Abstract
Coevolution of two species is typically thought to favour the evolution of faster evolutionary rates helping a species keep ahead in the Red Queen race, where ‘it takes all the running you can do to stay where you are’. In contrast, if species are in a mutualistic relationship, it was proposed that the Red King effect may act, where it can be beneficial to evolve slower than the mutualistic species. The Red King hypothesis proposes that the species which evolves slower can gain a larger share of the benefits. However, the interactions between the two species may involve multiple individuals. To analyse such a situation, we resort to evolutionary multiplayer games. Even in situations where evolving slower is beneficial in a two-player setting, faster evolution may be favoured in a multiplayer setting. The underlying features of multiplayer games can be crucial for the distribution of benefits. They also suggest a link between the evolution of the rate of evolution and group size.
Keywords: mutualism, evolutionary game theory, multiple players, rate of evolution
1. Introduction
Mutualistic relationships, interspecific interactions that benefit both species, have been empirically studied for many years [1–7] and also a considerable body of theory has been put forth trying to explain the evolution and maintenance of such relationships [8–15]. Many of these studies use evolutionary game theory for developing the models. The interactions in these models are usually pairwise. A representative of each species is chosen and the outcome of the interactions between these representatives determines the evolutionary dynamics within each of the two species. However, in many cases interactions between species cannot be reduced to such pairwise encounters [16].
For example, in the interaction between ants and aphids or butterfly larvae [17–19] many ants tend to these soft-bodied creatures, providing them with shelter and protection from predation and parasites in exchange for honeydew, a rich source of food for the ants [16,20]. This is not a one-to-one interaction between a larva and an ant, but rather a one-to-many interaction from the perspective of the larva. In this manuscript, we focus on this kind of—possibly—many-to-many interactions between two mutualistic species.
To analyse how the benefits are shared between the two mutualistic species, we make use of evolutionary game theory [21–23]. Following Bergstrom & Lachmann [12], we analyse the interactions between two species with a twist to the standard formulation. The two interacting species can have different evolutionary rates. In coevolutionary arms races, where species are locked in antagonistic relationships such as host–parasite interactions, we observe the Red Queen effect [24]. In these cases, a higher rate of evolution will be beneficial, as it would help the parasite infecting a host or a host escaping from a parasite. However, in mutualistic relationships a slower rate of evolution was predicted to be more favourable [12]. Hence, it is possible that the type of relationship between two species affects the evolution of the rate of evolution. As mentioned in [12,25] the different evolutionary rates could be owing to a multitude of factors ranging from different population sizes to the differing amount of segregating genetic variance. The implications of a difference in evolutionary rates are not limited to mutualism and antagonistic relationships. Epidemiological modelling and data have shown correlations between the rate of epidemic spreading and the evolutionary rate of the spreading pathogen [26,27].
We first recall the mutualistic relationship between two species in a two-player game, as proposed by Bergstrom and Lachmann. Then, we increase the number of players. Note that we do not increase the number of interacting species [28,29], but rather the number of interacting individuals between two species (see [30]). We include asymmetry in evolutionary rates and discuss its effect both in two-player and in multiplayer games. We find that when in a two-player setting it is beneficial to evolve at a slower rate, it can be detrimental in a multiplayer game.
2. Model and results
To set the stage, we first recapitulate the two-player model presented in [12] albeit with different notation. They considered two species each with two strategies, ‘Generous’ and ‘Selfish’. Each species is better off being Selfish as long as the other one is Generous. If both are Selfish, then no mutualistic benefit is generated and hence in that case it is better to be Generous.
Under these assumptions, the payoff matrices for the interactions describing the interactions of each type with one member of the other species are
| species 2 | species 1 | ||||||
| G2 | S2 | G1 | S1 | ||||
| species 1 | G1 | 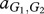 |
 |
species 2 | G2 | 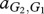 |
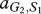 |
| S1 | 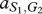 |
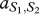 |
S2 | 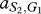 |
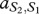 |
||
where, for example, a Generous member of species 1 obtains 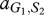 from an interaction with a Selfish member of species 2, whereas the latter obtains
from an interaction with a Selfish member of species 2, whereas the latter obtains 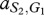 . In our case, we have
. In our case, we have 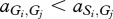 and
and 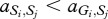 for i,j = 1,2. This ordering of payoffs corresponds to a snowdrift game [31] (see the electronic supplementary material) where there exists a point where the two strategies can coexist. This is because if both the players are Generous, then one can get away with being Selfish, but if both are Selfish, then it actually pays to be Generous and chip in. For a snowdrift game in a single species the coexistence point is stable, i.e. deviations from this point bring the system back to equilibrium, because the deviators would always be disfavoured by selection. However, for a snowdrift game between two species, this coexistence point is unstable as each species would be better off being Selfish, i.e. exploiting the deviation of the other species.
for i,j = 1,2. This ordering of payoffs corresponds to a snowdrift game [31] (see the electronic supplementary material) where there exists a point where the two strategies can coexist. This is because if both the players are Generous, then one can get away with being Selfish, but if both are Selfish, then it actually pays to be Generous and chip in. For a snowdrift game in a single species the coexistence point is stable, i.e. deviations from this point bring the system back to equilibrium, because the deviators would always be disfavoured by selection. However, for a snowdrift game between two species, this coexistence point is unstable as each species would be better off being Selfish, i.e. exploiting the deviation of the other species.
