Abstract
Migratory divides are contact zones between breeding populations that use divergent migratory routes and have been described in a variety of species. These divides are of major importance to evolution, ecology and conservation but have been identified using limited band recovery data and/or indirect methods. Data from band recoveries and mitochondrial haplotypes suggested that inland and coastal Swainson's thrushes (Catharus ustulatus) form a migratory divide in western North America. We attached light-level geolocators to birds at the edges of this contact zone to provide, to our knowledge, the first direct test of a putative divide using data from individual birds over the entire annual cycle. Coastal thrushes migrated along the west coast to Mexico, Guatemala and Honduras. Some of these birds used multiple wintering sites. Inland thrushes migrated across the Rocky Mountains, through central North America to Columbia and Venezuela. These birds migrated longer distances than coastal birds and performed a loop migration, navigating over the Gulf of Mexico in autumn and around this barrier in spring. These findings support the suggestion that divergent migratory behaviour could contribute to reproductive isolation between migrants, advance our understanding of their non-breeding ecology, and are integral to development of detailed conservation strategies for this group.
Keywords: migration, geolocators, songbird, migratory divide
1. Introduction
Migratory divides are contact zones between divergent populations that breed adjacent to one another but use different routes to get to their wintering grounds [1–3]. It is generally agreed that these divides formed following secondary contact between populations that were isolated in different glacial refugia during the Pliocene and/or Pleistocene [3–6]. During this time, these populations diverged in multiple traits, including migratory orientation. The migratory routes that these populations use today probably reflect the colonization routes their ancestral forms used following the last glacial maximum [4,7,8].
Migratory divides have been described in many taxonomic groups (e.g. fishes, mammals and insects [9–11]), although the majority of research has focused on songbirds. Of particular interest is the role differences in migratory behaviour play in maintaining genetic differentiation, local adaptation and reproductive isolation [12–15]. Research has also focused on the relevance of migratory divides to our understanding of the ecology and conservation of migrants; populations in divides encounter different sets of ecological conditions during migration and on the wintering grounds [16]. These differences could affect their reproduction on the breeding grounds [17,18] and suggest that these populations should be considered independent management units when establishing conservation strategies [13,19].
To date, migratory divides have been described using band recovery data and/or biological markers [13,14,20]. Band recovery data are often limited by sample size [21], can fail to include individuals from the area of interest (e.g. directly adjacent to a migratory divide) and provide only two location fixes over the entire annual cycle (initial capture and recapture). Biological markers are often restricted to describing broad-scale patterns and are indirect; individuals are not followed over the entire annual cycle [21–24]. By contrast, light-level geolocators, which are archival tags that record light intensity at specific time intervals, provide a means to directly track individuals. These devices are attached to birds on the breeding grounds and retrieved the following year. Through astronomical algorithms as well as atmospheric and movement models, the archived light intensity data can be analysed to produce positional data (i.e. latitude and longitude [25]). Geolocators were recently miniaturized, permitting their use on songbirds [26] and providing the opportunity to describe migratory divides using a more direct method.
The Swainson's thrush (Catharus ustulatus) is a Neotropical migrant that breeds throughout the boreal forests of North America and winters in southern Mexico, Central and South America [27]. Two subspecies groups have been described: the russet-backed, coastal group and olive-backed, inland group [28]. Ruegg [29] described a hybrid zone between these groups along the coast mountains of British Columbia. Data from band recoveries and mitochondrial haplotypes sampled at stopover and wintering sites suggested that these groups form a migratory divide [28]. The band recovery data reported by Ruegg & Smith [28] contained very little data from British Columbia, where the hybrid zone occurs (two birds from southeast British Columbia, far east of the hybrid zone and one from coastal British Columbia, west of the hybrid zone, see fig. 2 in Ruegg & Smith [28]). In addition, no band recovery data were included from the interior western USA. This region forms a large part of the inland group's range and raises the possibility that breeding populations just east of the hybrid zone migrate through the interior western USA, a route closely parallel to that of the coastal group. If this were the case, there would not be a strong migratory divide between birds on either side of the Swainson's thrush hybrid zone. We tested whether there is actually a narrow migratory divide here, using light-level geolocators attached to birds at the western and eastern edges of the hybrid zone between inland and coastal Swainson's thrushes.
2. Material and methods
We attached 39 geolocators to male Swainson's thrushes at three sites in British Columbia, Canada in June 2010 (Sunshine Coast (coastal), n = 10, 49.506 N, 123.751 W; Vancouver (coastal), n = 9, 49.277 N, 123.229 W; Kamloops (inland), n = 20, 50.861 N, 120.661 W). Birds were caught using song playback and mist nets and fitted with a Canadian Wildlife Service aluminium band. Body mass and wing, tarsus and tail length of each bird were measured. We also photographed each bird and obtained a tail feather (R4, the fourth right rectrix) for later genetic analyses. We used Mk12S geolocators (0.9 g, stalk length = 15 mm; British Antarctic Survey, Cambridge, UK) and attached these devices to birds using Rappole–Tipton [30] leg-loop backpack harnesses made of Teflon ribbon (Bally Ribbon), ethylene propylene diene monomer, or silicone cord (Budlar; 0.2–0.3 g). Total attachment weight was less than 4 per cent the average body mass of Swainson's thrushes (30.5 ± 1.5 g, K.E. Delmore 2011, unpublished data).
