity and diversity, tourism and recreation [120,132,133].
To summarize, the contrasting effects of various global change drivers, and the complexity of the responses to such drivers, challenge the elaboration of accurate projections on their consequences for dryland ecosystems. However, the evidence accumulated so far clearly indicates that global change will increase the degree of abiotic stress experience by multiple organisms living in drylands, will promote important changes in ecosystem structure and functioning and will negatively affect the food security, health and welfare of dryland human populations. While some of these changes may not necessarily be perceived as negative from the ecological point of view (e.g. increase in WUE of dryland vegetation and positive effects of shrub encroachment on carbon storage), the plethora of effects expected on both biotic and abiotic components, and the synergies that can be established between multiple global change drivers (e.g. land use and global change), will make drylands more vulnerable to disturbances, reducing their ability to provide goods and services to humans [7,19,35].
3. Research gaps and future directions
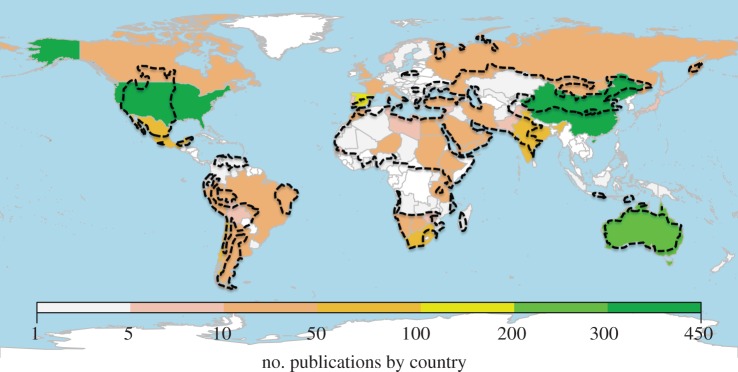
Understanding how global change will affect the composition, structure and functioning of dryland ecosystems, and how these changes might affect the livelihoods of the millions of people depending on their goods and services has been declared a top priority by the scientific community [25]. As such, it is not surprising that hundreds of studies on the topic have been published in the international, peer-reviewed literature (figure 2 and electronic supplementary material, appendix S2). Despite increasing research efforts, dryland regions such as the Guajira (Colombia), deserts such as the Thar (Pakistan, India), Sahara (Morocco, Argelia, Mauritania, Egypt, Mali, Chad, Libya, Niger, Sudan and Tunisia), Kavir (Iran) and Taklamakan (NW China, Kazakhstan, Uzbekistan and Turkmenistan) and countries such as Burkina Faso and Somalia remain poorly studied (figure 2), and deserve further attention in order to attain a better knowledge of drylands worldwide. Gaining this basic knowledge will further increase our ability to predict global change impacts. Naturally, we acknowledge that political and economic instability in certain regions does not warrant the feasibility of broader scale dryland research. Nonetheless, we suggest that logistic constrains can be solved in many places by involving local researchers.
Figure 2.
Research efforts on global change conducted in drylands (indicated by dashed borders), according to the country where the work was carried out. To obtain data for this map, we searched for peer-reviewed field studies on this topic in the ISI web of knowledge database (http://www.isiwebofknowledge.com) published between 1899 and 2011 (see electronic supplementary material, appendix S2 for details). Dashed areas represent drylands, as defined by UNEP [3]. The studies from countries such as the UK mostly reflect research based on paleo-scales, when these regions were drylands.
Beyond an overall call to action to increase future research efforts in those areas underexplored so far (figure 2), there are important issues that deserve special attention from dryland scientists. We list below some of important topics that require particular attention to achieve a better understanding of the impacts of global change in drylands, and illustrate the novel contributions of the articles included in this theme issue to fill in current gaps in our knowledge.
First, there is a need for more information on how global change drivers will affect key soil processes and stocks related to organic matter in drylands. This urgency arises from the implications of these processes for their role in soil fertility and the development of desertification. Such data are also needed to forecast possible feedback effects on the climate system promoted by expected increases in soil respiration, a component of the biosphere's C cycle representing more than 60 per cent of total ecosystem CO2 efflux [134]. This key process has been poorly studied in drylands in comparison to other biomes. For example, less than 8 per cent of case studies of a global soil respiration database accounting for more than 800 studies come from drylands [135], and many dryland regions are completely missing from this database (e.g. northern Africa, southern Spain, South Africa, Chile, Argentina, Kenya, Mali, Niger and the Arabian Peninsula, to name a few). Recent research efforts, such as the CARBOAFRICA network (http://www.carboafrica.net), are contributing to fill this gap by gathering information on the C cycle and other greenhouse gases in multiple sites across sub-Saharan Africa. In addition to these efforts, future research must evaluate how different global change drivers affect soil C stocks and fluxes in drylands, particularly in under-studied regions of Africa and Asia. Thomas [72] explores how grazing, a major land use in drylands worldwide, affects soil CO2 efflux and organic C in two sites in the Kalahari desert (southern Botswana; see electronic supplementary material, appendix S1 for geographical location of the study sites of this theme issue) with well-developed BSCs dominated by cyanobacteria. He found that in areas devoid of vascular plants, which dominate these savannah-like landscapes [136], organic C is not evenly distributed through the soil profile, as it concentrates under BSCs. Soil CO2 efflux was also significantly higher on sandy soils where the BSC was removed, and on calcrete soils where the BSC was buried under sand. These results confirm the importance of BSCs for C cycling in drylands, and show how intensive grazing negatively affects C sequestration and storage. This study also illustrates how land use affects key soil properties linked to the C cycle, and can be used to provide sound recommendations to manage grazing areas to maintain a positive C balance in African drylands.
In a survey of the published literature on global change and drylands (see the electronic supplementary material, appendix S2), we found only 84 studies with fieldwork in more than one continent (approx. 3.8% of the total of studies reviewed). A disproportionate number of these studies correspond to French–Algerian collaborations [137,138], which likely reflect colonial history. It is interesting to note, however, that the number of multi-continent studies has been increasing over recent years (number of multi-continent studies versus year of publication, Spearman correlation coefficient = 0.77, p < 0.001), suggesting that international collaborations are gaining importance [19,139,140]. Future avenues of dryland research should also take advantage of naturally occurring latitudinal gradients of temperature and precipitation, leaving aside geopolitical barriers whenever feasible, to better encompass the biology of deserts in a variety of regions. After all, species and ecological/socio-economical issues do not understand political frontiers [141], and scientists researching them should strive to act accordingly. In addition, large-scale studies can provide important insights to understand how dryland ecosystems function, and how global change may affect them. A good example of the benefits of multi-continent research is provided in this issue by Sala et al. [142], who analyse 16 datasets from multiple continents to analyse how precipitation controls ANPP in drylands. These authors found that the relative importance of current- versus previous-year precipitation as a driver of ANPP changes along precipitation gradients, suggesting that the ANPP of dryland ecosystems will respond to global change-driven alterations in water availability. Another multi-continental study included in this theme issue is that by Salguero-Gómez et al. [143], who introduce a novel approach—coupling robust climatic projections with stochastic, stage-structured models constructed from long-term demographic datasets—to examine the effects of precipitation shifts on populations of two desert plant species from the Colorado Plateau Desert (USA) and the Negev Desert (Israel). By using simulations based on long-term field data, they found that projected precipitation changes (wetter and drier growing seasons in the USA and Israel, respectively) will increase the population growth rates of both species. Their findings suggest that native desert plants, and thus the resources they provide, might be more resilient to global change than previously thought. These studies also illustrate the importance of carrying out research on multiple sites along wide environmental gradients to fully unravel basic principles underlying the functioning of dryland ecosystems, and thus provide insight into how global change will affect them.
A key feature of dryland ecosystems is the high inter-annual variability in their precipitation regime [115]. While the importance of precipitation as a key driver of ecosystem dynamics and socio-economical development in drylands is largely acknowledged [144,145], previous climate change research has mostly evaluated the impacts of changes in the mean climate variables (but see [61,146]). Therefore, it remains largely unknown how dryland ecosystems may respond to variation in the precipitation regime. This topic is of utmost importance in the dryland research agenda, given the evidence of an ongoing global increase in the interannual variability of precipitation, and the predicted intensification of extreme events by climate change models [25,147,148]. D'Odorico & Bhattachan [149] investigate current patterns of hydrologic variability in global drylands and review the implications of such fluctuations. These analyses highlight the complexities of the impacts of climate variability on drylands. While precipitation variability is often perceived as a disturbance for ecosystems and societies that makes difficult the provisioning of ecosystem services such as food production, the authors suggest that such variability may also enhance ecosystem resilience and promote the maintenance of biodiversity. This property, in turn, may allow dryland ecosystems to recover faster after severe disturbances, including those induced by extreme climatic events.