The frequency of players playing strategy Generous (G1) in species 1 is given by x and in species 2 (G2) by y. The frequencies of players playing strategy Selfish (S1 and S2) are given by 1 − x and 1 − y in species 1 and 2, respectively. The fitness of the Generous strategy in species 1, 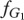 depends on the frequency y of Generous players in species 2,
depends on the frequency y of Generous players in species 2, 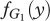 . Equivalently, the fitness of the Generous strategy in species 2,
. Equivalently, the fitness of the Generous strategy in species 2, 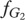 depends on the frequency x of Generous players in species 1,
depends on the frequency x of Generous players in species 1, 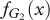 . The replicator dynamics assumes that the change in frequency of a strategy is proportional to the difference between the fitness of that strategy and the average fitness of the species
. The replicator dynamics assumes that the change in frequency of a strategy is proportional to the difference between the fitness of that strategy and the average fitness of the species 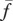 [23,32,33]. Thus, the time evolution of the frequencies of the Generous players in the two species are
[23,32,33]. Thus, the time evolution of the frequencies of the Generous players in the two species are
| 2.1 |
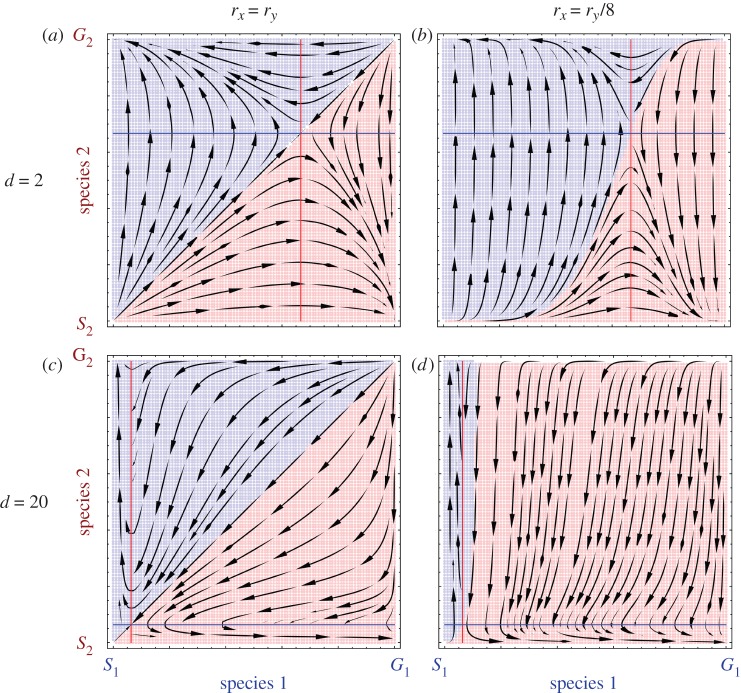
The parameters rx and ry are the evolutionary rates of the two species. We first recover the scenarios described in [12]. If the evolutionary rates are equal (rx = ry) and the evolutionary game is symmetric, then the basins of attraction of (S1, G2) and (G1, S2) are of equal size (figure 1a). For unequal evolutionary rates, the species which is evolving slower (in our case species 1 with the rate rx = ry/8) has a larger basin of attraction (figure 1b). This asymmetry where most of the initial conditions lead to an outcome favouring the slower evolving species has been termed as the Red King effect [12].
Figure 1.
The composition of both species can range from all Selfish (S) to all Generous (G). If the other species is sufficiently Generous, Selfish behaviour is favoured in both species. However, if the other species is Selfish, Generous behaviour is advantageous. This is captured by the snowdrift game discussed in the text. For equal evolutionary rates, rx = ry, the basins of attraction for the two outcomes (S1, G2) and (G1, S2) are of equal size (a,c). The colours illustrate the regions leading to the outcomes favourable to species 1 (blue-shaded area leading to (S1, G2)) and species 2 (red-shaded area leading to (G1, S2)). For a two-player game, d = 2, and rx = ry/8, the basin of attraction favourable to the slower evolving species 1 grows substantially (b) [12]. For a 20-player game, d = 20, the basins of attractions have identical size for equal evolutionary rates, but the position of the internal equilibrium is shifted (c). When species 1 evolves slower than species 2 in this situation, most of the initial conditions lead to a solution that is unfavourable to species 1 (d). Thus, for 20 players instead of two, the Red King effect is reversed (b = 2 and c = 1).
