Abstract
Extreme events have been suggested to play a disproportionate role in shaping ecological processes, but our understanding of the types of environmental conditions that elicit extreme consequences in natural ecosystems is limited. Here, we investigated the impact of a massive iceberg on the dynamics of a population of Weddell seals. Reproductive rates of females were reduced, but survival appeared unaffected. We also found suggestive evidence for a prolonged shift towards higher variability in reproductive rates. The annual number of females attending colonies showed unusual swings during the iceberg period, a pattern that was apparently the consequence of changes in sea-ice conditions. In contrast to the dramatic effects that were recorded in nearby populations of emperor penguins, our results suggest that this unusual environmental event did not have an extreme impact on the population of seals in the short-term, as they managed to avoid survival costs and were able to rapidly re-achieve high levels of reproduction by the end of the perturbation. Nevertheless, population projections suggest that even this modest impact on reproductive rates could negatively affect the population in the long run if such events were to occur more frequently, as is predicted by models of climate change.
Keywords: catastrophic event, demography, iceberg B-15, vital rates, Leptonychotes weddellii
1. Introduction
Extreme events are thought to play a disproportionate role in shaping ecological and evolutionary processes [1,2]. Previous empirical and theoretical work suggests that: (i) in many situations, maximum values taken by environmental covariates are better predictors of ecological patterns than are means and variances [3]; and (ii) strong episodes of natural selection principally occur during extreme events [4]. Since the intensification of extreme climatic events has been recognized as one of the most important facets of climate change [5,6], increasing attention has been dedicated to research on extreme events (i.e. ‘event-focused’ in contrast to ‘trend-focused’) [7]. Many aspects of this emerging field are, however, still poorly understood and deserve further investigation [8]. Climate extremes do not necessarily translate into an extreme ecological response (thereafter, EER), and one of the big challenges for ecologists will be to identify which conditions might potentially induce extreme reactions in biological systems [8]. An EER occurs when organisms cannot acclimate, at a relatively low fitness cost, to extreme or rare environmental conditions [2]. At the population level, a negative EER can thus be identified when conditions induce a strong reduction of the most important vital rates, which would otherwise be buffered against environmental variability [9–11], thus negatively affecting the growth and chances of persistence of the population. So far, our comprehension of the link between environmental extremes and EER remains very limited and case- or taxon-specific, with a predominance from studies on plants [2,8,12]. Most observational studies that have investigated the effects of climate extremes have reported an EER (e.g. animals [13–15], plants [16,17]). On the other hand, results of a number of recent experiments indicate that the response to climate extremes of numerous biological systems remains within their range of regular variability (see [8,12] and references therein), suggesting that EERs might occur less frequently than suggested by observational studies.
Extreme climatic events are usually thought of in terms of extreme values of a continuous environmental variable [2] (e.g. extreme temperatures, precipitation levels), but they can also take the form of discrete perturbations [18], such as unusually big fires [19], hurricanes [20], floods [14,21] or rain-on-snow events [22]. In polar regions, calving of massive icebergs, which are known to be influenced by worldwide weather systems [23] and expected to occur more frequently due to global climate change [24], are events that can strongly impact local ecosystems [25]. For instance, the occurrence of the unusually large iceberg B-15 (approx. 10 000 km²), which calved from the Ross Ice Shelf, Antarctica, in March 2000 [26], is known to have strongly disturbed the Ross Sea ecosystem, from primary productivity to upper levels of the trophic web [25,27–30]. In particular, local colonies of emperor penguins (Aptenodytes forsteri) experienced unusually high levels of breeding failure and of adult and chick mortality [29]. However, the potential impact of this iceberg event on a local population of Weddell seals (Leptonychotes weddellii), another long-lived marine vertebrate, has not been investigated yet. This population has been continuously monitored since 1969, including years of iceberg presence (2001–2005), and the data provide a unique opportunity to assess changes in local abundance and demographic rates in relation to the iceberg event and to compare the findings with responses seen in other local species. For that purpose, we estimated annual abundance of female seals in colonies (local abundance), as well as a complete set of annual vital rates (survival and reproduction probabilities), for a 29-year period and evaluated how the mean and variance of those demographic parameters changed during and after the onset of the iceberg. By doing so, we were able to assess whether this event induced an EER in a marine mammal species as it did in nearby populations of emperor penguins. Potential effects on process variance were explicitly considered in this study because any increase in the variability of demographic parameters decreases the long-term growth rate of a population, hence reducing its chances of persistence [31]. We then assessed by simulations how the growth rate of the population changed during the iceberg episode.
Here, the iceberg event is defined as the period from 2001 to 2005, during which four massive fragments of iceberg B-15 (see the electronic supplementary material, figure S1) were in the vicinity of the seals' breeding area. We expect the presence of these icebergs to have impacted the seal population through two different mechanisms. First, by creating challenging sea-ice conditions [32] (large extent of sea-ice and thick ice with massive pressure ridges likely making access to the ice surface difficult), these icebergs might have hampered access of seals to breeding colonies. We thus predict that local abundance was reduced during iceberg years. Second, because there is evidence that primary productivity and abundance of primary consumers were reduced during the perturbed period [25,27,30], the absolute amount of food available to seals might have been lower than usual. We therefore predict a negative impact on vital rates due to a limitation of available energy resources. However, because under such limiting conditions, long-lived iteroparous species are expected to allocate more energy to survival than to reproduction [33], we predict a more dramatic reduction in reproductive rates compared with survival rates [34]. Lastly, during challenging conditions, one might expect seals to emigrate and breed in colonies outside Erebus Bay, where ice and food conditions might be more favourable. However, previous evidence suggests that Weddell seals are highly philopatric, and seals breeding in Erebus Bay are very rarely observed breeding outside the primary study area (only 2% of all recorded breeding events occurred outside the study area) [35,36]. We therefore do not expect seals to have massively emigrated and bred in other colonies although we do assess this possibility based on the results of survey efforts conducted outside the study area during the iceberg event.
