Abstract
Desert species respond strongly to infrequent, intense pulses of precipitation. Consequently, indigenous flora has developed a rich repertoire of life-history strategies to deal with fluctuations in resource availability. Examinations of how future climate change will affect the biota often forecast negative impacts, but these—usually correlative—approaches overlook precipitation variation because they are based on averages. Here, we provide an overview of how variable precipitation affects perennial and annual desert plants, and then implement an innovative, mechanistic approach to examine the effects of precipitation on populations of two desert plant species. This approach couples robust climatic projections, including variable precipitation, with stochastic, stage-structured models constructed from long-term demographic datasets of the short-lived Cryptantha flava in the Colorado Plateau Desert (USA) and the annual Carrichtera annua in the Negev Desert (Israel). Our results highlight these populations' potential to buffer future stochastic precipitation. Population growth rates in both species increased under future conditions: wetter, longer growing seasons for Cryptantha and drier years for Carrichtera. We determined that such changes are primarily due to survival and size changes for Cryptantha and the role of seed bank for Carrichtera. Our work suggests that desert plants, and thus the resources they provide, might be more resilient to climate change than previously thought.
Keywords: demographic buffering, climate change, integral projection model, periodic population matrix model, precipitation, stochastic population growth rate (λS)
1. Introduction
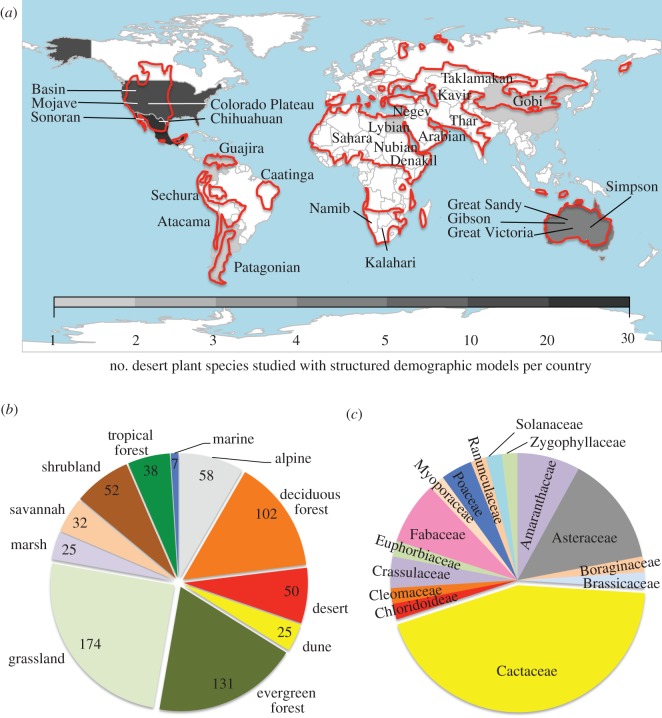
Drylands, the largest terrestrial ecosystem (figure 1a [2,3]), are considered among the most sensitive to projected global environmental change [4]. Global circulation models project temperature increases, precipitation decreases and an increase in interannual variability in precipitation for many deserts [4]. In them, even small changes in precipitation are expected to have large impacts on species composition and biodiversity [5]. However, a far-too-often overlooked fact is that desert flora have evolved a set of unique structures and mechanisms to withstand extensive periods of drought. These include succulence [6], deep roots [7], modified metabolic pathways [8], high modularity [9]) and bet hedging mechanisms such as seed dormancy [10], or extreme longevity [11], among others. These structures and mechanisms are thought to enable plants to buffer the enormous variability found in deserts. Owing to such adaptations, projected changes in abiotic conditions may still fall within the range of variation that desert plants are accustomed to without large consequences for long-term population viability.
Figure 1.
(a) Global distribution of desert plant demographic studies using structured population models. Red lines contain dryland regions defined by the UNEP [1]. (b) Number of plant species for which these models have been applied as a function of ecosystem. (c) Taxonomic family diversity of the aforementioned studies in deserts. Data in a,b from a database with ca 700 plant species (R. Salguero-Gómez 2012, unpublished data).
Deserts are a particularly suitable ecosystem for testing causal hypotheses of climate change. This is so because their biological responses are strongly controlled by water availability [12]. Climate change models applied to deserts have mostly used average temperature [13–15] and—less commonly—average precipitation [16,17] to explore future distributions of species. This approach is problematic because the frequency of climatic extremes is also projected to change [4], and because deserts' inherent variability in precipitation render average values uninformative of actual patterns [18,19]. Consequently, actual responses of desert species to global environmental change might differ from those generated by average-based approaches such as climatic envelopes. In addition, previous modelling approaches to determine responses of desert species to climate change have been highly correlative [20–22], and so our understanding of the causal mechanisms governing their responses is very limited.
A useful but largely unexplored approach towards a more mechanistic understanding of desert plants' responses to climate change couples long-term demographic models and high-resolution climatic projections. Size-/stage-structured demographic models explore population dynamics not as a function of their superficial characteristics (e.g. population size and population growth rate), but by directly examining the effects of a/biotic factors on underlying vital rates (e.g. survival, reproduction [23,24]). Furthermore, this demographic approach provides indirect information about past and current selection on crucial life-history traits [25]. Until recently, global climatic projections have been too coarse to predict precipitation at a specific population/site [4,26]. This is particularly relevant in deserts, where precipitation is highly spatially heterogeneous too [18]. A recent advance, a super-high-resolution global model [27], provides reliable monthly precipitation projections for any region around the globe down to a 20-km2-grid resolution. This model can be used in comparative demographic studies even on different continents. The remaining limitation for coupling demographic models and climatological data is long-term, individual-based demographic information from natural populations. Unfortunately, such datasets are extremely rare in drylands (but see [28]).
Coupling demographic and climatic data is particularly valuable when evaluating the response of short-lived, desert species, with relatively quick population turnover, to climate change. A meta-analysis using size-/stage-based demographic models concluded that species with short lifespans are more vulnerable to increased stochasticity than long-lived species [26]. Lewontin & Cohen [29] derived an approximation for the stochastic population growth rate (a = log(λS)) that points to the negative effect of variation in the long run. This long-term population measure, a, is directly proportional to the deterministic population growth rate (λ for a particular year, averaged over multiple years as 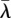 ), but it decreases with interannual variation in λ:
), but it decreases with interannual variation in λ:
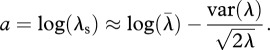 |
1.1 |
It is commonly assumed that increased variation in an abiotic factor (e.g. precipitation) will inevitably decrease a in natural populations. However, for this prediction to hold true, changes in λ must be strongly and positively coupled with variation in the abiotic factor. A few examples exist in the literature where this is not the case [30,31]. These studies highlight the importance of demographic buffering [32,33], that is, a population's ability to retain a more or less constant a despite varying climate by virtue of re-adjusting its vital rates. This can happen when, for instance, low fecundity is coupled with high survival, or when bet-hedging strategies reduce variance in fitness despite variance in abiotic factors across years. Therefore, whether or not short-lived desert plant species are vulnerable to climate change will depend not only on the climate change scenarios, but also on the life histories and potential for demographic buffering of the species [34].
The motivation for this manuscript is twofold. First, we provide an overview of how variation in precipitation might affect population dynamics of perennial and annual species and highlight promising avenues of climate change research. Secondly, we illustrate our suggested demographic approach for investigating the effect of high-resolution climatic projections on long-term population growth. We use unique long-term datasets from two different short-lived species from two contrasting deserts: the short-lived subshrub Cryptantha flava from Utah, USA and the annual herb Carrichtera annua from Israel. In Utah, USA, increases in precipitation are projected, and we hypothesized a consequent increase in demographic performance, mostly mediated by survival and growth. In Israel, more droughts are projected, and we hypothesized negative effects but demographic buffering via dormant seeds.
2. Precipitation, global environmental change and desert plant population dynamics
The consequences of global environmental change on desert plant populations are largely unknown, particularly with respect to precipitation. This is most likely due to the scarcity of long-term demographic data—a problem with many species but particularly pronounced for desert plants (figure 1b; but see [35]). Additionally, other factors such as biased taxonomic interest, geographical representation or selection of a particular methodology also play an important role in our limited knowledge. We present an overview of how variable precipitation affects the population dynamics of desert perennials and annuals, and offer suggestions for future avenues of research.
(a). Precipitation effects on perennial desert plant species
Demographic changes in aridland perennials are often viewed as episodic. Most recruitment and high survival occur in exceptionally favourable weather years [36,37]. This view is supported primarily by studies of woody shrubs and succulents, where populations' densities may be relatively static or slightly declining for decades [38–40] and plant longevity well exceeds the career—or even the lifespan—of the investigator.
Achieving a mechanistic understanding of the demography for any long-lived species is challenging. In arid systems, data often come from decadal or longer census intervals for mapped or historically photographed vegetation [39,41,42] or from estimating current population age structure based on yearly incremental growth of individuals, even those without distinct growth rings [36,40,43,44]. For some desert species, these approaches have led to estimates of remarkable longevity: ca 5000 years for Pinus longaeva (Pinaceae) [45], and over 10 000 years for Larrea tridentata (Zygophyllaceae) [46] (see [42] for more examples). Such estimates, as pointed out by Miriti et al. [37], may be woefully inaccurate if based on census intervals that do not bracket the rare drought events that trigger high mortality episodes.
Recent severe droughts in the southwestern US have provided rare opportunities to capture population responses [47]. In such cases, the link between lack of precipitation and mortality does appear strong. In a study of seven common shrub species in Joshua Tree National Park in the Colorado Desert, USA [37], six showed mortality rates ranging from 55 to 100 per cent, with negligible mortality only in L. tridentata. In Ambrosia dumosa (Asteraceae), Eriogonum fasciculatum (Polygonaceae), Simmondsia chinensis (Simmondsiaceae) and Tetracoccus hallii (Picrodendraceae), individuals weakened by an earlier drought, as judged from their growth rates, were more likely to die during a later one. Likewise, a regional study showed L. tridentata to have much higher survival at most locations than smaller drought-deciduous species and provided evidence for cumulative effects of multiple drought years [48].
Recruitment in desert perennials can, likewise, occur in episodes of variable duration [44]. Nonetheless, a causal link between precipitation and recruitment is not often clear [40], probably because seedling establishment may depend on multiple, carefully timed precipitation events [11,41], and be sensitive to numerous other factors [38]. Seed germination and seedling emergence are separated from seed production at least seasonally and potentially annually, especially if the seeds require stratification or otherwise form a seed bank [49]. It stands to reason that favourable precipitation conditions are then required for both events. There may be a range of impinging biotic factors, including the seedling's reliance on nurse plants [43], competition from established individuals [37] and predation on seed and seedlings [50]. High predation rates have led to the suggestion that mast fruiting may underlie episodic recruitment events in aridland perennial species [50,51]. Comprehensive investigations into rates of seed production, predation and seedling establishment as a function of both interannual and interseasonal variation in precipitation would be worthwhile.
