Abstract
Biological soil crusts (BSCs) are key biotic components of dryland ecosystems worldwide that control many functional processes, including carbon and nitrogen cycling, soil stabilization and infiltration. Regardless of their ecological importance and prevalence in drylands, very few studies have explicitly evaluated how climate change will affect the structure and composition of BSCs, and the functioning of their constituents. Using a manipulative experiment conducted over 3 years in a semi-arid site from central Spain, we evaluated how the composition, structure and performance of lichen-dominated BSCs respond to a 2.4°C increase in temperature, and to an approximately 30 per cent reduction of total annual rainfall. In areas with well-developed BSCs, warming promoted a significant decrease in the richness and diversity of the whole BSC community. This was accompanied by important compositional changes, as the cover of lichens suffered a substantial decrease with warming (from 70 to 40% on average), while that of mosses increased slightly (from 0.3 to 7% on average). The physiological performance of the BSC community, evaluated using chlorophyll fluorescence, increased with warming during the first year of the experiment, but did not respond to rainfall reduction. Our results indicate that ongoing climate change will strongly affect the diversity and composition of BSC communities, as well as their recovery after disturbances. The expected changes in richness and composition under warming could reduce or even reverse the positive effects of BSCs on important soil processes. Thus, these changes are likely to promote an overall reduction in ecosystem processes that sustain and control nutrient cycling, soil stabilization and water dynamics.
Keywords: climate change, lichens, mosses, biological soil crusts, Mediterranean, semi-arid
1. Introduction
There is ample scientific consensus that ongoing global environmental change (hereafter global change), which is promoted by multiple drivers (e.g. increases in atmospheric carbon dioxide concentration [CO2], changes in climate and nutrient deposition and in land use), will profoundly impact the structure, dynamics and functioning of terrestrial ecosystems, affecting a broad range of organisms (reviewed in [1–5]). The worldwide increase in temperature constitutes one of the clearest signals of climate change, and is one of the global change drivers most heavily studied to date (see [4–6] for reviews). Overall, the rate of warming during the last 100 years (1906–2005) has been approximately 0.74°C, with most years from 1995 ranking among the warmest years since the establishment of meteorological records [3]. As a consequence, important changes in the phenology and distribution of organisms, and in the composition and dynamics of communities are being documented in terrestrial ecosystems worldwide [7–10]. In addition, climate change will promote important modifications in rainfall patterns, such as the overall reduction in rainfall amounts and the increase in its intensity in many regions worldwide [3,11–13]. These rainfall changes can modulate the ability of plants and microbes to respond to warming [2,14,15]. Thus, the analysis of how organisms will respond to climate change must consider changes in both temperature and rainfall, particularly in ecosystems where rainfall is already scarce and unpredictable, such as in drylands [2]. These environments are of paramount importance at the global scale, as they occupy over 41 per cent of the terrestrial surface, are the home of 38 per cent of the global human population and provide ecosystem services that are critical for the maintenance of life on Earth [2,16].
One particularly important component of drylands, biological soil crusts (BSCs) are under-studied in terms of their response to global change. BSCs are a complex and highly specialized community composed of cyanobacteria, algae, mosses, liverworts, fungi, bacteria and lichens that live in the uppermost millimetres of the soil surface [17–19]. They are particularly prevalent in dry and/or extremely cold environments, where they may compose up to 70 per cent of the total living cover [20]. They play critical functional roles, as they contribute to atmospheric carbon and nitrogen fixation [21–23], control nitrogen mineralization and availability [24,25], stabilize the soil against erosion [26] and modulate infiltration and runoff processes [27,28]. In addition, they affect the abundance, diversity and performance of microbial [29,30], arthropod [31] and plant [32,33] communities.
Because BSC organisms are poikilohydric, their metabolism and physiological functions are highly dependent on ambient moisture and temperature [34–36]. Thus, changes in rainfall and temperature expected with climate change are likely to affect the functioning and dynamics of BSCs, as has been shown in a handful of experimental studies [37–40]. In dryland areas receiving a considerable portion of their rainfall during summer, such as some deserts of the southwestern USA, experimental field studies have shown that increased summer rainfall frequency negatively affects the functioning of moss- and lichen-dominated BSCs, leading to a reduction in their cover and to a replacement of these communities by cyanobacteria [37,39,40]. Interestingly, experimental increases of temperature up to 2°C in this region had negligible impacts on the development and physiology of these communities [39,40], although temperature can modulate negative effects of UV augmentation on their photosynthetic activity [38]. Rain frequency and duration of dry periods have been also found to be key factors controlling the development and composition of BSCs along a 2000 km natural transect in southwestern Africa [41]. While these studies point to the importance of changes in rainfall, results can be substantially different in ecosystems that do not receive summer rainfall, and in areas where dew is a key moisture source for BSC constituents [42,43]. For example, Pintado et al. [36] found that the BSC-forming lichen Diploschistes diacapsis (Ach.) Lumbsch, common across a semi-arid site from southeast Spain, was active only during the 20 per cent of the year that experienced dewfall [44], an activity that mainly occurred during conditions of relatively low temperature and photosynthetically active radiation. Increases in temperature can reduce the inputs of water through dew, and can also modify the number of days where BSC-forming lichens are active. Castillo-Monroy et al. [45] found that, over a 3.5 year period, soil CO2 efflux rates in lichen-dominated BSCs were significantly higher than those found in bare ground areas devoid of BSCs in a semi-arid site from central Spain, and that the rate of increase in soil respiration in response to increased temperature (Q10) was augmented with the degree of cover and development of BSCs. Similar results have been observed by Maestre et al. [46], who reported a significant increase in soil respiration in BSC-dominated microsites, but not in bare ground areas, in response to a 2.4°C experimental increase in temperature. Although these authors did not measure changes in the BSC constituents themselves, their results suggest that ongoing increases in temperature will affect their physiological activity.
Recent research has shown that the richness of BSC communities is positively linked to ecosystem functioning and multifunctionality, i.e. the ability of ecosystems to maintain multiple functions, such as carbon storage, productivity and the build-up of nutrient pools [47,48]. Other studies have found that the functional redundancy of BSC-forming species is very low [49,50]. Overall, these results indicate that maintaining species-rich BSC communities is crucial to maintain the overall functionality of ecosystems dominated by these organisms. Therefore, evaluating how the composition and diversity of BSCs will be affected by future changes in temperature and rainfall is crucial to fully understand how ongoing climate change will impact the structure and functioning of drylands. Observational studies carried out over large spatial scales have not reported consistent results to date. For example, Rogers [51] found that the number of lichens decreased with reductions in annual rainfall in drylands from Australia. Studies carried out along a Namibian–South African transect have found that the richness and cover of lichen species are positively related to lower temperature, higher altitude and increased water availability ([52], but see [41,53] for contrasting results along the same transect). Results from these studies may not, however, be directly applicable to smaller spatial scales because other abiotic factors that are important determinants of BSC distribution, such as soil type and texture [20,41], often covary with rainfall and temperature.
While drylands are known to be among the most sensitive biomes to global change [54,55], there are many uncertainties surrounding the ecological consequences of such change on these ecosystems [2]. This is particularly evident when considering climate change impacts on BSCs, as only a handful of experimental studies have explicitly evaluated how future climatic conditions affect the performance, dynamics and functioning of their constituents [38–40,46,56,57]. Furthermore, none of these have evaluated how simultaneous changes in temperature and rainfall jointly affect the composition, richness, diversity and physiological performance of BSC communities as a whole. We aimed to evaluate how different climate change drivers could affect BSCs by carrying out an experiment in central Spain. In this experiment, we evaluated responses of the BSC community and its main constituents to a 2.4°C increase in temperature, and to an approximately 30 per cent reduction of total annual rainfall, climatic conditions that mimic those forecasted for the last half of the twenty-first century in our study area [58]. Specifically, we tested the hypothesis that the transition to a more arid climate will reduce the physiological performance of BSCs, as warmer temperatures and lower rainfall will promote a more frequent and rapid desiccation of BSC constituents, which may impair their ability to function within a positive carbon balance [37,56,58,59]. As a result, we expected their growth to be reduced and important shifts in the composition of BSC communities to occur.
2. Material and methods
(a). Study area
This study was conducted in the Aranjuez Experimental Station, located in the centre of the Iberian Peninsula (40°02′ N–3°32′ W; 590 (m)a.s.l.). The climate is Mediterranean semi-arid, with a mean annual temperature and rainfall of 15°C and 349 mm, respectively (Aranjuez Meteorological Station, 40°02′ N–3°32′ W; 540 (m)a.s.l.; average data from the 1983–1988 and 1997–2011 periods). The soil is derived from gypsum, and is classified as Xeric Haplogypsid [60]. Perennial plant cover is below 40 per cent, and is dominated by the tussock grass Stipa tenacissima L. and the shrubs Helianthemum squamatum (L.) Dum. Cours and Retama sphaerocarpa (L.) Boiss. The open areas between perennial plants are colonized by well-developed BSCs dominated by lichens such as D. diacapsis (Ach.) Lumbsch, Squamarina lentigera (Weber) Poelt and Fulgensia subbracteata, and mosses such as Pleurochaete squarrosa (Brid.) Lindb. and Didymodon acutus (Brid.) K. Saito (see the electronic supplementary material, appendix S1 for a species checklist).