We now extend the above approach to multiplayer games. Extending the number of players from 2 to d adds a polynomial nonlinearity to the fitness functions of the strategies (see the electronic supplementary material). For a multiplayer game, we no longer have a 2 × 2 payoff matrix, but rather a payoff table. For this, we use the proposal from [34] for a d-player snowdrift game, where the costs of being Generous are divided among the Generous players. In addition, only if there are at least M Generous players, a benefit is produced. That is, for k < M Generous players, each one of them exhibits a loss of c/M and the Selfish players obtain nothing. If there are at least M Generous players, then a benefit is produced. The Generous obtain b−c/k and the Selfish obtain b at no cost. For species 2 we can write down a different payoff setup which could have different values for b, c, M, d, etc. thus creating a ‘bi-table’ game. For the time being, we assume that the payoff setups are symmetric for the two species and hence we just elucidate the details for species 1. The exact formulation of the payoffs and the calculations of fitness values are given in the electronic supplementary material.
Note that for d = 2, M = 1, b = 2 and c = 1, we recover the matrix used in [12]. Even for these new fitness functions for multiplayer games, the dynamics are still given by the replicator equations [35–37]. Also note that for two-player games with M = 1, there are four fixed points in which each species is either Selfish or Generous. In addition, there is an internal fixed point given by 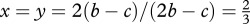 . The position and the stability of the fixed points is independent of the evolutionary rates, but as we will see, it depends on the number of players d. For a 20-player game (d = 20), the basins of attractions are still of the same size, but the dynamics leading to the stable points on the vertices are completely different (figure 1c, rx = ry). The internal equilibrium has now shifted to x = y = 0.063. As before, we introduce an asymmetry in the evolutionary rates. Interestingly, we find that for a 20-player game (figure 1d, rx = ry / 8) for the same asymmetric values of growth rates as in the two-player case, most of the initial conditions lead to a stable point where species 2 is Selfish and species 1 is Generous (G1, S2). This reverses the result which we got from d = 2. Everything else being the same, in the presence of multiple players, the Red King effect is not observed in this example.
. The position and the stability of the fixed points is independent of the evolutionary rates, but as we will see, it depends on the number of players d. For a 20-player game (d = 20), the basins of attractions are still of the same size, but the dynamics leading to the stable points on the vertices are completely different (figure 1c, rx = ry). The internal equilibrium has now shifted to x = y = 0.063. As before, we introduce an asymmetry in the evolutionary rates. Interestingly, we find that for a 20-player game (figure 1d, rx = ry / 8) for the same asymmetric values of growth rates as in the two-player case, most of the initial conditions lead to a stable point where species 2 is Selfish and species 1 is Generous (G1, S2). This reverses the result which we got from d = 2. Everything else being the same, in the presence of multiple players, the Red King effect is not observed in this example.
Next, we explore the process owing to which the Red King effect vanishes. The replicator solutions of the two species creates quadrants in the state space (0 ≤ x, y ≤ 1). Of these quadrants, the top right and the bottom left are of special interest as they contain the curve that separates the two basins of attraction (the blue and red sections in figure 1). Hence all points starting on one side of that curve lead to the same equilibrium. Consider the top-right quadrant. Species 2 is represented by the y-axis. A faster evolution by species 2 results in most of the initial conditions leading to the outcome favourable for species 2, i.e. (G1, S2). An exactly opposite scenario is taking place in the bottom-left quadrant. Hence in this quadrant as species 1 is evolving at a slower pace than species 2, most of the initial conditions here lead to an outcome favouring species 1, i.e. (S1, G2). As long as the internal equilibrium is on the diagonal, the Red King effect depends on the sizes of these quadrants. Changing the number of players alters the sizes of these two influential quadrants. For example, consider the case of the 20-player game (figure 1c,d). The size of the bottom-left quadrant is reduced to such an extent that almost the whole state space leads to the outcome favourable for the faster evolving species.
The bottom-left and the top-right quadrants have equal size when the internal equilibrium is at x = y = 0.5. For a fixed b and c, we cannot select any arbitrary number of players d to obtain this equilibrium, as d is not a continuous variable. If the equilibrium is above x = 0.5, then a decrease in the evolutionary rate can be beneficial as demonstrated by the Red King effect. Conversely, if the equilibrium is below x = 0.5, then an increase in the evolutionary rate might be favourable. Hence, if the number of players leads to an equilibrium at x < 0.5, then the faster evolving species would be favoured.
Asymmetries have been considered in mutualistic species at the level of species or other properties of the system such as interactions [38], interaction lengths [11] or growth rates [12]. Asymmetry in the number of interacting partners has only been recently tackled [30]. Going back to the example of ants and larvae, a single larva is tended to by multiple ants. Thus, while from each ant's point of view this is a two-player game, for the larva this would be a multiplayer game. Where such multiplayer games are feasible, it is also possible that a certain quorum needs to be fulfilled for the game to proceed. Client fish have been shown to choose cleaning stations with two cleaners over solitary cleaners [39]. A certain number of ants are required to save a caterpillar from its predator. It has been shown that the amount of secretions of a lycaenid larva is correlated to the number of attending ants [40]. In the following two paragraphs, we explore these two points, asymmetry in the number of players for the two species and different thresholds in either species to start off the benefits of mutualistic relations.