Precipitation deficits may additionally imprint the demography of perennials by negatively impacting individual plant size. A recent comparative study has shown a positive correlation between individual plant shrinkage and survival [52], but the importance of this phenomenon for population dynamics has often been overlooked. Plant shrinkage or dieback of shrub canopies is common during drought events [37,53,54], and negative size changes may occur over time even when severe drought is not apparent [31,39,55]. Sometimes the entire plant canopy dies, so dormant and dead plants are not immediately distinguishable [37]. The morphology of some desert shrubs [9], and at least one subshrub [56], where the stem fragments into separate hydraulic sectors as the plant ages, lends itself particularly to shrinkage, expressed through the death of any fragment(s). Thus, hydraulic fragmentation may enable the plant to slough off a part damaged or cavitated due to drought without resulting in the death of the entire individual [57]. The incidence of hydraulic fragmentation among [9] and within (R. Salguero-Gómez 2012, unpublished data) species is positively correlated with aridity, the most pronounced form being the splitting of the stem as in L. tridentata [58]. It may not be coincidental that this species often exhibits low mortality [59], even during severe droughts [37,39,48]. The demography of desert plants with multiple stems are normally treated as single individuals (i.e. genet), but the possible adaptive significance of stem splitting provides a good reason for individual stem/ramet demography as well [59,60].
While some desert perennials are famous for their longevity, we need more information about the demography of less charismatic species, including short-lived shrubs, subshrubs or herbaceous perennials whose populations may turn over in a shorter time than the return frequency of extreme climatic events. Practically, the demography of these species would be more amenable to modelling with annual or periodic projection matrix approaches [23,61]. To date, such models are most commonly applied to species in the Cactaceae (figure 1c), where emphasis is often placed on the relative importance of clonal versus sexual reproduction [49]. In general, we need to evaluate the importance of recruitment, mortality and growth responses to extreme weather patterns compared with background levels in more typical years [62].
We offer suggestions for building the most useful database of demographic studies to accomplish this goal. First, we need to choose study species more carefully, including sufficient numbers of common species that are neither invasive nor threatened/endangered. Several recent studies of cacti, for example, were motivated by the threatened status of the species [49]. Second, data are needed for a wider variety of perennial life forms and taxonomic groups, including other desert dominant families about which little is known (e.g. Agavaceae, Aizoaceae, Asclepiadaceae, Chenopodiaceae, Cupressaceae, Cyperaceae, Liliaceae, Mimosoidaceae, Rhamnaceae and Tamaricaceae). And finally, particularly because precipitation in arid systems is temporally variable, studies need to be (i) of sufficient duration to capture considerable natural variability, even if they cannot always encompass the extreme events of low recurrence interval, and/or (ii) to incorporate experimental manipulations of precipitation following climatic projections.
(b). Precipitation effects on annual desert plant species
Studies explicitly addressing demographic responses of dryland annual plants, a dominant component of warm deserts [6], to precipitation patterns are rare too. However, within the existing empirical body of evidence, three categories of studies can be distinguished: (i) comparison of plant population dynamics over a range of sites differing in mean precipitation (i.e. a ‘space-for-time’ approach), (ii) experimental manipulation of water availability, and (iii) observation of plant populations in the field over a range of years differing in precipitation, as is typically done also with perennials (see §2a above).
Comparing plant population dynamics over a range of sites differing in mean precipitation provides an evolutionary view of precipitation effects on plants because it shows the result of long-term selection regimes (i.e. precipitation). Consistent with theory, annual plant populations from drier habitats have been found to exhibit higher seed dormancy [63] and a stronger response to precipitation as a trigger of germination [64], compared with conspecifics from wetter, less variable habitats. In addition, they exhibit a faster life cycle [65] and larger reproductive effort when resources are abundant [30].
Experimental manipulation of water availability gives insight into the immediate (transient) effects of precipitation. Experiments of this nature are carried out either in the field or under common garden or greenhouse conditions, where water availability has been shown to affect integral elements of population dynamics. In particular, additional precipitation increases germination [63,64], biomass [10] and fecundity [64], while precipitation deficit leads to a shortened life cycle, expedited flowering [65] and higher reproductive effort [30]. However, multi-species experiments have shown that these responses are not ubiquitous and, often, coexisting species do not respond at all to changes in water availability [10,30].
Studies in which plant populations were observed over a longer time period, and therefore under different natural precipitation conditions show that responses are strongly species-specific. Still, two general patterns seem to emerge. First, fecundity is positively correlated with precipitation [66–68]. Second, drought years are associated with low population sizes and frequently result in complete reproductive failure. However, in wet years, plant populations can be remarkably insensitive to rainfall [66,68], suggesting a saturation-type response to precipitation. Levine et al. [68] found that in such years, the temperature associated with the first major precipitation event rather than total annual precipitation was the best predictor for plant density. The amount and timing of the first rains after the dry season serve as a germination cue for annual plants, and a number of studies have found them to be of high importance [69–71].
Another predictor of annual plants population performance in response to precipitation is species abundance. In a 4-year community study, Boeken & Shachak [72] found abundant species showing no response to variation in precipitation while rare species varied strongly with rainfall. Levine & Rees [73] reported a similar pattern when modelling the dynamics of an abundant grass and a rare forb. The latter did best in rainy years that were preceded by dry years. Only then was this species able to germinate from the seed bank while experiencing little competition from the grass competitor. Their study suggests that the interannual variation in precipitation may facilitate species coexistence because different species specialize on different types of years and thus avoid competition, similar to the storage effect described earlier [10].
A study by Fox et al. [67] found that germination of one species, Gilia tenuiflora arenaria (Polemoniaceae) was favoured by rainy, warm conditions of El Niño years. In such years, the species replenished its persistent seed bank, where the majority of seeds stayed dormant until the next, infrequent, rainy year. Another co-occurring species, Chorizanthe pungens pungens (Polygonaceae), germinated more in drier conditions and its population size was rather independent of weather conditions, suggesting demographic buffering [32]. The importance of rare, rainy years to restock the seedbank has also been reported for Lepidium pailliferum (Brassicaceae) [74]. This species apparently needs extreme variation in precipitation to persist because a long series of average precipitation years led to a monotonic decline and ultimately local extinction.
In summary, results from empirical studies indicate that interannual variation in precipitation is the major driver of annual plant population dynamics in arid ecosystems. Though annuals share the same overall dependence on precipitation with perennials (see §2a), the former may differ largely from the latter in their responses to rain via distinctive life-history strategies. Rather than leading to synchronized responses, the high variation in precipitation allows for multiple strategies and may be a requisite for population persistence [74] and species coexistence [73]. Seed germination has been identified as a key life stage for desert annual demography [75]. Even though seed dormancy has been studied relatively frequently, demographic records of annual plant populations still lack fine estimates of seed banks, probably owing to the difficulty of observing or estimating below-ground demographic stages. One possible solution could be a combination of long-term field surveys of annual plant populations with seed-bank experiments in the greenhouse or in situ. Similar to perennials, there is a need for long-term surveys that examine in-depth demographic processes to identify the mechanisms that control annual plant population responses to precipitation. Measuring single traits of single species under controlled conditions instead of all crucial vital rates seems insufficient. Finally, the study bias (figure 1) may be even more at the expense of annuals than perennials, because they are often an invisible component of desert plant communities, especially in drought years.
3. Precipitation shifts in two common desert short-lived species
In the following section, we address some of the above research gaps by evaluating demographic performance for two short-lived species in the light of projected shifts in precipitation. We do this by coupling long-term individual-based demographic information in the form of size-/stage-structured models with super-high-resolution climatic models for precipitation. The two native study species are the subshrub C. flava (Boraginaceae) from the Colorado Plateau in North America (Cryptantha hereafter), and the native, annual C. annua (Brassicaceae) from the Negev Desert in the Middle East (Carrichtera hereafter).
(a). Study species
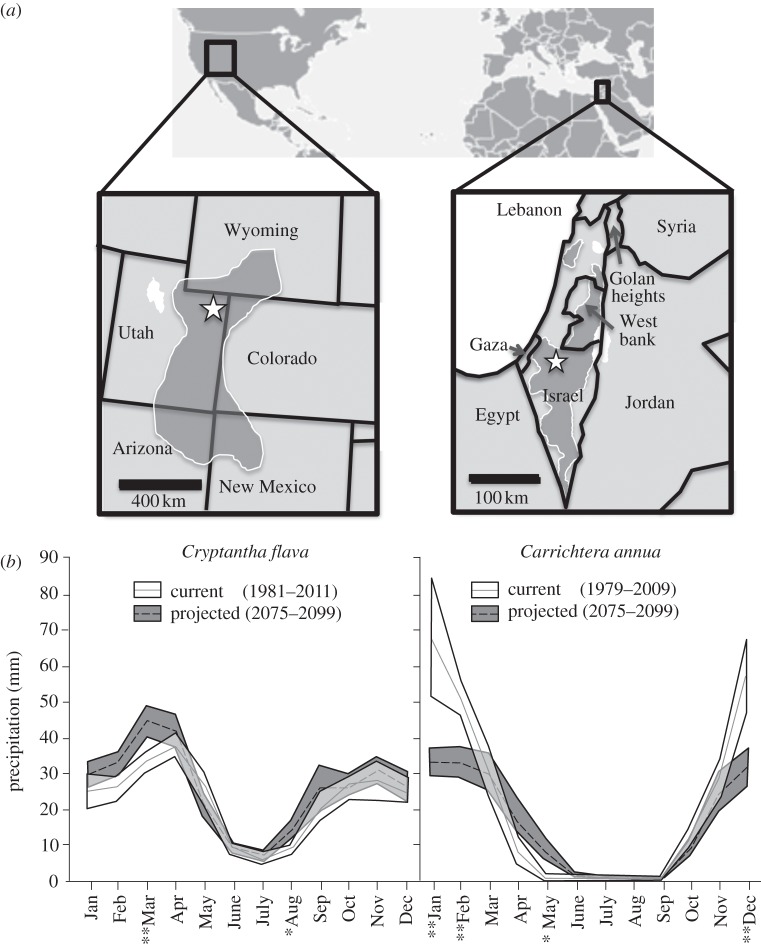
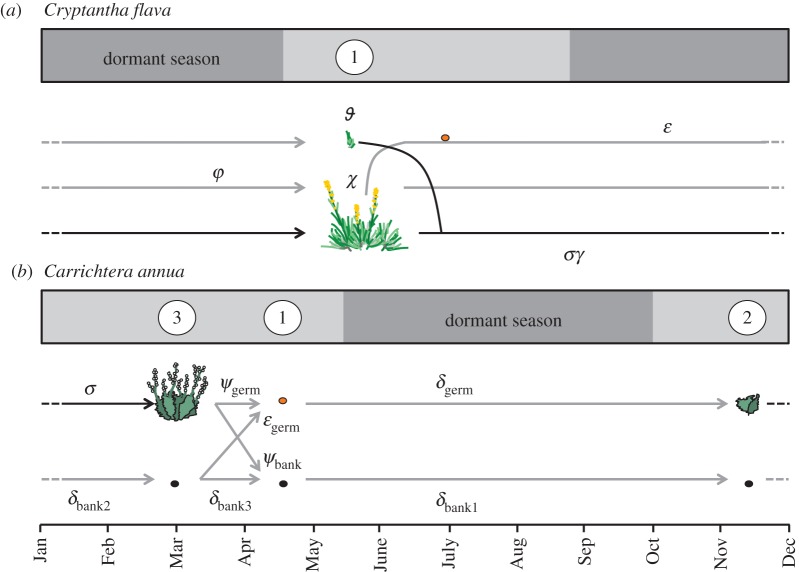
Cryptantha grows along the Colorado Plateau, USA (figure 2a; [77]). Its mean lifespan is 4.3 ± 2.1 (s.d.) years (R. Salguero-Gómez 2012, unpublished data). Individuals consist of one to approximately 200 leaf rosettes [77], organized into branches that emerge from a woody underground stem. The root system consists of a single, deep taproot and 5–15 lateral roots [78]. Leaf rosettes first appear in mid April (figure 3a), and new rosettes may develop throughout the growing season from axillary meristems of existing rosettes. Once induced to reproduce, a rosette bolts to approximately 25 cm and produces 20–70 flowers [79]. Flowering rosettes die as seeds ripen in late July. Seedlings may emerge in September–October or at the beginning of the next growing season, and there appears to be no permanent seed bank [79]. Individuals typically enter vegetative dormancy in early August. However, late growing season monsoons and experimental precipitation have been shown to prolong the growing season into September [80,81]. Individuals of Cryptantha possess great size plasticity, sometimes displaying extreme changes in size within 1 year, either positive (growth) or negative (shrinkage), as a function of the amount of precipitation [77]. Because individuals of Cryptantha become internally fragmented with development [56], some can decrease in size more than 80 per cent through the mortality of entire fragments.