Research should also focus on understanding the responses to global change of important yet traditionally under-studied organisms. Among them, special attention must be directed to BSCs, an integral biotic component of drylands [5]. These organisms strongly influence key functional processes, including C and N cycling, soil stabilization and infiltration [6,73,150]. Despite the multiple ecosystem processes and organisms affected by them, relatively few experimental studies have evaluated the response of BSC constituents to global change drivers, and most of them have been carried out in drylands from North America and Australia [64,151–154]. Escolar et al. [65] evaluated how the composition, structure and performance of lichen-dominated BSCs respond to predicted climatic changes in semi-arid, central Spain. Warming according to the Intergovernmental Panel for Climate Change projections [25] promoted a significant decrease in the richness and diversity of the whole BSC community, a result that was accompanied by important shifts in species composition. These results suggest that global change will strongly affect BSCs, with expected changes in richness and composition that could reduce or even reverse the positive effects of these organisms on multiple ecosystem functions.
In addition to expanding our efforts to under-studied organisms, future studies should also focus on biotic interactions across trophic levels. Their importance to predict future species distribution under climate change has already been highlighted [155]. Here, we emphasize the important research gaps in our knowledge on how dryland trophic interactions might be affected by global change. González-Megías & Menéndez [68] evaluated the effect of future changes in rainfall patterns on detritivore–plant–herbivore interactions in a semi-arid region from Spain. The authors found that changes in rainfall intensity modified the effect of below-ground detritivores on both plant traits and above-ground herbivore abundance. These results illustrate how global change will affect trophic levels and their interactions differentially. The authors also discuss the difficulties in predicting the responses of whole communities to global change when examining isolated organisms.
The importance of taking into account multiple drivers when evaluating global change impacts on ecosystems and the use of long-term experiments have been advocated many times [114]. Nonetheless, there are very few long-term experiments carried out in drylands that can inform us about how multiple simultaneous global change drivers can affect ecosystem structure and functioning (e.g. the Mojave Global Change Facility, where the impacts of changes in precipitation, nitrogen deposition and soil surface disturbance on the Mojave Desert are being explored; http://web.unlv.edu/Climate_Change_Research/MGCF/). Synthesizing data from one of these experiments, Ban & Lai [156] evaluated how N deposition affected plant community structure in a 10-year field experiment located in Inner Mongolia. The authors found significant reductions in species richness (up to 50–70%) after N addition. The responses of species richness and above-ground biomass to N inputs were greater in wet years than in dry years. Interestingly, N addition reduced the resistance of the semi-arid grassland studied to drought, diminishing ecosystem stability. Given the strong effects that N deposition has on the functioning of grasslands [157], these results have important implications for understanding the impacts of N deposition and climate change on the biodiversity and ecosystem services provided by these important ecosystems.
Owing to the multiple goods and services provided by drylands, and their importance to sustain life on the Earth [10], it is not surprising to find the large number of studies devoted to this topic in recent years [158–160]. Among them, those exploring how ecosystem services can be used to improve the livelihood of the poorest have been prioritized by scientists working in the natural and the social sciences alike. Community-based projects incorporating payments for ecosystem services (CBPES) allow individuals, governments, NGOs and private companies to pay for ecosystem services such as C storage and water conservation by supporting local-level projects promoting both community development and poverty alleviation [161]. These projects are being encouraged by international climate policies and investments [162]. In this issue, Dougill et al. [163] review multiple CBPES that deliver C and poverty reduction benefits in African forests. The authors discuss how CBPES can be successfully established in African rangelands, which have received little attention to date, despite their importance for the global C cycle and the maintenance of the livelihood of millions of persons in some of the poorest regions of the planet [164]. Through a literature review, Dougill and collaborators provide important insights and guidelines to design effective CBPES that can substantially contribute to poverty alleviation in drylands.
Most global change research carried out in drylands until now has been done on a unidisciplinary basis. When reviewing the literature on this topic (see the electronic supplementary material, appendix S2), we found very few studies that would fall in more than one of major research categories (ecology, biogeochemistry and sociology), and there is no overlapping among the three categories (figure 3a). Things are, however, starting to change, and some exemplary initiatives in this regard are GLOWA (http://www.glowa.org), a multidisciplinary team addressing water usage issues in Israel, or the International Network of Research on Coupled Human and Natural Systems (http://chans-net.org/), which facilitates communication and collaboration among scholars from around the world who are interested in coupled human and natural systems. The Central Arizona-Phoenix LTER (http://caplter.asu.edu/), where scientists from different disciplines and community partners work together to study the structure and function of an urban desert ecosystem, and how urban development in Phoenix (USA) affect ecosystem services in the Sonoran Desert, is also a noteworthy research programme. Calls for such multidisciplinary approaches have been made countless times in the past, regardless of biome. Because of the tight and intricate relationships between ecosystem services, biodiversity and human livelihoods in drylands [10], as well as the convergence of human conflicts and resource limitation, we cannot but further emphasize the need for the integration of multidisciplinary teams when conducting research on topics such as land degradation and desertification, global change and water management. Ultimately, we believe that a major breakthrough in our understanding of global change in drylands will come by the use of multidisciplinary approaches. Huber-Sannwald et al. [133] do so by combining the use of different conceptual frameworks with a thorough analysis of biophysical, socio-economical and historical data to assess the challenges and opportunities for livelihood development in drylands. Their results provide important insights to understand the complexities built into land degradation and desertification processes in socio-ecological dryland systems, and illustrate the potential of multidisciplinary studies to advance our knowledge of the links between human development, desertification and global change.
Figure 3.
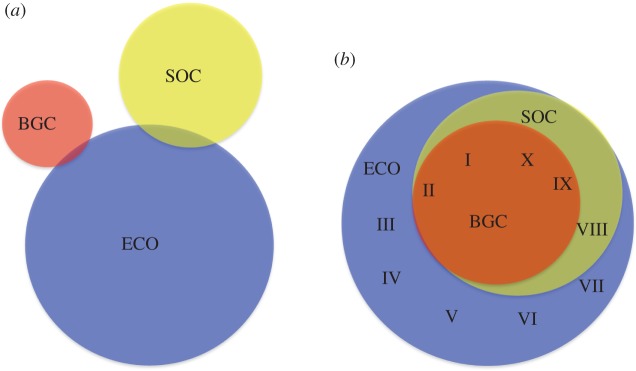
(a) Historically, there has been a lack of integration among biogeochemistry (BGC), sociology (SOC) and ecology (ECO) in global change research carried out in drylands, as shown in the Venn diagram for the number of publications on this topic. To obtain this diagram, we classified the dryland publication list obtained from our literature search (see the electronic supplementary material, appendix S2) into three disciplines—biogeochemistry, ecology and sociology—by adding those keywords and their possible seven combinations to this search. Areas are proportional to the number of publications, and the overlap between them indicates integrative efforts. (b) The present theme issue undertakes an integrative approach of biogeochemistry, ecology and sociology to offer a better understanding of how global environmental change will affect dryland ecosystems and their biota, including humans. Roman number position represents areas of expertise included in each manuscript of the theme issue: (I) Maestre et al. (this study), (II) Thomas [72], (III) Escolar et al. [65], (IV) Salguero-Gómez et al. [143], (V) González-Megías & Menéndez [68], (VI) Ban & Lai [156], (VII) Sala et al. [142], (VIII) D'Odorico et al. [149], (IX) Huber-Sannwald et al. [133] and (X) Dougill et al. [163].
4. Concluding remarks
There are important gaps in our knowledge of the ecological, biogeochemical and socio-economical impacts of global change in drylands, but also exciting challenges for future research on this topic. This theme issue aims to fill some of these gaps by including reviews and primary research articles illustrating the impacts of key global change drivers on fundamental ecosystem components and processes, and by examining the links between these impacts and the livelihood of human settlements in drylands from a multidisciplinary perspective (figure 3b).
Owing to the large number of matters involved in evaluating and determining global change impacts in drylands, we do not provide a complete, definitive overview of this topic in the present theme issue. Each of the topics treated would certainly require a theme issue by itself, and some important topics, such as global change effects on animals, are underrepresented in this issue simply due to lack of space (but see González-Megías & Menéndez [68]), and because they have recently been the subject of a theme issue of the journal [165]. The diverse contributions included in this theme issue are, however, highly timely in our opinion, as they deal with crucial, yet poorly understood issues on ecological impacts of global change. We believe that the topics treated here are in urgent need of conceptual advances in order to improve the livelihood of people living in drylands (e.g. desertification and management of natural resources to improve human well-being in developing areas [133,163]). We expect that the multidisciplinary, multi-organismal approach followed in this theme issue will advance our understanding of the projected effects of global change in drylands, and will stimulate further research on this important topic.
