Abstract
A 74-year-old man suffered from jejunal perforation and adhesion to sigmoid colon due to adenocarcinoma associated with intraductal papillary mucinous neoplasm (IPMN) arising in a jejunal heterotopic pancreas. The jejunal lesion showed direct extension to the sigmoid colon, which was mistaken as sigmoid colon cancer by surgeons. Malignant transformation is a rare complication of a heterotopic pancreas. About half of malignancies in reported cases were ductal adenocarcinoma arising in the stomach, and the jejunal location is extremely rare. Furthermore, IPMN is also uncommon finding in a heterotopic pancreas.
Keywords: Adenocarcinoma, Jejunum, Heterotopia, Pancreas
In 1727, Schilz first reported the example of a heterotopic pancreas and defined it as tissue histologically similar to normal pancreas but located somewhere other than its usual location, and without anatomic or vascular connection to the pancreas itself.1 A heterotopic pancreas is usually asymptomatic. The frequent sites of heterotopia are the stomach and duodenum, but a jejunal location is less common.1 Most pathologic changes of pancreas can occur in a heterotopic pancreas, but malignant transformation is rare. Furthermore, we have not found any report regarding an adenocarcinoma associated with intraductal papillary mucinous neoplasm (IPMN) in a heterotopic pancreas. In this case report, we describe a rare case of an adenocarcinoma arising in a jejunal heterotopic pancreas associated with IPMN, which was clinically misdiagnosed as perforated sigmoid colon cancer.
CASE REPORT
A 74-year-old man was admitted due to acute abdominal pain, which had started 3 hours earlier, with a stool problem since the previous month. He had a history of hypertension, cerebral infarction and benign prostatic hyperplasia under medical treatment. Physical examination revealed direct and rebound tenderness of the lower quadrants of the abdomen. Computed tomography of the abdomen showed multiple air bubbles and wall thickening of the sigmoid colon with fecal materials in the pericolic space. Laboratory results were within the normal ranges except for an elevated carbohydrate antigen 19-9 (88.39 U/mL). Under the impression of panperitonitis caused by perforation of sigmoid colon cancer an emergency operation was performed. During the operation, sigmoid colon showed a perforating tumor, which was severely adhered to the jejunum. Hartmann's operation and segmental resection of the small intestine were carried out.
Grossly, the specimen consisted of two separate segments of sigmoid colon and jejunum. Serosal surface of sigmoid colon showed a fibrotic torn area, which was considered to be a result of surgical detachment from jejunum. Inside was an irregular cauliflower-like mucosal lesion projected into the lumen. On section, a 3.0 cm-sized tumor with irregular infiltrating border was noted. The separately submitted jejunal segment demonstrated irregular serosal surface and intact mucosa. Sectioning of the jejunum revealed a 3 cm-sized gray-tan and rubbery tumor mainly located in the muscular layer, extending to the perijejunal soft tissue. Dilated duct-like spaces were seen in the involved muscular layer.
Histologically, the jejunal wall displayed irregularly dilated cystic areas, invasive carcinoma and focal heterotopic pancreatic tissue (Fig. 1). The foci of invasive carcinoma were composed of well-formed glands as well as individual cells and clusters, featuring well to poorly differentiated adenocarcinoma. The tumor was distributed through the muscular layer in a haphazard fashion, and infiltrated into the submucosal layer of jejunum and into the perijejunal soft tissue. The mucosa of jejunum was intact. The heterotopic pancreatic tissue was composed of ducts and acini without islet, intermingling with the tumor in the muscular layer of the jejunum (Fig. 2). Some ducts showed features of pancreatic intraepithelial neoplasia (PanIN) (Fig. 3). And more ectatic ducts with intraluminal papillary growth were present. The papillae were composed of mucin-secreting columnar epithelial cells with extensive pyloric metaplasia and variable degrees of cytologic atypia, being consistent with IPMN (Fig. 4). This lesion measured 7 mm in diameter. Transitional areas from high-grade PanIN and IPMN to adenocarcinoma were noted (Fig. 5). In the sigmoid colon, adenocarcinoma showing similar patterns to the jejunal tumor was present. Neoplastic glands were dispersed through the colonic wall and mesocolon (Fig. 6). Heterotopic pancreas, PanIN, and IPMN were not found in the sigmoid colon. Immunohistochemically, both jejunal and colonic tumors including IPMN showed positive reactions for cytokeratin 7, MUC5AC and MUC6 and negativity for cytokeratin 20, p53, and MUC2. By operative, pathologic and immunohistochemical findings, this adenocarcinoma was assumed to have arisen from a jejunal heterotopic pancreas via precancerous conditions (such as IPMN and PanIN), and invaded directly into the sigmoid colon.
Fig. 1.