Figure 2.
(a) Natural distribution of the herbaceous perennial C. flava (Boraginaceae; left) and distribution of the annual C. annua (Brassicaceae; here only shown for Israel; right) represented by dark grey area. Stars indicate location of the populations studied for 15 and 8 years, respectively. (b) Mean monthly precipitation ±95% CI at each population based on current precipitation patterns (last 30 years) and projections from the super-high-resolution model used in this paper [76]. Single (*) and double asterisks (**) represent monthly precipitation significant differences at p < 0.05 and p < 0.005, respectively, for current versus projected scenarios.
Figure 3.
Life cycles of (a) the perennial C. flava and (b) the annual C. annua. Light grey and dark grey areas in the rectangles represent periods of photosynthetic activity and vegetative dormancy, respectively. White circles represent timing and frequency of field censuses. (a) In Cryptantha, established individuals can survive (σ) to the next year and change in size (γ), become reproductive (φ), producing a given number of flowering stalks (χ) whose seeds may then establish (ɛ) and achieve a specific size the next year (ϑ). (b) In Carrichtera, reproductive individuals may produce seeds (ψgerm) that will germinate after the dry season if they survive (δgerm). Seedlings may then survive (σ) to become reproductive the next year. Reproductive individuals can also contribute to the seedbank (ψbank). Seeds in the seedbank may stay dormant during each period (δbank1,2,3) and emerge as germinable seeds in April (ɛgerm). Black arrows in both life cycles represent demographic events of established individuals, while grey arrows represent events during sexual reproduction.
Carrichtera is an annual herb native to shrub-steppe and desert habitats in most Mediterranean countries [82], but an invasive in semi-arid southern Australia [83]. In Israel, Carrichtera grows in Mediterranean and semi-arid shrublands and in deserts (figure 2a; [84]). Individuals germinate at the beginning of the rain season (October–December). Seedlings may become reproducers and create new seeds before dying towards the end of the rain season (March–May). Throughout the dry season (May–October), the population is ‘dormant’, being exclusively represented by seeds (figures 2b and 3b). Newly produced seeds are enclosed in lignified structures on the dried-out plant and thus are maintained as aerial seed banks until they are rain-dispersed [85]. Most seed loss is due to granivores during the dormant season, mainly harvester ants and rodents [83]. Carrichtera maintains a persistent seed bank, which means that a certain fraction of viable seeds do not germinate the first year, even if conditions are suitable. Seeds typically remain dormant until the second or later germination season.
(b). Experimental design
We studied the dynamics of a natural population of Cryptantha near its distribution centre over 14 years (1997–2011). The fieldsite located by the Redfleet State Park in Uintah County in northeastern Utah, USA (40°30′ N, 109°22′ 30″ W, 1730 m a.s.l.) is dominated by perennial woody species, such as Artemisia tridentata Nutt. (Asteraceae), Chrysothamnus nauseosus (Pallas) Britt. (Asteraceae) and Juniperus osteosperma (Torr.) Little (Cupressaceae). Cryptantha is the dominant subshrub species. Detailed descriptions of the experimental design are provided elsewhere [86]. Briefly, we established six 5 × 5 m2 permanent plots in early spring 1997, ensuring at least 20 m distance to encompass spatial heterogeneity. Each plot contained 13–1 × 1 m2 quadrats arranged in a chequerboard pattern. We conducted censuses each end of May, coinciding with the peak of the flowering for the study species (figure 2a). For each individual, we measured its Cartesian coordinates with reference to the plot to enable relocation each year and counted total number of living rosettes and number of reproductive rosettes. In addition, we carefully checked for the establishment of new recruits. During the length of this study, we followed 1232 individuals over 60 m2. The 2002 census was not carried out, and here we report the dynamics of the 2001–2003 annual period instead of 2001–2002 and 2002–2003 (see the electronic supplementary material, appendix SA).
We also studied the dynamics of a natural population of Carrichtera for 8 years (2001–2009). The perennial vegetation at the fieldsite located in the Northern Negev desert (31°23′ N, 34°54′ E, 590 m a.s.l.) consists of dwarf shrubs, mainly Sarcopoterium spinosum (L.) Spach (Rosaceae), associated with herbaceous, mainly annual, plant species [87]. Among the annual species, Carrichtera is one of the most abundant. Five 10 × 25 m2 permanent plots were established in the 2001 dry season. Each plot contained ten 20 × 20 cm2 permanent quadrats. We monitored them twice annually to quantify population size of seedlings (November–December) and adults (March) (figure 3b; see [88]). At the end of each growing season in all years except 2005, per capita biomass was measured by harvesting individuals in randomly chosen quadrats. In 2008, 29 randomly chosen individuals were harvested to examine the seed number–biomass relationship. The seed bank was inspected using 10 random 5 × 5 × 5 cm3 units per plot annually at the end of the dormant season, before the onset of autumn rains. Soil samples were spread in plastic trays and irrigated during winter in a net-house at the Botanical Gardens of Tel Aviv University (Israel). Soil collection was done each year and each year's collection was germinated in three consecutive years. Emerging seedlings were counted and continuously removed until no further emergence was observed (more details in [89]). Biomass and seed bank sampling, both invasive methods, were carried out in restricted areas within the permanent plots to avoid disturbance of natural population dynamics in the permanent quadrats.
(c). Size-/stage-structured demographic models
To explore the effects of projected shifts in precipitation regime (figure 2b) on the population of Cryptantha and Carrichtera, we used two well-established demographic approaches: integral projection models (IPMs hereafter; [24]) for Cryptantha and periodic matrix models (PMMs hereafter; [90]) for Carrichtera. The usage of each model was tailored to the biology and life history of each study species.
We applied an IPM framework to the long-term demographic data of Cryptantha. Briefly, an IPM links the size distributions of individuals in a population one year to the distribution the next year by explicitly incorporating the effect of individual size on the vital rates that affect survival and reproduction, and thus ultimately population growth rate (λ) [24]. The vital rates incorporated in our IPM to describe the life cycle of Cryptantha are described in figure 3a. We chose an IPM approach for the demographic dataset of Cryptantha, instead of classical matrix models where individuals are categorized into a small number of stages [23], because Cryptantha's vital rates of survival (σ), change in size (γ), reproduction (φ) or recruitment (χ; figure 3) were more adequately described by continuous functions of size than by the discrete step functions (not shown). In addition, IPMs represent a preferable approach over discrete projection matrices because the former (i) describe better large-size variations exhibited by individuals in the population [91], as in Cryptantha; (ii) are more robust when data are limiting [92,93], as it was here the case in some annual periods ( = 254.86 ± 44.78 (s.e.) individuals per annual period; range = 92–582); and (iii) are also applicable to data that are not truly continuous, as here (i.e. rosette numbers [94]).
= 254.86 ± 44.78 (s.e.) individuals per annual period; range = 92–582); and (iii) are also applicable to data that are not truly continuous, as here (i.e. rosette numbers [94]).
We applied a PMM approach to Carrichtera. PPMs describe cyclic temporal variation and account for the effects of multiple demographic processes [90,95] that typically govern annual species population dynamics. In this case, data were collected for four discrete plant stages and not as a function of plant size, as for Cryptantha. Namely, these are: reproductive individual, dormant seed, germinable seed and seedling; and during three time points, which defined three seasons: dormant, post-dormant and pre-dormant seasons. In the constructed demographic model, the life cycle of Carrichtera can be thought of as having two pathways: a vegetative pathway, where adults produce seeds that germinate the same year of their production, and a dormant pathway, where seeds remain in the seed bank (figure 3b). A description of the vital rates used in this model is provided in figure 3b. Details of both the IPMs and PMMs are described in electronic supplementary material, appendix SB.
(d). Projecting demographic effects of changes in precipitation
We explored how the population dynamics of Cryptantha and Carrichtera are likely to change from current to projected precipitation conditions. We first classified each annual period for which we had demographic information as an extreme precipitation or average precipitation period. We obtained annual precipitation records from the closest permanent weather station to both field sites for the 30 years previous to the last census: 1981–2011 for Cryptantha [86] and 1979–2009 for Carrichtera [87]. Then, we calculated annual precipitation as the sum of monthly precipitations from May of year t to April of t + 1. In this way, annual precipitation was temporally matched for later analyses with population growth rates, based on the timing of the field censuses (figure 3; see the electronic supplementary material, appendix SA). We used the 75th and 25th percentiles of these precipitation records as the thresholds to classify annual periods as wet, normal or dry. These percentiles were 399.35 and 288.16 mm for Cryptantha and 321.31 and 212.46 mm for Carrichtera (see the electronic supplementary material, appendix SD). Consequently, under current precipitation conditions, the probability of occurrence of a wet, normal or dry annual period was 0.25, 0.5 or 0.25, respectively, in both field sites (table 1). This discrete classification of annual periods in demographic models, instead of using continuous classifications of precipitation [96], is based on the deserts' wide variation in precipitation (precipitation coefficients of variation: 25.47% near Utah, USA field site, and 32.25% near Israel field site, based on permanent weather station records).
Table 1.