2. Methods
(a). Study area and study population
Erebus Bay is located in the southwestern part of Ross Sea, Antarctica (77.62°–77.87° S, 166.3°–167.0° E). Each year in spring, Weddell seal pupping colonies form on the fast ice within this study area. Pupping occurs from mid-October to late-November, and each mother remains on the ice with its pup for one to two weeks. Seals have been annually marked and resighted since 1969, and since 1982 every pup born in the study area has been individually marked shortly after birth. Mother–pup pairs are detected on the ice, with probability 1.0 [37], and many of the females that do not produce a pup in a given year (i.e. pre-breeders and skip-breeders; see below for details) also haul out in the study area and are highly detectable [38].
(b). Data collection and data analyses
Each year, during the pupping season, colonies were visited every two to three days to tag newborn pups and any other untagged seals. Also, beginning in early November of each year, five to eight surveys typically separated by 3–5 days were conducted throughout the study area. Every encountered animal (marked or unmarked) was recorded along with its sex and breeding state, which can be determined with certitude. Intense searching effort has also been conducted outside the study area since 1997 [35], and especially during the years of iceberg presence, to record breeding events occurring outside Erebus Bay.
The statistical analysis consisted of three components. In a first step, using mark-recapture data collected over the 29-years period (1982–2010), we estimated two types of annual demographic parameters: local abundance and vital rates (survival and reproductive rates). Annual estimates of local abundance were obtained using within-season resight data and the superpopulation approach of Schwarz & Arnason [39], while annual vital rates were estimated under a multi-state modelling approach [40], based on inter-annual resight data. Here, we define ‘local abundance’ as the number of females that were present in Erebus Bay colonies in a given year. As in any long-lived species, changes in abundance due to deaths and births are relatively slow, while immigration and emigration events can produce rapid and large annual changes. In our system, where local abundance is spatially defined by breeding colonies and temporally defined by the breeding season, annual variations are mainly linked to the pattern of utilization of colonies by females. In particular, some locally born seals may be absent from colonies during one or a few consecutive breeding seasons, while some seals born outside the study area may temporarily attend colonies. Whereas information on variation in death and birth processes is accurately investigated through the multi-state analyses of mark-recapture data (see below) on locally born individuals, annual variation in local abundance primarily informs us about possible changes in inter-annual utilization patterns.
In the second step, we estimated the mean and the process variance of the different demographic parameters, for different relevant periods, based on the decomposition of the total variance [41,42]. Two different pairs of periods were considered to make relevant comparisons: (i) non-iceberg years (1982–2000 and 2006–2010) versus iceberg years (2001–2005); and (ii) years before iceberg (period 1982–2000) versus years after iceberg (period 2001–2010). In the third step, we used simulations to estimate the population growth rate for the non-iceberg and the iceberg periods.
(i). Estimation of annual local abundance
Estimates of annual local abundance were obtained using methods recently developed for this population [38], based on data collected during multiple within-year surveys on both known-age females (tagged as pups) and unknown-age females (tagged as adults or untagged). Three different breeding classes were distinguished in this analysis: (i) pre-breeders (P), i.e. females not breeding that year and that never had a pup before; (ii) breeders, (B) i.e. females known to have had a pup that year; and (iii) skip-breeders (S), i.e. females without a pup that year but that were known to have produced a pup in a previous year. We first estimated the size of each class for known-age females, for which the breeding state can be assigned each year, given that the complete breeding history is known. Then, estimates of numbers in states P and S for known-age females were used to help inform the analysis of data from unknown-age females, for which states P and S are undistinguishable until a reproductive event has been observed. Full details of the estimation process were recently presented [38]. Estimates from data of known-age and unknown-age females were finally combined to obtain the total local abundance in each breeding class each year. Variances and model-selection uncertainty were carried forward at each step of this analysis, and when initial estimates were transformed into other quantities of interest, we used the delta method [43] to estimate variances of the new quantities.
(ii). Estimation of annual vital rates
A multi-state mark-recapture approach [40] was used to estimate apparent survival probabilities (ϕ) and reproductive rates (ψ; i.e. transition probabilities, conditional on survival from year t to t + 1, from any state in year t into an active breeder state in year t + 1), while accounting for imperfect detection. Four breeding states were distinguished in this analysis: (i) pre-breeder (P); (ii) first-time breeder (F); (iii) experienced breeder (E); and (iv) skip-breeder (S). Recruitment was defined as the transition from state P to F (ψPF) and reproduction as transition from state F, E or S to state E (ψFE, ψEE, ψSE). Several combinations of age and year effects were considered for each reproductive status [36,37,44] and evaluated with the Akaike information criterion (AIC) corrected for sample size (AICc) and overdispersion (QAICc). The estimates that were subsequently used in the analysis come from the most supported of these models. More details about the model selection procedure are provided in electronic supplementary material, appendix S1 and table S1. A total of 6373 individual histories from female Weddell seals, consisting in 5810 known-age individuals (i.e. tagged as newborn pups in the study area) and 563 unknown-age seals, were available and used in the analysis.
(iii). Decomposition of variance components
We used the method of moments [41,42] to (i) derive estimates of means and process variation and (ii) obtain shrunk annual estimates, along with their s.e. and 95% CIs, of local abundance and vital rates for each of the ensembles of years considered. For annual vital rates, which were all estimated simultaneously in the same multi-state models, this was done using the random effects module of program MARK [45]. Because vital rates are probabilities, their maximum possible variance is a function of the mean [10]. Therefore, following previous authors [9,10,15,44], we scaled the process variance by the maximum possible variance for the corresponding mean before making comparisons among different periods. For local abundance estimates, which were obtained independently for each year, the method of moments was performed in program R (v. 2.12.0, [46]), using the generalized weighted procedure [41]. No scaling of process variance was necessary as local abundances are not probabilities.
(iv). Estimation of population growth rates
Using estimates of the mean and process variance of vital rates, we estimated population growth rates for the non-iceberg and the iceberg periods. For each period, we randomly drew 5000 sets of vital rate values from their estimated distributions and calculated the dominant eigen value [47], or asymptotic population growth rate (λ), for each set. We then compared the distributions of λ between the two periods. The values of survival used in these simulations were adjusted for tag loss [37,44]. Because all vital rates are probabilities and thus belong to the interval [0;1], random values were generated at the scale of log odds using random normal deviates based on the estimated mean and variance of the log odds of each vital rate.