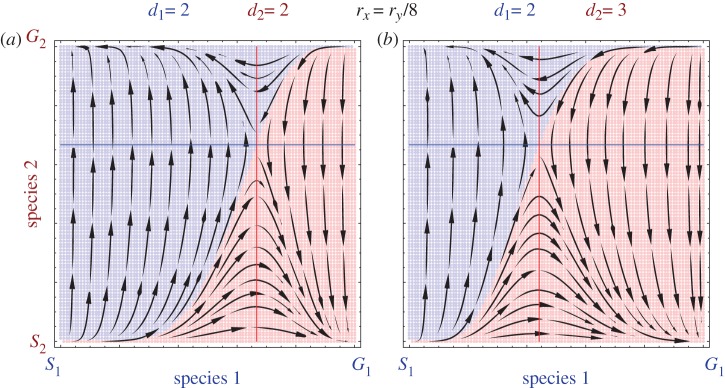
Instead of the single parameter d, now we have d1 and d2 as the number of players for the two species 1 and 2. For symmetric evolutionary rates, if the two species play different player games (d1 ≠ d2), then the basins of attraction become asymmetric. Hence, if a species is currently at a disadvantage, a modification of the number of players or the evolutionary rate may put it on equal footing with the other species. Owing to an asymmetric number of players the sizes of the basins of attraction depends not only on the sizes of the quadrants but also on the shape of the curve separating the basins of attraction. Thus, it is possible to counter the Red King effect by changing the number of interacting agents (figure 2).
Figure 2.
The Red King effect can be neutralized and/or even reversed if the number of players increases. Here, we show the scenario explored in [12] on (a); a two-player game (d1 = d2 = 2) with rx =ry/8. Most of the initial conditions lead towards the state favourable for species 1, (S1, G2). This changes when the number of players in species 2 increases from d2 = 2 to d2 = 3, i.e. now one individual of species 2 interacts with two individuals of species 1 (b). The horizontal and vertical lines denote the positions where the change in the strategy frequency is zero for species 1 and 2, respectively. The solution for species 2 (vertical line) moves towards smaller x, increasing the size of the top-right quadrant. For d1 = 2 and d2 = 3, it is given by 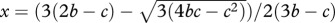 , whereas the solution for species 2 is still y = 2(b−c)/2b−c (see the electronic supplementary material). Thus, the quadrant favouring the species with a faster evolutionary rate grows. Since the number of players affects the size of these quadrants, it can eliminate or magnify the Red King effect.
, whereas the solution for species 2 is still y = 2(b−c)/2b−c (see the electronic supplementary material). Thus, the quadrant favouring the species with a faster evolutionary rate grows. Since the number of players affects the size of these quadrants, it can eliminate or magnify the Red King effect.
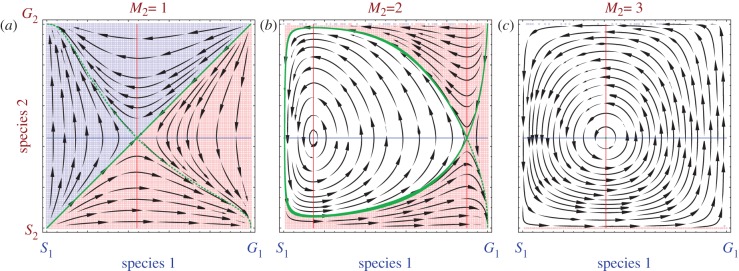
Until now, we have considered that a single Generous individual can generate the benefit of mutualism (M = 1). To begin with the simplest multiplayer case, we consider a symmetric three-player game with different thresholds in either species to start off the benefits of mutualistic relations (say for species 1 the threshold is M1 and similarly for species 2 is M2 where in general Mi can range from 1 to di).
The payoff matrices become asymmetric owing to the different thresholds for the two species. Here, it matters which dynamics we are studying, the usual replicator dynamics or the modified replicator equations (figure 3 and see the electronic supplementary material) as they can result in different sizes of the basins of attraction. The choice of dynamics depends on the details of the model system under consideration. Ultimately, the macroscopic dynamics can be derived from the underlying microscopic process [41,42]. Manipulating the thresholds can also change the nature of the game from coexistence to coordination [34], which implies that different social dilemmas arise in multiplayer games.
Figure 3.