Probability of occurrence of wet, normal and dry annual periods at both field sites based on current (1981–2011) in situ precipitation records, and projected (2075–2099) scenario under the super-high-resolution climate change model used in this manuscript [76]. Superscript ‘m.s.’ denotes marginally significant at 0.05 < p < 0.10.
|
Cryptantha flava (UT, USA) |
Carrichtera annua (Israel) |
|||
|---|---|---|---|---|
| current | projected | current | projected | |
| wet | 0.25 | 0.33 | 0.25 | 0.21 |
| normal | 0.50 | 0.50 | 0.50 | 0.25m.s. |
| dry | 0.25 | 0.17 | 0.25 | 0.54* |
*Significant differences at p < 0.05.
Next, we calculated the probability of occurrence of a wet, normal or dry annual precipitation period in the future. We obtained high-resolution precipitation data for both field sites from the MRI-AGCM3.2S super-high-resolution climate model ([76]; R. Mizuta 2012, personal communication). The models were run for the four 20 km2 grids around the GPS coordinates of the centre of both populations. The model output consists of monthly precipitation into the past (1979–2003) using the AMIP scenario (back-projected precipitation, hereafter; see the electronic supplementary material, appendix SC) and into the future (2075–2099; projected precipitation, hereafter) using the A1B scenario of the IPCC [4]. We classified wet, normal and dry annual periods using the 75th and 25th percentiles from the back-projected precipitation. We then applied the percentile values from the back-projected to the projected model to get the probabilities for wet, normal and dry annual periods in the future. We used back-projected model values, instead of the permanent weather stations, because precipitation means were lower for the back-projected precipitation values (Cryptantha:  = 350.75 ± 16.31 mm (s.e.),
= 350.75 ± 16.31 mm (s.e.), 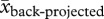 = 267.67 ± 10.09 mm, t46.79 = 4.33, p < 0.001; Carrichtera:
= 267.67 ± 10.09 mm, t46.79 = 4.33, p < 0.001; Carrichtera: 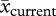 = 264.43 ± 15.57 mm,
= 264.43 ± 15.57 mm, 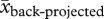 = 238.09 ± 13.00 mm, t51.78 = 1.30, p = 0.20). Thus, imposing the percentiles of the current weather would have resulted in future projections for a higher frequency of dry periods in Utah, USA, when in fact a significant increase in precipitation has been recorded during the last 75 years at the field site (R. Salguero-Gómez 2012, unpublished data). With this approach, we also secured that the percentile thresholds would result in 0.25, 0.50 and 0.25 probabilities for wet, normal and dry annual periods under back-projected precipitation conditions (see the electronic supplementary material, appendix SC). Finally, we compared whether the frequency of wet, normal and dry annual periods changed significantly between current and projected scenarios using z-tests for two proportions of independent groups [97].
= 238.09 ± 13.00 mm, t51.78 = 1.30, p = 0.20). Thus, imposing the percentiles of the current weather would have resulted in future projections for a higher frequency of dry periods in Utah, USA, when in fact a significant increase in precipitation has been recorded during the last 75 years at the field site (R. Salguero-Gómez 2012, unpublished data). With this approach, we also secured that the percentile thresholds would result in 0.25, 0.50 and 0.25 probabilities for wet, normal and dry annual periods under back-projected precipitation conditions (see the electronic supplementary material, appendix SC). Finally, we compared whether the frequency of wet, normal and dry annual periods changed significantly between current and projected scenarios using z-tests for two proportions of independent groups [97].
To explore short-term effects of precipitation on the population of both species, we quantified the overall demographic performance for each annual period of our studies. We calculated the deterministic population growth rates (λ) as the eigenvalue of each discretized kernel K resulting from the IPMs of Cryptantha, and annual projection matrix A resulting from the back-multiplication of the periodic matrices of Carrichtera. Briefly, the kernel K and the annual projection matrix A describe the population dynamics in each population. A detailed description of their construction and analyses is available in the electronic supplementary material, appendix SD. The deterministic population growth rate describes the asymptotic growth of a population under constant environmental conditions without density-dependence [23]. We jack-knifed the individual data for both species 999 times and re-calculated the K kernel and A matrix for each annual period. Next, we used Spearman's rank correlation coefficient (ρ) to correlate annual period precipitation with the jack-knifed population growth rate values.
We accounted for the large variation in precipitation at the Colorado Plateau and Negev deserts by quantifying stochastic population growth rates (a = log(λS)) of both populations. A set of discretized, annual kernels K for Cryptantha, and a set of PMMs Bi (the matrix that describes the population dynamics within each season in the life cycle; see the electronic supplementary material, appendix SD) for Carrichtera were then randomly chosen, after having been classified into dry, normal or wet annual periods under the aforementioned current and projected precipitation scenarios. This approach treats the variability within each period as crude estimates of the variation in the vital rates [98]. The model abstracts a continuous environmental factor into a finite set of discrete environmental states [23]. The sampling frequency of each type of kernel/matrix depends on the frequency of wet, normal and dry annual periods described in the electronic supplementary material, appendix SA. We estimated λS using the mean population size over T = 4000 iterations, but discarding the first 250 projections in order to avoid transient dynamics [98]. This simulation was replicated 100 times to quantify variation. The population growth rate was calculated as
| 3.1 |
where N(0) is the mean population size at time t = 0 and N(T) the mean population size 3750 iterations later.
We obtained a more mechanistic understanding of potential differences in the stochastic population growth rates during current versus projected scenarios by calculating their stochastic elasticities. In its simplest form, deterministic elasticities inform about the relative importance of a given demographic process (e.g. reproduction) on the deterministic population growth rate, λ [23,99]. A stochastic elasticity (ES) takes into account the variation in vital rate values through time [100]. However, ES can be difficult to interpret because it includes the effect of mean and variation in values of the vital rates on a. Consequently, we also calculated the stochastic elasticity ESμ for perturbations onto mean vital rate values and the stochastic elasticity ESσ for perturbations onto vital rate variances. We performed these calculations in R [101] modifying available programming [100].
We implemented stochastic elasticities analyses tailored to the two demographic models employed. For the IPMs in Cryptantha, we perturbed (i.e. increased by an infinitesimal amount, 0.0001) the intercept of the vital rates of its life cycle (figure 3a; see the electronic supplementary material, appendix SC). Our approach affected all individuals equally, as opposed to individually as a function of their sizes, which would be difficult to justify from a biological point of view. For instance, this infinitesimal increase in the intercept of the regression for changes in size γ resulted in bigger individuals, regardless of initial size [96]. For the PMPs in Carrichtera, instead of perturbing the elements of the annual matrix A (see the electronic supplementary material, appendix SB), which would blur different biological processes, we carried out vital rate perturbations in the periodic matrices following methods described elsewhere [95].
(e). Results
Precipitation monthly regimes and annual amounts are projected to change in opposite directions in the two regions. In the case of Cryptantha in northeast Utah, USA, the super-high-resolution climatic model projects an increase in frequency of wet periods in comparison to the actual historical records, which results in fewer dry periods while normal precipitation periods will remain the same (table 1). However, we must note that these shifts are not statistically significant. Projections also report a lengthening of the growing season of Cryptantha, with more precipitation in March and August (figure 2b). In contrast, in the case of Carrichtera in Israel, the climatic model projects more frequent dry periods (z = −2.20, p = 0.03), halving the frequency of normal precipitation annual periods (z = 1.90, p = 0.058), and slightly decreasing the frequency of dry periods (table 1). In this region, winters (December, January and February) are expected to become drier with an increase in precipitation in May, that is, after desert annuals have shed their seeds (figure 2b)
The deterministic population growth rates (λ) of both species were strongly affected by annual current precipitation. In Cryptantha, the effect of precipitation on λ was positive (Spearman's rank correlation coefficient ρ = 0.17, p < 0.001). For Carrichtera, when all annual sampled periods were included, there was a negative correlation between precipitation and λ (ρ = −0.11, p < 0.001). However, this correlation was mostly driven by the high λ jack-knifed values during 2008–2009 (see the electronic supplementary material, appendix SA). Treating this annual period as an outlier resulted in a positive correlation between λ and annual precipitation (ρ = 0.77, p < 0.001).
The projected precipitation scenarios increased population growth rates of both species, even though an increase in precipitation was projected for Cryptantha and a decrease for Carrichtera. The stochastic population growth rate (a; equation (3.1)) of Cryptantha under current precipitation conditions was significantly lower than zero (acurrent = −0.1752 ± 0.04 (95% CI)), implying demographic inviability under the present precipitation regime. In contrast, its aprojected = −0.044 ± 0.05 for the projected precipitation scenario was statistically greater than Acurrent (t23896.59 = 106.15, p < 0.001), and overlapped with zero, implying demographic viability (figure 4). In Carrichtera, the fitness values under current and projected conditions indicated demographic viability (acurrent = 0.015 ± 0.03, aprojected = 0.138 ± 0.04), but the projected demographic performance was also significantly greater under projected than under current conditions (t15997.81 = 7.58, p < 0.001; figure 4).
Figure 4.
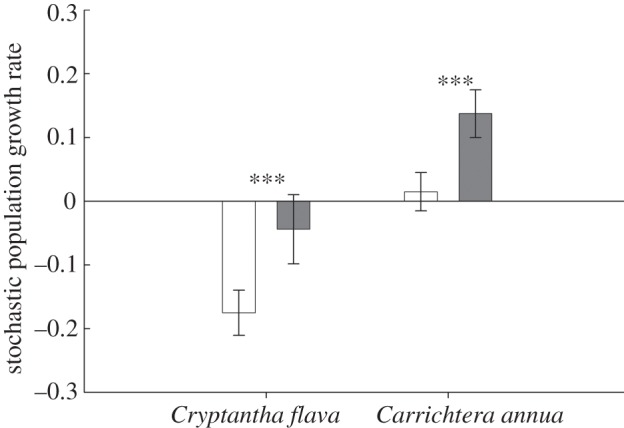
Stochastic population growth rates (a = log(λS) ± 95% CI; number of jack-knife iterations = 999) under current and projected changes in frequency of wet, normal and dry annual periods (table 1) in the studied populations of the perennial C. flava and the annual C. annua. Three asterisks (***) represents significant differences at p < 0.001. Filled bars denote projected, unfilled bars, current.