(b). Experimental design
We established a factorial experimental design with three factors, each with two levels: BSC cover (poorly developed BSC communities with cover less than 25% versus well-developed communities with cover greater than 75%), warming (control versus a 2.4°C annual temperature increase) and rainfall exclusion (control versus an approx. 30% rainfall reduction in total annual rainfall). The working plots (1.2 × 1.2 m) were randomly placed on either bare ground (8.6 ± 0.8% of BSC cover; mean ± s.e., n = 40; hereafter Bare plots) or BSC-dominated (73.8 ± 1.7% of BSC cover; mean ± s.e., n = 40; hereafter Crust plots) microsites. A minimum separation distance between plots of 1 m was ensured to minimize the risk of sampling non-independent areas. The different combinations of treatments were randomly assigned to Bare and Crust plots. Ten replicates per combination of treatments were established, resulting in a total of 80 plots.
The warming treatment aimed to simulate the average of predictions derived from six atmosphere–ocean general circulation models for the second half of the twenty-first century (2040–2070) in central Spain [58]. These models predict an increment of annual temperature ranging from 2.6°C (B2 IPCC scenario) to 2.8°C (A2 IPCC scenario). This increment ranges between 2.1–2.3°C during winter months (B2 and A2 scenarios, respectively) and 3.2–3.5°C during summer months (B2 and A2 scenarios, respectively). To achieve a temperature increase within this range, we used open-top chambers (OTCs) similar to those employed in warming experiments carried out in arctic [61] and dryland [57] areas. OTCs were built with methacrylate plates, which have a high transmittance in the visible spectrum and a very low emission of the infrared wavelength, using a hexagonal design with sloping sides of 40 × 50 × 32 cm. The chambers are open on the top to allow rainfall and air to enter. The bottom edge of all chambers was situated 5 cm above the surface, to allow air flow and avoid excessive temperatures (see the electronic supplementary material, appendix S2). OTCs were installed in the field in July 2008.
Forecasted changes in rainfall for our study area are subject to a high degree of uncertainty, but all models predict a significant reduction of rainfall, mostly during existing wet months (spring and fall; the number of days with rainfall higher than 1 mm is predicted to be reduced between 10 and 50% during these seasons [58]). To achieve a rainfall reduction similar to that forecasted, we set up passive rainfall shelters (RSs) based upon the design described by Yahdjian & Sala [62]. Each RS has an area of 1.44 m2 (1.2 × 1.2 m), and a mean height of 1 m. Each roof has an inclination of 20° and is composed of three gutters of methacrylate that cover approximately 37 per cent of the surface, connected to containers that collect the excluded water (see the electronic supplementary material, appendix S2). The RS did not modify the frequency of rainfall events, which has been shown to be an important component of climate change in other regions and to strongly affect BSC functioning and dynamics [37–40], but effectively reduced the size of individual rain events and the total amount of rainfall reaching the soil surface (see the electronic supplementary material, appendix S3). The RSs were set up during November 2008.
The effects of the OTCs and RSs on air temperature and humidity, and on soil temperature, were monitored using automated sensors (HOBO Pro v.2 Temp/RH and H8 Data Loggers, Onset corporation, Bourne, MA, USA).
(c). Biological soil crust measurements
In each plot, and prior to the setup of the OTCs, we placed a permanent circular plot (20 cm diameter) to monitor changes in the cover, diversity, composition and physiological performance of BSCs. In each plot, we estimated the composition of the main visible components of the BSC community (mosses and lichens) in June 2008 and May 2011 using the point-sampling method (1 × 1 cm grid; 120 sampling points per plot). With these data, we calculated the total cover of the BSC community, species richness, diversity (using the exponential Shannon diversity index [63]) and evenness (using Pielou's index [64]). To assess the changes in these variables through time, we estimated a difference index (Dif) as Rfinal − Rinitial, where R is the value of the variable of interest in May 2011 (final) and June 2008 (initial). We preferred using this index over other relative indices commonly employed, such as Relative Interaction Index [65] or Relative Neighbour Effect [66], to avoid the extreme values in the relative difference created by the presence of zeros in some of the variables measured at the beginning of the experiment. We obtained Dif values for the cover, diversity, richness and evenness of the whole BSC communities and lichens, as well as for the cover of the dominant lichen species (S. lentigera and D. diacapsis; electronic supplementary material, appendix S1) and mosses.
The physiological performance of BSCs was evaluated in the Crust plots by measuring the maximum photochemical efficiency of photosystem II (Fv/Fm) as an overall indicator of the status of this photosystem, and as a measure of the efficiency of the photosynthetic process [21,39,43,57,67]. Measurements were taken seasonally at midday from November 2008 until November 2011 on sunny days by using an FMS 2 Pulse Modulated Chlorophyll Fluorometer (Hansatech Instruments Ltd, King's Lynn, UK). Measurements taken at this moment of the day are commonly used when evaluating the physiological performance of BSC-forming organisms [21,68]. We found these measurements to be representative of those obtained in other moments of the day such as during early morning (see the electronic supplementary material, appendix S4), when BSC-forming lichens are physiologically most active in environments such as those studied [36]. Fv/Fm was calculated as the ratio between the variable (Fv) and the maximum (Fm) fluorescence signal. Lichens were dark adapted for 30 min prior to measurements by using dark cloth. Fluorometer measurements were made in six replicated plots per combination of treatments for the whole community, and for S. lentigera and D. diacapsis. Six measurements per plot were taken in all cases, which were averaged for further analyses.
(d). Statistical analyses
Changes in cover and diversity metrics (richness, diversity and evenness) between 2008 and 2011, as measured with Dif, did not follow a normal distribution, nor did they show homogeneity of variances, in most cases. Thus, we evaluated the effects of the warming (WA) and rainfall exclusion (RE) treatments (fixed factors), and their interaction, on these data using permutational multivariate analysis of variance (PERMANOVA [69]). This method is based on the use of permutation tests to obtain p-values, does not rely on the normality assumption of ANOVA and can handle experimental designs such as those used here. For these analyses, the Euclidean distance and 10 000 permutations (permutation of raw data [70]) were used to analyse our data. To investigate higher-order interactions, data were divided into subsets based on one of the factors of the interaction, and then were subject to PERMANOVA. In addition to PERMANOVA analyses, we evaluated whether median Dif values obtained for each treatment and variable were different from zero using the non-parametric Wilcoxon signed-rank test.
To evaluate how the treatments affected community composition during the study period, we conducted a PERMANOVA analysis with warming (WA), rainfall exclusion (RE) and year (2008 or 2011) as fixed factors. Prior to these analyses, which were carried out using the Bray–Curtis distance, we square-root transformed the data. As an additional interpretive tool, we determined individual species contributions to average Bray–Curtis dissimilarity from the beginning to the end of the experiment in each treatment using the SIMPER approach [71]. This method allows consideration of which species along the treatments are primarily responsible for any observed difference in abundance between the 2 years.
Seasonal Fv/Fm data were analysed by a three-way (WA, RE and time) ANOVA with repeated measures of one of the factors (time). Analyses were carried out separately for the whole BSC community, S. lentigera and D. diacapsis. Community and S. lentigera data did not follow the sphericity assumption (Mauchly's test < 0.010, p < 0.011), and thus we used the Greenhouse–Geisser estimate to evaluate the significance of within-subjects tests in both cases [72].
PERMANOVA analyses were carried out with the PERMANOVA+ for the PRIMER statistical package (PRIMER-E Ltd, Plymouth Marine Laboratory, UK). SIMPER and repeated-measures ANOVA analyses were carried out using PRIMER and SPSS v. 15 (SPSS Inc, Chicago, IL, USA), respectively. As suggested by Gotelli & Ellison [73], the experiment-wide error rate was not adjusted, and all the interpretations of the effects of the different treatments were performed by evaluating the exact p-values. The data used in this article and not included in the appendices are deposited in the Dryad repository: http://dx.doi.org/10.5061/dryad.c2pd5.
3. Results
Throughout the experimental period, our warming treatment promoted an average increase of 2.4°C and 2.7°C per year in air and soil temperatures, respectively (see the electronic supplementary material, appendix S5). Such an increase was particularly evident during summer, with increases in daily averages in OTC plots relative to control plots higher than 3 ± 0.44°C and 4 ± 0.87°C (means ± s.e.) in air and soil temperatures, respectively. During winter months, such increases were of 1.5 ± 0.18°C and 1.7 ± 0.16°C (means ± s.e.) for air and soil temperatures, respectively. RSs did not substantially modify air and soil temperatures, as differences in annual temperature between control and RS plots were below 0.4°C on average. These shelters were effective in reducing the amount of rainfall reaching the soil, as they excluded between 7 and 50 per cent of the incoming rainfall depending on the event (approx. 30% on average; electronic supplementary material, appendix S3).