A three-player game with asymmetric thresholds, but rx = ry. Species 1 and 2 are both playing a three-player game. (a) For species 1, it is enough if one individual is Generous to produce the benefit (M1 = 1). For species 2, however, the minimum number of Generous players required to produce any benefit strongly affects the replicator dynamics. For M2 = M1 = 1, we observe symmetric basins of attraction. The manifolds for the saddle point plotted forward in time (dashed green lines) and backward in time (solid green lines) can be used to define the basins of attraction. (b) For M2 = 2, we observe a region with closed orbits in the interior (white background), almost all initial conditions outside this region lead to (G1, S2). (c) For M2 = 3, we observe closed orbits in almost the whole state space. To avoid negative payoffs and to facilitate the comparison with the modified dynamics (see the electronic supplementary material), we have added a background fitness of 1.0 to all payoffs, but this does not alter the dynamics here.
3. Discussion
Interspecific relationships are exceedingly complex [43]. The development of a game theoretical approach for such multiplayer mutualisms requires an approach beyond that arising typically in multiplayer social dilemmas [44]. In a mutualistic framework, it is best for the two species to cooperate with each other. We do not question how these mutualisms arise. Rather when they do, what is the best strategy to contribute towards the common benefit [7,45]? It would be possible to include the interactions between the individuals of the same species, as has been recently explored experimentally [30]. It has also been shown [40] that the amount of larval secretions is also influenced by the quality of the other larvae in the group. But then, we would be shifting our focus from the problem of interspecific mutualism to intraspecific cooperation [15]. Here, we have focused on the interspecific interactions, where the interacting partners are always picked from the other species [46]. Bergstrom and Lachmann have shown that in such a mutualistic scenario, the species that evolves slower can get away with being Selfish and force the other species to make a Generous contribution. They termed this as the Red King effect. If we include multiple players, then the Red King effect is much more complex.
For simplicity, usually pairwise interactions are assumed in game theoretical arguments. For modelling collective phenomena [47,48], multiplayer games may be necessary. The exact number of players is a matter of choice, though. Group-size distributions give us an idea about the mean group size of a species. Instead of using pairwise interactions or an arbitrary number of individuals to form a group, we could use the mean group sizes as the number of interacting individuals. Group size is known to be of importance in mutualisms [3]. As we have seen here, it can be an influential factor in deciding how the benefits are shared. Countering the Red King or enhancing its effect is possible by altering the group size. Hence, can the group size itself be an evolving strategy? The study of group-size distributions has been tackled theoretically [49–56] and empirically in various species ranging from house sparrows to humans [48,49,57,58]. In our example of ants and butterfly larvae, it has been observed that a larva was most successful in getting more ant attendants in a group of four larvae [18]. It would be interesting to see if the distributions in mutualistic species peak at the group size which is the best response to their symbiont partners choices. This brings forth another of our assumptions also implicit in [12]. The rate at which strategies evolve is assumed to be much faster than the rate at which the evolutionary rates or as just mentioned, the group size evolve. If these traits are genetically determined, then this assumption may no longer hold. The rate of evolution is typically assumed to be constant, but it could well be a variable, subject to evolution. We have seen that the number of players can affect whether evolving slower or faster is favourable. It would then be interesting to determine the interplay between the evolving group size and the evolving evolutionary rate and what effect it has on the dynamics of strategy evolution.
Another method of introducing asymmetry is to have different payoff tables for the two species (i.e. different benefits and costs for the two species). Also we have only considered two strategies per species. An asymmetric number of strategies can induce further asymmetries in the interaction [46]. The intricacies of multiplayer games lend themselves to study such systems, but they also show that mutualistic interactions may be far more complex than often envisioned. Applying multiplayer game theory to mutualism unravels this dynamics between species and can be used to understand the complexity of these non-linear systems.
Acknowledgments
We thank Noémie Erin, Christian Hilbe, Aniek Ivens, Martin Kalbe, Michael Lachmann, Eric Miller and Istvàn Scheuring for helpful discussions and suggestions. We also thank the referees and the editor for their detailed and constructive input. Financial support from the Emmy-Noether program of the DFG and from the Max Planck Society is gratefully acknowledged.