We used BASTrak software (BAS) to download light intensity data and estimate daily latitude and longitude at local noon. Mk12S geolocators measure light intensity every minute and record the maximum measurement every 2 min. Light intensity is recorded on an arbitrary scale between 0 and 64 and is used in combination with a sun elevation (altitude) angle to define light transitions (i.e. sunrise and sunset). Lower values of light intensity are recommended for most birds, especially those spending time in shaded environments. Accordingly, we used a value of 1 and determined the equivalent sun elevation calibration value from data when birds were on the breeding grounds (i.e. a known location). Calculated sun elevation angles ranged between −4.5° and −3° and we used the average of these values (−3.7) for the analysis of the entire dataset (see the electronic supplementary material for one exception). We rejected transitions with high levels of noise and evidence of shading (34% of days). We also rejected latitudinal estimates within 15 days of the autumn and spring equinoxes as well as obvious outliers, most of which occurred near the equinoxes (3% of days). Latitude and longitude can be estimated at local midnight and local noon. Assuming the birds migrated only at night, we used noon location fixes only (without movement compensation) as these should be more accurate, being uncomplicated by analysis errors induced by movement (e.g. movement in longitude causes an apparent increase or decrease in day length). We evaluated the accuracy of our estimates using the standard deviation of longitude and latitude while birds were on the breeding grounds and the distance between the true deployment location and the location estimated using geolocators.
We plotted daily estimates of latitude and longitude in ArcGIS (Esri) and calculated average locations for days when we assumed birds were stationary (at stopover sites on the breeding or wintering grounds). Following Stutchbury et al. [26], we defined stopover sites as locations where birds remained stationary for greater than or equal to 2 days. We mapped points that fell over water to the closest point on land and used the most direct route when connecting points. It should be noted that by using the most direct route, we are probably underestimating the distance travelled by each bird. In addition, we were unable to obtain latitude estimates during a large portion of the autumn migration owing to the equinox (see §3). Rather than connecting the points before and after the equinox using straight lines, we used information from estimates of longitude to draw our lines. More specifically, we identified three stopover sites used by birds using longitude data during this period and used this information to interpolate between points.
3. Results
We recovered and successfully downloaded data from 10 out of 39 devices in 2011. The light stalk fell off one of these devices on 27 July 2010 and was not used in our analyses. The battery on one of the devices stopped working on 16 May 2011. Fortunately, the bird had almost completed its spring migration, allowing us to include this device in our analyses. Average standard deviation for location estimates on the breeding grounds was 106.77 km for latitude and 37.67 km for longitude. Average distance between the true deployment location and the estimate from geolocator data was 117 km.
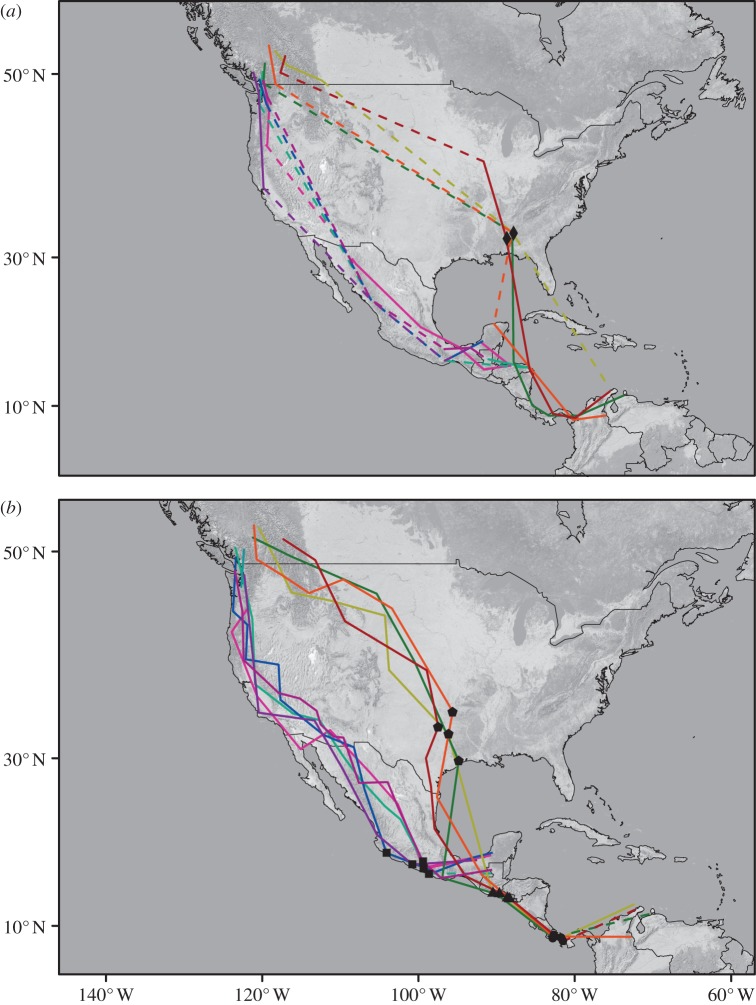
Figure 1 depicts the annual cycle of the thrushes we recaptured. Coastal birds migrated along the western coast of North America on autumn and spring migration and wintered in southern Mexico and Central America (Guatemala and Honduras). Inland birds used more eastern routes, passing over the Rocky Mountains and through the central United States. These birds migrated over the Gulf of Mexico on autumn migration, wintered in South America (Colombia and Venezuela), and migrated around the Gulf through Central America and Mexico on spring migration (see the electronic supplementary material, table S1 and figures S1 and S2 include graphs for individual birds and daily estimates of longitude and latitude ± s.d.).