3. Results
We predicted that the challenging sea-ice conditions created by the presence of the icebergs would hamper the access of seals to breeding colonies and thus decrease local abundance. Moreover, in relation to a probable reduction in food resources, we also expected seals to adopt a strategy in keeping with conserving and allocating more energy to survival at the expense of reproduction. We therefore also predicted a decrease in reproductive rates, but not in survival, during the iceberg period. To test these predictions, we estimated local abundance as well as rates of survival and reproduction for a series of 29 years of available data and assessed how these parameters changed during the iceberg episode. Moreover, given that we expected that the temporal variability of these demographic parameters might also have been affected by the iceberg event, we assessed potential changes in process variance.
(a). Local abundance
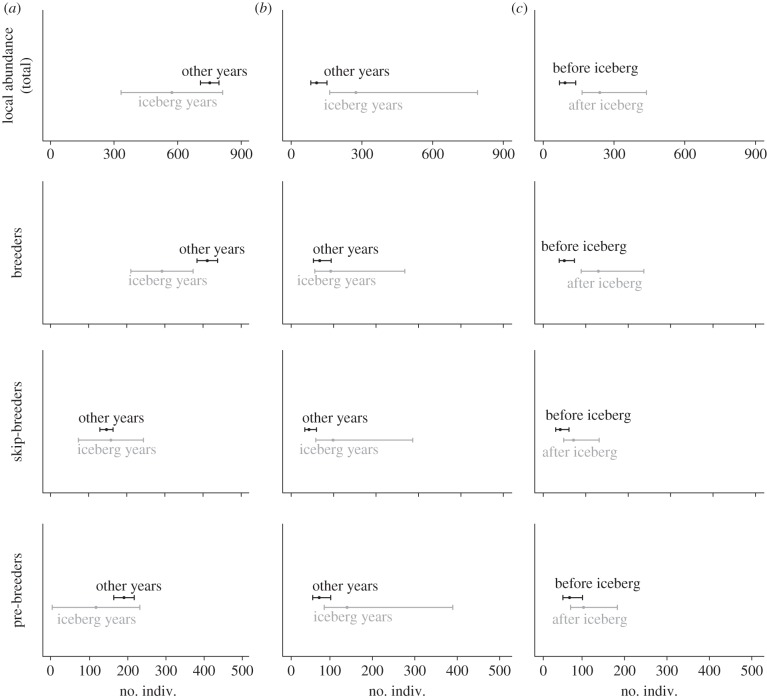
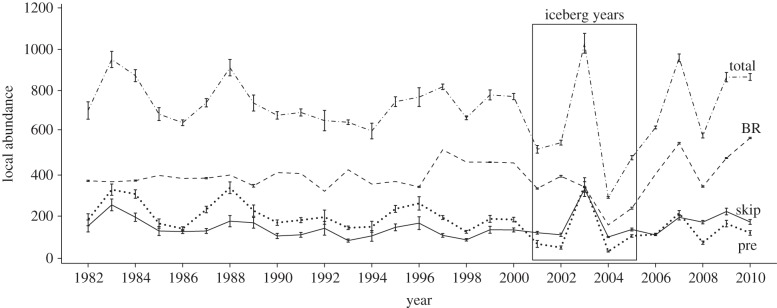
We found suggestive evidence for a reduction of total local abundance during iceberg years (figure 1a; see also electronic supplementary material, table S2), although the details of this reduction were not as simple as predicted. Indeed, while the number of breeders was consistently lower than usual during all iceberg years (figures 1a and 2), the number of pre-breeders and skip-breeders was more variable within this period (figure 2). In fact, for these two latter classes of seals, the estimated local abundance for a single year (2003) was in strong contrast with estimates for all other iceberg years. Local abundance in 2003 was the largest of the entire time series 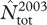 (1028.9, SE = 24.2; see the electronic supplementary material, table S3) and was associated with peaks in the estimated numbers of skip-breeders and pre-breeders (figure 2). Nevertheless, of females that attended the breeding grounds in 2003, only about 33 per cent produced a pup, which is the smallest proportion of breeders ever estimated. By contrast, the subsequent year (2004) had the lowest estimate of both total local abundance
(1028.9, SE = 24.2; see the electronic supplementary material, table S3) and was associated with peaks in the estimated numbers of skip-breeders and pre-breeders (figure 2). Nevertheless, of females that attended the breeding grounds in 2003, only about 33 per cent produced a pup, which is the smallest proportion of breeders ever estimated. By contrast, the subsequent year (2004) had the lowest estimate of both total local abundance 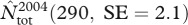 and number of breeders ever recorded
and number of breeders ever recorded 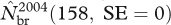 . There is also some evidence that process variance in local abundance changed over the years of study (figure 1b,c; see the electronic supplementary material, table S4). The estimated process s.d. (
. There is also some evidence that process variance in local abundance changed over the years of study (figure 1b,c; see the electronic supplementary material, table S4). The estimated process s.d. (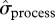 ) of total local abundance, as well as
) of total local abundance, as well as 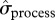 of skip-breeder and pre-breeder numbers, was higher during the iceberg episode than in other years (figure 1b). This increased variance was due to a large difference in local abundance between 2003 and other iceberg years (see above). When estimates for the pre-iceberg period were compared with those for all years following the iceberg onset (2001–2010), we found evidence that
of skip-breeder and pre-breeder numbers, was higher during the iceberg episode than in other years (figure 1b). This increased variance was due to a large difference in local abundance between 2003 and other iceberg years (see above). When estimates for the pre-iceberg period were compared with those for all years following the iceberg onset (2001–2010), we found evidence that 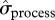 increased for all population components (figure 1c), and especially for breeders (figure 2).
increased for all population components (figure 1c), and especially for breeders (figure 2).
Figure 1.
Summary of the results obtained for the mean and process s.d. of the different components (breeding classes) of local abundance. (a) Comparison of the mean between iceberg years and during other years; (b) comparison of process s.d. between iceberg and non-iceberg years; and (c) comparison of process s.d. after and before the iceberg event. Error bars represent 95% CIs (i.e. error due to sampling variation). Note that for optimal scaling of CIs, x-axis ranges are not necessarily the same among individual graphs.
Figure 2.