Acknowledgements
We wish to thank H. Eaton, A. Ashford and C. Rawlinson in the editorial office at the Royal Society for their assistance and patience during the preparation of this theme issue, and M. A. Bowker and two reviewers for their multiple and constructive comments and suggestions on a previous version of this manuscript. The foundation of the present Theme Issue resided in the symposium ‘Impacts of Global Environmental Change on the Structure and Functioning of Dryland Ecosystems’, organized at the 2012 European Ecological Federation Meeting, in Ávila (Spain) by F.T.M. and R.S.-G. This work was made possible thanks to the support of the European Research Council under the European Community's Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 242658 (BIOCOM) awarded to F.T.M. R.S.-G. acknowledges support by the Evolutionary Biodemography laboratory of the Max Planck Institute for Demographic Research. J.L.Q was supported by the BIOCOM project.
References
- 1.Reynolds J. F., Maestre F. T., Kemp P. R., Stafford-Smith D. M., Lambin E. 2007. Natural and human dimensions of land degradation in drylands: causes and consequences. In Terrestrial ecosystems in a changing world (eds Canadell J., Pataki D., Pitelka L. F.), pp. 247–258 Berlin, Germany: Springer [Google Scholar]
- 2.Ward D. 2009. The biology of deserts. Oxford, UK: Oxford University Press [Google Scholar]
- 3.United Nations Environment Programme 1992. World atlas of desertification. London, UK: Edward Arnold [Google Scholar]
- 4.Li S. X., Wang Z. H., Hu T. T., Gao Y. J., Stewart B. A. 2009. Nitrogen in dryland soils of China and its management. Adv. Agron. 101, 123–181 10.1016/S0065-2113(08)00803-1 (doi:10.1016/S0065-2113(08)00803-1) [DOI] [Google Scholar]
- 5.Whitford W. G. 2002. Ecology of desert systems. London, UK: Academic Press [Google Scholar]
- 6.Belnap J., Lange O. L. 2003. Biological soil crusts: structure, function and management. Berlin, Germany: Springer [Google Scholar]
- 7.Reynolds R., Belnap J., Reheis M., Lamothe P., Luiszer F. 2001. Aeolian dust in Colorado Plateau soils: nutrient inputs and recent change in source. Proc. Natl Acad. Sci. USA 98, 7123–7127 10.1073/pnas.121094298 (doi:10.1073/pnas.121094298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds J. F., et al. 2007. Global desertification: building a science for dryland development. Science 316, 847–851 10.1126/science.1131634 (doi:10.1126/science.1131634) [DOI] [PubMed] [Google Scholar]
- 9.Mortimore M., et al. 2009. Dryland opportunities: a new paradigm for people, ecosystems and development. IUCN, Gland, Switzerland; IIED, London, UK and UNDP/DDC, Nairobi, Kenya [Google Scholar]
- 10.Safirel U., Adeel Z. 2005. Dryland systems. In Ecosystems and human well-being: current state and trends, vol. 1 (eds Hassan R., Scholes R., Neville A.), pp. 623–662 Washington, DC: Island Press [Google Scholar]
- 11.Millennium Ecosystem Assessment 2005. Ecosystems and human well-being: biodiversity synthesis. Washington, DC: World Resources Institute [Google Scholar]
- 12.White R. P., Nackoney J. 2003. Drylands, people, and ecosystem goods and services: a web-based geospatial analysis. Washington, DC: World Resources Institute; (www.wri.org/publication/content/8241) [Google Scholar]
- 13.Kier G., Mutke J., Dinerstein E., Ricketts T. H., Küper W., Kreft H., Barthlott W. 2005. Global patterns of plant diversity and floristic knowledge. J. Biogeogr. 32, 1–10 10.1111/j.1365-2699.2005.01272.x (doi:10.1111/j.1365-2699.2005.01272.x) [DOI] [Google Scholar]
- 14.Field C. B., Behrenfeld M. J., Randerson J. T., Falkowski P. 1998. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 282, 237–240 10.1126/science.281.5374.237 (doi:10.1126/science.281.5374.237) [DOI] [PubMed] [Google Scholar]
- 15.Fierer N., Jackson R. B. 2006. The diversity and biogeography of soil bacterial communities. Proc. Natl Acad. Sci. USA 103, 626–631 10.1073/pnas.0507535103 (doi:10.1073/pnas.0507535103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Housman D. C., Yeager C. M., Darby B. J., Sanford R. L., Kuske C. R., Neher D. A., Belnap J. 2007. Heterogeneity of soil nutrients and subsurface biota in a dryland ecosystem. Soil Biol. Biochem. 39, 2138–2149 10.1016/j.soilbio.2007.03.015 (doi:10.1016/j.soilbio.2007.03.015) [DOI] [Google Scholar]
- 17.Martínez-Palacios A., Eguiarte L. E., Furnier G. E. 1999. Genetic diversity of the endangered endemic Agave victoriae-reginae (Agavaceae) in the Chihuahuan Desert. Am. J. Bot. 86, 1093–1098 10.2307/2656971 (doi:10.2307/2656971) [DOI] [PubMed] [Google Scholar]
- 18.Zavaleta E. S., Pasari J. R., Hulvey K. B., Tilman G. D. 2010. Sustaining multiple ecosystem functions in grassland communities requires higher biodiversity. Proc. Natl Acad. Sci. USA 107, 1443–1446 10.1073/pnas.0906829107 (doi:10.1073/pnas.0906829107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maestre F. T., et al. 2012. Plant species richness and ecosystem multifunctionality in global drylands. Science 335, 214–218 10.1126/science.1215442 (doi:10.1126/science.1215442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maestre F. T., et al. 2010. Do biotic interactions modulate ecosystem functioning along abiotic stress gradients? Insights from semi-arid Mediterranean plant and biological soil crust communities. Phil. Trans. R. Soc. B 365, 2057–2070 10.1098/rstb.2010.0016 (doi:10.1098/rstb.2010.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowker M. A., Maestre F. T., Escolar C. 2010. Biological crusts as a model system for examining the biodiversity–ecosystem function relationship in soils. Soil Biol. Biochem. 42, 405–417 10.1016/j.soilbio.2009.10.025 (doi:10.1016/j.soilbio.2009.10.025) [DOI] [Google Scholar]
- 22.Bowker M. A., Mau B. L., Maestre F. T., Escolar C., Castillo-Monroy A. P. 2011. Functional profiles reveal unique ecological roles of various biological soil crust organisms. Funct. Ecol. 25, 787–795 10.1111/j.1365-2435.2011.01835.x (doi:10.1111/j.1365-2435.2011.01835.x) [DOI] [Google Scholar]
- 23.Organization of the Petroleum Exporting Countries 2010. Annual statistical bulletin 2010/2011. Vienna, Austria: OPEC [Google Scholar]
- 24.Munasinghe M. 2009. Sustainable development in practice: sustainomics methodology and applications. Cambridge, UK: Cambridge University Press [Google Scholar]
- 25.Intergovernmental Panel for Climate Change 2007. Climate Change 2007: synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Core Writing Team, Pachauri R. K., Reisinger A.), pp. 104 Geneva, Switzerland: United Nations [Google Scholar]
- 26.Körner C. H. 2000. Biosphere responses to CO2-enrichment. Ecol. Appl. 10, 1590–1619 10.1890/1051-0761(2000)010[1590:BRTCE]2.0.CO;2 (doi:10.1890/1051-0761(2000)010[1590:BRTCE]2.0.CO;2) [DOI] [Google Scholar]
- 27.Le Houérou H. N. 1996. Climate change, drought and desertification. J. Arid Environ. 34, 133–185 10.1006/jare.1996.0099 (doi:10.1006/jare.1996.0099) [DOI] [Google Scholar]
- 28.Sala O. E., et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774 10.1126/science.287.5459.1770 (doi:10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 29.Boberg F., Christensen J. H. 2012. Overestimation of Mediterranean summer temperature projections due to model deficiencies. Nat. Clim. Change 2, 433–436 10.1038/nclimate1454 (doi:10.1038/nclimate1454) [DOI] [Google Scholar]