Figure 1.
(a) Autumn and (b) spring migration of nine Swainson's thrushes. Routes for thrushes from the inland subspecies group shown in warm colours (red, orange, yellow, green); routes for thrushes from coastal subspecies group shown in cool colours (blue, purple, pink, turquoise, maroon). Dashed lines link locations where latitude could not be estimated around the equinox periods. Long-term stopover sites are shown in the United States (Alabama, diamonds; Texas, pentagons), Central America (Panama, circles; El Salvador and Guatemala, triangles), southern Mexico (squares).
All of the coastal birds used a common stopover site in the Sierre Madre mountain ranges of southern Mexico on spring migration. These birds spent a prolonged period of time at this stopover site (average = 19 days, range = 8–29 days; figure 1b). Inland birds also used common stopover sites for extended periods of time. On autumn migration, these birds stopped at a site north of the Gulf of Mexico, in Alabama (average = 20 days, range = 11–29 days; figure 1a). On spring migration, these birds stopped at sites south of the Gulf of Mexico, in Panama (average = 11 days, range = 7–16 days) and Guatemala (average = 11 days, range = 3–17 days), and at one site north of the Gulf, in Texas (range = 9–15 days, average = 13 days; figure 1b).
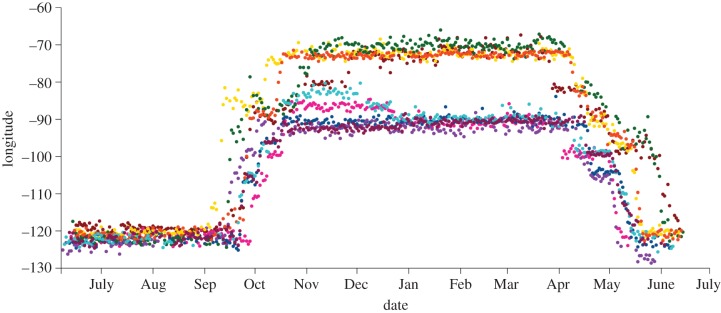
Latitude is estimated using day length. There is little variation in this variable around the equinoxes and, as a result, we were unable to estimate latitude for approximately 30 days around each of these periods. These missing days are illustrated in figure 1 as dashed lines and overlap substantially with autumn migration routes. One consequence of this overlap is that, for two of the inland birds, we could not determine precise locations of major autumn stopover sites north of the Gulf Coast. Estimates of longitude are unaffected by the equinoxes, however, and a plot of longitude by date suggests that these birds may have spent time at the same stopover site, as all four birds remained at roughly the same longitude in late October (approx. 85°; figure 2). This plot also suggests that all four coastal birds spent a substantial amount of time at one or two stopover sites between 106° and 96° longitude on autumn migration. These sites may be located in the Mexican monsoon region of western North America, where many western breeding birds stop between the months of July and October to moult [31].
Figure 2.
Change in longitude during the annual cycle of nine Swainson's thrushes. Colours correspond to those used in figure 1, with data for thrushes from the inland subspecies group shown in warm colours (red, orange, yellow, green) and from the coastal subspecies group shown in cool colours (blue, purple, pink, turquoise, maroon).
Table 1 summarizes differences in the timing and distance travelled by birds from coastal and inland subspecies groups. Spring migration was shorter in duration than autumn migration for birds from both groups (paired t-test, t7 = −6.36, p = 0.0004). Significant differences between inland and coastal birds were observed in the distance travelled on both spring and autumn migration, with inland birds migrating longer distances on each leg. Significant differences were not observed between these groups in any of the other variables measured, including departure and arrival date, duration of migration and number of days spent at stopover sites. Nevertheless, the data suggest that coastal birds left their breeding grounds earlier than inland birds. Coastal birds also appear to have spent less time on spring migration, leaving their wintering grounds later than inland birds but arriving on the breeding grounds earlier.
Table 1.