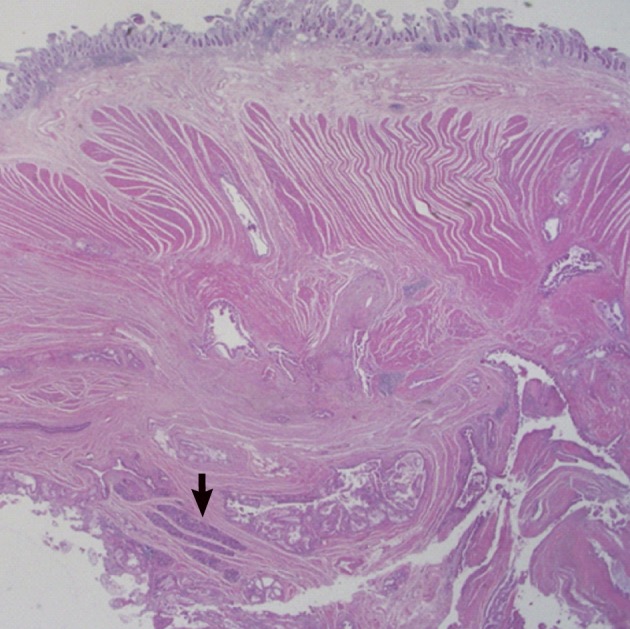
The jejunal wall shows areas of intraductal papillary mucinous neoplasm, invasive adenocarcinoma and heterotopic pancreas (arrow).
Fig. 2.
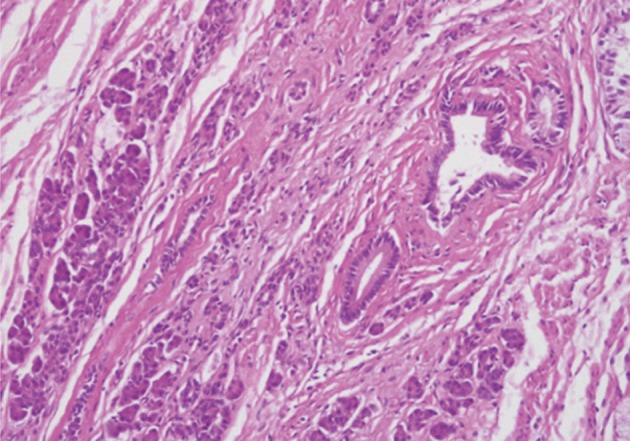
The heterotopic pancreas in jejunum demonstrates aggregates of ducts and acini.
Fig. 3.
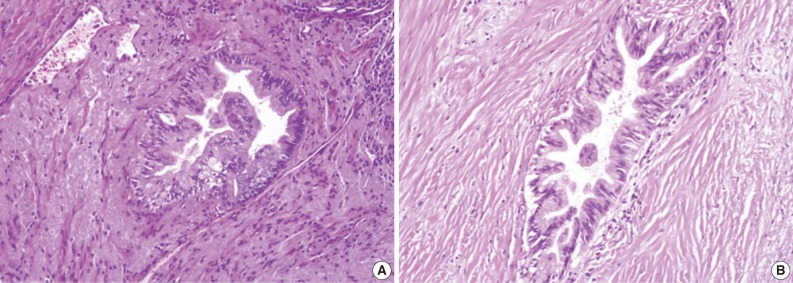
Grades of pancreatic intraepithelial neoplasia (PanIN). (A) A duct involved by PanIN2 shows pseudostratification of nuclei with mild to moderate cytologic abnormalities. (B) In PanIN3, budding of cellular tufts into the duct lumen and moderate to severe nuclear atypia are noted.
Fig. 4.

A cystically dilated duct is containing intraluminal papillae. The papillae are lined by tall columnar cells with abundant apical cytoplasmic mucin and basally oriented nuclei with mild to moderate dysplasia, consistent with intraductal papillary mucinous neoplasm.
Fig. 5.
(A) A transitional area from high grade pancreatic intraepithelial neoplasia to adenocarcinoma is identified. (B) A transitional area from intraductal papillary mucinous neoplasm to adenocarcinoma is present.
Fig. 6.
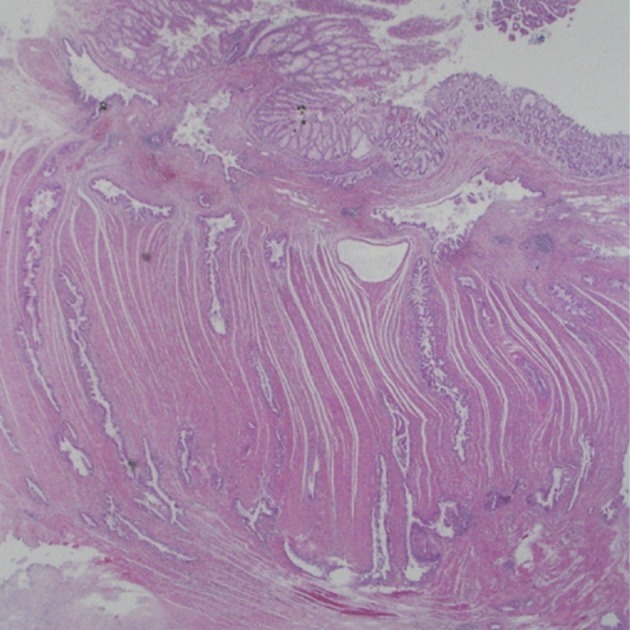
The sigmoid colon shows transmural invasion by adenocarcinoma.