- 30.Bates B. C., Kundzewicz Z. W., Wu S., Palutikof J. P. 2008. Climate change and water. Technical Paper of the Intergovernmental Panel on Climate Change. Secretariat, Geneva, Switzerland: IPCC [Google Scholar]
- 31.Gao Y., Leung L. R., Salathé E. P., Jr, Domínguez F., Nijssen B., Lettenmaier D. P. 2012. Moisture flux convergence in regional and global climate models: implications for droughts in the southwestern United States under climate change. Geophys. Res. Lett. 39, L09711. 10.1029/2012GL051560 (doi:10.1029/2012GL051560) [DOI] [Google Scholar]
- 32.Lelieveld J., et al. 2012. Climate change and impacts in the Eastern Mediterranean and the Middle East. Clim. Change (doi:10.1007/s10584-012-0418-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guobin F., Charles S. P., Yu J., Liu C. 2009. Decadal climatic variability, trends, and future scenarios for the North China Plain. J. Climate 22, 2111–2123 10.1175/2008JCLI2605.1 (doi:10.1175/2008JCLI2605.1) [DOI] [Google Scholar]
- 34.Christensen J. H., Hewitson B. 2007. Regional climate projections, climate change: the physical science basis. In Contribution of working group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L.), pp. 847–940 Cambridge, UK: Cambridge University Press [Google Scholar]
- 35.Schwinning S., Sala O. E. 2004. Hierarchy of responses to resource pulses in arid and semi-arid ecosystems. Oecologia 141, 211–220 10.1007/s00442-004-1520-8 (doi:10.1007/s00442-004-1520-8) [DOI] [PubMed] [Google Scholar]
- 36.Heisler-White J. L., Blair J. M., Kelly E.F., Harmoney K., Knapp A. K. 2009. Contingent productivity responses to more extreme rainfall regimes across a grassland biome. Glob. Change Biol. 15, 2894–2904 10.1007/s00442-011-1948-6 (doi:10.1007/s00442-011-1948-6) [DOI] [Google Scholar]
- 37.Thomey M. L., Collins S. L., Vargas R., Johnson J. E., Brown R. F., Natvig D. O., Friggens M. T. 2011. Effect of precipitation variability on net primary production and soil respiration in a Chihuahuan Desert grassland. Glob. Change Biol. 17, 1505–1515 10.1111/j.1365-2486.2010.02363.x (doi:10.1111/j.1365-2486.2010.02363.x) [DOI] [Google Scholar]
- 38.Seager R., et al. 2007. Model projections of an imminent transition to a more arid climate in southwestern North America. Science 316, 1181–1184 10.1126/science.1139601 (doi:10.1126/science.1139601) [DOI] [PubMed] [Google Scholar]
- 39.Mariotti A., Zeng N., Yoon J. H., Artale V., Navarra A., Alpert P., Li L. Z. X. 2008. Mediterranean water cycle changes: transition to drier 21st century conditions in observations and CMIP3 simulations. Environ. Res. Lett. 3, 044001. 10.1088/1748-9326/3/4/044001 (doi:10.1088/1748-9326/3/4/044001) [DOI] [Google Scholar]
- 40.Haensler A., Hagemann S., Jacob D. 2011. The role of the simulation setup in a long-term high-resolution climate change projection for the southern African region. Theor. Appl. Clim. 106, 153–169 10.1007/s00704-011-0420-1 (doi:10.1007/s00704-011-0420-1) [DOI] [Google Scholar]
- 41.Hughes L. 2011. Climate change and Australia: key vulnerable regions. Reg. Environ. Change 11, S189–S195 10.1007/s10113-010-0158-9 (doi:10.1007/s10113-010-0158-9) [DOI] [Google Scholar]
- 42.Squeo F. A., Holmgren M., Jiménez M., Albán L., Reyes J., Gutiérrez J. R. 2007. Tree establishment along an ENSO experimental gradient in the Atacama Desert. J. Veg. Sci. 18, 195–202 10.1111/j.1654-1103.2007.tb02530.x (doi:10.1111/j.1654-1103.2007.tb02530.x) [DOI] [Google Scholar]
- 43.Haase P., Pugnaire F. I., Clark S. C., Incoll L. D. 1999. Environmental control of canopy dynamics and photosynthetic rate in the evergreen tussock grass Stipa tenacissima. Plant Ecol. 145, 327–339 10.1023/A:1009892204336 (doi:10.1023/A:1009892204336) [DOI] [Google Scholar]
- 44.Xu Z., Zhou G. 2011. Responses of photosynthetic capacity to soil moisture gradient in perennial rhizome grass and perennial bunchgrass. BMC Plant Biol. 11, 21–32 10.1186/1471-2229-11-21 (doi:10.1186/1471-2229-11-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L., D'Odorico P., O'Halloran L. R., Caylor K., Macko S. 2009. Combined effects of soil moisture and nitrogen availability variations on grass productivity in African savannas. Plant Soil 328, 95–108 10.1007/s11104-009-0085-z (doi:10.1007/s11104-009-0085-z) [DOI] [Google Scholar]
- 46.Woodward F. I., Kelly C. K. 2008. Responses of global plant diversity capacity to changes in carbon dioxide concentration and climate. Ecol. Lett. 11, 1229–1237 10.1111/j.1461-0248.2008.01240.x (doi:10.1111/j.1461-0248.2008.01240.x) [DOI] [PubMed] [Google Scholar]
- 47.Morgan J. A., et al. 2011. C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi-arid grassland. Nature 476, 202–206 10.1038/nature10274 (doi:10.1038/nature10274) [DOI] [PubMed] [Google Scholar]
- 48.Maseyk K., Hemming D., Angert A., Leavitt S. W., Yakir D. 2011. Increase in water-use efficiency and underlying processes in pine forests across a precipitation gradient in the dry Mediterranean region over the past 30 years. Oecologia 167, 573–85 10.1007/s00442-011-2010-4 (doi:10.1007/s00442-011-2010-4) [DOI] [PubMed] [Google Scholar]
- 49.Tietjen B., Jeltsch F., Zehe E., Classen N., Groengroeft A., Schiffers K., Oldeland J. 2010. Effects of climate change on the coupled dynamics of water and vegetation in drylands. Ecohydrology 3, 226–237 10.1002/eco.70 (doi:10.1002/eco.70) [DOI] [Google Scholar]
- 50.Peñuelas J., et al. 2004. Nonintrusive field experiments show different plant responses to warming and drought among sites, seasons, and species in a North–South European gradient. Ecosystems 7, 598–612 10.1007/s10021-004-0179-7 (doi:10.1007/s10021-004-0179-7) [DOI] [Google Scholar]
- 51.Miranda J. D., Armas C., Padilla F. M., Pugnaire F. I. 2011. Climatic change and rainfall patterns: effects on semi-arid plant communities of the Iberian Southeast. J. Arid Environ. 75, 1302–1309 10.1016/j.jaridenv.2011.04.022 (doi:10.1016/j.jaridenv.2011.04.022) [DOI] [Google Scholar]
- 52.De la Maza M., Lima M., Meserve P. L., Gutiérrez J. R., Jaksic F. M. 2009. Primary production dynamics and climate variability: ecological consequences in semiarid Chile. Glob. Change Biol. 15, 1116–1126 10.1111/j.1365-2486.2008.01796.x (doi:10.1111/j.1365-2486.2008.01796.x) [DOI] [Google Scholar]
- 53.Shinoda M., Nachinshonhor G. U., Nemoto M. 2010. Impact of drought on vegetation dynamics of the Mongolian steppe: a field experiment. J. Arid Environ. 74, 63–69 10.1016/j.jaridenv.2009.07.004 (doi:10.1016/j.jaridenv.2009.07.004) [DOI] [Google Scholar]
- 54.Vargas R., Collins S. L., Thomey M. L., Johnson J. E., Brown R. F., Natvig D. O., Friggens M. T. 2012. Precipitation variability and fire influence the temporal dynamics of soil CO2 efflux in an arid grassland. Glob. Change Biol. 18, 1401–1411 10.1111/j.1365-2486.2011.02628.x (doi:10.1111/j.1365-2486.2011.02628.x) [DOI] [Google Scholar]
- 55.Jenerette G. D., Scott R. L., Huxman T. E. 2008. Whole ecosystem metabolic pulses following precipitation events. Funct. Ecol. 22, 924–930 10.1016/j.jaridenv.2012.01.007 (doi:10.1016/j.jaridenv.2012.01.007) [DOI] [Google Scholar]