Details on the autumn and spring migration of nine Swainson's thrushes, including departure and arrival dates, total duration, distance travelled, days spent at stopover sites, migration pace and flight speed. (Colours correspond to those used in figure 1 (inland subspecies group: red, orange, yellow, green; coastal subspecies group: blue, purple, pink, turquoise, maroon). Averages for each subspecies are shown in bold. Significant results from paired t-tests comparing values for these groups are also bold.)
| autumn migration |
spring migration |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| departure | arrival | duration (days) | stopover (days) | distance (km) | pace (km per day) | speed (km per day) | departure | arrival | duration (days) | stopover (days) | distance (km) | pace(km per day) | speed (km per day) | ||
| coastal | |||||||||||||||
| maroon | 12 July | 17 Oct | 94 | 83 | 3470 | 37 | 315 | 16 Apr | 01 July | 46 | 31 | 4253 | 92 | 284 | |
| turquoise | 25 July | 18 Oct | 85 | 54 | 4366 | 51 | 141 | 06 Apr | 28 May | 52 | 39 | 4441 | 85 | 342 | |
| blue | 28 July | 14 Oct | 78 | 71 | 3759 | 48 | 537 | 18 Apr | 23 May | 36 | 21 | 4564 | 127 | 304 | |
| pinka | 24 July | 20 Oct | 88 | 72 | 4466 | 51 | 279 | 01 Apr | |||||||
| purple | 26 July | 02 Oct | 68 | 35 | 4067 | 60 | 123 | 11 Apr | 17 May | 36 | 28 | 4041 | 112 | 505 | |
| average | 23 July | 14 Oct | 83 | 63 | 4026 | 49 | 279 | 10 Apr | 25 May | 44 | 33 | 4325 | 104 | 359 | |
| inland | |||||||||||||||
| red | 31 July | 27 Nov | 119 | 98 | 5468 | 46 | 260 | 26 Mar | 09 June | 75 | 57 | 5552 | 74 | 308 | |
| green | 20 July | 03 Nov | 96 | 85 | 6081 | 63 | 553 | 04 Apr | 06 June | 63 | 38 | 6333 | 101 | 253 | |
| orange | 31 July | 17 Oct | 78 | 66 | 5519 | 71 | 460 | 07 Apr | 22 May | 45 | 31 | 6083 | 135 | 435 | |
| yellow | 28 Aug | 08 Oct | 41 | 32 | 5122 | 125 | 569 | 10 Apr | 21 May | 41 | 32 | 5532 | 135 | 615 | |
| average | 06 Aug | 29 Oct | 84 | 70 | 5547 | 76 | 461 | 04 Apr | 30 May | 57 | 44 | 5875 | 111 | 403 | |
| p-value | 0.073 | 0.18 | 0.95 | 0.66 | 0.0008 | 0.13 | 0.13 | 0.20 | 0.43 | 0.18 | 0.21 | 0.0001 | 0.71 | 0.66 | |
aBattery stopped working 16 May 2011.
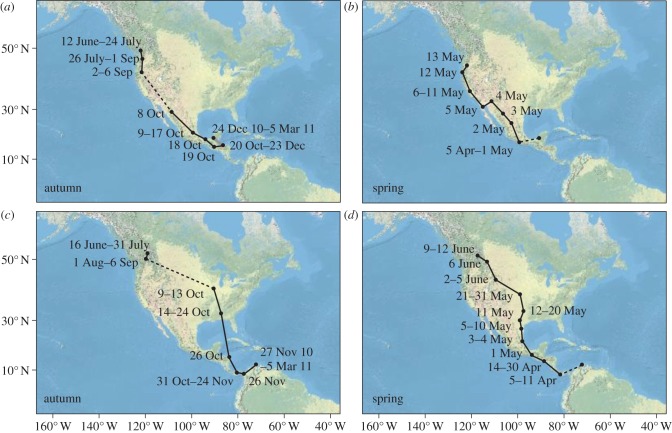
The tracks of individual birds revealed additional details from the annual cycle of these groups. First, three of the coastal birds used two distinct sites on the wintering grounds, moving from their first site at the end of December. Two birds moved east from Honduras to Guatemala and southern Mexico (figure 3a,b and see the electronic supplementary material, figure S1a,b), one bird moved west from southern Mexico to Guatemala (see the electronic supplementary material, figure S1g,h). Average distance between these sites was 421 km. Second, as mentioned briefly earlier, two of the inland birds appear to have flown over the Gulf of Mexico on autumn migration, leaving from Alabama and arriving in Honduras. These birds flew around the Gulf on spring migration, taking a land route through Central America and Mexico (figure 3c,d and see the electronic supplementary material, figure S2c,d). It is likely that the other two inland birds followed a similar path, as these birds appear to have spent time at the same stopover site in the southeastern United States (described earlier; figure 2).
Figure 3.
Annual cycle of two Swainson's thrushes, one from the coastal subspecies group ((a,b) shown in pink on figure 1) and one from the inland subspecies group ((c,d) shown in red on figure 1). Dates, stopover sites, breeding and wintering locations are shown. Autumn migration for both birds shown in (a,c); spring migration shown in (b,d). Missing dates indicate periods for which the location of the bird could not be estimated (e.g. because of shading). Dashed lines link locations where latitude could not be estimated around the equinox periods. The bird in (a) and (b) used two wintering sites; movement between these sites is shown using a thick grey line.