Temporal variation in the size of the different breeding classes occupying colonies from 1982 to 2010. The dashed-dotted line represents the total local abundance (total), the dashed line represents the number of breeders (BR), the solid line represents the number of skip-breeders (skip) and the dotted line represents the number of pre-breeders (pre). The period during which the iceberg was present in the vicinity of seal colonies is showed by a solid frame. Error bars represent 95% CIs (i.e. error due to sampling variation).
To summarize, the iceberg event seems to have impacted both the mean and variance of local abundance, but in a more complex way than predicted. It reduced the mean number of breeders, increased 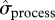 for the non-breeding components of the population during iceberg years and increased
for the non-breeding components of the population during iceberg years and increased 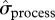 for breeders for a more prolonged period (figures 1 and 2).
for breeders for a more prolonged period (figures 1 and 2).
(b). Vital rates
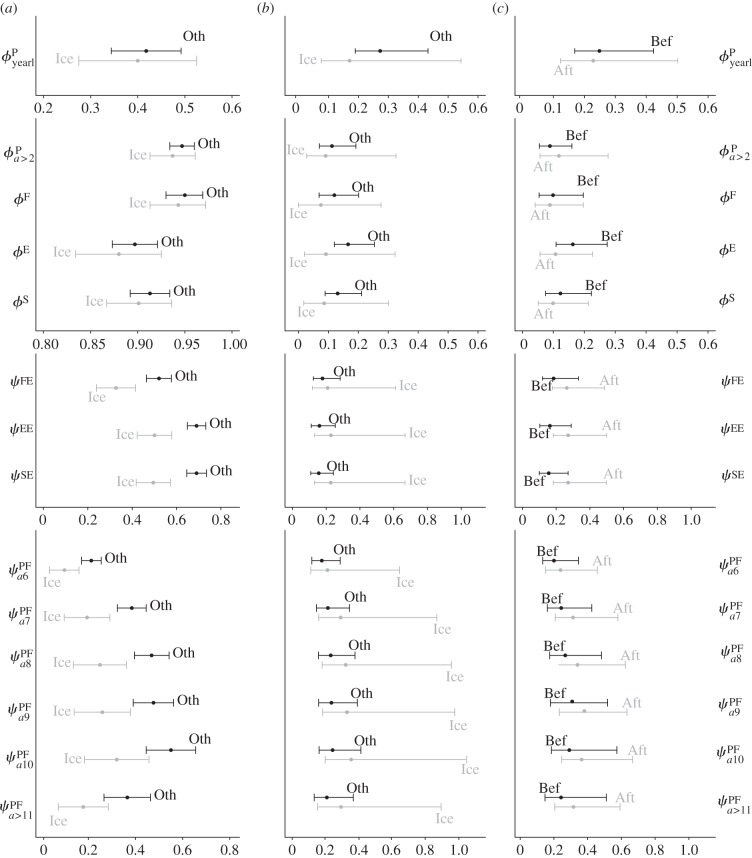
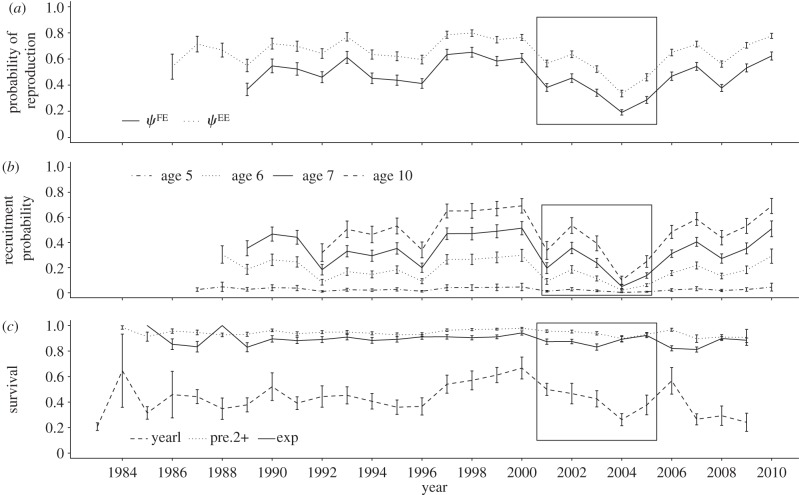
As predicted, probabilities of recruitment and probabilities of reproduction substantially decreased during iceberg years (figure 3a; see also figure 4a,b and electronic supplementary material, table S5). In particular, the reduction of recruitment rates (ψPF) ranged from 42 per cent for 10-year-old females (a drop from 0.54 to 0.31) to 59 per cent for 5-year-old females (from 0.03 to 0.01), and the reduction of reproductive rates ranged from 27 per cent for ψEE (from 0.69 to 0.50) to 37 per cent for ψFE (from 0.52 to 0.33). It was however unclear whether rates declined more for younger or less-experienced animals than for others (figure 3a). The temporal projection of annual reproduction and recruitment rates (figure 4a,b) shows that these parameters followed a decreasing trend until 2004, when they reached the lowest values ever recorded in 29 years, but then rebounded and stayed relatively high after the icebergs were gone.
Figure 3.
Summary of the results obtained for the mean and process s.d. of vital rates. (a) Comparison of the mean between iceberg years (Ice) and during other years (Oth); (b) comparison of process s.d. between iceberg and non-iceberg years; and (c) comparison of process s.d. after (Aft) and before (Bef) the iceberg event. Error bars represent 95% CIs (i.e. error due to sampling variation). Note that for optimal scaling of CIs, x-axis ranges are not necessarily the same among individual graphs. Demographic parameters are represented by lowercase Greek letters: ‘ϕ’ (survival) and ‘ψ’ (reproductive rates, i.e. transition among breeding states). Superscripts represent states specificities of these parameters: ‘P’ (pre-breeder), ‘F’ (first-time breeder), ‘E’ (experienced breeder) and ‘S’ (skip-breeder). For transition parameters ψ, the first superscript represents state of departure (at time t) and the second superscript represents state of arrival (at t + 1). Subscripts represent age-specificity for the pre-breeder state: ‘yearl’ (yearling), ‘a ≥ 2’ (older than two), ‘a5’, ‘a6’, ‘a7’, ‘a8’, ‘a9’ and ‘a10’ (age 5, 6, 7, 8, 9 and 10, respectively), and ‘a ≥ 11’ (older than eleven). Note that yearling survival corresponds to survival from 1-year-old to 2-year-old.