(a). Changes in biological soil crust cover and composition
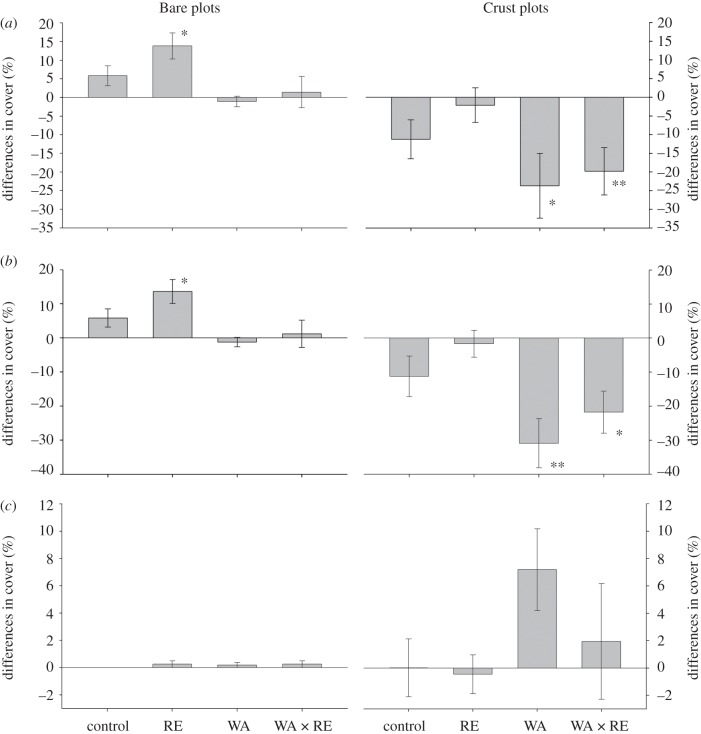
Changes in total BSC cover during the first 3 years of our experiment varied with the initial BSC cover (p = 0.022, figure 1a; electronic supplementary material, appendix S6). Averaged across all treatments, there was a 5 per cent increase and 14 per cent decrease in BSC cover in Bare and Crust plots, respectively. The magnitude of the effect of the warming treatment differed between Bare and Crust plots, as indicated by a significant WA × BSC interaction (p = 0.018, electronic supplementary material, appendix S6). In Bare plots, total BSC cover increased by 5 per cent in the control, while it decreased by 1 per cent in plots subjected to warming (figure 1a, PERMANOVA, FWA = 9.29, p = 0.004). In Crust plots, total cover decreased in both the control and WA treatment, but this reduction was substantially higher in the latter (figure 1a, PERMANOVA, FWA = 5.29, p = 0.029). Overall, we found no significant effects of RE, although a marginally significant RE × BSC interaction (p = 0.076, electronic supplementary material, appendix S6) was found when analysing variations in total cover. However, some responses to RE must be noted, as we found a significant increase in total cover in the Bare plots when rainfall was excluded (figure 1a).
Figure 1.
Differences in the total cover of the whole BSC community (including lichens and mosses) (a), lichens (b) and bryophytes (c) in areas without (Bare plots) and with well-developed biological soil crusts (Crust plots) between June 2008 and May 2011. Data represent means ± s.e. (n = 9–10). RE, rainfall exclusion; WA, warming; and WA × RE, warming and rainfall exclusion. Asterisks indicate p-values from the Wilcoxon test: *p < 0.05, **p < 0.01, ***p < 0.001. See the electronic supplementary material, appendices S13–15 for raw data.
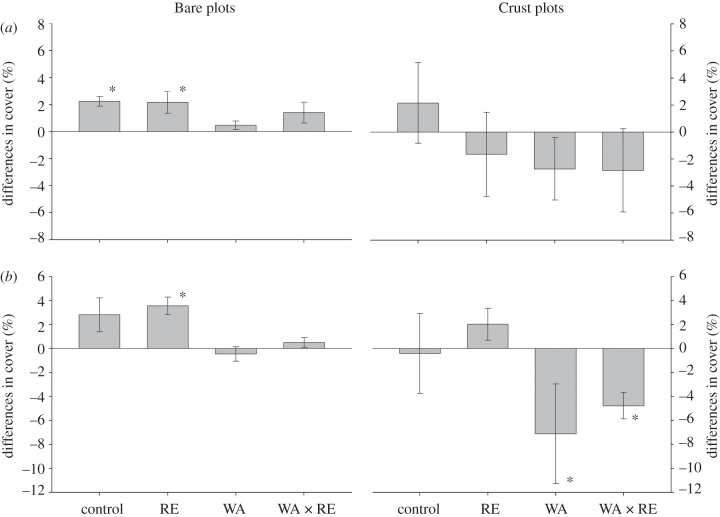
The analysis of variations in cover for lichens (figure 1b) yielded similar results to those described for the whole BSC community, but without any significant effects of either WA or RE treatments when all the data were analysed together (see the electronic supplementary material, appendix S7). However, the increase in cover observed in the Bare plots in the RE treatment was significant, as well as the reductions in this variable observed in the WA and WA × RE treatments in the Crust plots (figure 1b). Significant increases in the cover of S. lentigera were observed in the Bare plots (control and RE treatment, figure 2a), albeit we did not find significant effects of WA or RE (see the electronic supplementary material, appendix S8). Overall, the cover of D. diacapsis decreased under warming (figure 2b, p = 0.001, electronic supplementary material, appendix S8), but increased when rainfall was excluded (figure 2b, p = 0.001; electronic supplementary material, appendix S8). A significant increase in the cover of bryophytes was observed in the WA treatment (figure 1c, p < 0.001; electronic supplementary material, appendix S8), albeit this effect was largely observed in the Crust plots (FWA × BSC = 4.19, p = 0.037).
Figure 2.
Differences in total cover of S. lentigera (a) and D. diacapsis (b) in areas without (Bare plots) and with well-developed biological soil crusts (Crust plots) from June 2008 until May 2011. Data represent means ± s.e. (n = 9–10). See the electronic supplementary material, appendix S15 for raw data. Rest of legend as in figure 1.
At the beginning of the experiment, the BSC community was clearly dominated by lichens, which constituted over 96 per cent and almost 100 per cent of the total BSC cover in Crust and Bare plots, respectively (see the electronic supplementary material, appendix S1). During the first 3 years of the experiment, we found important changes in the composition of the BSC community (p = 0.011, electronic supplementary material, appendices S1 and S9), which also varied between Crust and Bare plots (p < 0.001, electronic supplementary material, appendix S9). Significant RE × BSC (p = 0.017) and RE × WA (p = 0.011) interactions were also found when analysing composition data (see the electronic supplementary material, appendix S9). Overall, and after the 3 years of our experiment, the main species of lichens that contributed to the observed changes in community composition in Bare plots were D. diacapsis, S. lentigera, F. subbracteata and Psora decipiens (Hedw.) Hoffm. (see the electronic supplementary material, appendix S10). Together with Buellia zoharyi Galun., they were the most important species driving the changes in community composition in the Crust plots observed (see the electronic supplementary material, appendix S10). The contribution of the different species to these changes, however, varied depending on the treatment considered. For example, in Crust plots subjected to warming, a strong decrease in the abundance of Toninia sedifolia (Scop.) Timdal. largely determined changes in community composition, while increases in the abundance of Collema crispum (Huds.) F. H. Wigg. in the RE treatment were the main driver of such changes in this treatment (see the electronic supplementary material, appendix S10). In the case of bryophytes, D. acutus (Brid.) K. Saito is the only moss species contributing to increase the dissimilarity between years in all the Crust plots, increasing its abundance in all of them (see the electronic supplementary material, appendix S10).
(b). Changes in biological soil crust diversity
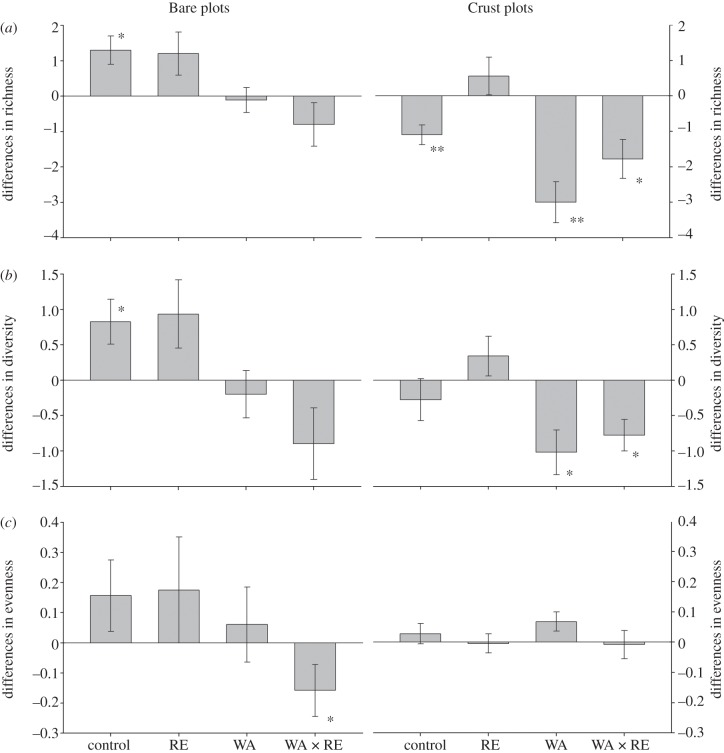
At the beginning of the experiment, a total of 21 species of lichens and mosses were identified (see the electronic supplementary material, appendix S1). Three years later, species richness of the whole BSC community increased and decreased in the control treatment at Bare and Crust plots, respectively (figure 3a). Warming significantly reduced species richness at both plot types (PERMANOVA, FBare = 11.10, p = 0.002; FCrust = 18.20, p < 0.001), although it was particularly evident in the Crust plots, where differences in richness were significantly lower than zero (figure 3a). A significant RE × BSC interaction (p = 0.013, electronic supplementary material, appendix S6) was also found when analysing richness data. Separate analyses for Bare and Crust plots revealed that, overall, richness was reduced in the RE treatment in the later plots (PERMANOVA, F = 8.48, p = 0.006). When evaluating changes in richness for lichens only, results mimicked those obtained for the whole BSC community (see the electronic supplementary material, appendices S7 and S11).
Figure 3.