DISCUSSION
A heterotopic pancreas is assumed to be a congenital anomaly, although its origin is not clear. Although several theories are suggested, none are conclusive. Separation from the main pancreatic structure during embryonic rotation and fusion of the dorsal and ventral anlagen2 is the most convincing hypothesis. Warthin stated that a heterotopic pancreas is formed from lateral budding of the rudimentary pancreatic ducts as they penetrate the intestinal wall, the mass of pancreatic tissue thus formed being snared off and carried by the longitudinal growth of the intestine, either upward and downward.3 Commonly reported locations of the heterotopic pancreas are the stomach (25-28.2%), duodenum (17-36.3%), and jejunum (15-21.7%), which are relatively near to the embryologic origin of a normal pancreas.1 The heterotopic pancreas has rarely been detected in the ileum, colon, spleen, liver, biliary tract, mesentery, lymph node, or rectum.4-6 Most cases are discovered incidentally during other procedures; endoscopy, surgery, or autopsy. Every pathologic change occurring in an eutopic pancreas can also occur in its heterotopic counterpart, such as chronic pancreatitis, pseudocyst, abscess formation and malignant transformation.7
A malignant change in the heterotopic pancreas is uncommon. It has been reported in about 32 cases.7 Appoximately half of these were ductal adenocarcinomas in the gastric heterotopia. The other cases included mucinous cystadenocarcinoma, neuroendocrine carcinoma, anaplastic carcinoma, acinar cell carcinoma, solid pseudopapillary neoplasm, and mixed acinar endocrine carcinoma arising from heterotopic pancreatic tissue in the duodenum, jejunum, spleen, esophagus, ampulla of Vater, and mesocolon.7 Only five cases of IPMN arising in heterotopic pancreas of stomach (four cases) and Meckel's diverticulum (one case) have been reported.8-12 Neoplasms in jejunal heterotopic pancreas are extremely rare. We found only six cases of neoplasms arising in jejunal heterotopic pancreas. Three were reports of ductal adenocarcinoma, and the others were acinar cell carcinoma, borderline mucinous cystic tumor, and solid pseudopapillary tumor.13-17
Histological determination that a carcinoma has developed from pre-existing heterotopic pancreatic tissue may be difficult. Guillou et al.18 proposed minimal diagnostic criteria.18 Firstly, the tumor must be found within or close to the heterotopic pancreatic tissue. Secondly, the transitional area between pancreatic structures and carcinoma must be observed (i.e., duct-cell dysplasia and/or carcinoma in situ). Finally, the non-neoplastic pancreatic tissue must comprise at least fully developed acini and/or ductal structures. In addition, direct extension or metastasis from another site must be excluded. Our case met all of these criteria.
Interestingly, our case disclosed the features of varying degrees of PanIN and IPMN, which are known as precancerous lesions of invasive ductal adenocarcinoma. When both PanIN and IPMN are present in a single pancreas, differentiation between small IPMN from PanIN may be difficult. However, the morphological features such as relatively large duct lesion more than 5 mm in diameter, cystically dilated duct-like spaces, intraluminal papillary excrescence with fibrovascular cores in our case strongly favor the diagnosis of IPMN over PanIN. Although MUC2 is more frequently expressed in IPMNs than in PanINs, absence of MUC2 expression cannot rule out an IPMN.19 This rare case showing precancerous lesions associated invasive adenocarcinoma demonstrates that a heterotopic pancreas can develop pathological changes similar to the normal pancreas.
In conclusion, although most cases of heterotopic pancreas are asymptomatic and remain undiagnosed until incidentally found, malignant transformations can occur within it. When heterotopic pancreatic tissue is detected (incidentally or not), efforts should be made to find any pathologic change. As precancerous conditions, such as PanIN and/or IPMN, arising in a heterotopic pancreas may be a warning of an associated invasive cancer, IPMN and/or PanIN in a heterotopic pancreas should be entirely submitted for histologic examination to search any invasive foci.