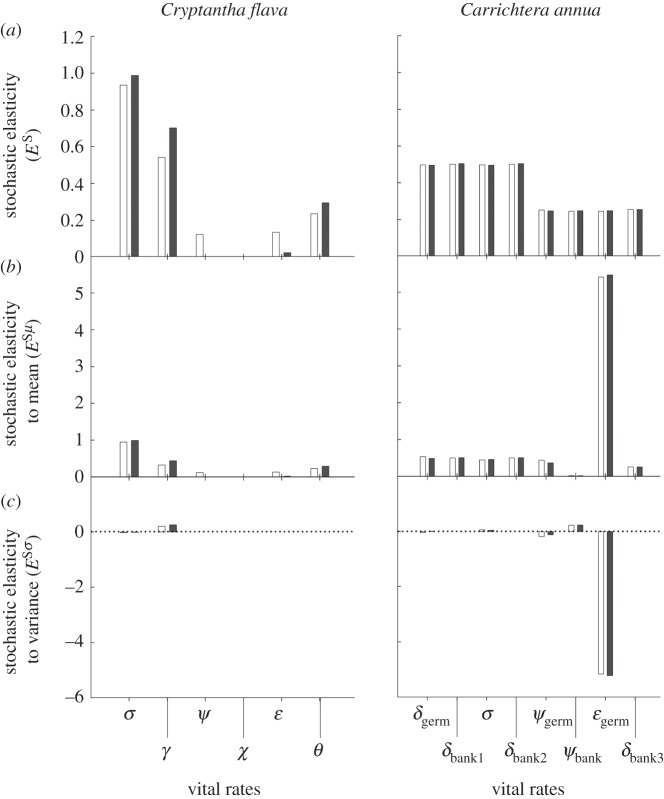
Neither for Cryptantha nor for Carrichtera were the magnitudes of total stochastic elasticities (ES), mean stochastic elasticities (ESμ) and variance stochastic elasticities (ESσ) to vital rates drastically different under current and projected precipitation conditions (figure 5). For Cryptantha, population dynamics under projected conditions relied more on survival (σ) and changes in individual size (γ; both growth and shrinkage), as well as greater sizes of new seedlings (ϑ), and less on reproduction (ψ) and establishment (ɛ) probabilities. The effects of variation in all vital rates on the stochastic population growth rate a were negligible, with the exception of changes in individual size (figure 5c); the ability to rapidly change in size had a positive effect on the population growth rate. For Carrichtera, differences in the relative impact of the vital rates on a were negligible, despite the significant differences in a values shown in figure 4 under current and projected conditions. All its vital rates had an overall similar effect on a when considering effects of mean and variance in vital rates simultaneously, as depicted in ES values (note that for each season ΣES = 1). However, the effect of mean values (ESμ) of emergence of seeds from the seed bank (ɛgerm) was one order of magnitude greater than the effects of mean values for all other vital rates. The impact of variation of ɛgerm (ESσ) was greatly negative. The impacts of ESσ for contributions of adults were positive if the seed went into the seed bank (ψbank), and negative when seeds germinated the same year as that of production (ψgerm).
Figure 5.
(a) Stochastic elasticity of the stochastic growth rate (a = log(λS)), and stochastic elasticity of a to (b) the mean and (c) variance of each of the vital rates involved in the life cycle of the perennial C. flava and the annual C. annua (figure 3) under current (open bar) and projected (filled bar) precipitation regimes. Negative values indicate that increasing variability of a vital rate decreases a. Vital rates for Cryptantha: survival (σ), change in size (γ), probability of reproduction (φ), production of flowering stalks (χ), seedling establishment (ɛ) and seedling size (ϑ). Vital rates for Carrichtera: germination probability (δgerm), seedlings survival (σ), germinable seed production (ψgerm), survival in seed bank (δbank1, δbank2 and δbank3), seed-bank contribution (ψbank) and seed-bank emergence probability (ɛgerm).
4. Climate change, precipitation and desert plant demography
We found a surprising pattern of increased population growth for both study species when we compared population dynamics in the future to current conditions, consistent with increasing precipitation in Utah, USA and despite decreasing precipitation in Israel. Our findings support our hypotheses for C. flava's improved demographic performance in wetter years and highlight the potential of the annual C. annua to buffer stochasticity of drier years via its seed bank (i.e. demographic buffering [33,34]). This is the first usage of a comparative demographic approach studying desert plant responses to climate change, and our conclusions challenge the commonly held perception based on correlative approaches (e.g. bioclimatic envelope approaches) suggesting that desert organisms may be particularly vulnerable to climate change [14,20–22].
The tools we have used here, size-/stage-structured population models [23,24], provide a robust, quantitative link between the overall characteristics of the population (e.g. population size and growth rate), their underlying vital rates (e.g. survival, changes in size, germination, seed dormancy), and evolutionary pressures [96,99]. In particular, our stochastic simulations based on high-resolution projections of precipitation for 2075–2099, as well as on long-term, field-collected demographic data, allowed us to discern the vital rates underlying the demographic improvement. Interestingly, the significant increases in stochastic population growth rates took place with relatively little change in the relative impact of the vital rates. In the case of the population of Cryptantha in Utah, USA, where more frequent rainy years and a resulting longer growing season are projected, population growth rates are expected to increase, mediated through more drastic changes in size (growth and shrinkage), increases in survival and in size of new recruits. The fact that such changes in size, when examined in a stochastic, long-term perspective, currently and in the future have positive impact on the stochastic population growth rate might provide further support for the hypothesized adaptive value of hydraulic fragmentation [9]. Internally fragmented individuals in desert species typically experience rapid shrinkage under dry conditions [56,81]. We believe that the linkage of this phenomenon to population dynamics deserves further attention [52].
Our analyses highlight the crucial role of seed dormancy in C. annua in Israel as a bet-hedging mechanism buffering extreme precipitation years. In both current and future scenarios, the vital rate elasticity to seed bank emergence was the most important one to population growth. In this population, we were able to discern a complex pattern behind the almost identical vital rate stochastic elasticities under current versus projected conditions: reducing only the frequency of wet years in our models led to a decrease in the relative importance of the vegetative pathway, with seeds germinating the same year of their production (figure 3b). On the other hand, increasing only the frequency of dry annual periods had the opposite effect: the relative impact of the dormant pathway, with seeds remaining in the seed bank, decreased (W. Siewert 2012, unpublished data). Since wet periods will become less frequent and dry periods more frequent in the climate change projection, the effects cancel each other out, resulting in negligible difference, but still producing a higher growth rate than under current conditions. However, it must be noted that a single and rather exceptional year is responsible for the increase in stochastic population growth rate. In the 2008–2009 annual period, the driest one in our study, we measured an extremely high population growth rate owing to very high fecundity. If we excluded this year from our simulation, we found a decreasing growth rate with increasing frequency of dry years. Still, the correctness of the measured fecundity was confirmed by a record number of individuals in the following 2009–2010 growing season (J. Kigel 2012, unpublished data). Very high fecundity associated with a drought year is unusual in desert annual demography and inconsistent with general findings of reproductive failure (see §2b). A possible explanation for this pattern may be reduced competition from coexisting plant species. Indeed, population size of Trisetaria macrochaeta (Poaceae), the most abundant species at the site, was the lowest during 2008–2009 (W. Siewert 2012, unpublished data). Nonetheless, future work will re-visit the present demographic projections with higher temporal replication in the estimates of seedbank dynamics.
Our study contributes two notable exceptions to the accepted view that short-lived species, regardless of habitat, are particularly vulnerable to climate change [26]. We argue that for both species, the potential for demographic buffering is high, in the light of the resulting higher stochastic population growth rates. However, other plausible explanations, such as interannual co-variance of vital rates [102], deserve further exploration. The simplicity of our modelling approach, whenever long-term demographic data is available, together with the robustness of the climatic projections, calls for its application to other species, particularly when considering the urgent need to understand responses to climate change of more desert species that might prove important in alleviating poverty. If our findings are applicable to other desert plant species, the implications for carbon storage [103,104], particularly below-ground, exploitation of natural resources [105], and self-sustainability should be re-evaluated in the context of the large amount of dryland human inhabitants [2], a number that is only expected to increase owing to desertification [3].
(a). Future directions
Clearly, we are still far from achieving a detailed understanding of how climate change will affect desert plant species in general, or whether it will at all, given their potential for buffering abiotic stochasticity. More and longer studies with more frequent censuses will help in this task by encompassing a large range of probable precipitation situations to which desert plants are exposed. Demographic studies in which plots exposed only to natural precipitation are revisited every decade [39,40,44] may not offer enough resolution to examine the underlying vital rates that govern the population dynamics, especially if large positive and negative changes in individual size may occur [77], or provide a mechanistic understanding of the effects of precipitation. We see great value in simulating expected precipitation pulses experimentally, as in physiological studies [106–108], but at the whole-population level. This approach can drastically decrease the amount of years necessary to provide robust demographic projections (R. Salguero-Gómez 2012, unpublished data). The coupling of field-based studies with greenhouse experiments to explore more obscure demographic processes, such as seed bank and vegetative dormancy, will also be beneficial here [49–51]. Along the same lines, the conclusions of our field study where only one population was studied per species must be carefully evaluated. Demographic heterogeneity has been shown in different populations within the same species [109] in other ecosystems and is likely not an exception in deserts. Better spatially replicated studies are needed in deserts, perhaps including populations from recently desertified versus historically desert regions.
Demographic studies that reach beyond charismatic taxa (e.g. Cactaceae; figure 1c) and field sites close to research groups (e.g. USA, Australia; figure 1a) need to be carried out. As a suggestion, the demography of dominant desert families, such as the Agavaceae, Aizoaceae, Cyperaceae and Rhamnaceae remain completely unexplored. Likewise, despite the crucial role that biological soil crusts play in ecosystem functioning and structure [110], no demographic model has studied these lichen-moss-cyanobacterial symbiotic assemblies. It is also puzzling that so much plant demography has been done in other ecosystems (figure 1b), despite deserts' species richness and extension [2]. Geographically, it seems logical that research efforts should prioritize demographically unexplored real deserts (less than 100 mm yr−1) such as the Patagonian, Sahara, Namib, Kalahari, Karakum, Kavir, Taklamakan and Gobi (figure 1a). Finally, we call for the importance of comparing underlying vital rates of populations of the same species under different aridity gradient values. Work in this regard has shown drastic changes in seed production and seedling establishment [111]. We hope that a greater interest in deserts will generate more long-term demographic datasets to which super-high-resolution climatic models [76] can be applied. Such efforts should result in a more comprehensive understanding of how deserts will fare in the future.
Acknowledgements
The authors are most grateful to R. Mizuta, A. Kitoh and M. Hosaka for providing the super-high-resolution precipitation projections used in this manuscript. Long-term research on C. flava was supported by grant IBN95–27833 (National Science Foundation) to B.B.C., and by CTL Fellowship, Binns-Williams Funds, Forrest Shreve Desert Ecology Award (Ecological Society of America), Grant-In-Aid of Research (Sigma Xi), Lewis & Clark Funds for Exploratory Field Research (American Philosophical Society), and the Max Planck Institute for Demographic Research to R.S.-G. Long-term research on C. annua by W.S. and K.T. is part of the GLOWA Jordan River project funded by the German Federal Ministry of Education and Research (BMBF). R.S.-G. and B.B.C. thank H. and P. Kempenich, C. Plunkett, J. Sinclear, J. Salix and T. Faircloth from the US Forest Service and Bureau of Land Management in Vernal, UT, for logistical support. W.S and K.T. thank J. Metz for doing most censuses, and J. Kigel and M. Sternberg for sharing data on biomass and seed bank dynamics, as well as for logistical support.