Total differences in richness (a), diversity (b) and evenness (c) of the whole BSC community in areas without (Bare plots) and with well-developed biological soil crusts (Crust plots) from June 2008 until May 2011. Data represent means ± s.e. (n = 9–10). See the electronic supplementary material, appendix S13 for raw data. Rest of legend as in figure 1.
Community diversity, like richness, was negatively affected by warming, albeit differences were only significantly lower than zero in Crust plots (figure 3b). When all the data were analysed together, no significant effects were found for the treatments evaluated (electronic supplementary material, appendix S6). Results obtained for lichens were very similar (see the electronic supplementary material, appendix S11), but a significant BSC × RE interaction was found (p < 0.05, electronic supplementary material, appendix S7). Species evenness decreased in response to warming (figure 3c, p < 0.001; electronic supplementary material, appendices S6 and S7). It is interesting to note how differences in species evenness were significantly lower than zero in Bare plots subjected to both RE and WA treatments (figure 3c).
(c). Changes in biological soil crust performance
During the course of the experiment, Fv/Fm of both the BSC community and the dominant lichens was significantly higher under warming (figure 4a–c, p < 0.001; electronic supplementary material, appendix S12). However, this response was not consistent throughout the study period, as indicated by significant time × WA interactions (repeated-measures ANOVA, community, F5.2,108.7 = 4.59, p = 0.001; D. diacapsis, F10,210, p < 0.001; S. lentigera, F5.7,119.0 = 5.61, p < 0.001).
Figure 4.
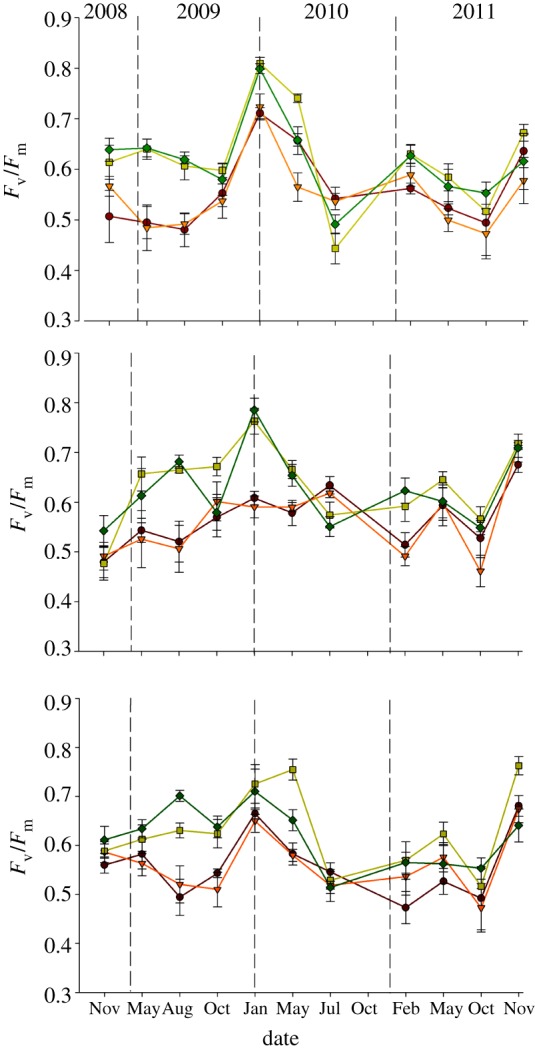
Maximum photochemical efficiency of PSII (Fv/Fm) of the whole BSC community (a), S. lentigera (b), and D. diacapsis (c) between November 2008 and November 2011. Data represent means ± s.e. (n = 6). Brown lines, control; orange lines, rainfall exclusion; light green lines, warming; dark green lines, warming and rainfall exclusion.
4. Discussion
We found important changes in the composition, structure, dynamics and functioning of the studied BSC community in response to changes in climate, and to warming in particular. Overall, and after 3 years, warming had negative effects on the cover, richness and diversity of lichens in well-developed BSC communities, and reduced notably the degree of colonization of bare ground areas by lichens, which was observed in control plots. We also found evidence of significant increases in the cover of mosses in those places where lichens were declining (e.g. Crust plots under warming). The effects of warming were largely independent of those of rainfall, which were also negligible on many of the variables evaluated. The strong and negative effects of warming, and the lack of important effects of rainfall reduction, on BSC composition and structure have not been previously reported, and contrast with climate change studies conducted in other regions [39,40]. These contrasting results highlight the need to conduct studies at multiple locations before BSC responses to climate change can be generalized.
(a). Responses of biological soil crust communities to simulated climate change
Warming promoted a dramatic decrease in the cover of lichens in Crust plots during the first 3 years of our experiment, a response that was not modulated by changes in the total rainfall amount. At the same time, this treatment promoted an increase in the cover of mosses in these plots. These findings contrast with the general impression that lichens are long-lived and extremely stress-tolerant organisms that have a limited ability to respond quickly to changes in environmental conditions because of their slow growth rate [74]. However, despite that BSC-forming lichens are long-lived organisms, they can be quite dynamic, and can quickly respond to changes in environmental conditions and disturbances of the soil surface. Lázaro et al. [75] and Dojani et al. [76] have reported significant increments in the cover of BSC-forming lichens in the semi-arid areas from Spain and South Africa, respectively, in a few years, with increments in the cover of a particular species (e.g. S. lentigera) above 20 per cent in a year [75]. Belnap et al. [77] found decreases in the cover of some lichen species in the Colorado Plateau in response to an increase in monthly maximum temperatures over 8 years. Grote et al. [56] used a physiological argument to suggest that in cyanobacteria-dominated BSCs, warming is likely to reduce carbon uptake and growth because it will increase respiration rates without a comparable increase in photosynthetic rates. An interesting exception is that BSCs dominated by mosses were relatively unaffected by experimental warming [39], suggesting a lower sensitivity than lichens or cyanobacteria to increases in temperature. Warming-induced decreases in lichen abundance are not restricted to drylands, as similar results have also been detected in arctic ecosystems [78]. However, the primary stress factor in these ecosystems is cold temperatures, rather than low water availability. Studies conducted in these areas have attributed decreases in lichens or mosses to an increase in vascular vegetation with warming [79,80]. However, this is an unlikely explanation for our study because BSC plots were located in areas where vascular vegetation was excluded and plant litter was nearly non-existent. Furthermore, annual plants were carefully clipped from the plots surveyed every spring.
The large declines in lichen cover observed under warming could be promoted by higher respiration rates in this treatment [56], which would lead to increases in mortality and decreases in cover if the photosynthetic rate cannot compensate these carbon losses [40]. In this direction, soil respiration in BSC-dominated areas is clearly enhanced by experimental warming in our experiment [46], albeit we cannot separate the fraction of this respiration corresponding to the BSCs themselves. At first glance, our Fv/Fm data would not fully support this potential mechanism underlying declines in lichen cover; Fv/Fm was significantly higher in plots subjected to warming, suggesting higher efficiency of the photosynthetic process (figure 4). We must highlight, however, that the decrease in cover observed with warming was mainly attributable to species such as B. zoharyi, T. sedifolia and F. subbracteata (see the electronic supplementary material, appendix S10), while the species where Fv/Fm was specifically measured either decreased slightly in cover (D. diacapsis) or increased it (S. lentigera) in response to this treatment (see the electronic supplementary material, appendix S10). As these species were the most abundant at our study site in the crust plots subjected to warming (see the electronic supplementary material, appendices S1 and S10), and the community measurements of Fv/Fm consisted of random measurements over each sampling plot, the later measurements reflected mostly the behaviour of the dominant species (figure 4), and thus did not capture properly the physiological status of the species that were mostly affected by warming. Indeed, significant reductions in the Fv/Fm of BSC-forming lichens with a 2.1–3.8°C warming and an approximately 30 per cent reduction in dew and fog inputs have been found in South Africa [57].
Why do mosses respond differently to warming than lichens? Possibly, this is because an early break of dormancy and reactivation with warming promoted initiation of new stems, mosses being more adapted for the new conditions than lichens [81,82]. Such a response would probably occur during the favourable seasons in terms of humidity and soil moisture, such as during autumn and the early part of the winter, where bryophytes in the warming plots could encounter a rare convergence of warm temperatures and adequate moisture. In this regard, mosses may be more phenotypically plastic than lichens in their ability to change their seasonal activity in order to increase their cover [83]. This could also reduce competition for living space with lichens, which is likely to be very intense in the studied communities [84], increasing the abundance and cover of mosses at the expense of lichens.
Another potential mechanism that could explain our results is mediation by pigments such as zeaxanthin, which is formed to protect chlorophyll during the desiccation process (normally in darkness) and to promote faster acclimation when conditions return to the optimal situation [85]. In this context, recent studies [85,86] suggest that if desiccation occurs faster than in normal conditions, photoprotective mechanisms do not work properly, and, as a consequence, structural damages are likely to occur. This mechanism has already been demonstrated in the lichen Lobaria pulmonaria (L.) Hoffm. [86], and could explain declines in lichen cover with warming, as desiccation should occur much faster because of the temperature increase in this treatment (see the electronic supplementary material, appendix S5). Mosses usually show more structural complexity than lichens [83,87], and this may allow mosses to have quicker responses to desiccation [88]. Very few data on desiccation tolerance of BSC constituents are available, and most of the studies on the topic have been carried out on bryophytes [83]. This work suggests that mosses could be more tolerant to desiccation than lichens [89], but there is not enough evidence to affirm this with confidence. Because of the known structural limitation of the lichen thallus, if bryophytes desiccate more slowly than lichens at our study site, mosses could have the opportunity to activate protective mechanisms against warming, and thus to be well prepared to compete against lichens and increase their abundance and cover under warming. This mechanism, however, cannot be proved by our results, and further studies are needed to evaluate its role in the changes in the cover of lichens and mosses observed in our experiment.