References
- 1.Boucher D. H. 1985. The idea of mutualism, past and future. In The biology of mutualism (ed. Boucher D. H.), pp. 1–28 New York, NY: Oxford University Press [Google Scholar]
- 2.Hinton H. E. 1951. Myrmecophilous Lycaenidae and other Lepidoptera—a summary. Proc. Trans. Soc. London Entomol. Nat. Hist. Soc. 1949–1950, 111–175 [Google Scholar]
- 3.Wilson D. S. 1983. The effect of population structure on the evolution of mutualism: a field test involving burying beetles and their phoretic mites. Am. Nat. 121, 851–870 10.1086/284108 (doi:10.1086/284108) [DOI] [Google Scholar]
- 4.Bronstein J. L. 1994. Our current understanding of mutualism. Q. Rev. Biol. 69, 31–51 10.1086/418432 (doi:10.1086/418432) [DOI] [Google Scholar]
- 5.Pierce N. E., Braby M. F., Heath A., Lohman D. J., Mathew J., Rand D.B., Travassos M. A. 2002. The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu. Rev. Entomol. 47, 733–770 10.1146/annurev.ento.47.091201.145257 (doi:10.1146/annurev.ento.47.091201.145257) [DOI] [PubMed] [Google Scholar]
- 6.Kiers E. T., Rousseau R. A., West S. A., Denison R. F. 2003. Host sanctions and the legume–rhizobium mutualism. Nature 425, 78–81 10.1038/nature01931 (doi:10.1038/nature01931) [DOI] [PubMed] [Google Scholar]
- 7.Bshary R. S., Noë R. 2003. Biological markets: the ubiquitous influence of partner choice on the dynamics of cleaner fish–client reef fish interactions. In Genetic and cultural evolution of cooperation (ed. Hammerstein P.), pp. 167–184 Cambridge, MA: MIT Press [Google Scholar]
- 8.Poulin R., Vickery W. L. 1995. Cleaning symbiosis as an evolutionary game: to cheat or not to cheat? J. Theoret. Biol. 175, 63–70 10.1006/jtbi.1995.0121 (doi:10.1006/jtbi.1995.0121) [DOI] [PubMed] [Google Scholar]
- 9.Doebeli M., Knowlton N. 1998. The evolution of interspecific mutualisms. Proc. Natl Acad. Sci. USA 95, 8676–8680 10.1073/pnas.95.15.8676 (doi:10.1073/pnas.95.15.8676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noë R. 2001. Biological markets: partner choice as the driving force behind the evolution of mutualisms. In Economics in nature: social dilemmas, mate choice and biological markets (eds Noë R., van Hooff J. A. R. A. M., Hammerstein P.). Cambridge, UK: Cambridge University Press [Google Scholar]
- 11.Johnstone R. A., Bshary R. 2002. From parasitism to mutualism: partner control in asymmetric interactions. Ecol. Lett. 5, 634–639 10.1046/j.1461-0248.2002.00358.x (doi:10.1046/j.1461-0248.2002.00358.x) [DOI] [Google Scholar]
- 12.Bergstrom C. T., Lachmann M. 2003. The Red King effect: when the slowest runner wins the coevolutionary race. Proc. Natl Acad. Sci. USA 100, 593–598 10.1073/pnas.0134966100 (doi:10.1073/pnas.0134966100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoeksema J. D., Kummel M. 2003. Ecological persistence of the plant–mycorrhizal mutualism: a hypothesis from species coexistence theory. Am. Nat. 162, S40–S50 10.1086/378644 (doi:10.1086/378644) [DOI] [PubMed] [Google Scholar]
- 14.Akçay E., Roughgarden J. 2007. Negotiation of mutualism: rhizobia and legumes. Proc. R. Soc. B 274, 25–32 10.1098/rspb.2006.3689 (doi:10.1098/rspb.2006.3689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bshary R., Grutter A. S., Willener A. S. T., Leimar O. 2008. Pairs of cooperating cleaner fish provide better service quality than singletons. Nature 455, 964–967 10.1038/nature07184 (doi:10.1038/nature07184) [DOI] [PubMed] [Google Scholar]
- 16.Stadler B., Dixon A. F. G. 2008. Mutualism: ants and their insect partners. Cambridge, UK: Cambridge University Press; 10.1017/CBO9780511542176 (doi:10.1017/CBO9780511542176) [DOI] [Google Scholar]
- 17.Kunkel H. 1973. [Defecation of aphids (Aphidina, Hemiptera) influenced by ants.] Die Kotabgabe der Aphiden (Aphidina, Hemiptera) unter Einfluss von Ameisen. Bonner Zoologische Beiträg.) 24, 105–121 [Google Scholar]
- 18.Pierce N. E., Kitching R. L., Buckley R. C., Taylor M. F. J., Benbow K. F. 1987. The costs and benefits of cooperation between the Australian lycaenid butterfly, Jalmenus evagoras, and its attendant ants. Behav. Ecol. Sociobiol. 21, 237–248 10.1007/BF00292505 (doi:10.1007/BF00292505) [DOI] [Google Scholar]
- 19.Hölldobler B., Wilson E. O. 1990. The ants. Cambridge, MA: Belknap Press. [Google Scholar]