References
- 1.United Nations Environmental Programme 1992. Global biodiversity strategy. Guidelines for action to save, study and use earth's biotic wealth sustainably and equitably. Washington, DC: World Resources Institute [Google Scholar]
- 2.Maestre F. T., Salguero-Gómez R., Quero J. L. 2012. It's getting hotter in here: determining and projecting the impacts of global environmental change on drylands. Phil. Trans. R. Soc. B 367, 3062–3075 10.1098/rstb.2011.0323 (doi:10.1098/rstb.2011.0323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds J. F., et al. 2007. Global desertification: building a science for dryland development. Science 316, 847–851 10.1126/science.1131634 (doi:10.1126/science.1131634) [DOI] [PubMed] [Google Scholar]
- 4.Intergovernmental Panel for Climate Change 2007. Climate change 2007: synthesis report. Contribution of working groups I, II and III to the fourth assessment report of the intergovernmental panel on climate change. Geneva, Switzerland: IPCC [Google Scholar]
- 5.Sala O. E., Lauenroth W. K. 1982. Small rainfall events: an ecological role in semiarid regions. Oecologia 53, 301–304 10.1007/BF00389004 (doi:10.1007/BF00389004) [DOI] [PubMed] [Google Scholar]
- 6.Smith W. K., Monson R. K., Anderson J. E. 1997. Physiological ecology of North American desert plants. New York, NY: Springer [Google Scholar]
- 7.Canadell J., Jackson R. B., Ehleringer J. R., Mooney H. A., Sala O. E., Schulze E. D. 1996. Maximum rooting depth of vegetation types at the global scale. Oecologia 108, 583–595 10.1007/BF00329030 (doi:10.1007/BF00329030) [DOI] [PubMed] [Google Scholar]
- 8.Dodd A. N., Borland A. M., Haslam R. P., Griffiths H., Maxwell K. 2002. Crassulacean acid metabolism: plastic, fantastic. J. Exp. Bot. 53, 569–580 10.1093/jexbot/53.369.569 (doi:10.1093/jexbot/53.369.569) [DOI] [PubMed] [Google Scholar]
- 9.Schenk H. J., Espino S., Goedhart C. M., Nordenstahl M., Cabrera H. I. M., Jones C. S. 2008. Hydraulic integration and shrub growth form linked across continental aridity gradients. Proc. Natl Acad. Sci. USA 105, 11 248–11 253 10.1073/pnas.0804294105 (doi:10.1073/pnas.0804294105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angert A. L., Horst J. L., Huxman T. E., Venable D. L. 2010. Phenotypic plasticity and precipitation response in Sonoran desert winter annuals. Am. J. Bot. 97, 405–411 10.3732/ajb.0900242 (doi:10.3732/ajb.0900242) [DOI] [PubMed] [Google Scholar]
- 11.Bowers J. E., Webb R. H., Rondeau R. J. 1995. Longevity, recruitment and mortality of desert plants in Grand Canyon, Arizona, USA. J. Veg. Sci. 6, 551–564 10.2307/3236354 (doi:10.2307/3236354) [DOI] [Google Scholar]
- 12.Schwinning S., Sala O. E., Loik M. E., Ehleringer J. R. 2004. Thresholds, memory, and seasonality: understanding pulse dynamics in arid/semi-arid ecosystems. Oecologia 141, 191–193 10.1007/s00442-004-1683-3 (doi:10.1007/s00442-004-1683-3) [DOI] [PubMed] [Google Scholar]
- 13.Easterling D. R., Meehl G. A., Parmesan C., Changnon S. A., Karl T. R., Mearns L. O. 2000. Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074 10.1126/science.289.5487.2068 (doi:10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 14.Parmesan C., et al. 1999. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399, 579–583 10.1038/21181 (doi:10.1038/21181) [DOI] [Google Scholar]
- 15.Parmesan C., Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 10.1038/nature01286 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 16.Zou S. B., Cheng G. D., Xiao H. L., Xu B. R., Feng Z. D. 2009. Holocene natural rhythms of vegetation and present potential ecology in the Western Chinese Loess Plateau. Quat. Int. 194, 55–67 10.1016/j.quaint.2008.09.009 (doi:10.1016/j.quaint.2008.09.009) [DOI] [Google Scholar]
- 17.Huxman T. E., et al. 2004. Convergence across biomes to a common rain-use efficiency. Nature 429, 651–654 10.1038/nature02561 (doi:10.1038/nature02561) [DOI] [PubMed] [Google Scholar]
- 18.Noy-Meir I. 1973. Desert ecosystems: environment and producers. Annu. Rev. Ecol. Evol. Syst. 4, 25–51 10.1038/nature02561 (doi:10.1038/nature02561) [DOI] [Google Scholar]
- 19.Reynolds J. F., Kemp P. R., Ogle K., Fernández R. J. 2004. Modifying the ‘pulse-reserve’ paradigm for deserts of North America: precipitation pulses, soil water, and plant responses. Oecologia 141, 194–210 10.1007/s00442-004-1524-4 (doi:10.1007/s00442-004-1524-4) [DOI] [PubMed] [Google Scholar]
- 20.Midgley G. F., Thuiller W. 2007. Potential vulnerability of Namaqualand plant diversity to anthropogenic climate change. J. Arid Environ. 70, 615–628 10.1016/j.jaridenv.2006.11.020 (doi:10.1016/j.jaridenv.2006.11.020) [DOI] [Google Scholar]
- 21.Munson S. M., Webb R. H., Hubbard J. A. 2011. A comparison of methods to assess long-term changes in Sonoran Desert vegetation. J. Arid Environ. 75, 1228–1231 10.1016/j.jaridenv.2011.04.032 (doi:10.1016/j.jaridenv.2011.04.032) [DOI] [Google Scholar]
- 22.Miranda J. D., Armas C., Padilla F. M., Pugnaire F. I. 2011. Climatic change and rainfall patterns: effects on semi-arid plant communities of the Iberian Southeast. J. Arid Environ. 75, 1302–1309 10.1016/j.jaridenv.2011.04.022 (doi:10.1016/j.jaridenv.2011.04.022) [DOI] [Google Scholar]
- 23.Caswell H. 2001. Matrix population models: construction, analysis, and interpretation, 2nd edn. Sunderland, MA: Sinauer Associates [Google Scholar]
- 24.Easterling M. R., Ellner S., Dixon P. 2000. Size-specific sensitivity: applying a new structured population model. Ecology 81, 694–708 10.1890/0012-9658(2000)081[0694:SSSAAN]2.0.CO;2 (doi:10.1890/0012-9658(2000)081[0694:SSSAAN]2.0.CO;2) [DOI] [Google Scholar]
- 25.van Tienderen P. H. 2000. Elasticities and the link between demographic and evolutionary dynamics. Ecology 81, 666–679 10.1890/0012-9658(2000)081[0666:EATLBD]2.0.CO;2 (doi:10.1890/0012-9658(2000)081[0666:EATLBD]2.0.CO;2) [DOI] [Google Scholar]
- 26.Morris W. F., et al. 2008. Longevity can buffer plant and animal populations against changing climatic variability. Ecology 89, 19–25 10.1890/07-0774.1 (doi:10.1890/07-0774.1) [DOI] [PubMed] [Google Scholar]
- 27.Murakami H., Wang B. 2010. Future change of North Atlantic tropical cyclone tracks: projection by a 20-km-mesh global atmospheric model. J. Climate 23, 2699–2721 10.1175/2010JCLI3338.1 (doi:10.1175/2010JCLI3338.1) [DOI] [Google Scholar]
- 28.Huxman T. E., Barron-Gafford G., Gerst K. L., Angert A. L., Tyler A. P., Venable D. L. 2008. Photosynthetic resource-use efficiency and demographic variability in desert winter annual plants. Ecology 89, 1554–1563 10.1890/06-2080.1 (doi:10.1890/06-2080.1) [DOI] [PubMed] [Google Scholar]
- 29.Lewontin R. C., Cohen D. 1969. On population growth in a randomly varying environment. Proc. Natl Acad. Sci. USA 62, 1056–1060 10.1073/pnas.62.4.1056 (doi:10.1073/pnas.62.4.1056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aronson J., KIigel J., Shmida A. 1993. Reproductive allocation strategies in desert and Mediterranean populations of annual plants grown with and without water-stress. Oecologia 93, 336–342 10.1007/BF00317875 (doi:10.1007/BF00317875) [DOI] [PubMed] [Google Scholar]
- 31.Toft C. A. 1995. A 10-year demographic study of rabbitbrush (Chrysothamnus nauseosus): growth, survival and water limitation. Oecologia 101, 1–12 10.1007/BF00328893 (doi:10.1007/BF00328893) [DOI] [PubMed] [Google Scholar]
- 32.Koons D. N., Pavard S., Baudisch A., Metcalf C. J. E. 2009. Is life-history buffering or liability adaptive in stochastic environments? Oikos 118, 972–980 10.1111/j.1600-0706.2009.16399.x (doi:10.1111/j.1600-0706.2009.16399.x) [DOI] [Google Scholar]
- 33.Morris W. F., Doak D. F. 2004. Buffering of life histories against environmental stochasticity: accounting for a spurious correlation between the variabilities of vital rates and their contributions to fitness. Am. Nat. 163, 579–90 10.1086/382550 (doi:10.1086/382550) [DOI] [PubMed] [Google Scholar]
- 34.Doak D. F., Morris W. F. 2010. Demographic compensation and tipping points in climate-induced range shifts. Nature 467, 959–962 10.1038/nature09439 (doi:10.1038/nature09439) [DOI] [PubMed] [Google Scholar]
- 35.Bowers J. E. 2005. Effects of drought on shrub survival and longevity in the northern Sonoran Desert. J. Torrey Bot. Soc. 132, 421–431 10.3159/1095-5674(2005)132[421:EODOSS]2.0.CO;2 (doi:10.3159/1095-5674(2005)132[421:EODOSS]2.0.CO;2) [DOI] [Google Scholar]
- 36.Bowers J. E., Turner R. M. 2001. Dieback and episodic mortality of Cercidium microphyllum (foothill paloverde), a dominant Sonoran Desert tree. J. Torrey Bot. Soc. 128, 128–140 10.2307/3088735 (doi:10.2307/3088735) [DOI] [Google Scholar]
- 37.Miriti M. N., Rodríguez-Buritica S., Wright S. J., Howe H. F. 2007. Episodic death across species of desert shrubs. Ecology 88, 32–36 10.1890/0012-9658(2007)88[32:EDASOD]2.0.CO;2 (doi:10.1890/0012-9658(2007)88[32:EDASOD]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 38.Bowers J. E., Turner R. M. 2002. The influence of climatic variability on local populations dynamics of Cercidium microphyllum (foothill paloverde). Oecologia 130, 105–113 10.1007/s004420100779 (doi:10.1007/s004420100779) [DOI] [PubMed] [Google Scholar]
- 39.Goldberg D. E., Turner R. M. 1986. Vegetation change and plant demography in permanent plots in the Sonoran Desert. Ecology 67, 695–712 10.2307/1937693 (doi:10.2307/1937693) [DOI] [Google Scholar]
- 40.Pierson E. A., Turner R. M. 1998. An 85-year study of saguaro (Carnegiea gigantea) demography. Ecology 79, 2676–2693 10.1890/0012-9658(1998)079[2676:AYSOSC]2.0.CO;2 (doi:10.1890/0012-9658(1998)079[2676:AYSOSC]2.0.CO;2) [DOI] [Google Scholar]
- 41.Bullock S. H., Martijena N. E., Webb R. H., Turner R. M. 2005. Twentieth century demographic changes in cirio and cardon in Baja California, Mexico. J. Biogeogr. 32, 127–143 10.1111/j.1365-2699.2004.01152.x (doi:10.1111/j.1365-2699.2004.01152.x) [DOI] [Google Scholar]
- 42.Cody M. L. 2000. Slow-motion population dynamics in Mojave Desert perennial plants. J. Veg. Sci. 11, 351–358 10.2307/3236627 (doi:10.2307/3236627) [DOI] [Google Scholar]