Given the proven importance of rainfall to lichens and mosses, it was surprising to find that, unlike warming, rainfall reduction did not have negative effects on the cover and performance of BSCs, although some negative effects on species richness were found. Our results could be due to the fact that their apparent sensitivity to changes in rainfall is more conditioned by the size, duration and timing of rainfall events than on average rainfall [37,39]. We expect that more so than with warming, the response of BSCs will be strongly dependent on the timing and characteristics of rainfall pulses in a given locality, and thus may vary widely from place to place. Carbon balance in BSC-forming mosses of the Mojave Desert (a winter-rainfall desert) seems to largely depend on the size of individual events (and thus the length of the hydration period), in addition to the length of desiccation periods in between them and seasonality of the event [90]. Our manipulation did not influence seasonality, but influenced event size, and in consequence indirectly affected length of desiccation period because smaller events hydrate BSCs for less time. In our study region, autumn and spring rainfall events can be both large and frequent compared with the much more arid Mojave Desert. Thus, we can only hypothesize that an approximately 30 per cent reduction in the natural event size is insufficient to induce change in only a few years, though we cannot rule out longer term changes.
The different treatments (warming and rainfall exclusion) promoted important differences in the dynamics of the BSC community studied during the first 3 years of our experiment. We found increases in the abundance of species such as D. diacapsis, S. lentigera, F. subbracteata and P. decipiens in all treatments in the Bare plots. This suggests that these species may be some of the first colonizing species after disturbance situations or in natural conditions [75,91]. Overall, BSC richness and diversity, but not evenness, decreased with warming. In the Crust plots, reductions in rainfall also promoted a reduction in species richness, albeit it was mainly evident under warming. Similar decreases in the richness and diversity of BSC components have been reported in arctic ecosystems subjected to warming [78].
(b). Consequences of biological soil crust decline in semi-arid Mediterranean regions
The observed changes in the diversity, richness and cover of BSCs, and in the abundance of particular lichen and moss species could have profound consequences on ecosystem functioning. Previous studies carried out in BSC-dominated ecosystems have found positive effects of species richness and other components of biodiversity in maintaining processes important for ecosystem structure and functioning, such as soil stability [47,92], dust trapping [47] and N cycling [46]. Bowker et al. [49] suggest that there may be a high degree of functional singularity among different BSC mosses and lichens in Spanish drylands, indicating that a species loss is not likely to be compensated for by another species.
Changes in the composition of BSC species with warming could also have important implications on the water balance and the maintenance of plant patches in semi-arid areas. Studies carried out in our study site have found overall positive and negative effects of mosses and lichens, respectively, on infiltration [27]. Thus, decreases in the cover of lichens and increases in that of mosses suggested by our results would increase the infiltration in BSC-dominated areas, reducing the amount of runoff that would normally be redistributed and captured by the plants [93]. Given the dependence of plants such as S. tenacissima on water inputs coming from runoff [94], promoted by the concentration of the roots of this species under its canopy, such an effect would further exacerbate the negative effects on the performance and growth of semi-arid Mediterranean vegetation expected with ongoing climate change [95].
Recent research has found that BSC-dominated microsites are the main contributor to soil CO2 efflux in our study site [45]. In areas with well-developed BSCs, increases in such flux in response to increases in temperature during spring and autumn are higher than in areas without or with low BSC cover [45]. In the Kalahari Desert, the same pattern was found, whereby soil respiration was enhanced with increases in air temperature, which could represent a net loss of carbon storages and a potential process of soil deterioration [22,96]. Ongoing measurements of soil CO2 efflux at our site indicate that this flux was higher in warmed plots throughout most of the study period, and that this effect is particularly evident in Crust plots (C. Escolar & F. T. Maestre 2012, unpublished data) Overall, these results suggest that warming would promote C losses in BSC-dominated areas. Given that these areas are also losing lichen cover, and that the BSC communities are becoming less diverse, this response would be exacerbated in the future because of reduced photosynthetic capacity of BSCs.
(c). Concluding remarks
According to our results, increases in air temperature such as those expected by the middle of the twenty-first century will have profound negative impacts on the cover, composition and diversity of BSCs in Mediterranean regions. Contrary to the common vision about these organisms, BSCs respond quickly (in terms of years) to the environmental changes created, and seem to be less tolerant to drought than they are usually considered to be. These results add to existing studies, which in most cases suggest that BSCs are most likely to be negatively affected by projected warming. We are much farther from a generalization about the response of BSCs to reduced rainfall. Our results suggest that BSCs of the Mediterranean may be slow to change based on rainfall reduction alone, but there is evidence from various localities around the world that suggest that timing, frequency and individual event size may exert very rapid effects [39,40].
Our findings indicate that the expected changes in total cover, richness and composition under warming would reduce or even negate the positive effects of BSCs on important functional variables, promoting an overall reduction in ecosystem functioning in terms of carbon fixation, nutrient pools, water infiltration and soil stabilization. These changes could also exacerbate direct effects of climate change on processes such as soil CO2 efflux, and could also propagate beyond BSC communities to affect plant patches, and thus the overall structure of drylands. Future studies aiming to evaluate climate change effects on these regions must explicitly consider the importance of biological soil crusts, as this is crucial for a full understanding of the role of these organisms and their attributes as drivers of ecosystem functioning in drylands, and of their responses to ongoing climate change.
Acknowledgements
We thank the Instituto Madrileño de Investigación y Desarrollo Rural, Agrario y Alimentario (IMIDRA) for allowing us to work in the Aranjuez Experimental Station (Finca de Sotomayor), the AEMET for providing climatic information, Katia Cezón (Real Jardín Botánico de Madrid) for her help with identification of bryophytes and Victoria Ochoa, Patricia Alonso, Rebecca L. Mau, Beatriz Gozalo and Miguel Berdugo for their help in the field. Rob Salguero-Gómez and two anonymous reviewers provided multiple comments that helped to improve previous versions of this manuscript. This research was funded by the European Research Council (ERC) under the European Community's Seventh Framework Programme (FP7/2007–2013)/ERC Grant agreement no. 242658 (BIOCOM), and by the British Ecological Society, which supported C.E. through the Studentship 231/1975. F.T.M. acknowledges support from the Spanish Ministerio de Educación (Salvador de Madariaga programme, PR2010-0230) during the writing of the manuscript.
References
- 1.Le Houeroun H. N. 1996. Climate change, drought and desertification. J. Arid Environ. 34, 133–185 10.1006/jare.1996.0099 (doi:10.1006/jare.1996.0099) [DOI] [Google Scholar]
- 2.Maestre F. T., Salguero-Gómez R., Quero J. L. 2012. It is getting hotter in here: determining and projecting the impacts of global environmental change on drylands. Phil. Trans. R. Soc. B 367, 3062–3075 10.1098/rstb.2011.0323 (doi:10.1098/rstb.2011.0323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IPCC 2007. Observed changes in climate and their effects. In Climate Change 2007: Synthesis Report (eds Core Writing Team, Pachauri R. K., Reisinger A.), 104 pp Geneva, Switzerland: IPCC [Google Scholar]
- 4.McCarty J. P. 2001. Ecological consequences of recent climate change. Conserv. Biol. 15, 320–331 10.1046/j.1523-1739.2001.015002320.x (doi:10.1046/j.1523-1739.2001.015002320.x) [DOI] [Google Scholar]
- 5.Walther G. R., et al. 2010. Community and ecosystem responses to recent climate change. Phil. Trans. R. Soc. B. 365, 2019–2024 10.1098/rstb.2010.0021 (doi:10.1098/rstb.2010.0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walther G. R., Post E., Convey P., Menzel A., Parmesank C., Beebee T. J. C., Fromentin J-M., Hoegh-Guldberg O., Bairlein F. 2002. Ecological responses to recent climate change. Science 416, 389–395 10.1038/416389a (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 7.González-Megías A., Menéndez R. 2012. Climate change effects on above- and below-ground interactions in a dryland ecosystem. Phil. Trans. R. Soc. B 367, 3115–3124 10.1098/rstb.2011.0346 (doi:10.1098/rstb.2011.0346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson R. J., Gutiérrez D., Gutiérrez J., Martínez D., Agudo R., Monserrat V. J. 2005. Changes to the elevational limits and extent of species ranges associated with climate change. Ecol. Lett. 8, 1138–1146 10.1111/j.1461-0248.2005.00824.x (doi:10.1111/j.1461-0248.2005.00824.x) [DOI] [PubMed] [Google Scholar]
- 9.Salguero-Gómez R., Siewert W., Casper B. B., Tielbörger K. 2012. A demographic approach to study effects of climate change in desert plants. Phil. Trans. R. Soc. B 367, 3100–3114 10.1098/rstb.2012.0074 (doi:10.1098/rstb.2012.0074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visser M. E., Both C. 2005. Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2569 10.1098/rspb.2005.3356 (doi:10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bates B. C., Kundzewicz Z. W., Wu S., Palutikof J. P. Climate change and water. Technical Paper of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: IPCC: 2008. [Google Scholar]
- 12.Mariotti A., Zeng N., Yoon J. H., Artale V., Navarra A., Alpert P., Li L. Z. X. 2008. Mediterranean water cycle changes: transition to drier 21st century conditions in observations and CMIP3 simulations. Environ. Res. Lett. 3, 044001. 10.1088/1748-9326/3/4/044001 (doi:10.1088/1748-9326/3/4/044001) [DOI] [Google Scholar]
- 13.Haensler A., Hagemann S., Jacob D. 2011. The role of the simulation setup in a long-term high-resolution climate change projection for the southern African region. Theor. Appl. Clim. 106, 153–169 10.1007/s00704-011-0420-1 (doi:10.1007/s00704-011-0420-1) [DOI] [Google Scholar]
- 14.Zavaleta E. S., Shaw M. R., Chiariello N. R., Thomas B. D., Cleland E. E., Field C. B., Mooney H. A. 2003. Grassland responses to three years of elevated temperature, CO2, precipitation, and N deposition. Ecol. Monogr. 73, 585–604 10.1890/02-4053 (doi:10.1890/02-4053) [DOI] [Google Scholar]
- 15.Landesman W. J., Dighton J. 2011. Shifts in microbial biomass and the bacteria:fungi ratio occur under field conditions within 3 h after rainfall. Microb. Ecol. 62, 228–236 10.1007/s00248-011-9811-1 (doi:10.1007/s00248-011-9811-1) [DOI] [PubMed] [Google Scholar]
- 16.Safirel U., Adeel Z. 2005. Dryland systems. In Ecosystems and human well-being: current state and trends, vol. 1 (eds. Hassan R., Scholes R., Neville A.), pp. 623–662 Washington, DC: Island Press [Google Scholar]
- 17.Belnap J., Lange O. L. 2003. Biological soil crusts: structure, function, and management. Ecological Studies Series, no. 150, 2nd edn Berlin, Germany: Springer [Google Scholar]
- 18.Castillo-Monroy A. P., Maestre F. T. 2011. La costra biológica del suelo: Avances recientes en el conocimiento de su estructura y función ecológica. Rev. Chil. Hist. Nat. 84, 1–21 10.4067/S0716-078X2011000100001 (doi:10.4067/S0716-078X2011000100001) [DOI] [Google Scholar]
- 19.Eldridge D. J., Greene R. S. B. 1994. Microbiotic soil crusts: a review of their roles in soil and ecological processes in the rangelands of Australia. Aust. J. Soil. Res. 32, 389–415 10.1071/SR9940389 (doi:10.1071/SR9940389) [DOI] [Google Scholar]
- 20.Belnap J., Kaltenecker J. H., Rosentreter R., Williams J., Leonard S., Eldridge D. Biological soil crusts: ecology and management. Technical Reference No. 1730-2. Denver, CO: USDI. 2001.