References
- 1.Chen HL, Chang WH, Shih SC, Bair MJ, Lin SC. Changing pattern of ectopic pancreas: 22 years of experience in a medical center. J Formos Med Assoc. 2008;107:932–936. doi: 10.1016/S0929-6646(09)60016-4. [DOI] [PubMed] [Google Scholar]
- 2.Cattell RB, Warren KW. Surgery of the pancreas. Philadelphia: W. B. Saunders Co.; 1953. pp. 26–37. [Google Scholar]
- 3.Pearson S. Aberrant pancreas: review of the literature and report of three cases, one of which produced common and pancreatic duct obstruction. AMA Arch Surg. 1951;63:168–184. [PubMed] [Google Scholar]
- 4.Chen HL, Lin SC, Chang WH, Yang TL, Chen YJ. Identification of ectopic pancreas in the ileum by capsule endoscopy. J Formos Med Assoc. 2007;106:240–243. doi: 10.1016/S0929-6646(09)60246-1. [DOI] [PubMed] [Google Scholar]
- 5.Chetty R, Weinreb I. Gastric neuroendocrine carcinoma arising from heterotopic pancreatic tissue. J Clin Pathol. 2004;57:314–317. doi: 10.1136/jcp.2003.013557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan BG, Zhang FB, Gan MF, Andrén-Sandberg A. Image of the month: heterotopic (ectopic) pancreas of ileum. Arch Surg. 2005;140:911–913. doi: 10.1001/archsurg.140.9.911. [DOI] [PubMed] [Google Scholar]
- 7.Goodarzi M, Rashid A, Maru D. Invasive ductal adenocarcinoma arising from pancreatic heterotopia in rectum: case report and review of literature. Hum Pathol. 2010;41:1809–1813. doi: 10.1016/j.humpath.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Tsapralis D, Charalabopoulos A, Karamitopoulou E, et al. Pancreatic intraductal papillary mucinous neoplasm with concomitant heterotopic pancreatic cystic neoplasia of the stomach: a case report and review of the literature. Diagn Pathol. 2010;5:4. doi: 10.1186/1746-1596-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park HS, Jang KY, Kim YK, Yu HC, Cho BH, Moon WS. Cystic lesion mimicking intraductal papillary mucinous tumor arising in heterotopic pancreas of the stomach and synchronous intraductal papillary mucinous adenocarcinoma of the pancreas. Int J Surg Pathol. 2008;16:324–328. doi: 10.1177/1066896907313536. [DOI] [PubMed] [Google Scholar]
- 10.Patel N, Berzin T. Intraductal papillary mucinous neoplasm arising in a heterotopic pancreas: a case report. Am J Gastroenterol. 2010;105:2513–2514. doi: 10.1038/ajg.2010.298. [DOI] [PubMed] [Google Scholar]
- 11.Phillips J, Katz A, Zopolsky P. Intraductal papillary mucinous neoplasm in an ectopic pancreas located in the gastric wall. Gastrointest Endosc. 2006;64:814–815. doi: 10.1016/j.gie.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Cates JM, Williams TL, Suriawinata AA. Intraductal papillary mucinous adenoma that arises from pancreatic heterotopia within a meckel diverticulum. Arch Pathol Lab Med. 2005;129:e67–e69. doi: 10.5858/2005-129-e67-IPMATA. [DOI] [PubMed] [Google Scholar]
- 13.Naqvi A, de la Roza G. Borderline mucinous cystic tumor in jejunal pancreatic heterotopia. Ann Diagn Pathol. 2004;8:151–155. doi: 10.1016/j.anndiagpath.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Slidell MB, Schmidt EF, Jha RC, Rossi CT, Becker TE, Guzzetta PC. Solid pseudopapillary tumor in a pancreatic rest of the jejunum. J Pediatr Surg. 2009;44:E25–E27. doi: 10.1016/j.jpedsurg.2009.01.074. [DOI] [PubMed] [Google Scholar]
- 15.Fujita K, Hirakawa K, Matsumoto T, et al. Small-intestinal cancer arising from heterotopic pancreas. Endoscopy. 2008;40(Suppl 2):E240–E241. doi: 10.1055/s-2008-1077693. [DOI] [PubMed] [Google Scholar]
- 16.Arao J, Fukui H, Hirayama D, et al. A case of aberrant pancreatic cancer in the jejunum. Hepatogastroenterology. 1999;46:504–507. [PubMed] [Google Scholar]
- 17.Makhlouf HR, Almeida JL, Sobin LH. Carcinoma in jejunal pancreatic heterotopia. Arch Pathol Lab Med. 1999;123:707–711. doi: 10.5858/1999-123-0707-CIJPH. [DOI] [PubMed] [Google Scholar]
- 18.Guillou L, Nordback P, Gerber C, Schneider RP. Ductal adenocarcinoma arising in a heterotopic pancreas situated in a hiatal hernia. Arch Pathol Lab Med. 1994;118:568–571. [PubMed] [Google Scholar]
- 19.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]