Figure 4.
Temporal variation of probabilities of (a) reproduction (ψFE, ψEE), (b) recruitment (ψPF) and (c) survival. The iceberg period is represented by a solid frame. Error bars represent s.e. (i.e. error due to sampling variation). Not all states and age classes are displayed on these graphs for better readability. ‘yearl’, yearling, which corresponds to second-year survival (i.e. survival from age 1 to age 2); ‘pre2+’, pre-breeders of age ≥ 2; ‘exp’, experienced breeders.
Process variation of recruitment and reproduction did not appear to be different in iceberg years versus non-iceberg years (figure 3b). However, when estimates were compared for the pre-iceberg period versus the period after iceberg onset (2001–2010), we found evidence of increased process variation (figure 3c). Despite the substantial uncertainty associated with estimates, due to the small number of iceberg years, the evidence is relatively strong for probabilities of reproduction, for which 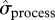 increased by 42 per cent, 65 per cent and 73 per cent for ψFE, ψEE and ψSE, respectively, after the onset of the iceberg (see the electronic supplementary material, table S6).
increased by 42 per cent, 65 per cent and 73 per cent for ψFE, ψEE and ψSE, respectively, after the onset of the iceberg (see the electronic supplementary material, table S6).
Finally, as predicted, we found no evidence of change in the mean and the process variance of survival rates during or after the iceberg event (figures 3 and 4c). These results indicate that the iceberg event did not have extreme consequences on the dynamic of this population, as females managed to avoid survival costs and were able to achieve high reproductive rates immediately after the iceberg episode.
(c). Population growth rate
For the non-iceberg period, the average value of population growth rate (λ) was found as 1.00, and λ as greater than 1.0 in about 37.7 per cent of simulations (see the electronic supplementary material, figure S2). For the iceberg period, λ averaged 0.96 and only 1.3 per cent of simulated λ were greater than 1.0. Therefore, we conclude that, while the population was typically stable during the non-iceberg period, it was most likely in a declining phase (λ < 1) during the iceberg episode, because of the decrease in vital rates.
4. Discussion
In this study, we assessed whether a large-scale environmental perturbation, represented by the occurrence of massive icebergs, had extreme consequences on a population of Weddell seals breeding in the southwestern Ross Sea. Previous studies of this well-known iceberg event reported dramatic effects on lower-level taxa [25,27] and on two local populations of emperor penguins [29], but the potential impact on the population of Weddell seals was unknown. Our goal was to evaluate whether this event would (i) exceed the acclimation capacity of the species and (ii) be a threat for the population at a mid-term time scale. The comparison of our results for seals with those from a previous study on emperor penguins [29] allowed us to compare the responses of two high-level predators that depend on similar food resources but have substantial differences in their feeding and breeding habits. Of particular note, female Weddell seals store resources in the months before giving birth and then essentially stop feeding and remain at the breeding colony for the entire pup-rearing period. By contrast, to successfully rear their chick, emperor penguin parents have to maintain movements between breeding colonies and foraging areas, and these movements were known to have been hampered by the presence of the icebergs [29].
First, our results on vital rates reveal that seals were much less affected than emperor penguins in the short-term. Indeed, Weddell seals did not suffer any apparent cost of survival, while emperor penguin colonies incurred very high adult and chick mortality [29]. Moreover, the magnitude of the decline in reproductive rates was lower for this population of seals than for nearby populations of emperor penguins (e.g. 100% of breeding attempts failed at the Cape Crozier colony in 2001, [29]). The impact on penguins' reproduction was partly linked to the fact that their travelling routes from breeding colonies to foraging areas were partially blocked by the presence of the iceberg [29], making chick feeding much more difficult. Seals probably avoided such a large impact on their reproduction because, as lactating mammals and capital breeders, they are not dependent on such foraging trips to feed their pups. Second, as mature seals were able to survive at high rates during perturbed years, both local abundance and breeding rates returned to previous levels by the end of this episode, and they have remained relatively high since then. We therefore conclude that this iceberg event did not represent an EER for this population, as it does not appear to have exceeded the acclimation abilities of individual seals or to have represented a threat for the persistence of the population at a mid-term time scale.
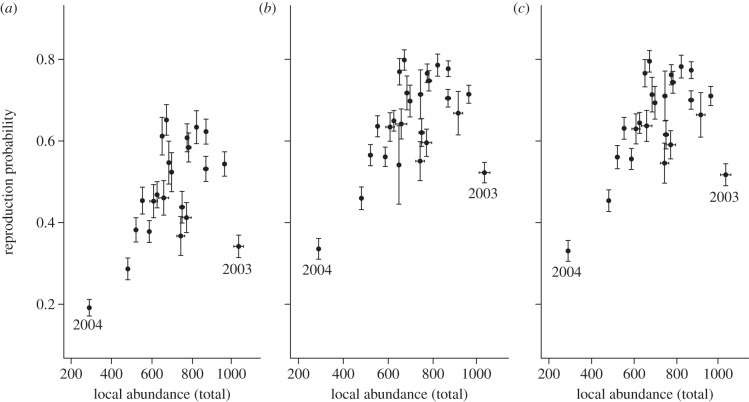
In terms of local abundance, we found a substantial reduction during most iceberg years, but in contrast with our predictions, this pattern was not consistent across the entire period. Indeed, the most extreme positive and negative peaks ever recorded occurred during this event, in 2003 and 2004, respectively. These large fluctuations in colony attendance contrast with the consistent pattern of low reproductive rates within the iceberg period, indicating that these two demographic parameters probably responded to different environmental factors. On the one hand, because Weddell seal females strongly depend on stored energy reserves during the pup-rearing period [48,49], we can hypothesize that the consistent decrease in reproductive rates might have primarily been a consequence of the overall reduction in primary production and prey availability [25,27,30]. On the other hand, it seems that variations in local abundance, and notably the contrast between 2003 and other iceberg years, primarily resulted from changes in sea-ice conditions [35]. Indeed, while most iceberg years were characterized by extensive sea ice, in 2003 open water extended far south, close to Erebus Bay colonies, and sea-ice extent was low (see the electronic supplementary material, figure S1). It is also interesting to note that the high local abundance observed in 2003 was associated with unusually low reproductive rates, a pattern in contrast with the positive correlation observed between these two demographic parameters in all other years (figure 5). Therefore, seals massively attended Erebus colonies in 2003, but not principally to breed. Moreover, the detection rate of skip-breeders, which provides a relevant measure of colonies utilization (see the electronic supplementary material, figure S3) given that resighting effort is high and consistent every year, displays the same temporal pattern as local abundance (see the electronic supplementary material, figure S4). It indicates that skip-breeders have a flexible pattern of colony utilization, depending on current environmental conditions, and clearly shows that such females were much more likely to be in Erebus Bay colonies in 2003 than in other iceberg years. Furthermore, the high local abundance of 2003 was associated with an upsurge in the number of untagged and unknown-age adult females (i.e. females that were born outside the study area), indicating the presence of many temporary immigrants this year (see the electronic supplementary material, figure S5). These observations suggest that environmental conditions