- 56.Collins S. L., Sinsabaugh R. L., Crenshaw C., Green L., Porras-Alfaro A., Stursova M., Zeglin L. H. 2008. Pulse dynamics and microbial processes in aridland ecosystems. J. Ecol. 96, 413–420 10.1111/j.1365-2745.2008.01362.x (doi:10.1111/j.1365-2745.2008.01362.x) [DOI] [Google Scholar]
- 57.Ogle K., Reynolds J. F. 2004. Plant responses to precipitation in desert ecosystems: integrating functional types, pulses, thresholds, and delays. Oecologia 141, 282–294 10.1007/s00442-004-1507-5 (doi:10.1007/s00442-004-1507-5) [DOI] [PubMed] [Google Scholar]
- 58.Reynolds J. F., Kemp P. R., Ogle K., Fernández R. J. 2004. Modifying the ‘pulse–reserve’ paradigm for deserts of North America: precipitation pulses, soil water, and plant responses. Oecologia 141, 194–210 10.1007/s00442-004-1524-4 (doi:10.1007/s00442-004-1524-4) [DOI] [PubMed] [Google Scholar]
- 59.Knapp A. K., et al. 2008. Consequences of more extreme precipitation regimes for terrestrial ecosystems. BioScience 58, 1–11 10.1641/B580908 (doi:10.1641/B580908) [DOI] [Google Scholar]
- 60.Wolf B. O., Walsberg G. E. 1996. Respiratory and cutaneous evaporative water loss at high environmental temperatures in a small bird. J. Exp. Biol. 199, 451–457 [DOI] [PubMed] [Google Scholar]
- 61.McCluney K. E., et al. 2012. Shifting species interactions in terrestrial dryland ecosystems under altered water availability and climate change. Biol. Rev. 87, 563–582 10.1111/j.1469-185X.2011.00209.x (doi:10.1111/j.1469-185X.2011.00209.x) [DOI] [PubMed] [Google Scholar]
- 62.McCluney K. E., Date R. C. 2008. The effects of hydration on growth of the House Cricket, Acheta domesticus. J. Insect Sci. 8, 1–9 10.1673/031.008.3201 (doi:10.1673/031.008.3201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McKechnie A. E., Wolf B. O. 2010. Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol. Lett. 6, 253–256 10.1098/rsbl.2009.0702 (doi:10.1098/rsbl.2009.0702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zelikova T. J., Housman D. C., Grote E. D., Neher D., Belnap J. 2012. Biological soil crusts show limited response to warming but larger response to increased precipitation frequency: implications for soil processes on the Colorado Plateau. Plant Soil 355, 265–282 10.1007/s11104-011-1097-z (doi:10.1007/s11104-011-1097-z) [DOI] [Google Scholar]
- 65.Escolar C., Martínez I., Bowker M. A., Maestre F. T. 2012. Warming reduces the growth and diversity of biological soil crusts in a semi-arid environment: implications for ecosystem structure and functioning. Phil. Trans. R. Soc. B 367, 3087–3099 10.1098/rstb.2011.0344 (doi:10.1098/rstb.2011.0344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang W., Parker K., Luo Y., Wan S., Wallace L., Hu S. 2005. Soil microbial responses to experimental warming and clipping in a tallgrass prairie. Glob. Change Biol. 11, 266–277 10.1111/j.1365-2486.2005.00902.x (doi:10.1111/j.1365-2486.2005.00902.x) [DOI] [Google Scholar]
- 67.Sheik C. S., Beasley W. H., Elshahed M. S., Zhou X., Luo Y., Krumholz L. R. 2011. Effect of warming and drought on grassland microbial communities. ISME J. 5, 1692–1700 10.1038/ismej.2011.32 (doi:10.1038/ismej.2011.32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.González-Megías A., Menéndez R. 2012. Climate change effects on above- and below-ground interactions in a dryland ecosystem. Phil. Trans. R. Soc. B 367, 3115–3124 10.1098/rstb.2011.0346 (doi:10.1098/rstb.2011.0346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown J. H., Valonem T. J., Curtin C. G. 1997. Reorganization of an arid ecosystem in response to recent climate change. Proc. Natl Acad. Sci. USA 94, 9729–9733 10.1073/pnas.94.18.9729 (doi:10.1073/pnas.94.18.9729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Austin A. T., Yahdjian L., Stark J. M., Belnap J., Porporato A., Norton U., Ravetta D. A., Schaeffer S. M. 2004. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141, 221–235 10.1007/s00442-004-1519-1 (doi:10.1007/s00442-004-1519-1) [DOI] [PubMed] [Google Scholar]
- 71.Schwinning S., Sala O. E., Loik M. E., Ehleringer J. R. 2004. Thresholds, memory, and seasonality: understanding pulse dynamics in arid/semi-arid ecosystems. Oecologia 141, 191–193 10.1007/s00442-004-1683-3 (doi:10.1007/s00442-004-1683-3) [DOI] [PubMed] [Google Scholar]
- 72.Thomas A. D. 2012. Impact of grazing intensity on seasonal variations in soil organic carbon and soil CO2 efflux in two semiarid grasslands in southern Botswana. Phil. Trans. R. Soc. B 367, 3076–3086 10.1098/rstb.2012.0102 (doi:10.1098/rstb.2012.0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castillo-Monroy A. P., Maestre F. T. 2011. La costra biológica del suelo: avances recientes en el conocimiento de su estructura y función ecológica. Rev. Chil. Hist. Nat. 84, 1–21 10.4067/S0716-078X2011000100001 (doi:10.4067/S0716-078X2011000100001) [DOI] [Google Scholar]
- 74.Carrillo Y., Pendall E., Dijkstra F. A., Morgan J. A., Newcomb J. M. 2011. Response of soil organic matter pools to elevated CO2 and warming in a semi-arid grassland. Plant Soil 347, 339–350 10.1007/s11104-011-0853-4 (doi:10.1007/s11104-011-0853-4) [DOI] [Google Scholar]
- 75.Dijkstra F. A., Morgan J. A., Blumenthal D., Follett R. F. 2010. Water limitation and plant inter-specific competition reduce rhizosphere-induced C decomposition and plant N uptake. Soil Biol. Biochem. 42, 1073–1082 10.1016/j.soilbio.2010.02.026 (doi:10.1016/j.soilbio.2010.02.026) [DOI] [Google Scholar]
- 76.Yahdjian L., Sala O. E., Austin A. T. 2006. Differential controls of water input on litter decomposition and nitrogen dynamics in the Patagonian steppe. Ecosystems 9, 128–141 10.1007/s10021-004-0118-7 (doi:10.1007/s10021-004-0118-7) [DOI] [Google Scholar]
- 77.Kemp P. R., Reynolds J. F., Virginiaz R. A., Whitford W. G. 2003. Decomposition of leaf and root litter of Chihuahuan desert shrubs: effects of three years of summer drought. J. Arid Environ. 53, 21–39 10.1006/jare.2002.1025 (doi:10.1006/jare.2002.1025) [DOI] [Google Scholar]
- 78.Santos P. F., Elkins N. Z., Steinberger Y., Whitford W. G. 1984. A comparison of surface and buried Larrea tridentata leaf litter decomposition in North American hot deserts. Ecology 65, 278–284 10.2307/1939480 (doi:10.2307/1939480) [DOI] [Google Scholar]
- 79.Reynolds J. F., Virginia R. A., Kemp P. R., deSoyza A. G., Tremmel D. C. 1999. Impact of drought on desert shrubs: effects of seasonality and degree of resource island development. Ecol. Monogr. 69, 69–106 10.1890/0012-9615(1999)069[0069:IODODS]2.0.CO;2 (doi:10.1890/0012-9615(1999)069[0069:IODODS]2.0.CO;2) [DOI] [Google Scholar]
- 80.Mazzarino M. J., Bertiller M. B., Sain C. L., Satti P., Coronato F. R. 1998. Soil nitrogen dynamics in northeastern Patagonia steppe under different precipitation regimes. Plant Soil 202, 125–131 10.1023/A:1004389011473 (doi:10.1023/A:1004389011473) [DOI] [Google Scholar]
- 81.Lellei-Kovács E., Kovács-Láng E., Kalapos T., Botta-Dukát Z., Barabás S., Beier C. 2008. Experimental warming does not enhance soil respiration in a semiarid temperate forest-steppe ecosystem. Commun. Ecol. 9, 29–37 10.1556/ComEc.9.2008.1.4 (doi:10.1556/ComEc.9.2008.1.4) [DOI] [Google Scholar]
- 82.Sponseller R. A. 2007. Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Glob. Change Biol. 13, 426–436 10.1111/j.1365-2486.2006.01307.x (doi:10.1111/j.1365-2486.2006.01307.x) [DOI] [Google Scholar]