- 20.Hill C. J., Pierce N. E. 1989. The effect of adult diet on the biology of butterflies 1. The common imperial blue, Jalmenus evagoras. Oecologia 81, 249–257 [DOI] [PubMed] [Google Scholar]
- 21.Weibull J. W. 1995. Evolutionary game theory. Cambridge, MA: MIT Press [Google Scholar]
- 22.Hofbauer J. 1996. Evolutionary dynamics for bimatrix games: a Hamiltonian system? J. Math. Biol. 34, 675–688 10.1007/BF02409754 (doi:10.1007/BF02409754) [DOI] [PubMed] [Google Scholar]
- 23.Hofbauer J., Sigmund K. 1998. Evolutionary games and population dynamics. Cambridge, UK: Cambridge University Press [Google Scholar]
- 24.van Valen L. 1973. A new evolutionary law. Evol. Theory 1, 1–30 10.1017/CBO9781139173179 (doi:10.1017/CBO9781139173179) [DOI] [Google Scholar]
- 25.Dawkins R., Krebs J. R. 1979. Arms races between and within species. Proc. R. Soc. Lond. B 205, 489–511 10.1098/rspb.1979.0081 (doi:10.1098/rspb.1979.0081) [DOI] [PubMed] [Google Scholar]
- 26.Berry I. M., Ribeiro R., Kothari M., Athreya G., Daniels M., Lee H. Y., Bruno W., Leitner T. 2007. Unequal evolutionary rates in the human immunodeficiency virus type 1 (HIV-1) pandemic: the evolutionary rate of HIV-1 slows down when the epidemic rate increases. J. Virol. 81, 10 625–10 635 10.1128/JVI.00985-07 (doi:10.1128/JVI.00985-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zehender G., Maddalena C. D., Giambelli C., Milazzo L., Schiavini M., Bruno R., Tanzi E., Galli M. 2008. Different evolutionary rates and epidemic growth of hepatitis B virus genotypes A and D. Virology 380, 84–90 10.1016/j.virol.2008.07.009 (doi:10.1016/j.virol.2008.07.009) [DOI] [PubMed] [Google Scholar]
- 28.Mack K. M. L., Rudgers J. A. 2008. Balancing multiple mutualists: asymmetric interactions among plants, arbuscular mycorrhizal fungi, and fungal endophytes. OIKOS 117, 310–320 10.1111/j.2007.0030-1299.15973.x (doi:10.1111/j.2007.0030-1299.15973.x) [DOI] [Google Scholar]
- 29.Damore J. A., Gore J. 2011. A slowly evolving host moves first in symbiotic interactions. Evolution 65, 2391–2398 10.1111/j.1558-5646.2011.01299.x (doi:10.1111/j.1558-5646.2011.01299.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang R.-W., Sun B.-F., Zheng Q., Shi L., Zhu L. 2011. Asymmetric interaction and indeterminate fitness correlation between cooperative partners in the fig–fig wasp mutualism. J. R. Soc. Interface 7, 1487–1496 10.1098/rsif.2011.0063 (doi:10.1098/rsif.2011.0063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doebeli M., Hauert C. 2005. Models of cooperation based on the Prisoner's Dilemma and the Snowdrift game. Ecol. Lett. 8, 748–766 10.1111/j.1461-0248.2005.00773.x (doi:10.1111/j.1461-0248.2005.00773.x) [DOI] [Google Scholar]
- 32.Taylor P. D., Jonker L. 1978. Evolutionary stable strategies and game dynamics. Math. Biosci. 40, 145–156 10.1016/0025-5564(78)90077-9 (doi:10.1016/0025-5564(78)90077-9) [DOI] [Google Scholar]
- 33.Hofbauer J., Sigmund K. 2003. Evolutionary game dynamics. Bull. Amer. Math. Soc. 40, 479–519 10.1090/S0273-0979-03-00988-1 (doi:10.1090/S0273-0979-03-00988-1) [DOI] [Google Scholar]
- 34.Souza M. O., Pacheco J. M., Santos F. C. 2009. Evolution of cooperation under N-person snowdrift games. J.Theoret. Biol. 260, 581–588 10.1016/j.jtbi.2009.07.010 (doi:10.1016/j.jtbi.2009.07.010) [DOI] [PubMed] [Google Scholar]
- 35.Hauert C., Michor F., Nowak M. A., Doebeli M. 2006. Synergy and discounting of cooperation in social dilemmas. J. Theoret. Biol. 239, 195–202 10.1016/j.jtbi.2005.08.040 (doi:10.1016/j.jtbi.2005.08.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacheco J. M., Santos F. C., Souza M. O., Skyrms B. 2009. Evolutionary dynamics of collective action in N-person stag hunt dilemmas. Proc. R. Soc. B 276, 315–321 10.1098/rspb.2008.1126 (doi:10.1098/rspb.2008.1126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gokhale C. S., Traulsen A. 2010. Evolutionary games in the multiverse. Proc. Natl Acad. Sci. USA 107, 5500–5504 10.1073/pnas.0912214107 (doi:10.1073/pnas.0912214107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noë R., van Schaik C. P., van Hooff J. A. R. A. M. 1991. The Markert Effect: an explanation for pay-off asymmetries among collaborating animals. Ethology 87, 97–118 10.1111/j.1439-0310.1991.tb01192.x (doi:10.1111/j.1439-0310.1991.tb01192.x) [DOI] [Google Scholar]