might have forced seals to move further south and thus massively attend Erebus Bay in 2003 but not primarily for the purpose of giving birth. This environmental forcing was probably linked to the large extent of open water to the north for the following reasons. First, Erebus Bay, which is the southern-most breeding site for the species, might have been one of the few available places suitable for hauling out in the Ross Sea this year. Second, the reduced sea-ice extent allowed the formation of the McMurdo Polynya, thus providing a relatively abundant source of food near colonies this year. Finally, the distribution of seals' competitors and predators, which cannot occupy areas of consolidated ice, might have been shifted further south in the Ross Sea [50], such that Weddell seals could have moved further south to reduce interspecific competition and predation risk. In contrast, the sea-ice conditions that prevailed in all other iceberg years could have prevented a large number of seals from using Erebus Bay because (i) the access to colonies might have been relatively hard and (ii) sites further north had more food available, enough fast ice for hauling out, and relatively few competitors and predators. This pattern might have been particularly strong in 2004, when both local abundance and reproductive rates reached the lowest values ever recorded. However, although the extent of sea-ice cover was indeed particularly large this year, it was not dramatically different from 2002 or 2005 (see the electronic supplementary material, figure S1). We thus speculate that these physical conditions have acted in combination to particularly unfavourable food conditions [30] to cause such a poor situation in 2004.
Figure 5.
Relationship between local abundance (x-axis) and the three state-specific reproductive rates (y-axis): (a) first-time breeders (ψFE), (b) experienced-breeders (ψEE) and (c) skip-breeders (ψSE). Years 2003 and 2004 are marked on each graph. Error bars represent s.e. (i.e. error due to sampling variation).
Taken together, the results of the present study suggest that Weddell seals were able to handle the challenging conditions created by the iceberg mainly by skipping reproduction and occupying the most favourable available environment, probably balancing between energy expenditure (reproductive effort, availability of haul-out sites), resource intake (proximity of food resources and competitors) and predation risk. An alternative explanation would be that seals massively emigrated from Erebus Bay, except in 2003, to breed elsewhere. However, this appears unlikely, as during all surveys conducted outside the study area at other seal-aggregation sites during iceberg years, almost no females known to have been born in Erebus Bay were found with pups outside the study area despite intensive searches [36]. Moreover, given the unusually high local abundance, but still relatively low level of reproduction, recorded in 2003, such a hypothesis seems untenable. The apparent behavioural response of seals was in keeping with a strategy of saving and allocating a larger proportion of energy to survival, as would be expected in a long-lived organism [9,33]. The ability to buffer adult survival against environmental variability by adjusting reproductive effort has already been shown in this population [44], but previous work did not investigate the particulars of the iceberg event. Here, by explicitly assessing how demography during the iceberg period contrasted with that recorded in years with typical conditions, we demonstrated that the iceberg event had a substantial impact on reproduction, but that survival was well buffered from environmental variation. Two previous studies on Adélie penguins (Pygoscelis adeliae) [32,34] reported similar results, as they showed that this iceberg event induced an increase in foraging effort and of dispersal rates of these birds but had no apparent effect on their survival. It is, however, important to recognize that all these analyses, including ours, are based on a single event, such that their scope of inference remains limited to this particular event, and as a consequence, any extrapolation must be considered with caution.
Although we found a moderate impact, in the short- and mid-term, of this single perturbing event on the seal population, more substantial negative effects might be expected in the long-term, especially as the frequency of such events is predicted to increase [24,25]. First, we found that the average population growth rate dropped from 1.00 during non-iceberg years to 0.96 during iceberg years, meaning that the population was temporarily in a declining phase. A higher frequency of such events would therefore be detrimental for the population in the long run. Second, our results also suggest that the process variance of reproduction and recruitment probabilities might have been increased for a prolonged period after the iceberg onset. Although analyses of data of future years will be required to assess the consistency and potential persistence of this pattern, this is in direct contrast to the long-lasting stability of vital rates that was observed pre-iceberg. It is especially interesting to note the quick rebound, in 2006 and 2007, in the abundance of breeders on colonies and in rates of reproduction and recruitment, after a decreasing trend during the iceberg period. By skipping reproduction or delaying recruitment for several years during the iceberg period, many females avoided a major energy expense. Therefore, when conditions went back to normal after 2005, these ‘resting’ females were likely in a good condition to recruit or reproduce again. The temporary decline in these variables the following year (2008) might indicate that fewer inexperienced females recruited that year, perhaps because many had recruited the previous two years, as well as that many females that produced pups in 2006 and 2007 were unable to compensate for energy expenditures made in those two years and breed again in 2008. After 2008, reproductive rates went back to high values again. This post-iceberg pattern suggests that the delay in reproduction and recruitment of many individuals caused by the perturbation could have shifted the reproductive regime of the population towards greater variation, a pattern that would have negative consequences on the long-term growth rate of the population [31]. These types of regime-shift pattern have indeed been suggested as one of the main consequences of extreme events [12,51,52].
Our understanding of the potential ecological importance of extreme environmental events is still in its infancy but can be expected to rapidly improve, given growing interest in the topic among ecologists [7,8], especially in relation to climate change predictions [5,6]. Extreme events have often been considered as necessarily having immediate and overwhelming effects [14,25,29], but growing evidence suggests that their effects might often be more complicated and more indirect than previously assumed [8]. As illustrated in this study and previous ecological studies on the same iceberg, some events might induce EER in some species but not in others. Moreover, even if they do not cause an immediate EER, extreme events might still have important longer-term consequences through cumulative or recurring effects. The investigation of extreme events is, by definition, relatively difficult, as such events occur rarely in nature, but the growing number of long-term ecological monitoring programmes, as well as the development of experiments directly imposing extreme conditions on a biological system, should allow for improved understanding.