- 43.Nobel P. S., Zutta B. R. 2005. Morphology, ecophysiology, and seedling establishment for Fouquieria splendens in the northwestern Sonoran Desert. J. Arid Environ. 62, 251–265 10.1016/j.jaridenv.2004.11.002 (doi:10.1016/j.jaridenv.2004.11.002) [DOI] [Google Scholar]
- 44.Danzer S., Drezner T. D. 2010. Demographics of more than 12,000 individuals of a keystone species in the northern Sonoran Desert since the mid-1800s. Int. J. Plant Sci. 171, 538–546 10.1086/652013 (doi:10.1086/652013) [DOI] [Google Scholar]
- 45.Flanary B. E., Kletetschka G. 2006. Analysis of telomere length and telomerase activity in tree species of various lifespans, and with age in the bristlecone pine Pinus longaeva. Rejuv. Res. 9, 61–3 10.1089/rej.2006.9.61 (doi:10.1089/rej.2006.9.61) [DOI] [PubMed] [Google Scholar]
- 46.Vasek F. C. 1980. Creosote bush: long-lived clones in the Mojave Desert. Am. J. Bot. 67, 246–255 10.2307/2442649 (doi:10.2307/2442649) [DOI] [Google Scholar]
- 47.Hereford R., Webb R. H., Longpre C. I. 2006. Precipitation history and ecosystem response to multidecadal precipitation variability in the Mojave Desert region, 1893–2001. J. Arid Environ. 67, 13–34 10.1016/j.jaridenv.2006.09.01 (doi:10.1016/j.jaridenv.2006.09.01) [DOI] [Google Scholar]
- 48.McAuliffe J. R., Hamerlynck E. P. 2010. Perennial plant mortality in the Sonoran and Mojave deserts in response to severe, multi-year drought. J. Arid Environ. 74: 885–896 10.1016/j.jaridenv.2010.01.001 (doi:10.1016/j.jaridenv.2010.01.001) [DOI] [Google Scholar]
- 49.Godínez-Álvarez H., Valverde T., Ortega-Baes P. 2003. Demographic trends in the Cactaceae. Bot. Rev. 69, 173–203 10.1663/0006-8101(2003)069[0173:DTITC]2.0.CO;2 (doi:10.1663/0006-8101(2003)069[0173:DTITC]2.0.CO;2) [DOI] [Google Scholar]
- 50.Meyer S. E., Pendleton B. K. 2005. Factors affecting seed germination and seedling establishment of a long-lived desert shrub (Coleogyne ramosissima: Rosaceae). Plant Ecol. 178, 171–187 10.1007/s11258-004-3038-x (doi:10.1007/s11258-004-3038-x) [DOI] [Google Scholar]
- 51.Herrera C. M. 1998. Population-level estimates of interannual variability in seed production: what do they actually tell us? Oikos 82, 612–616 10.2307/3546384 (doi:10.2307/3546384) [DOI] [Google Scholar]
- 52.Salguero-Gómez R., Casper B. B. 2010. Keeping plant shrinkage in the demographic loop. J. Ecol. 98, 312–323 10.1111/j.1365-2745.2009.01616.x (doi:10.1111/j.1365-2745.2009.01616.x) [DOI] [Google Scholar]
- 53.Davis S. D., Ewers F. W., Sperry J. S., Portwood K. A., Crocker M. C., Adams G. C. 2002. Shoot dieback during prolonged drought in Ceanothus (Rhamnaceae) chaparral of California: a possible case of hydraulic failure. Am. J. Bot. 89, 820–828 10.3732/ajb.89.5.820 (doi:10.3732/ajb.89.5.820) [DOI] [PubMed] [Google Scholar]
- 54.Kozlowski T. T. 1973. Shedding of plant parts. New York, NY: Academic Press [Google Scholar]
- 55.Casper B. B., Forseth I. N., Wait D. A. 2006. A stage-based study of drought response in Cryptantha flava (Boraginaceae): gas exchange, water use efficiency, and whole plant performance. Am. J. Bot. 93, 977–987 10.3732/ajb.93.7.978 (doi:10.3732/ajb.93.7.978) [DOI] [PubMed] [Google Scholar]
- 56.Salguero-Gómez R., Casper B. B. 2010. A hydraulic explanation for size-specific plant shrinkage: developmental hydraulic sectoriality. New Phytol. 189, 229–240 10.1111/j.1469-8137.2010.03447.x (doi:10.1111/j.1469-8137.2010.03447.x) [DOI] [PubMed] [Google Scholar]
- 57.Zanne A. E., Sweeney K., Sharma M., Orians C. M. 2006. Patterns and consequences of differential vascular sectoriality in 18 temperate tree and shrub species. Funct. Ecol. 20, 200–206 10.1111/j.1365-2435.2006.01101.x (doi:10.1111/j.1365-2435.2006.01101.x) [DOI] [Google Scholar]
- 58.Schenk H. J. 1999. Clonal splitting in desert shrubs. Plant Ecol. 141, 41–52 10.1023/A:1009895603783 (doi:10.1023/A:1009895603783) [DOI] [Google Scholar]
- 59.Miller R. E., Huenneke L. F. 1996. Size decline in Larrea tridentata (creosotebush). Southwest. Nat. 41, 248–250 10.2151/jmsj.2012-A12 (doi:10.2151/jmsj.2012-A12) [DOI] [Google Scholar]
- 60.Colling G., Matthies D. 2006. Effects of habitat deterioration on population dynamics and extinction risk of an endangered, long-lived perennial herb (Scorzonera humilis). J. Ecol. 94, 959–972 10.1111/j.1365-2745.2006.01147.x (doi:10.1111/j.1365-2745.2006.01147.x) [DOI] [Google Scholar]
- 61.Verhulst J., Montana C., Mandujano M. C., Franco M. 2008. Demographic mechanisms in the coexistence of two closely related perennials in a fluctuating environment. Oecologia 156, 95–105 10.1007/s00442-008-0980-7 (doi:10.1007/s00442-008-0980-7) [DOI] [PubMed] [Google Scholar]
- 62.Watson I. W., Westoby M., Holm A. M. 1997. Continuous and episodic components of demographic change in arid zone shrubs: models of two Eremophila species from Western Australia compared with published data on other species. J. Ecol. 85, 833–846 10.2307/2960605 (doi:10.2307/2960605) [DOI] [Google Scholar]
- 63.Clauss M., Venable D. 2000. Seed germination in desert annuals: an empirical test of adaptive bet hedging. Am. Nat. 155, 168–186 10.1086/303314 (doi:10.1086/303314) [DOI] [PubMed] [Google Scholar]
- 64.Kadmon R. 1993. Population dynamic consequences of habitat heterogeneity: an experimental study. Ecology 74, 816–825 10.2307/1940808 (doi:10.2307/1940808) [DOI] [Google Scholar]
- 65.Aronson J., Kigel J., Shmida A., Klein J. 1992. Adaptive phenology of desert and Mediterranean populations of annual plants grown with and without water-stress. Oecologia 89, 17–26 10.1007/BF00319010 (doi:10.1007/BF00319010) [DOI] [PubMed] [Google Scholar]
- 66.Evans M. E. K., Ferriere R., Kane M. J., Venable D. L. 2007. Bet hedging via seed banking in desert evening primroses (Oenothera, Onagraceae): demographic evidence from natural populations. Am. Nat. 169, 184–194 10.1086/510599 (doi:10.1086/510599) [DOI] [PubMed] [Google Scholar]
- 67.Fox L. R., Steele H. N., Holl K. D., Fusari M. H. 2006. Contrasting demographies and persistence of rare annual plants in highly variable environments. Plant Ecol. 183, 157–170 10.1007/s11258-005-9014-2 (doi:10.1007/s11258-005-9014-2) [DOI] [Google Scholar]
- 68.Levine J. M., McEachern A. K., Cowan C. 2008. Rainfall effects on rare annual plants. J. Ecol. 96, 795–806 10.1111/j.1365-2745.2008.01375.x (doi:10.1111/j.1365-2745.2008.01375.x) [DOI] [Google Scholar]
- 69.Adondakis S., Venable D. 2004. Dormancy and germination in a guild of Sonoran Desert annuals. Ecology 85, 2582–2590 10.1890/03-0587 (doi:10.1890/03-0587) [DOI] [Google Scholar]
- 70.Brown G. 2002. Community composition and population dynamics in response to artificial rainfall in an undisturbed desert annual community in Kuwait. Basic Appl. Ecol. 3, 145–156 10.1078/1439-1791-00097 (doi:10.1078/1439-1791-00097) [DOI] [Google Scholar]
- 71.Sher A., Goldberg D., Novoplansky A. 2004. The effect of mean and variance in resource supply on survival of annuals from Mediterranean and desert environments. Oecologia 141, 353–362 10.1007/s00442-003-1435-9 (doi:10.1007/s00442-003-1435-9) [DOI] [PubMed] [Google Scholar]
- 72.Boeken B., Shachak M. 1998. The dynamics of abundance and incidence of annual plant species during colonization in a desert. Ecography 21, 63–73 10.1111/j.1600-0587.1998.tb00394.x (doi:10.1111/j.1600-0587.1998.tb00394.x) [DOI] [Google Scholar]
- 73.Levine J., Rees M. 2004. Effects of temporal variability on rare plant persistence in annual systems. Am. Nat. 164, 350–363 10.1086/422859 (doi:10.1086/422859) [DOI] [PubMed] [Google Scholar]
- 74.Meyer S., Quinney D., Weaver J. 2006. A stochastic population model for Lepidium papilaferum (Brassicaceae), a rare desert ephemeral with a persistent seed bank. Am. J. Bot. 93, 891–902 10.3732/ajb.93.6.891 (doi:10.3732/ajb.93.6.891) [DOI] [PubMed] [Google Scholar]
- 75.Cohen D. 1966. Optimizing reproduction in a randomly varying environment when a correlation may exist between the conditions at the time a choice has to be made and the subsequent outcome. J. Theor. Biol. 16, 1–14 10.1016/0022-5193(67)90050-1 (doi:10.1016/0022-5193(67)90050-1) [DOI] [PubMed] [Google Scholar]
- 76.Mizuta R., et al. 2012. Climate simulations using MRI-AGCM3.2 with 20-km grid. J. Meteorol. Soc. Jpn. 90A, 235–360 [Google Scholar]
- 77.Casper B. B. 1996. Demographic consequences of drought in the herbaceous perennial Cryptantha flava: effects of density, associations with shrubs, and plant size. Oecologia 106, 144–152 10.1007/BF00328593 (doi:10.1007/BF00328593) [DOI] [PubMed] [Google Scholar]
- 78.Peek M. S., Forseth I. N. 2005. Non-destructive estimation of lateral root distribution in an aridland perennial. Plant Soil 273, 211–217 10.1007/s11104-004-7600-z (doi:10.1007/s11104-004-7600-z) [DOI] [Google Scholar]
- 79.Casper B. B. 1981. Fixed rates of random ovule abortion in Cryptantha flava (Boraginaceae) and its possible relation to seed dispersal. Ecology 62, 866–869 10.2307/1937752 (doi:10.2307/1937752) [DOI] [Google Scholar]
- 80.Casper B. B., Forseth I. N., Kempenich H., Seltzer S., Xavier K. 2001. Drought prolongs leaf life span in the herbaceous desert perennial Cryptantha flava. Funct. Ecol. 15, 740–747 10.1046/j.0269-8463.2001.00583.x (doi:10.1046/j.0269-8463.2001.00583.x) [DOI] [Google Scholar]
- 81.Salguero-Gómez R., Casper B. B. 2010. Introducing short roots in a desert perennial: anatomy and spatiotemporal foraging responses to increased precipitation. New Phytol. 191, 173–183 10.1111/j.1469-8137.2011.03679.x (doi:10.1111/j.1469-8137.2011.03679.x) [DOI] [PubMed] [Google Scholar]
- 82.Marhold K. 2011. Brassicaceae. In Euro+Med Plantbase: the information resource for Euro-Mediterranean plant diversity. See http://www.emplantbase.org/home.html. [Google Scholar]
- 83.Cooke J., Groves R. H., Ash J. 2011. The distribution of Carrichtera annua in Australia: introduction, spread and probable limits. Rangeland J. 33, 23–35 10.1071/RJ10001 (doi:10.1071/RJ10001) [DOI] [Google Scholar]
- 84.Danin A. 2006. Flora of Israel online. See http://flora.huji.ac.il/browse.asp?lang=en.