4. Discussion
Data from light-level geolocators deployed in our study revealed dramatic differences in the migratory routes, stopover sites and wintering grounds used by inland and coastal Swainson's thrushes. To the best of our knowledge, this is the first study in which data from individual birds over their entire annual cycle have been used to characterize long-distance migration routes across a narrow migratory divide. Our results confirm and expand on conclusions presented in Ruegg & Smith [28] based on band recovery data and genetic markers. More specifically, geolocators provided location estimates for most days over the entire annual cycle of each bird. This additional resolution allowed us to document several novel aspects of the routes used by Swainson's thrushes, including the circum-Gulf route used by inland birds and long-term stopover sites used by both groups. Geolocator data also suggest that the trans-Gulf route used by these birds is not as far east as was suggested with the banding data, which provided only two locations per bird; geolocators show birds crossing between Alabama and the Yucatan, whereas the banding data suggested crossing between Florida and Columbia. Finally, the banding data presented by Ruegg & Smith [28] included long-distance movements from the breeding range to Central America for only two birds. The present study provides five cases, and much detail on those movements. Below, we discuss the relevance of our results to understanding the evolution, ecology and conservation of migratory species.
(a). Evolution of migratory routes and their role as reproductive isolating barriers
Migratory routes are believed to reflect historical range shifts, with populations migrating along the same routes their ancestral forms used to expand out of glacial refugia [3,4,7,8]. Our results partially support this suggestion in Swainson's thrushes. Population genetic analyses and palaeodistribution modelling suggested that inland and coastal thrushes were isolated in separate eastern (inland) and western (coastal) refugia during the last glacial maximum. Both groups expanded northwards out of these refugia; once the inland group reached the eastern boreal forest, it expanded west into the western boreal forest [32]. Coastal thrushes migrated along a north–south axis in our study, supporting the suggestion that they are retracing their post-glacial colonization routes. Routes used by inland thrushes on autumn migration also support this suggestion; these birds migrated southeast to their ancestral range in the eastern United States before continuing south to their wintering grounds.
Migratory routes have also been implicated in the maintenance of reproductive isolation between populations. Specifically, migratory orientation is largely genetically determined in many groups, including songbirds [33], and often involves navigation around geographical areas that are difficult to migrate over and/or have little suitable habitat for refuelling [34]. Accordingly, hybrids between populations at these divides are expected to use intermediate routes that will be inferior to those of parental forms [1,3,6,14]. Inland and coastal thrushes tracked in our study used divergent migratory routes that took them east or west of several large geographical features in western North America, including three mountain ranges (Cascade, Sierra Nevada and Rocky Mountains) and deserts in the southwestern United States. Inland and coastal thrushes are genetically differentiated from one another, and an analysis of hybrid populations suggests that they exhibit some degree of reproductive isolation [28,29].
(b). Contributions to our knowledge of the non-breeding season ecology of migratory species
Migratory connectivity has been defined as the link between an individual's breeding and wintering grounds [21]. This link appears to be relatively strong for inland thrushes; these birds migrated to sites within 2° longitude in Columbia and Venezuela. Connectivity was lower in coastal birds; these birds migrated to sites within 8° longitude in southern Mexico, Honduras and Guatemala. Three coastal birds used two wintering sites, contributing to lower levels of connectivity in this group. This behaviour probably represents an example of intratropical migration [35,36]. To the best of our knowledge, this is only the second time this behaviour has been documented in a Neotropical migrant; Heckscher et al. [36] documented a similar pattern in Veeries, in which birds wintering in the Amazon Basin moved to a second site between the months of January and March. Together, these results suggest that migratory connectivity may not be as straightforward as was once believed; studies of this phenomenon should not be constructed to assign birds to single wintering sites.
Two additional details relevant to our understanding of the non-breeding season of Swainson's thrushes were revealed in our study. First, at least two of the inland birds we tracked exhibited seasonal differences in the migratory routes they used, using a trans-Gulf route on autumn migration and a more western circum-Gulf route on spring migration. This behaviour has been termed loop migration and has been described using data from bird-monitoring stations and band recoveries for other Neotropical migrants (Selasphorus hummingbirds [36] and blackpoll warblers [37]). This behaviour was also described in two recent studies using light-level geolocators in the western Palaearctic (European hoopoe [38] and red-backed shrike [39]). Very little is known about loop migration but it is probably related to variation in food availability and/or prevailing winds [35,37,38]. Second, individuals from both inland and coastal groups used similar long-term stopover sites. These sites were located near large geographical barriers, including the Gulf of Mexico and the Sierra Madre mountain ranges and were probably used to acquire the resources necessary to cross these barriers; birds spent prolonged periods of time at these sites and crossed these barriers immediately after. Previous studies using data from bird-monitoring stations and radar technology have highlighted the importance of these sites for migratory songbirds [40–44]. Veeries and wood thrushes fitted with geolocators in eastern North America also relied on similar stopover sites around the Gulf of Mexico [26,36].
(c). Relevance for establishing conservation strategies for migratory species
Details from the non-breeding season of Swainson's thrushes described earlier can be used to inform year-round conservation strategies for songbirds. For example, the long-term stopover sites identified are probably important for completing migration along eastern and western routes; all of the birds from each group stopped at these sites and, in many cases spent more than a week at each. We can use these data to establish sets of stopover sites along these routes that should be the focus of conservation efforts. Data collected in our study can also be used to inform conservation strategies in the future. For example, migratory connectivity appears to be relatively high within subspecies groups. If a decline is observed in one group, we should focus management efforts on the specific breeding, wintering and stopover sites used by individuals from this group. It should be noted that the data we presented are only from one species. Nevertheless, similar east–west genetic differentiation has been described in several other species of North American songbirds and data from biological markers suggests that many of these populations may follow similar routes [13,20,22,24]. Accordingly, we should be able to apply results obtained in this single species study to the conservation of many migratory species.