- 21.Housman D. C., Powers H. H., Collins A. D., Belnap J. 2006. Carbon and nitrogen fixation differ between successional stages of biological soil crusts in the Colorado Plateau and Chihuahuan Desert. J. Arid Environ. 66, 620–634 10.1016/j.jaridenv.2005.11.014 (doi:10.1016/j.jaridenv.2005.11.014) [DOI] [Google Scholar]
- 22.Thomas A. D. 2012. Impact of grazing intensity on seasonal variations of soil organic carbon and soil CO2 efflux in two semiarid grasslands in southern Botswana. Phil. Trans. R. Soc. B 367, 3076–3086 10.1098/rstb.2012.0102 (doi:10.1098/rstb.2012.0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belnap J. 2002. Nitrogen fixation in biological soil crust from southeast Utah, USA. Biol. Fert. Soils 35, 128–135 10.1007/s00374-002-0452-x (doi:10.1007/s00374-002-0452-x) [DOI] [Google Scholar]
- 24.Castillo-Monroy A. P., Maestre F. T., Delgado-Baquerizo M., Gallardo A. 2010. Biological soil crusts modulate nitrogen availability in semi-arid ecosystems: insights from a Mediterranean grassland. Plant Soil 333, 21–34 10.1007/s11104-009-0276-7 (doi:10.1007/s11104-009-0276-7) [DOI] [Google Scholar]
- 25.Delgado-Baquerizo M., Castillo-Monroy A. P., Maestre F. T., Gallardo A. 2010. Plants and biological soil crusts modulate the dominance of N forms in a semi-arid grassland. Soil Biol. Biochem. 42, 376–378 10.1016/j.soilbio.2009.11.003 (doi:10.1016/j.soilbio.2009.11.003) [DOI] [Google Scholar]
- 26.Chaudhary V. B., Bowker M. A., O'Dell T. E., Grace J. B., Redman A. E., Rillig M. C., Johnson N. C. 2009. Untangling the biological contributions to soil stability in semiarid shrublands. Ecol. Appl. 19, 110–122 10.1890/07-2076.1 (doi:10.1890/07-2076.1) [DOI] [PubMed] [Google Scholar]
- 27.Eldridge D.J., Bowker M. A., Maestre F. T., Alonso P., Mau R. L., Papadopolous J. 2010. Interactive effects of three ecosystem engineers on infiltration in a semi-arid Mediterranean grassland. Ecosystems 13, 499–510 10.1007/s10021-010-9335-4 (doi:10.1007/s10021-010-9335-4) [DOI] [Google Scholar]
- 28.Chamizo S., Cantón Y., Lázaro R., Solé-Benet A., Domingo F. 2012. Crust composition and disturbance drive infiltration through biological soil crusts in semiarid ecosystems. Ecosystems 15, 148–161 10.1007/s10021-011-9499-6 (doi:10.1007/s10021-011-9499-6) [DOI] [Google Scholar]
- 29.Bates S. T., García-Pichel F. 2009. A culture-independent study of free-living fungi in biological soil crusts of the Colorado Plateau: their diversity and relative contribution to microbial biomass. Environ. Microbiol. 11, 56–67 10.1111/j.1462-2920.2008.01738.x (doi:10.1111/j.1462-2920.2008.01738.x) [DOI] [PubMed] [Google Scholar]
- 30.Castillo-Monroy A. P., Bowker M. A., Maestre F. T., Rodríguez-Echeverría S., Martinez I., Barraza-Zepeda C. E., Escolar C. 2011. Relationships between biological soil crusts, bacterial diversity and abundance, and ecosystem functioning: insights from a semi-arid Mediterranean environment. J. Veg. Sci. 22, 165–174 10.1111/j.1654-1103.2010.01236.x (doi:10.1111/j.1654-1103.2010.01236.x) [DOI] [Google Scholar]
- 31.Neher D. A., Lewins S. A., Weicht T. R., Darby B. J. 2009. Microarthropod communities associated with biological soil crusts in the Colorado Plateau and Chihuahuan deserts. J. Arid Environ. 73, 672–677 10.1016/j.jaridenv.2009.01.013 (doi:10.1016/j.jaridenv.2009.01.013) [DOI] [Google Scholar]
- 32.De Falco L. A., Detling J. K., Tracy C. R., Warren S. D. 2001. Physiological variation among native and exotic winter annual plants associated with microbiotic crusts in the Mojave Desert. Plant Soil 234, 1–14 10.1023/A:1010323001006 (doi:10.1023/A:1010323001006) [DOI] [Google Scholar]
- 33.Green L. E., Porras-Alfaro A., Sinsabaugh R. L. 2008. Translocation of nitrogen and carbon integrates biotic crust and grass production in desert grassland. J. Ecol. 96, 1076–1085 10.1111/j.1365-2745.2008.01388.x (doi:10.1111/j.1365-2745.2008.01388.x) [DOI] [Google Scholar]
- 34.Lange O. L., Belnap J., Reichenberger H. 1998. Photosynthesis of the cyanobacterial soil-crust lichen Collema tenax from arid lands in southern Utah, USA: role of water content on light and temperature responses of CO2 exchange. Funct. Ecol. 12, 195–202 10.1046/j.1365-2435.1998.00192.x (doi:10.1046/j.1365-2435.1998.00192.x) [DOI] [Google Scholar]
- 35.Lange O. L. 2003. Photosynthetic productivity of the epilithic lichen Lecanora muralis: long-term field monitoring of CO2 exchange and its physiological interpretation II. Diel and seasonal patterns of net photosynthesis and respiration. Flora 198, 55–70 10.1016/S0367-2530(04)70052-3 (doi:10.1016/S0367-2530(04)70052-3) [DOI] [Google Scholar]
- 36.Pintado L. G., Sancho J. M., Blanquer T. G., Green A., Lázaro R. 2010. Microclimatic factors and photosynthetic activity of crustose lichens from the semiarid southeast of Spain: long-term measurements for Diploschistes diacapsis. Bibl. Lichenol. 105, 211–224 [Google Scholar]
- 37.Belnap J., Phillips S. L., Miller M. E. 2004. Response of desert biological soil crusts to alterations in precipitation frequency. Oecologia 141, 306–316 10.1007/s00442-003-1438-6 (doi:10.1007/s00442-003-1438-6) [DOI] [PubMed] [Google Scholar]
- 38.Belnap J., Phillips S. L., Flint S., Money J., Caldwell M. 2008. Global change and biological soil crusts: effects of ultraviolet augmentation under altered precipitation regimes and nitrogen additions. Global Change Biol. 14, 670–686 10.1111/j.1365-2486.2007.01509.x (doi:10.1111/j.1365-2486.2007.01509.x) [DOI] [Google Scholar]
- 39.Zelikova T. J., Housman D. C., Grote E. D., Neher D., Belnap J. 2012. Biological soil crusts show limited response to warming but larger response to increased precipitation frequency: implications for soil processes on the Colorado Plateau. Plant Soil 355, 265–282 10.1007/s11104-011-1097-z (doi:10.1007/s11104-011-1097-z) [DOI] [Google Scholar]
- 40.Reed S. C., Coe K. K., Sparks J. P., Housman D. C., Zelikova T. J., Belnap J. In press Changes to dryland rainfall result in rapid moss mortality and altered soil fertility. Nat. Clim. Change. 10.1038/nclimate1596 (doi:10.1038/nclimate1596) [DOI] [Google Scholar]
- 41.Büdel B., Darienko T., Deutschewitz K., Dojani S., Friedl T., Mohr K. I., Salisch M., Reisser W., Weber B. 2009. Southern African biological soil crusts are ubiquitous and highly diverse in drylands, being restricted by rainfall frequency. Microbial Ecol. 57, 229–247 10.1007/s00248-008-9449-9 (doi:10.1007/s00248-008-9449-9) [DOI] [PubMed] [Google Scholar]