Acknowledgements
We are grateful to J. D. Nichols and 2 anonymous reviewers for helpful comments and to the many individuals who have worked on projects associated with the Erebus Bay Weddell seal population since the 1960s. The project was supported by the National Science Foundation, Division of Polar Programs (grant no. ANT-1141326 to R.A.G., J.J.R. and D. B. Siniff) and prior NSF grants to R.A.G., J.J.R., D. B. Siniff and J. W. Testa. Logistical support for fieldwork in Antarctica was provided by Raytheon Polar Services Company, Antarctic Support Associates, the United States Navy and Air Force, and Petroleum Helicopters Incorporated. Animal handling protocol was approved by Montana State University's Animal Care and Use Committee (Protocol no. 41–05).
References
- 1.Parmesan C., Root T. L., Willig M. R. 2000. Impacts of extreme weather and climate on terrestrial biota. Bull. Am. Meteorol. Soc. 81, 443–450 (doi:10.1175/1520-0477(2000)081<0443:IOEWAC>2.3.CO;2) [DOI] [Google Scholar]
- 2.Gutschick V. P., BassiriRad H. 2010. Biological extreme events: a research framework. Eos (Washington, D.C.) 91, 85–87 [Google Scholar]
- 3.Gaines S. D., Denny M. W. 1993. The largest, smallest, highest, lowest, longest, and shortest: extremes in ecology. Ecology 74, 1677–1692 10.2307/1939926 (doi:10.2307/1939926) [DOI] [Google Scholar]
- 4.Gutschick V. P., BassiriRad H. 2003. Extreme events as shaping physiology, ecology, and evolution of plants: toward a unified definition and evaluation of their consequences. New Phytol. 160, 21–42 10.1046/j.1469-8137.2003.00866.x (doi:10.1046/j.1469-8137.2003.00866.x) [DOI] [PubMed] [Google Scholar]
- 5.Easterling D. R., Meehl G. A., Parmesan C., Changnon S. A., Karl T. R., Mearns L. O. 2000. Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074 10.1126/science.289.5487.2068 (doi:10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 6.Meehl G. A., Zwiers F., Evans J., Knutson T., Mearns L., Whetton P. 2000. Trends in extreme weather and climate events: issues related to modeling extremes in projections of future climate change. Bull. Am. Meteorol. Soc. 81, 427–436 (doi:10.1175/1520-0477(2000)081<0427:TIEWAC>2.3.CO;2) [DOI] [Google Scholar]
- 7.Jentsch A., Kreyling J., Beierkuhnlein C., Jenuch A. 2007. A new generation of climate-change experiments: events, not trends. Front. Ecol. Environ. 5, 365–374 10.1890/1540-9295(2007)5[365:ANGOCE]2.0.CO;2 (doi:10.1890/1540-9295(2007)5[365:ANGOCE]2.0.CO;2) [DOI] [Google Scholar]
- 8.Smith M. D. 2011. The ecological role of climate extremes: current understanding and future prospects. J. Ecol. 99, 651–655 10.1111/j.1365-2745.2011.01833.x (doi:10.1111/j.1365-2745.2011.01833.x) [DOI] [Google Scholar]
- 9.Gaillard J.-M., Yoccoz N. G. 2003. Temporal variation in survival of mammals: a case of environmental canalization? Ecology 84, 3294–3306 10.1890/02-0409 (doi:10.1890/02-0409) [DOI] [Google Scholar]
- 10.Morris W. F., Doak D. F. 2004. Buffering of life histories against environmental stochasticity: accounting for a spurious correlation between the variabilities of vital rates and their contributions to fitness. Am. Nat. 163, 579–590 10.1086/382550 (doi:10.1086/382550) [DOI] [PubMed] [Google Scholar]
- 11.Pfister C. A. 1998. Patterns of variance in stage-structured populations: Evolutionary predictions and ecological implications. Proc. Natl Acad. Sci. USA 95, 213–218 10.1073/pnas.95.1.213 (doi:10.1073/pnas.95.1.213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith M. D. 2011. An ecological perspective on extreme climatic events: a synthetic definition and framework to guide future research. J. Ecol. 99, 656–663 10.1111/j.1365-2745.2011.01798.x (doi:10.1111/j.1365-2745.2011.01798.x) [DOI] [Google Scholar]
- 13.Altwegg R., Roulin A., Kestenholz M., Jenni L. 2006. Demographic effects of extreme winter weather in the barn owl. Oecologia 149, 44–51 10.1007/s00442-006-0430-3 (doi:10.1007/s00442-006-0430-3) [DOI] [PubMed] [Google Scholar]
- 14.Wilson J., Peach W. 2006. Impact of an exceptional winter flood on the population dynamics of bearded tits (Panurus biarmicus). Anim. Conserv. 9, 463–473 10.1111/j.1469-1795.2006.00063.x (doi:10.1111/j.1469-1795.2006.00063.x) [DOI] [Google Scholar]
- 15.Frederiksen M., Daunt F., Harris M. P., Wanless S. 2008. The demographic impact of extreme events: stochastic weather drives survival and population dynamics in a long-lived seabird. J. Anim. Ecol. 77, 1020–1029 10.1111/j.1365-2656.2008.01422.x (doi:10.1111/j.1365-2656.2008.01422.x) [DOI] [PubMed] [Google Scholar]
- 16.Allen C. D., Breshears D. D. 1998. Drought-induced shift of a forest–woodland ecotone: rapid landscape response to climate variation. Proc. Natl Acad. Sci. USA 95, 14 839–14 842 10.1073/pnas.95.25.14839 (doi:10.1073/pnas.95.25.14839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breshears D. D., et al. 2005. Regional vegetation die-off in response to global-change-type drought. Proc. Natl Acad. Sci. USA 102, 15 144–15 148 10.1073/pnas.0505734102 (doi:10.1073/pnas.0505734102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner M. G., Dale V. H. 1998. Comparing large, infrequent disturbances: what have we learned? Ecosystems 1, 493–496 10.1007/s100219900045 (doi:10.1007/s100219900045) [DOI] [Google Scholar]