Avian migration is perhaps the most geographically widespread of all biological phenomena, as it ties together the ecology of locations separated by great distances, often on different continents. Migratory divides are particularly interesting in this regard, because they show that neighbouring breeding populations can be widely separated geographically at other times throughout the year. As discussed earlier, these large migratory differences can have large consequences for evolution (e.g. by contributing to reproductive isolation), ecology (e.g. because the two groups depend on different wintering environments) and conservation (e.g. by revealing important stopover sites). Geolocators now provide us with a tool to reconstruct detailed pathways of individual birds, allowing researchers to examine a wide variety of questions in these fields. We anticipate that great advances in understanding patterns of migratory connectivity will soon be made by combining analyses of trackways used by individual birds with population-level analyses of genetic and feather isotope variation.
Acknowledgements
This study was approved by the Animal Care Committee at the University of British Columbia.
We thank Kristen Ruegg for advice regarding Swainson's thrushes, Wendy Easton and Heather Baines for help establishing our system and Ryan Germain and Stephanie Cavaghan for field assistance. We also thank Bridget Stutchbury and Allison Alvarado for advice on the use of geolocators, and Richard Phillips and Bridget Connelly for help with the analysis of our data. Helpful comments on the manuscript were provided by Ryan Germain, Ken Norris, Ryan Norris and one anonymous reviewer. This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (discovery grant no. 311931-2005 to D.E.I. and C.G.S-D. to K.E.D.), Wilson Ornithological Society (Paul A. Stewart Research Award to K.E.D.) and Environment Canada (Science Horizons Community Funding Programme to K.E.D.). We are grateful to Metro Vancouver Regional Parks Environment Canada for providing permits (master banding permit no. 10746 and scientific permit no. BC-10-0035).
References
- 1.Helbig A. 1996. Genetic basis, mode of inheritance and evolutionary changes of migratory directions in Palaearctic warblers (Aves: Sylviidae). J. Exp. Biol. 199, 49–55 [DOI] [PubMed] [Google Scholar]
- 2.Bensch S., Andersson T., Åkesson S. 1999. Morphological and molecular variation across a migratory divide in willow warblers, Phylloscopus trochilus. Evolution 53, 1925–1935 10.2307/2640451 (doi:10.2307/2640451) [DOI] [PubMed] [Google Scholar]
- 3.Irwin D. E., Irwin J. H. 2005. Siberian migratory divides: the role of seasonal migration in speciation. In Birds of two worlds: the ecology and evolution of migration (eds Greenberg R., Marra P. P.), pp. 27–40 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 4.Berthold P. 2001. Bird migration: a general survey. New York, NY: Oxford University Press [Google Scholar]
- 5.Møller A. P., Garamszegi L. Z., Peralta-Sánchez J. M., Soler J. J. 2011. Migratory divides and their consequences for dispersal, population size and parasite–host interactions. J. Evol. Biol. 24, 1744–1755 10.1111/j.1420-9101.2011.02302.x (doi:10.1111/j.1420-9101.2011.02302.x) [DOI] [PubMed] [Google Scholar]
- 6.Rohwer S., Irwin D. E. 2011. Molt, orientation, and avian speciation. Auk 128, 419–425 10.1525/auk.2011.10176 (doi:10.1525/auk.2011.10176) [DOI] [Google Scholar]
- 7.Rappole J. 1995. The ecology of migrant birds. Washington, DC: Smithsonian Institution Press [Google Scholar]
- 8.Newton I. 2008. The migration ecology of birds. London, UK: Academic Press [Google Scholar]
- 9.Gagnaire P. A., Albert V., Jónsson B., Bernatchez L. 2009. Natural selection influences AFLP intraspecific genetic variability and introgression patterns in Atlantic eels. Mol. Ecol. 18, 1678–1691 10.1111/j.1365-294X.2009.04142.x (doi:10.1111/j.1365-294X.2009.04142.x) [DOI] [PubMed] [Google Scholar]
- 10.McDevitt A. D., Mariani S., Hebblewhite M., Decesare N. J., Morgantini L., Seip D., Weckworth B. V., Musiani M. 2009. Survival in the Rockies of an endangered hybrid swarm from diverged caribou (Rangifer tarandus) lineages. Mol. Ecol. 18, 665–679 10.1111/j.1365-294X.2008.04050.x (doi:10.1111/j.1365-294X.2008.04050.x) [DOI] [PubMed] [Google Scholar]