- 42.Kidron G. J., Herrnstadt I., Barzilay E. 2002. The role of dew as a moisture source for sand microbiotic crusts in the Negev Desert, Israel. J. Arid Environ. 52, 517–533 10.1006/jare.2002.1014 (doi:10.1006/jare.2002.1014) [DOI] [Google Scholar]
- 43.Del Prado R., Sancho L. G. 2007. Dew as a key factor for the distribution pattern of Teloschistes lacunosus in the Tabernas Desert (Almería, Spain). Flora 202, 417–428 10.1016/j.flora.2006.07.007 (doi:10.1016/j.flora.2006.07.007) [DOI] [Google Scholar]
- 44.Moro M. J., Were A., Villagarcía L., Cantón Y., Domingo F. 2007. Dew measurement by Eddy covariance and wetness sensor in a semiarid ecosystem of SE Spain. J. Hydrol. 335, 295–302 10.1016/j.jhydrol.2006.11.019 (doi:10.1016/j.jhydrol.2006.11.019) [DOI] [Google Scholar]
- 45.Castillo-Monroy A. P., Maestre F. T., Rey A., Soliveres S., García-Palacios P. 2011. Biological soil crusts are the main contributor to soil CO2 efflux and modulate its spatio-temporal variability in a semi-arid ecosystem. Ecosystems 14, 835–847 10.1007/s10021-011-9449-3 (doi:10.1007/s10021-011-9449-3) [DOI] [Google Scholar]
- 46.Maestre F. T., et al. 2010. Do biotic interactions modulate ecosystem functioning along abiotic stress gradients? Insights from semi-arid Mediterranean plant and biological soil crust communities. Phil. Trans. R. Soc. B 365, 2057–2070 10.1098/rstb.2010.0016 (doi:10.1098/rstb.2010.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowker M. A., Maestre F. T., Escolar C. 2010. Biological crusts as a model system for examining the biodiversity–ecosystem function relationship in soils. Soil Biol. Biochem. 42, 405–417 10.1016/j.soilbio.2009.10.025 (doi:10.1016/j.soilbio.2009.10.025) [DOI] [Google Scholar]
- 48.Maestre F. T., Castillo-Monroy A. P., Bowker M. A., Ochoa-Hueso R. 2012. Species richness effects on ecosystem multifunctionality depend on evenness, composition and spatial pattern. J. Ecol. 100, 317–330 10.1111/j.1365-2745.2011.01918.x (doi:10.1111/j.1365-2745.2011.01918.x) [DOI] [Google Scholar]
- 49.Bowker M. a., Mau R. L., Maestre F. T., Escolar C., Castillo-Monroy A. P. 2011. Functional profiles reveal unique ecological roles of various biological soil crust organisms. Funct. Ecol. 25, 787–795 10.1111/j.1365-2435.2011.01835.x (doi:10.1111/j.1365-2435.2011.01835.x) [DOI] [Google Scholar]
- 50.Gotelli N. J., Ulrich W., Maestre F. T. 2011. Randomization tests for quantifying species importance to ecosystem function. Methods Ecol. Evol. 2, 634–642 10.1111/j.2041-210X.2011.00121.x (doi:10.1111/j.2041-210X.2011.00121.x) [DOI] [Google Scholar]
- 51.Rogers R.W. 1972. Soil surface lichens in arid and sub-arid southeastern Australia. II. Phytosociology and geographic zonation. Aust. J. Bot. 20, 215–227 10.1071/BT9720215 (doi:10.1071/BT9720215) [DOI] [Google Scholar]
- 52.Zedda L., Gröngröft A., Schultz M., Petersen A., Mills A., Rambold G. 2010. Patterns of soil lichen diversity along the BIOTA transects in relation to climate and soil features. In Biodiversity in southern Africa. Volume 2: Patterns and processes at regional scale (eds Schmiedel U., Jürgens N.), pp. 100–106 Göttingen, Germany/Windhoek, Namibia: Klaus Hess Publishers [Google Scholar]
- 53.Zedda L., Gröngröft A., Schultz M., Petersen A., Mills A., Rambold G. 2011. Distribution patterns of soil lichens across the principal biomes of southern Africa. J. Arid Environ. 75, 215–220 10.1016/j.jaridenv.2010.10.007 (doi:10.1016/j.jaridenv.2010.10.007) [DOI] [Google Scholar]
- 54.West N. E., Stark J. M., Johnson D. W., Abrams M. M., Wight J. R., Heggem D., Peck S. 1994. Effects of climatic change on the edaphic features of arid and semiarid lands of western North America. Arid Soil Res. Rehab. 8, 307–351 10.1080/15324989409381408 (doi:10.1080/15324989409381408) [DOI] [Google Scholar]
- 55.Körner C. 2000. Biosphere responses to CO2 enrichment. Ecol. Appl. 10, 1590–1619 10.1890/1051-0761(2000)010[1590:BRTCE]2.0.CO;2 (doi:10.1890/1051-0761(2000)010[1590:BRTCE]2.0.CO;2) [DOI] [Google Scholar]
- 56.Grote E. E., Belnap J., Housman D. C., Sparks J. P. 2010. Carbon exchange in biological soil crust communities under differential temperatures and soil water contents: implications for global change. Global Change Biol. 16, 2763–2774 10.1111/j.1365-2486.2010.02201.x (doi:10.1111/j.1365-2486.2010.02201.x) [DOI] [Google Scholar]
- 57.Maphangwa K.W., Musil C.F., Raitt L., Zedda L. 2012. Experimental climate warming decreases photosynthetic efficiency of lichens in an arid South African ecosystem. Oecologia 169, 257–268 10.1007/s00442-011-2184-9 (doi:10.1007/s00442-011-2184-9). [DOI] [PubMed] [Google Scholar]
- 58.De Castro M., Martín-Vide J., Alonso S. 2005. El clima de España: pasado, presente y escenarios de clima para el siglo XXI. In Evaluación Preliminar de los Impactos en España por Efecto del Cambio Climático (ed. Moreno J. M.), pp. 1–64 Madrid, Spain: Ministerio Medio Ambiente. [Google Scholar]
- 59.Barker D. H., Stark L. R., Zimpfer J. F., Mcletchie N. D., Smith S. D. 2005. Evidence of drought-induced stress on biotic crust moss in the Mojave Desert. Plant Cell Environ. 28, 939–947 10.1111/j.1365-3040.2005.01346.x (doi:10.1111/j.1365-3040.2005.01346.x) [DOI] [Google Scholar]
- 60.Marques M. J., Bienes R., Jiménez L. 2008. Soil degradation in Central Spain due to sheet water erosion by low-intensity rainfall events. Earth Surf. Process. Landforms 33, 414–423 10.1002/esp.1564 (doi:10.1002/esp.1564) [DOI] [Google Scholar]
- 61.Arft A. M., et al. 1999. Responses of tundra plants to experimental warming: meta-analysis of the international tundra experiment. Ecol. Monogr. 69, 491–511 10.1890/0012-9615(1999)069[0491:ROTPTE]2.0.CO;2 (doi:10.1890/0012-9615(1999)069[0491:ROTPTE]2.0.CO;2) [DOI] [Google Scholar]
- 62.Yahdjian L., Sala O. 2002. A rainout shelter design for intercepting different amounts of rainfall. Oecologia 133, 95–101 10.1007/s00442-002-1024-3 (doi:10.1007/s00442-002-1024-3) [DOI] [PubMed] [Google Scholar]
- 63.Colwell R. K.EstimateS: statistical estimation of species richness and shared species from samples, 2011. Version 8.2.0. See http://viceroy.eeb.uconn.edu/estimates/index.html .