- 83.Anderson-Teixeira K., Delong J., Fox A., Brese D., Litvak M. 2011. Differential responses of production and respiration to temperature and moisture drive the carbon balance across a climatic gradient in New Mexico. Glob. Change Biol. 17, 410–424 10.1111/j.1365-2486.2010.02269.x (doi:10.1111/j.1365-2486.2010.02269.x) [DOI] [Google Scholar]
- 84.Shen W., Reynolds J. F., Hui D. 2009. Responses of dryland soil respiration and soil carbon pool size to abrupt versus gradual and individual versus combined changes in soil temperature, precipitation, and atmospheric [CO2]: a simulation analysis. Glob. Change Biol. 15, 2274–2294 10.1111/j.1365-2486.2009.01857.x (doi:10.1111/j.1365-2486.2009.01857.x) [DOI] [Google Scholar]
- 85.Sardans J., Peñuelas J., Estiarte M. 2008. Changes in soil enzymes related to C and N cycle and in soil C and N content under prolonged warming and drought in a Mediterranean shrubland. Appl. Soil Ecol. 39, 223–235 10.1016/j.apsoil.2007.12.011 (doi:10.1016/j.apsoil.2007.12.011) [DOI] [Google Scholar]
- 86.Ochoa-Hueso R., et al. 2011. Nitrogen deposition effects on Mediterranean-type ecosystems: an ecological assessment. Environ. Pollut. 159, 2265–2279 10.1016/j.envpol.2010.12.019 (doi:10.1016/j.envpol.2010.12.019) [DOI] [PubMed] [Google Scholar]
- 87.Sardans J., Rodà F., Peñuelas J. 2004. Phosphorus limitation and competitive capacities of Pinus halepensis and Quercus ilex subsp. rotundifolia on different soils. Plant Ecol. 174, 305–317 10.1023/B:VEGE.0000049110.88127.a0 (doi:10.1023/B:VEGE.0000049110.88127.a0) [DOI] [Google Scholar]
- 88.Pausas J. G. 2004. Changes in fire and climate in the eastern Iberian Peninsula (Mediterranean basin). Clim. Change 63, 337–350 10.1023/B:CLIM.0000018508.94901.9c (doi:10.1023/B:CLIM.0000018508.94901.9c) [DOI] [Google Scholar]
- 89.Williams A. A. J., Karoly D. J., Tapper N. 2001. The sensitivity of Australian fire danger to climate change. Clim. Change 49, 171–191 10.1023/A:1010706116176 (doi:10.1023/A:1010706116176) [DOI] [Google Scholar]
- 90.Christian J. M., Wilson S. D. 1999. Long-term ecosystem impacts of an introduced grass in the northern Great Plains. Ecology 80, 2397–2407 10.1890/0012-9658(1999)080[2397:LTEIOA]2.0.CO;2 (doi:10.1890/0012-9658(1999)080[2397:LTEIOA]2.0.CO;2) [DOI] [Google Scholar]
- 91.Smith S. D., Huxman T. E., Zitzer S. F., Charlet T. N., Housman D. C., Coleman J. S., Fenstermaker L. K., Seemann J. R., Nowak R. S. 2000. Elevated CO2 increases productivity and invasive species success in an arid ecosystem. Nature 408, 79–82 10.1038/35040544 (doi:10.1038/35040544) [DOI] [PubMed] [Google Scholar]
- 92.Pasquini S. C., Vourlitis G. L. 2010. Post-fire primary production and plant community dynamics in chaparral stands exposed to varying levels of nitrogen deposition. J. Arid Environ. 74, 310–314 10.1016/j.jaridenv.2009.08.013 (doi:10.1016/j.jaridenv.2009.08.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Okin G. S., Parsons A. J., Wainwright J., Herrick J. E., Bestelmeyer B. T., Peters D. C., Fredrickson E. D. L. 2009. Do changes in connectivity explain desertification? BioScience 59, 237–244 10.1525/bio.2009.59.3.8 (doi:10.1525/bio.2009.59.3.8) [DOI] [Google Scholar]
- 94.Craine J. M., Nippert J. B., Towne E. G., Tucker S., Kembel S. W., Skibbe A., McLauchlan K. K. 2011. Functional consequences of climate change-induced plant species loss in a tallgrass prairie. Oecologia 165, 1109–1117 10.1007/s00442-011-1938-8 (doi:10.1007/s00442-011-1938-8) [DOI] [PubMed] [Google Scholar]
- 95.García-Fayos P., Bochet E. 2009. Indication of antagonistic interaction between climate change and erosion on plant species richness and soil properties in semiarid Mediterranean ecosystems. Glob. Change Biol. 15, 306–318 10.1111/j.1365-2486.2008.01738.x (doi:10.1111/j.1365-2486.2008.01738.x) [DOI] [Google Scholar]
- 96.Van Vuuren D. P., Sala O. E., Pereira H. M. 2006. The future of vascular plant diversity under four global scenarios. Ecol. Soc. 11, 25–44 [Google Scholar]
- 97.Maestre F. T., et al. 2009. Shrub encroachment can reverse desertification in Mediterranean semiarid grasslands. Ecol. Lett. 12, 930–941 10.1111/j.1461-0248.2009.01352.x (doi:10.1111/j.1461-0248.2009.01352.x) [DOI] [PubMed] [Google Scholar]
- 98.Eisenhauer N., et al. 2011. Plant diversity surpasses plant functional groups and plant productivity as driver of soil biota in the long term. PLoS ONE 6, e16055. 10.1371/journal.pone.0016055 (doi:10.1371/journal.pone.0016055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Auken O. W. 2009. Causes and consequences of woody plant encroachment into western North American grasslands. J. Environ. Manag. 90, 1–12 10.1016/j.jenvman.2009.04.023 (doi:10.1016/j.jenvman.2009.04.023) [DOI] [PubMed] [Google Scholar]
- 100.Eldridge D. J., Bowker M. A., Maestre F. T., Reynolds J. F., Roger E., Whitford W. G. 2011. Impacts of shrub encroachment on ecosystem structure and functioning: towards a global synthesis. Ecol. Lett. 14, 709–722 10.1111/j.1461-0248.2011.01630.x (doi:10.1111/j.1461-0248.2011.01630.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ratajczak Z., Nippert J. B., Collins S. L. 2012. Woody encroachment decreases diversity across North American grasslands and savannas. Ecology 93, 697. 10.1890/11-1199.1 (doi:10.1890/11-1199.1) [DOI] [PubMed] [Google Scholar]
- 102.Archer S. A., Schimel D. S., Holland E. A. 1995. Mechanisms of shrubland expansion: land use or CO2? Clim. Change 29, 91–99 10.1007/BF01091640 (doi:10.1007/BF01091640) [DOI] [Google Scholar]
- 103.Gao Q., Reynolds J. F. 2003. Historical shrub–grass transitions in the northern Chihuahuan Desert: modeling the effects of shifting rainfall seasonality and event size over a landscape gradient. Glob. Change Biol. 9, 1475–1493 10.1046/j.1365-2486.2003.00676.x (doi:10.1046/j.1365-2486.2003.00676.x) [DOI] [Google Scholar]
- 104.Shen W., Wu J., Grimm N., Hope D. 2008. Effects of urbanization-induced environmental changes on ecosystem functioning in the Phoenix metropolitan region, USA. Ecosystems 11, 138–155 10.1007/s10021-007-9085-0 (doi:10.1007/s10021-007-9085-0) [DOI] [Google Scholar]
- 105.Farley K. A., Jobbágy E. G., Jackson R. B. 2005. Effects of afforestation on water yield: a global synthesis with implications for policy. Glob. Change Biol. 11, 1565–1576 10.1111/j.1365-2486.2005.01011.x (doi:10.1111/j.1365-2486.2005.01011.x) [DOI] [Google Scholar]
- 106.Downward S. R., Taylor R. 2007. An assessment of Spain's program AGUA and its implications for sustainable water management in the province of Almería, southeast Spain. J. Environ. Manag. 82, 277–289 10.1016/j.jenvman.2005.12.015 (doi:10.1016/j.jenvman.2005.12.015) [DOI] [PubMed] [Google Scholar]
- 107.Fearnside P. M. 2001. Soybean cultivation as a threat to the environment in Brazil. Environ. Conserv. 28, 23–38 10.1017/S0376892901000030 (doi:10.1017/S0376892901000030) [DOI] [Google Scholar]
- 108.Steininger M. K., Tucker C. J., Townshend J. R. G., Killeen T. R., Desch A. 2001. Tropical deforestation in Bolivian Amazon. Environ. Conserv. 28, 127–134 10.1117/12.381717 (doi:10.1117/12.381717) [DOI] [Google Scholar]