- 39.Bshary R., Schäffer D. 2002. Choosy reef fish select cleaner fish that provide high-quality service. Anim. Behav. 63, 557–564 10.1006/anbe.2001.1923 (doi:10.1006/anbe.2001.1923) [DOI] [Google Scholar]
- 40.Axén A. H., Pierce N. E. 1998. Aggregation as a cost-reducing strategy for lycaenid larvae. Behav. Ecol. 9, 109–115 10.1093/beheco/9.2.109 (doi:10.1093/beheco/9.2.109) [DOI] [Google Scholar]
- 41.Traulsen A., Claussen J. C., Hauert C. 2005. Coevolutionary dynamics: from finite to infinite populations. Phys. Rev. Lett. 95, 238701. 10.1103/PhysRevLett.95.238701 (doi:10.1103/PhysRevLett.95.238701) [DOI] [PubMed] [Google Scholar]
- 42.Black A. J., McKane A. J. 2012. Stochastic formulation of ecological models and their applications. Trends Ecol. Evol. 27, 337–345 10.1016/j.tree.2012.01.014 (doi:10.1016/j.tree.2012.01.014) [DOI] [PubMed] [Google Scholar]
- 43.Blaser M. J., Kirschner D. 2007. The equilibria that allow bacterial persistence in human hosts. Nature 449, 843–849 10.1038/nature06198 (doi:10.1038/nature06198) [DOI] [PubMed] [Google Scholar]
- 44.Bshary R. S., Bronstein J. L. 2004. Game structures in mutualisms: what can the evidence tell us about the kinds of models we need? Adv. Study Behav. 34, 59–104 10.1016/S0065-3454(04)34002-7 (doi:10.1016/S0065-3454(04)34002-7) [DOI] [Google Scholar]
- 45.Bowles S., Gintis H. 2003. The origins of human cooperation. In The genetic and cultural origins of cooperation (ed. Hammerstein P.) pp. 429–443 Cambridge, MA: MIT Press [Google Scholar]
- 46.Schuster P., Sigmund K., Hofbauer J., Wolff R. 1981. Selfregulation in behaviour in animal societies. II. Games between two populations without selfinteractions. Biol. Cybernet. 40, 9–15 10.1007/BF00326676 (doi:10.1007/BF00326676) [DOI] [Google Scholar]
- 47.Couzin I. D., Krause J., Franks N. R., Levin S. A. 2005. Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516 10.1038/nature03236 (doi:10.1038/nature03236) [DOI] [PubMed] [Google Scholar]
- 48.Sumpter D. J. T. 2010. Collective animal behavior. Princeton, NJ: Princeton University Press [Google Scholar]
- 49.Krause J., Ruxton G. 2002. Living in groups. Oxford, UK: Oxford University Press [Google Scholar]
- 50.Hauert C., De Monte S., Hofbauer J., Sigmund K. 2002. Volunteering as Red Queen mechanism for cooperation in public goods games. Science 296, 1129–1132 10.1126/science.1070582 (doi:10.1126/science.1070582) [DOI] [PubMed] [Google Scholar]
- 51.Niwa H. S. 2003. Power-law versus exponential distributions of animal group sizes. J. Theoret. Biol. 224, 451–457 10.1016/S0022-5193(03)00192-9 (doi:10.1016/S0022-5193(03)00192-9) [DOI] [PubMed] [Google Scholar]
- 52.Hauert C., Holmes M., Doebeli M. 2006. Evolutionary games and population dynamics: maintenance of cooperation in public goods games. Proc. R. Soc. B 273, 2565–2570 10.1098/rspb.2006.3600 (doi:10.1098/rspb.2006.3600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hauert C., Traulsen A., Brandt H., Nowak M. A., Sigmund K. 2008. Public goods with punishment and abstaining in finite and infinite populations. Biol. Theory 3, 114–122 10.1162/biot.2008.3.2.114 (doi:10.1162/biot.2008.3.2.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Veelen M., Garcia J., Aviles L. 2010. It takes grouping and cooperation to get sociality. J. Theoret. Biol. 264, 1240–1253 10.1016/j.jtbi.2010.02.043 (doi:10.1016/j.jtbi.2010.02.043) [DOI] [PubMed] [Google Scholar]
- 55.Brännström Å., Gross T., Blasius B., Dieckmann U. 2011. Consequences of fluctuating group size for the evolution of cooperation. J. Math. Biol. 63, 263–281 10.1007/s00285-010-0367-3 (doi:10.1007/s00285-010-0367-3) [DOI] [PubMed] [Google Scholar]
- 56.Peña J. 2012. Group size diversity in public goods games. Evolution 66, 623–636 10.1111/j.1558-5646.2011.01504.x (doi:10.1111/j.1558-5646.2011.01504.x) [DOI] [PubMed] [Google Scholar]
- 57.Zipf G. K. 1949. Human behaviour and the principle of least effort. Cambridge, UK: Addison-Wesley [Google Scholar]
- 58.Griesser M., Ma Q., Webber S., Bowgen K., Sumpter D. J. T. 2011. Understanding animal group-size distributions. PLoS ONE 6, e23438. 10.1371/journal.pone.0023438 (doi:10.1371/journal.pone.0023438) [DOI] [PMC free article] [PubMed] [Google Scholar]