- 85.Gutterman Y., Gendler T., Huang Z.-Y. 2007. Plant dispersal strategies, seed bank distribution and germination of Neger Desert species. In Seeds: biology, development and ecology (eds Adkins S., Ashmore S., Navie S. C.), pp. 407–415 Brisbane, Australia: CAB International [Google Scholar]
- 86.Lucas R. W., Forseth I. N., Casper B. B. 2008. Using rainout shelters to evaluate climate change effects on the demography of Cryptantha flava. J. Ecol. 96, 514–522 10.1111/j.1365-2745.2007.01350.x (doi:10.1111/j.1365-2745.2007.01350.x) [DOI] [Google Scholar]
- 87.Sternberg M., et al. 2011. The use and misuse of climatic gradients for evaluating climate impact on dryland ecosystems–an example for the solution of conceptual problems. In Gradients in drylands: linking patterns and processes and their consequences for biodiversity (eds Veste M., Linstädter A., Breckle S. W.), pp. 361–374 New York, NY: Springer [Google Scholar]
- 88.Metz J., Liancourt P., Kigel J., Harel D., Sternberg M., Tielbörger K. 2010. Plant survival in relation to seed size along environmental gradients: a long-term study from semi-arid and Mediterranean annual plant communities. J. Ecol. 98, 697–704 10.1111/j.1365-2745.2010.01652.x (doi:10.1111/j.1365-2745.2010.01652.x) [DOI] [Google Scholar]
- 89.Harel D., Holzapfel C., Sternberg M. 2011. Seed mass and dormancy of annual plant populations and communities decreases with aridity and rainfall predictability. Basic Appl. Ecol. 12, 674–684 10.1016/j.baae.2011.09.003 (doi:10.1016/j.baae.2011.09.003) [DOI] [Google Scholar]
- 90.Caswell H., Trevisan M. C. 1994. Sensitivity analysis of periodic matrix models. Ecology 75, 1299–1303 10.2307/1937455 (doi:10.2307/1937455) [DOI] [Google Scholar]
- 91.Li S. L., Yu F. H., Werger M. J. A., Dong M., Zuidema P. A. 2011. Habitat-specific demography across dune fixation stages in a semi-arid sandland: understanding the expansion, stabilization and decline of a dominant shrub. J. Ecol. 99, 610–620 10.1111/j.1365-2745.2010.01777.x (doi:10.1111/j.1365-2745.2010.01777.x) [DOI] [Google Scholar]
- 92.Ramula S., Rees M., Buckley Y. M. 2009. Integral projection models perform better for small demographic data sets than matrix projection models: a case study of two perennial herbs. J. Appl. Ecol. 46, 1048–1053 10.1111/j.1365-2664.2009.01706.x (doi:10.1111/j.1365-2664.2009.01706.x) [DOI] [Google Scholar]
- 93.Salguero-Gómez R., Plotkin J. B. 2010. Matrix dimensions bias demographic inferences: implications for comparative plant demography. Am. Nat. 176, 710–722 10.1086/657044 (doi:10.1086/657044) [DOI] [PubMed] [Google Scholar]
- 94.Ellner S. P., Rees M. 2006. Integral projection models for species with complex demography. Am. Nat. 167, 410–428 10.1086/499438 (doi:10.1086/499438) [DOI] [PubMed] [Google Scholar]
- 95.Smith M., Caswell H., Mettler-Cherry P. 2005. Stochastic flood and precipitation regimes and the population dynamics of a threatened floodplain plant. Ecol. Appl. 15, 1036–1052 10.1890/04-0434 (doi:10.1890/04-0434) [DOI] [Google Scholar]
- 96.Ellner S. P., Rees M. 2007. Stochastic stable population growth in integral projection models: theory and application. J. Math. Biol. 54, 227–256 10.1007/s00285-006-0044-8 (doi:10.1007/s00285-006-0044-8) [DOI] [PubMed] [Google Scholar]
- 97.Sokal R. R., Rohlf F. J. 1995. Biometry: the principles and practices in statistics in biological sciences. New York, NY: WH Freeman [Google Scholar]
- 98.Hunter C. M., Caswell H., Runge M. C., Regehr E. V., Amstrup S. C., Stirling I. 2010. Climate change threatens polar bear populations: a stochastic demographic analysis. Ecology 91, 2883–2897 10.1890/09-1641.1 (doi:10.1890/09-1641.1) [DOI] [PubMed] [Google Scholar]
- 99.de Kroon H., Plaisier A., van Groenendael J., Caswell H. 1986. Elasticity: the relative contribution of demographic parameters to population growth rate. Ecology 67, 1427–1431 10.2307/1938700 (doi:10.2307/1938700) [DOI] [Google Scholar]
- 100.Horvitz C. C., Ehrlén J., Matlaga D. 2010. Context-dependent pollinator limitation in stochastic environments: can increased seed set overpower the cost of reproduction in an understorey herb. J. Ecol. 98, 268–278 10.1111/j.1365-2745.2009.01628.x (doi:10.1111/j.1365-2745.2009.01628.x) [DOI] [Google Scholar]
- 101.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing [Google Scholar]
- 102.Tuljapurkar S. 1989. An uncertain life: demography in random environments. Theor. Popul. Biol. 35, 227–294 10.1016/0040-5809(89)90001-4 (doi:10.1016/0040-5809(89)90001-4) [DOI] [PubMed] [Google Scholar]
- 103.Smart K., Archibald S., Scholes R. J., Reyers B., Nickless A., Knowles T. In press. Ecosystem service trade-offs in drylands: examples from the Eastern Cape of South Africa. Phil. Trans. R. Soc. B. [Google Scholar]
- 104.Dougill A. J., Stringer L. C., Leventon J., Riddell M., Rueff H., Spracklen D. V., Butt E. 2012. Lessons from community-based payment for ecosystem service schemes: from forests to rangelands. Phil. Trans. R. Soc. B 367, 3178–3190 10.1098/rstb.2011.0418 (doi:10.1098/rstb.2011.0418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grace O. M. 2011. Current perspectives on the economic botany of the genus Aloe L. (Xanthorrhoeaceae). S Afr. J. Bot. 77, 980–987 10.1016/j.sajb.2011.07.002 (doi:10.1016/j.sajb.2011.07.002) [DOI] [Google Scholar]
- 106.Hultine K. R., Cable W. L., Burgess S. S. O., Williams D. G. 2003. Hydraulic redistribution by deep roots of a Chihuahuan Desert phreatophyte. Tree Physiol. 23, 353–360 10.1093/treephys/23.5.353 (doi:10.1093/treephys/23.5.353) [DOI] [PubMed] [Google Scholar]
- 107.Schwinning S., Starr B. I., Ehleringer J. R. 2003. Dominant cold desert plants do not partition warm season precipitation by event size. Oecologia 136, 252–260 10.1007/s00442-003-1255-y (doi:10.1007/s00442-003-1255-y) [DOI] [PubMed] [Google Scholar]
- 108.Sperry J. S., Perry A. H., Sullivan J. E. M. 1991. Pit membrane degradation and air-embolism formation in ageing xylem vessels of Populus tremuloides Michx. J. Exp. Bot. 42, 1399–406 10.1093/jxb/42.11.1399 (doi:10.1093/jxb/42.11.1399) [DOI] [Google Scholar]
- 109.Jongejans E., de Kroon H. 2005. Space versus time variation in the population dynamics of three co-occurring perennial herbs. J. Ecol. 93, 681–692 10.1111/j.1365-2745.2005.01003.x (doi:10.1111/j.1365-2745.2005.01003.x) [DOI] [Google Scholar]
- 110.Escolar C., Martínez I., Bowker M. A., Maestre F. T. 2012. Warming reduces the growth and diversity of biological soil crusts in a semi-arid environment: implications for ecosystem structure and functioning. Phil. Trans. R. Soc. B 367, 3087–3099 10.1098/rstb.2011.0344 (doi:10.1098/rstb.2011.0344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gomaa N. H., Pico X. F. 2011. Seed germination, seedling traits, and seed bank of the tree Moringa peregrina (Moringaceae) in a hyper-arid environment. Am. J. Bot. 98, 1024–1030 10.3732/ajb.1000051 (doi:10.3732/ajb.1000051) [DOI] [PubMed] [Google Scholar]