- 11.Altizer S., Davis A. K. 2010. Populations of monarch butterflies with different migratory behaviors show divergence in wing morphology. Evolution 64, 1018–1028 10.1111/j.1558-5646.2010.00946.x (doi:10.1111/j.1558-5646.2010.00946.x) [DOI] [PubMed] [Google Scholar]
- 12.Bearhop S., Fiedler W., Furness R. W., Votier S. C., Waldron S., Newton J., Bowen G. J., Berthold P., Farnsworth K. 2005. Assortative mating as a mechanism for rapid evolution of a migratory divide. Science 310, 502–504 10.1126/science.1115661 (doi:10.1126/science.1115661) [DOI] [PubMed] [Google Scholar]
- 13.Boulet M., Gibbs H. L., Hobson K. A. 2006. Integrated analysis of genetic, stable isotope, and banding data reveal migratory connectivity and flyways in the northern yellow warbler (Dendroica petechia; Aestiva group). Ornithol. Monogr. 61, 29–78 10.1642/0078-6594(2006)61[29:IAOGSI]2.0.CO;2 (doi:10.1642/0078-6594(2006)61[29:IAOGSI]2.0.CO;2) [DOI] [Google Scholar]
- 14.Bensch S., Grahn M., Müller N., Gay L., Akesson S. 2009. Genetic, morphological, and feather isotope variation of migratory willow warblers show gradual divergence in a ring. Mol. Ecol. 18, 3087–3096 10.1111/j.1365-294X.2009.04210.x (doi:10.1111/j.1365-294X.2009.04210.x) [DOI] [PubMed] [Google Scholar]
- 15.Rolshausen G., Segelbacher G., Hobson K. A., Schaefer H. M. 2009. Contemporary evolution of reproductive isolation and phenotypic divergence in sympatry along a migratory divide. Curr. Biol. 19, 2097–2101 10.1016/j.cub.2009.10.061 (doi:10.1016/j.cub.2009.10.061) [DOI] [PubMed] [Google Scholar]
- 16.Kelly J. F., Hutto R. L. 2005. An east–west comparison of migration in North American wood warblers. Condor 107, 197–211 10.1650/7805 (doi:10.1650/7805) [DOI] [Google Scholar]
- 17.Marra P. P., Hobson K. A., Holmes R. T. 1998. Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282, 1884–1886 10.1126/science.282.5395.1884 (doi:10.1126/science.282.5395.1884) [DOI] [PubMed] [Google Scholar]
- 18.Norris D. R., Marra P. P., Montgomerie R., Kyser T. K., Ratcliffe L. M. 2004. Reproductive effort, molting latitude, and feather color in a migratory songbird. Science 306, 2249–2250 10.1126/science.1103542 (doi:10.1126/science.1103542) [DOI] [PubMed] [Google Scholar]
- 19.Faaborg J., et al. 2010. Conserving migratory land birds in the New World: do we know enough? Ecol. Appl. 20, 398–418 10.1890/09-0397.1 (doi:10.1890/09-0397.1) [DOI] [PubMed] [Google Scholar]
- 20.Irwin D. E., Irwin J. H., Smith T. B. 2011. Genetic variation and seasonal migratory connectivity in Wilson's warblers (Wilsonia pusilla): species-level differences in nuclear DNA between western and eastern populations. Mol. Ecol. 20, 3102–3115 10.1111/j.1365-294X.2011.05159.x (doi:10.1111/j.1365-294X.2011.05159.x) [DOI] [PubMed] [Google Scholar]
- 21.Webster M. S., Marra P. P., Haig S. M., Bensch S., Holmes R. T. 2002. Links between worlds: unraveling migratory connectivity. Trends Ecol. Evol. 17, 76–83 10.1016/S0169-5347(01)02380-1 (doi:10.1016/S0169-5347(01)02380-1) [DOI] [Google Scholar]
- 22.Lovette I. J., Clegg S. M., Smith T. B. 2004. Limited utility of mtDNA markers for determining connectivity among breeding and overwintering locations in three Neotropical migrant birds. Conserv. Biol. 18, 156–166 10.1111/j.1523-1739.2004.00239.x (doi:10.1111/j.1523-1739.2004.00239.x) [DOI] [Google Scholar]
- 23.Hobson K. A. 2005. Stable isotopes and the determination of avian migratory connectivity and seasonal interactions. Auk 122, 1037–1048 10.1642/0004-8038(2005)122[1037:SIATDO]2.0.CO;2 (doi:10.1642/0004-8038(2005)122[1037:SIATDO]2.0.CO;2) [DOI] [Google Scholar]
- 24.Smith T. B., Clegg S. M., Kimura M., Ruegg K., Mila B., Lovette I. 2005. Molecular genetic approaches to linking breeding and overwintering areas in five Neotropical migrant passerines. In Birds of two worlds: the ecology and evolution of migration (eds Greenburg R., Marra P.), pp. 222–234 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 25.Hill R. 1994. Theory of geolocation by light levels. In Elephant seals: population ecology, behaviour and physiology (eds Boeuf B. J., Laws R. M.), pp. 227–236 Berkeley, CA: University of California Press [Google Scholar]
- 26.Stutchbury B. J. M., Tarof S. A., Done T., Gow E., Kramer P. M., Tautin J., Fox J. W., Afanasyev V. 2009. Tracking long-distance songbird migration by using geolocators. Science 323, 896. 10.1126/science.1166664 (doi:10.1126/science.1166664) [DOI] [PubMed] [Google Scholar]