- 64.Pielou E. C. 1966. The measurement of diversity in different types of biological collections. J. Theor. Biol. 13, 131–144 10.1016/0022-5193(66)90013-0 (doi:10.1016/0022-5193(66)90013-0) [DOI] [Google Scholar]
- 65.Armas C., Pugnaire F. I., Ordiales R. 2004. Measuring plant interactions: a new comparative index. Ecology 85, 2682–2686 10.1890/03-0650 (doi:10.1890/03-0650) [DOI] [Google Scholar]
- 66.Markham J. H., Chanway C. P. 1996. Measuring plant neighbor effects. Funct. Ecol. 10, 548–549 [Google Scholar]
- 67.Bowker M. A., Reed S. C., Belnap J., Phillips S. L. 2002. Temporal variation in community composition, pigmentation, and F(v)/F(m) of desert cyanobacterial soil crusts. Microbial Ecol. 43, 13–25 10.1007/s00248-001-1013-9 (doi:10.1007/s00248-001-1013-9) [DOI] [PubMed] [Google Scholar]
- 68.Lan S., Wu L., Zhang D., Hu C. 2011. Composition of photosynthetic organisms and diurnal changes of photosynthetic efficiency in algae and moss crusts. Plant Soil 351, 325–336 10.1007/s11104-011-0966-9 (doi:10.1007/s11104-011-0966-9) [DOI] [Google Scholar]
- 69.Anderson M. J. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46 10.1111/j.1442-9993.2001.01070.pp.x (doi:10.1111/j.1442-9993.2001.01070.pp.x) [DOI] [Google Scholar]
- 70.Anderson M. J., Ter Braak C. J. F. 2003. Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Sim. 73, 85–113 10.1080/00949650215733 (doi:10.1080/00949650215733) [DOI] [Google Scholar]
- 71.Clarke K. R., Warwick R. M. 1994. Change in marine communities: an approach to statistical analysis and interpretation. Plymouth, UK: Plymouth Marine Laboratory [Google Scholar]
- 72.Quinn G. P., Keough M. J. 2002. Experimental design and data analysis for biologists. Cambridge, UK: Cambridge University Press [Google Scholar]
- 73.Gotelli N. J., Ellison A. M. 2004. A primer of ecological statistics. Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- 74.Becket R. P., Minibayeva F. V. 2007. Dessication tolerance in lichens. In Plant dessication tolerance (eds. Jenks M. A., Wood A. J.), pp. 91–109 Oxford, UK: Blackwell [Google Scholar]
- 75.Lázaro R., Canton Y., Solé-Benet A., Bevan J., Alexander R., Sancho L., Puigdefabregas J. 2008. The influence of competition between lichen colonization and erosion on the evolution of soil surfaces in the Tabernas badlands (SE Spain) and its landscape effects. Geomorphology 102, 252–266 10.1016/j.geomorph.2008.05.005 (doi:10.1016/j.geomorph.2008.05.005) [DOI] [Google Scholar]
- 76.Dojani S., Büdel B., Deutschewitz K., Weber B. 2011. Rapid succession of biological soil crusts after experimental disturbance in the Succulent Karoo, South Africa. App. Soil Ecol. 48, 263–269 10.1016/j.apsoil.2011.04.013 (doi:10.1016/j.apsoil.2011.04.013) [DOI] [Google Scholar]
- 77.Belnap J., Phillips S., Troxler T. 2006. Soil lichen and moss cover and species richness can be highly dynamic: the effects of invasion by the annual exotic grass Bromus tectorum, precipitation, and temperature on biological soil crusts in SE Utah. Appl. Soil Ecol. 32, 63–76 10.1016/j.apsoil.2004.12.010 (doi:10.1016/j.apsoil.2004.12.010) [DOI] [Google Scholar]
- 78.Lang S. I., Cornelissen J. H. C., Shaver G. R., Ahrens M., Callaghan T. V., Molau U., Ter Braak C. J. F., Hölzer A., Aerts R. 2011. Arctic warming on two continents has consistent negative effects on lichen diversity and mixed effects on bryophyte diversity. Global Change Biol. 18, 1096–1107 10.1111/j.1365-2486.2011.02570.x (doi:10.1111/j.1365-2486.2011.02570.x) [DOI] [Google Scholar]
- 79.Cornelissen J. H. C., et al. 2001. Global change and arctic ecosystems: is lichen decline a function of increases in vascular plant biomass? J. Ecol. 89, 984–994 10.1046/j.1365-2745.2001.00625.x (doi:10.1046/j.1365-2745.2001.00625.x) [DOI] [Google Scholar]
- 80.Wahren C-H. A., Walker M. D., Bret-Harte M. S. 2005. Vegetation responses in Alaskan arctic tundra after 8 years of a summer warming and winter snow manipulation experiment. Global Change Biol. 11, 537–552 10.1111/j.1365-2486.2005.00927.x (doi:10.1111/j.1365-2486.2005.00927.x) [DOI] [Google Scholar]
- 81.Kappen L., Valladares F. 2007. Opportunistic growth and desiccation tolerance: the ecological success of poikilohydrous autotrophs. In Functional plant ecology (eds Pugnaire F., Valladares F.), pp. 7–65 New York, NY: Taylor and Francis Group, CRC Press [Google Scholar]
- 82.Bjerke J. W., Bokhorst S., Zielke M., Callaghan T. V., Bowles F. W., Phoenix G. K. 2011. Contrasting sensitivity to extreme winter warming events of dominant sub-Arctic heathland bryophyte and lichen species. J. Ecol. 99, 1481–1488 10.1111/j.1365-2745.2011.01859.x (doi:10.1111/j.1365-2745.2011.01859.x) [DOI] [Google Scholar]
- 83.Green T.G.A., Sancho L.G., Pintado A. 2011. Ecophysiology of desiccation/rehydration cycles in mosses and lichens. In Plant desiccation tolerance (eds Luttge U., Beck E., Bartels D.), pp. 89–120 Ecological Studies 215, Part 2 Berlin, Germany: Springer-Verlag; 10.1007/978-3-642-19106-0_6 (doi:10.1007/978-3-642-19106-0_6) [DOI] [Google Scholar]
- 84.Bowker M. A., Soliveres S., Maestre F. T. 2010. Competition increases with abiotic stress and regulates the diversity of biological soil crusts. J. Ecol. 98, 551–560 10.1111/j.1365-2745.2010.01647.x (doi:10.1111/j.1365-2745.2010.01647.x) [DOI] [Google Scholar]
- 85.Fernández-Marín B., Balaguer L., Esteban R., Becerril J. M., García-Plazaola J. I. 2009. Dark induction of the photoprotective xanthophyll cycle in response to dehydration. J. Plant Physiol. 166, 1734–1744 10.1016/j.jplph.2009.04.019 (doi:10.1016/j.jplph.2009.04.019) [DOI] [PubMed] [Google Scholar]
- 86.Fernández-Marín B., Becerril J. M., García-Plazaola J. I. 2010. Unravelling the roles of desiccation-induced xanthophyll cycle activity in darkness: a case study in Lobaria pulmonaria. Planta 231, 1335–1342 10.1007/s00425-010-1129-6 (doi:10.1007/s00425-010-1129-6) [DOI] [PubMed] [Google Scholar]
- 87.Green T. G. A., Lange O. L. 1994. Photosynthesis in poikilohydric plants: a comparison of lichens and bryophytes. In Ecophysiology of photosynthesis (eds Schulze E.Z., Caldwell M. C.), pp. 319–341 Ecological Studies Berlin, Germany: Springer-Verlag [Google Scholar]
- 88.Proctor M. 2001. Patterns of desiccation tolerance and recovery in bryophytes. Plant Growth Regul. 35, 147–156 10.1023/A:1014429720821 (doi:10.1023/A:1014429720821) [DOI] [Google Scholar]
- 89.Tuba Z., Proctor M. C. F., Csintalan Z. 1998. Ecophysiological responses of homoiochlorophyllous and poikilochlorophyllous desiccation tolerant plants: a comparison and an ecological perspective. Plant Growth Regul. 24, 211–217 10.1023/A:1005951908229 (doi:10.1023/A:1005951908229) [DOI] [Google Scholar]
- 90.Coe K. K., Belnap J., Sparks J. P. 2012. Precipitation-driven carbon balance controls survivorship of desert biocrust mosses. Ecology 93, 1626–1636 10.1890/11-2247.1 (doi:10.1890/11-2247.1) [DOI] [PubMed] [Google Scholar]
- 91.Martínez I., Escudero A., Maestre F., de la Cruz A., Guerrero C., Rubio A. 2006. Small scale abundance of mosses and lichens forming soil biological crusts in two semi-arid gypsum environments. Aust. J. Bot. 54, 339–348 10.1071/BT05078 (doi:10.1071/BT05078) [DOI] [Google Scholar]
- 92.Maestre F. T., Escudero A., Martínez I., Guerrero C., Rubio A. 2005. Does spatial pattern matter to ecosystem functioning? Insights from biological soil crusts. Funct. Ecol. 19, 566–573 10.1111/j.1365-2435.2005.01000.x (doi:10.1111/j.1365-2435.2005.01000.x) [DOI] [Google Scholar]
- 93.Eldridge D. J., Zaady E., Schachak M. 2000. Infiltration through three contrasting biological soil crusts in patterned landscapes in the Negev, Israel. Catena 40, 323–336 10.1016/S0341-8162(00)00082-5 (doi:10.1016/S0341-8162(00)00082-5) [DOI] [Google Scholar]
- 94.Puigdefábregas J., Solé-Benet A., Gutierrez L., Barrio G., Boer M. 1999. Scales and processes of water and sediment redistribution in drylands: results from the Rambla Honda field site in Southeast Spain. Earth Sci. Rev. 48, 39–70 10.1016/S0012-8252(99)00046-X (doi:10.1016/S0012-8252(99)00046-X) [DOI] [Google Scholar]
- 95.Haase P., Pugnaire F. I., Clark S. C., Incoll L. D. 1999. Environmental control of canopy dynamics and photosynthetic rate in the evergreen tussock grass Stipa tenacissima. Plant Ecol. 145, 327–339 10.1023/A:1009892204336 (doi:10.1023/A:1009892204336) [DOI] [Google Scholar]
- 96.Thomas A. D., Hoon S. R., Dougill A. J. 2011. Soil respiration at five sites along the Kalahari transect: effects of temperature, precipitation pulses and biological soil crust cover. Geoderma 167–168, 284–294 10.1016/j.geoderma.2011.07.034 (doi:10.1016/j.geoderma.2011.07.034) [DOI] [Google Scholar]