- 109.Zak M. R., Cabido M., Hodgson J. G. 2004. Do subtropical seasonal forests in the Gran Chaco, Argentina, have a future? Biol. Conserv. 120, 589–593 10.1016/j.biocon.2004.03.034 (doi:10.1016/j.biocon.2004.03.034) [DOI] [Google Scholar]
- 110.Schofield N. J. 1992. Tree-planting for dryland salinity control in Australia. Agrofor. Syst. 20, 1–23 10.1007/BF00055303 (doi:10.1007/BF00055303) [DOI] [Google Scholar]
- 111.Leblanc M. J., Favreau G., Massuel S., Tweed S. O., Loireau M., Cappelaere B. 2008. Land clearance and hydrological change in the Sahel: SW Niger. Glob. Planet. Change 61, 135–150 10.1016/j.gloplacha.2007.08.011 (doi:10.1016/j.gloplacha.2007.08.011) [DOI] [Google Scholar]
- 112.Nosetto M. D., Jobbágy E. G., Brizuela A. B., Jackson R. B. 2012. The hydrologic consequences of land cover change in central Argentina. Agric. Ecosyst. Environ. 154, 2–11 10.1016/j.agee.2011.01.008 (doi:10.1016/j.agee.2011.01.008) [DOI] [Google Scholar]
- 113.Pouyat R. V., Pataki D. E., Belt K. T., Groffman P. M., Hom J., Band L. E. 2007. Effects of urban land-use change on biogeochemical cycles. In Terrestrial ecosystems in a changing world (eds Canadell J., Pataki D., Pitelka L.), pp. 45–58 New York, NY: Springer [Google Scholar]
- 114.Norby R. J., Rustad L. E., Dukes J. S., Ojima D. S., Parton W. J., Del Grosso S. J., McMurtrie R. E., Pepper D. A. 2007. Ecosystem responses to warming and interacting global change factors. In Terrestrial ecosystems in a changing world (eds Canadell J., Pataki D., Pitelka L.), pp. 23–36 New York, NY: Springer [Google Scholar]
- 115.Le Houérou H. N. 2001. Biogeography of the arid steppeland north of the Sahara. J. Arid Environ. 48, 103–128 10.1006/jare.2000.0679 (doi:10.1006/jare.2000.0679) [DOI] [Google Scholar]
- 116.Jones P. G., Thornton P. K. 2003. The potential impacts of climate change in tropical agriculture: the case of maize in Africa and Latin America in 2055. Glob. Environ. Change 13, 51–59 10.1016/S0959-3780(02)00090-0 (doi:10.1016/S0959-3780(02)00090-0) [DOI] [Google Scholar]
- 117.International Livestock Research Institute 2006. Mapping climate vulnerability and poverty in Africa. Nairobi, Kenya: ILRI [Google Scholar]
- 118.Stetter S., Herschinger E., Teichler T., Albert M. 2011. Conflicts about water: securitizations in a global context. Cooperat. Confl. 46, 441–459 10.1177/0010836711422462 (doi:10.1177/0010836711422462) [DOI] [Google Scholar]
- 119.Fischhendler I., Dinar S., Katz D. 2011. The politics of unilateral environmentalism: cooperation and conflict over water management along the Israeli–Palestinian border. Glob. Environ. Polit. 11, 36–61 10.1162/GLEP_a_00042 (doi:10.1162/GLEP_a_00042) [DOI] [Google Scholar]
- 120.Webster M., Ginnetti J., Walker P., Coppard D., Kent R. 2008. The humanitarian costs of climate change. Medford, MA: Tufts University, Feinstein International Center. [Google Scholar]
- 121.Chaves L. F., Koenraadt C. J. M. 2010. Climate change and highland malaria: fresh air for a hot debate. Q. Rev. Biol. 85, 27–55 10.1086/650284 (doi:10.1086/650284) [DOI] [PubMed] [Google Scholar]
- 122.Hales S., de Wet N., Maindonald J., Woodward A. 2002. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet 360, 830–834 10.1016/S0140-6736(02)09964-6 (doi:10.1016/S0140-6736(02)09964-6) [DOI] [PubMed] [Google Scholar]
- 123.Patz J. A., Confalonieri U. E. C. 2005. Human health: ecosystem regulation of infectious diseases. In Ecosystems and human well-being: current state and trends, vol. 1 (eds Hassan R., Scholes R., Neville A.), pp. 393–415 Washington, DC: Island Press [Google Scholar]
- 124.Meze-Hausken E. 2000. Migration caused by climate change: how vulnerable are people in dryland areas? Mitig. Adapt. Strat. Glob. Change 5, 379–406 10.1023/A:1026570529614 (doi:10.1023/A:1026570529614) [DOI] [Google Scholar]
- 125.Morton J. F. 2007. The impact of climate change on smallholder and subsistence agriculture. Proc. Natl Acad. Sci. USA 104, 19 680–19 685 10.1073/pnas.0701855104 (doi:10.1073/pnas.0701855104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kniveton D. R., Smith C. D., Black R. 2012. Emerging migration flows in a changing climate in dryland Africa. Nat. Clim. Change 2, 444–447 10.1038/nclimate1447 (doi:10.1038/nclimate1447) [DOI] [Google Scholar]
- 127.Biermann F., Boas I. 2008. Protecting climate refugees: the case for a global protocol. Environment 50, 8–16 [Google Scholar]
- 128.Burkea M. B., Miguel E., Satyanath S., Dykema J. A., Lobell D. B. 2009. Warming increases the risk of civil war in Africa. Proc. Natl Acad. Sci. USA 106, 20 670–20 674 10.1073/pnas.0907998106 (doi:10.1073/pnas.0907998106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hsiang S. M., Meng K. C., Cane M. A. 2001. Civil conflicts are associated with the global climate. Nature 476, 438–441 10.1038/nature10311 (doi:10.1038/nature10311) [DOI] [PubMed] [Google Scholar]
- 130.Cao M., Prince S. D., Small J., Goetz S. J. 2004. Remotely sensed interannual variations and trends in terrestrial net primary productivity 1981–2000. Ecosystems 7, 232–242 10.1007/s10021-003-0189-x (doi:10.1007/s10021-003-0189-x) [DOI] [Google Scholar]
- 131.Egoh B. E., et al. 2010. Safeguarding biodiversity and ecosystem services in the Little Karoo, South Africa. Conserv. Biol. 24, 1021–1030 10.1111/j.1523-1739.2009.01442.x (doi:10.1111/j.1523-1739.2009.01442.x) [DOI] [PubMed] [Google Scholar]
- 132.Reyers B., O'Farrell P. J., Cowling R. M., Egoh B. N., Le Maitre D. C., Vlok J. H. J. 2009. Ecosystem services, land-cover change, and stakeholders: finding a sustainable foothold for a semiarid biodiversity hotspot. Ecol. Soc. 14, 38 (http://www.ecologyandsociety.org/vol14/iss1/art38/) [Google Scholar]
- 133.Huber-Sannwald E., Palacios M. R., Arredondo J. T., Braasch M., Martínez-Peña R. M., García de Alba Verduzco J., Monzalvo Santos K. 2012. Navigating challenges and opportunities of land degradation and sustainable livelihood development in dryland social–ecological systems: a case study from Mexico. Phil. Trans. R. Soc. B 367, 3158–3177 10.1098/rstb.2011.0349 (doi:10.1098/rstb.2011.0349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Luo Y., Zhou Z. 2006. Soil respiration and the environment, 1st edn Burlington, MA: Academic Press [Google Scholar]
- 135.Bond-Lamberty B., Thomson A. 2010. A global database of soil respiration data. Biogeosciences 7, 1915–1926 10.5194/bg-7-1915-2010 (doi:10.5194/bg-7-1915-2010) [DOI] [Google Scholar]
- 136.Thomas A. D., Dougill A. J. 2006. Distribution and characteristics of cyanobacterial soil crusts in the Molopo Basin, South Africa. J. Arid Environ. 64, 270–283. 10.1016/j.jaridenv.2005.04.011 (doi:10.1016/j.jaridenv.2005.04.011) [DOI] [Google Scholar]
- 137.Slimani H., Aidoud A., Roze F. 2010. 30 Years of protection and monitoring of a steppic rangeland undergoing desertification. J. Arid Environ. 74, 685–691 10.1016/j.jaridenv.2009.10.015 (doi:10.1016/j.jaridenv.2009.10.015) [DOI] [Google Scholar]
- 138.Hirche A., Salamani M., Abdellaoui A., Benhouhou S., Valderrama J. M. 2011. Landscape changes of desertification in arid areas: the case of south-west Algeria. Environ. Monit. Assess. 179, 403–420 10.1007/s10661-010-1744-5 (doi:10.1007/s10661-010-1744-5) [DOI] [PubMed] [Google Scholar]