- 27.Mack D., Yong W., Poole A. 2001. Swainson's thrush (Catharus ustulatus). In Birds of North America online. New York, NY: Cornell Laboratory of Ornithology [Google Scholar]
- 28.Ruegg K. C., Smith T. B. 2002. Not as the crow flies: a historical explanation for circuitous migration in Swainson's thrush (Catharus ustulatus). Proc. R. Soc. Lond. B 269, 1375–1381 10.1098/rspb.2002.2032 (doi:10.1098/rspb.2002.2032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruegg K. 2008. Genetic, morphological, and ecological characterization of a hybrid zone that spans a migratory divide. Evolution 62, 452–466 10.1111/j.1558-5646.2007.00263.x (doi:10.1111/j.1558-5646.2007.00263.x) [DOI] [PubMed] [Google Scholar]
- 30.Rappole J. H., Tipton A. R. 1991. New harness design for attachment of radio transmitters to small passerines. J. Field Ornithol. 62, 335–337 [Google Scholar]
- 31.Rohwer S., Butler L., Froehlich D. 2005. Ecology and demography of east-west differences in molt scheduling of Neotropical migrant passerines. In Birds of two worlds: the ecology and evolution of migration (eds Greenburg R., Marra P. P.), pp. 87–105 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 32.Ruegg K. C., Hijmans R. J., Moritz C. 2006. Climate change and the origin of migratory pathways in the Swainson's thrush, Catharus ustulatus. J. Biogeogr. 33, 1172–1182 10.1111/j.1365-2699.2006.01517.x (doi:10.1111/j.1365-2699.2006.01517.x) [DOI] [Google Scholar]
- 33.Pulido F. 2007. The genetics and evolution of avian migration. Bioscience 57, 165–174 10.1641/B570211 (doi:10.1641/B570211) [DOI] [Google Scholar]
- 34.Alerstam T. 2001. Detours in bird migration. J. Theor. Biol. 209, 319–331 10.1006/jtbi.2001.2266 (doi:10.1006/jtbi.2001.2266) [DOI] [PubMed] [Google Scholar]
- 35.Faaborg J., et al. 2010. Recent advances in understanding migration systems of New World land birds. Ecol. Monogr. 80, 3–48 10.1890/09-0395.1 (doi:10.1890/09-0395.1) [DOI] [Google Scholar]
- 36.Heckscher C. M., Taylor S. M., Fox J. W., Afanasyev V. 2011. Veery (Catharus fuscescens) wintering locations, migratory connectivity, and a revision of its winter range using geolocator technology. Auk 128, 531–542 10.1525/auk.2011.10280 (doi:10.1525/auk.2011.10280) [DOI] [Google Scholar]
- 37.Phillips A. R. 1975. The migrations of Allen's and other hummingbirds. Condor 77, 196–205 10.2307/1365790 (doi:10.2307/1365790) [DOI] [Google Scholar]
- 38.Hunt P. D., Eliason B. C., Poole A. 1999. Blackpoll warbler (Dendroica striata). In Birds of North America online. New York, NY: Cornell Laboratory of Ornithology [Google Scholar]
- 39.Bächler E., Hahn S., Schaub M., Arlettaz R., Jenni L., Fox J. W., Afanasyev V., Liechti F. 2010. Year-round tracking of small trans-Saharan migrants using light-level geolocators. PLoS ONE 5, e9566. 10.1371/journal.pone.0009566 (doi:10.1371/journal.pone.0009566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gauthreaux S. A., Jr 1999. Neotropical migrants and the Gulf of Mexico: the view from aloft. In Gatherings of angels: migrating birds and their ecology (ed. Able K. P.), pp. 27–49 Ithaca, NY: Cornell University Press [Google Scholar]
- 41.Moore F. R. 1999. Cheniers of Louisiana and the stopover ecology of migrant landbirds. In Gatherings of angels: migrating birds and their ecology (ed. Able K. P.), pp. 51–62 Ithaca, NY: Cornell University Press [Google Scholar]
- 42.Felger R. S., Wilson M. 1994. Northern Sierra Madre Occidental and its Apachian outliers: a neglected center of biodiversity. In Biodiversity and the management of the Madrean Archipelago: the sky Islands of Southwestern United States and Northwestern Mexico (eds DeBano F. L., Ffolliott P. F.), pp. 36–59 Tucson, Arizona: U.S. Forest Service, Rocky Mountain Forest and; Range Experiment Station [Google Scholar]
- 43.Skagen S. K., Kelly J. F., van Riper C., III, Hutto R. L., Finch D. M., Krueper D. J., Melcher C. P. 2005. Geography of spring landbird migration through riparian habitats in southwestern North America. Condor 107, 212–227 10.1650/7807 (doi:10.1650/7807) [DOI] [Google Scholar]
- 44.Gauthreaux S. A., Belser C. G., Welch C. M. 2006. Atmospheric trajectories and spring bird migration across the Gulf of Mexico. J. Ornithol. 147, 317–325 10.1007/s10336-006-0063-7 (doi:10.1007/s10336-006-0063-7) [DOI] [Google Scholar]