- 139.Díaz S., et al. 2004. The plant traits that drive ecosystems: evidence from three continents. J. Veg. Sci. 15, 295–304 10.1111/j.1654-1103.2004.tb02266.x (doi:10.1111/j.1654-1103.2004.tb02266.x) [DOI] [Google Scholar]
- 140.Reynolds J. F., et al. 2011. Scientific concepts for an integrated analysis of desertification. Land Degrad. Dev. 22, 166–183 10.1002/ldr.1104 (doi:10.1002/ldr.1104) [DOI] [Google Scholar]
- 141.Hannah L. 2011. A global conservation system for climate-change adaptation. Conserv. Biol. 24, 70–77 10.1111/j.1523-1739.2009.01405.x (doi:10.1111/j.1523-1739.2009.01405.x) [DOI] [PubMed] [Google Scholar]
- 142.Sala O. E., Gherardi L. A., Reichmann L., Jobbágy E., Peters D. 2012. Legacies of precipitation fluctuations on primary production: theory and data synthesis. Phil. Trans. R. Soc. B 367, 3135–3144 10.1098/rstb.2011.0347 (doi:10.1098/rstb.2011.0347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Salguero-Gómez R., Siewert W., Casper B. B., Tielbörger K. 2012. A demographic approach to study effects of climate change in desert plants. Phil. Trans. R. Soc. B 367, 3100–3114 10.1098/rstb.2012.0074 (doi:10.1098/rstb.2012.0074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Borgogno F., D'Odorico P., Laio F., Ridolfi L. 2007. Effect of rainfall interannual variability on the stability and resilience of dryland plant ecosystems. Water Resour. Res. 43, 1–9 10.1029/2006wr005314 (doi:10.1029/2006wr005314)20300476 [DOI] [Google Scholar]
- 145.D'Odorico P., Laio F., Ridolfi L. 2005. Noise-induced stability in dryland plant ecosystems. Proc. Natl Acad. Sci. USA 102, 10 819–10 822 10.1073/pnas.0502884102 (doi:10.1073/pnas.0502884102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Weltzin J. F., et al. 2003. Assessing the response of terrestrial ecosystems to potential changes in precipitation. BioScience 53, 941–952 10.1641/0006-3568(2003)053[0941:atrote]2.0.co;2 (doi:10.1641/0006-3568(2003)053[0941:atrote]2.0.co;2) [DOI] [Google Scholar]
- 147.Easterling D. R., Evans J. L., Groisman P. Y., Karl T. R., Kunkel K. E., Ambenje P. 2000. Observed variability and trends in extreme climate events: a brief review. Bull. Am. Meteorol. Soc. 81, 417–425 (doi:10.1175/1520-0477(2000)081<0417:ovatie>2.3.co;2) [DOI] [Google Scholar]
- 148.Allen M. R., Ingram W. J. 2002. Constraints on future changes in climate and the hydrologic cycle. Nature 419, 224. 10.1038/nature01092 (doi:10.1038/nature01092) [DOI] [PubMed] [Google Scholar]
- 149.D'Odorico P., Bhattachan A. 2012. Hydrologic variability in dryland regions: impacts on ecosystem dynamics and food security. Phil. Trans. R. Soc. B 367, 3145–3157 10.1098/rstb.2012.0016 (doi:10.1098/rstb.2012.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Belnap J. 2006. The potential roles of biological soil crusts in dryland hydrologic cycles. Hydrol. Process. 120, 3159–3178 10.1002/hyp.6325 (doi:10.1002/hyp.6325) [DOI] [Google Scholar]
- 151.Belnap J., Phillips S. L., Miller M. E. 2004. Response of desert biological soil crusts to alterations in precipitation frequency. Oecologia 141, 306–316 10.1007/s00442-003-1438-6 (doi:10.1007/s00442-003-1438-6) [DOI] [PubMed] [Google Scholar]
- 152.Belnap J., Phillips S. L., Flint S. D., Money J., Caldwell M. M. 2008. Global change and biological soil crusts: effects of ultraviolet augmentation under altered precipitation regimes and nitrogen additions. Glob. Change Biol. 14, 670–686 10.1111/j.1365-2486.2007.01509.x (doi:10.1111/j.1365-2486.2007.01509.x) [DOI] [Google Scholar]
- 153.Grote E. E., Belnap J., Housman D. C., Sparks J. P. 2010. Carbon exchange in biological soil crust communities under differential temperatures and soil water contents: implications for global change. Glob. Change Biol. 16, 2763–2774 10.1111/j.1365-2486.2010.02201.x (doi:10.1111/j.1365-2486.2010.02201.x) [DOI] [Google Scholar]
- 154.Eldridge D. J., Leys J. F. 2003. Exploring some relationships between biological soil crusts, soil aggregation and wind erosion. J. Arid Environ. 53, 457–466 10.1006/jare.2002.1068 (doi:10.1006/jare.2002.1068) [DOI] [Google Scholar]
- 155.Van der Putten W. H., Macel M., Visser M. E. 2010. Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels? Phil. Trans. R. Soc. B 365, 2025–2034 10.1098/rstb.2010.0037 (doi:10.1098/rstb.2010.0037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lan Z., Bai Y. 2012. Testing mechanisms of N-enrichment induced species loss in a semiarid Inner Mongolia grassland: critical thresholds and implications for long-term ecosystem responses. Phil. Trans. R. Soc. B 367, 3125–3134 10.1098/rstb.2011.0352 (doi:10.1098/rstb.2011.0352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee M., Manning P., Rist J., Power S. A., Marsh C. 2010. A global comparison of grassland biomass responses to CO2 and nitrogen enrichment. Phil. Trans. R. Soc. B 365, 2047–2056 10.1098/rstb.2010.0028 (doi:10.1098/rstb.2010.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Birch J. C., Newton A. C., Aquino C. A., Cantarello E., Echeverría C., Kitzberger T., Schiappacasse I., Garavito N. T. 2010. Cost-effectiveness of dryland forest restoration evaluated by spatial analysis of ecosystem services. Proc. Natl Acad. Sci. USA 107, 21 925–21 930 10.1073/pnas.1003369107 (doi:10.1073/pnas.1003369107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schiappacasse I., Nahuelhual L., Vásquez F., Echeverría C. 2012. Assessing the benefits and costs of dryland forest restoration in central Chile. J. Environ. Manag. 97, 38–45 10.1016/j.jenvman.2011.11.007 (doi:10.1016/j.jenvman.2011.11.007) [DOI] [PubMed] [Google Scholar]
- 160.Qi J., Chen J., Wan S., Ai L. 2012. Understanding the coupled natural and human systems in dryland East Asia. Environ. Res. Lett. 7, 015202. 10.1088/1748-9326/7/1/015202 (doi:10.1088/1748-9326/7/1/015202) [DOI] [Google Scholar]
- 161.Thomas R. J. 2008. Opportunities to reduce the vulnerability of dryland farmers in Central and West Asia and North Africa to climate change. Agric. Ecosyst. Environ. 126, 36–45 10.1016/j.agee.2008.01.011 (doi:10.1016/j.agee.2008.01.011) [DOI] [Google Scholar]
- 162.Farley J., Aquino A., Daniels A., Moulaert A., Lee D., Krause A. 2010. Global mechanisms for sustaining and enhancing PES schemes. Ecol. Econ. 69, 2075–2084 10.1016/j.ecolecon.2010.02.016 (doi:10.1016/j.ecolecon.2010.02.016) [DOI] [Google Scholar]
- 163.Dougill A. J., Stringer L. C., Leventon J., Riddell M., Rueff H., Spracklen D. V., Butt E. 2012. Lessons from community-based payment for ecosystem service schemes: from forests to rangelands. Phil. Trans. R. Soc. B 367, 3178–3190 10.1098/rstb.2011.0418 (doi:10.1098/rstb.2011.0418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Williams C. A., Hanan N. P., Neff J. C., Scholes R. J., Berry J. A., Denning A. S., Baker D. F. 2007. Africa and the global carbon cycle. Carbon Balance Manag. 2, 3–16 10.1186/1750-0680-2-3 (doi:10.1186/1750-0680-2-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Milligan S. R., Holt W. V., Lloyd R. 2009. Impacts of climate change and environmental factors on reproduction and development in wildlife. Phil. Trans. R. Soc. B 364, 3313–3319 10.1098/rstb.2009.0175 (doi:10.1098/rstb.2009.0175) [DOI] [PMC free article] [PubMed] [Google Scholar]