- 19.Moritz M. A. 1997. Analyzing extreme disturbance events: fire in Los Padres National Forest. Ecol. Appl. 7, 1252–1262 10.1890/1051-0761(1997)007[1252:AEDEFI]2.0.CO;2 (doi:10.1890/1051-0761(1997)007[1252:AEDEFI]2.0.CO;2) [DOI] [Google Scholar]
- 20.Ackerman J. D., Walker L. R., Scatena F. N., Wunderle J. 1991. Ecological Effects of Hurricanes. Bull. Ecol. Soc. Am. 72, 178–180 [Google Scholar]
- 21.Parsons M., McLoughlin C. A., Kotschy K. A., Rogers K. H., Rountree M. W. 2005. The effects of extreme floods on the biophysical heterogeneity of river landscapes. Front. Ecol. Environ. 3, 487–494 10.1890/1540-9295(2005)003[0487:TEOEFO]2.0.CO;2 (doi:10.1890/1540-9295(2005)003[0487:TEOEFO]2.0.CO;2) [DOI] [Google Scholar]
- 22.Hansen B. B., Aanes R., Herfindal I., Kohler J., Sæther B.-E. 2011. Climate, icing, and wild arctic reindeer: past relationships and future prospects. Ecology 92, 1917–1923 10.1890/11-0095.1 (doi:10.1890/11-0095.1) [DOI] [PubMed] [Google Scholar]
- 23.MacAyeal D. R., et al. 2006. Transoceanic wave propagation links iceberg calving margins of Antarctica with storms in tropics and Northern Hemisphere. Geophys. Res. Lett. 33, L17502. 10.1029/2006GL027235 (doi:10.1029/2006GL027235) [DOI] [Google Scholar]
- 24.Scambos T. A., Hulbe C., Fahnestock M., Bohlander J. 2000. The link between climate warming and break-up of ice shelves in the Antarctic Peninsula. J. Glaciol. 46, 516–530 10.3189/172756500781833043 (doi:10.3189/172756500781833043) [DOI] [Google Scholar]
- 25.Arrigo K. R., van Dijken G. L., Ainley D. G., Fahnestock M. A., Markus T. 2002. Ecological impact of a large Antarctic iceberg. Geophys. Res. Lett. 29, 6–9 10.1029/2001GL014160 (doi:10.1029/2001GL014160) [DOI] [Google Scholar]
- 26.MacAyeal D. R., Okal M. H., Thom J. E., Brunt K. M., Kim Y.-J., Bliss A. K. 2008. Tabular iceberg collisions within the coastal regime. J. Glaciol. 54, 371–386 10.3189/002214308784886180 (doi:10.3189/002214308784886180) [DOI] [Google Scholar]
- 27.Seibel B. A., Dierssen H. M. 2003. Cascading trophic impacts of reduced biomass in the Ross Sea, Antarctica: just the tip of the iceberg? Biol. Bull. 205, 93–97 10.2307/1543229 (doi:10.2307/1543229) [DOI] [PubMed] [Google Scholar]
- 28.Arrigo K. R., van Dijken G. L. 2004. Annual changes in sea-ice, chlorophyll a, and primary production in the Ross Sea, Antarctica. Deep Sea Res. II. Top. Stud. Oceanogr. 51, 117–138 10.1016/j.dsr2.2003.04.003 (doi:10.1016/j.dsr2.2003.04.003) [DOI] [Google Scholar]
- 29.Kooyman G. L., Ainley D. G., Ballard G., Ponganis P. J. 2007. Effects of giant icebergs on two emperor penguin colonies in the Ross Sea, Antarctica. Antarctic Sci. 19, 31–38 10.1017/S0954102007000065 (doi:10.1017/S0954102007000065) [DOI] [Google Scholar]
- 30.Thrush S. F., Cummings V. J. 2011. Massive icebergs, alteration in primary food resources and change in benthic communities at Cape Evans, Antarctica. Mar. Ecol. 32, 289–299 10.1111/j.1439-0485.2011.00462.x (doi:10.1111/j.1439-0485.2011.00462.x) [DOI] [Google Scholar]
- 31.Lewontin R. C., Cohen D. 1969. On population growth in a randomly varying environment. Proc. Natl Acad. Sci. USA 62, 1056–1060 10.1073/pnas.62.4.1056 (doi:10.1073/pnas.62.4.1056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dugger K. M., Ainley D. G., Lyver P. O., Barton K., Ballard G. 2010. Survival differences and the effect of environmental instability on breeding dispersal in an Adélie penguin meta-population. Proc. Natl Acad. Sci. USA 107, 12 375–12 380 10.1073/pnas.1000623107 (doi:10.1073/pnas.1000623107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erikstad K. E., Fauchald P., Tveraa T., Steen H. 1998. On the cost of reproduction in long-lived birds: the influence of environmental variability. Ecology 79, 1781–1788 10.1890/0012-9658(1998)079[1781:OTCORI]2.0.CO;2 (doi:10.1890/0012-9658(1998)079[1781:OTCORI]2.0.CO;2) [DOI] [Google Scholar]
- 34.Lescroël A., Dugger K. M., Ballard G., Ainley D. G. 2009. Effects of individual quality, reproductive success and environmental variability on survival of a long-lived seabird. J. Anim. Ecol. 78, 798–806 10.1111/j.1365-2656.2009.01542.x (doi:10.1111/j.1365-2656.2009.01542.x) [DOI] [PubMed] [Google Scholar]
- 35.Cameron M. F., Siniff D. B. 2004. Age-specific survival, abundance, and immigration rates of a Weddell seal (Leptonychotes weddellii) population in McMurdo Sound, Antarctica. Can. J. Zool. 82, 601–615 10.1139/z04-025 (doi:10.1139/z04-025) [DOI] [Google Scholar]
- 36.Hadley G. L., Rotella J. J., Garrott R. A. 2007. Evaluation of reproductive costs for Weddell seals in Erebus Bay, Antarctica. J. Anim. Ecol. 76, 448–458 10.1111/j.1365-2656.2007.01219.x (doi:10.1111/j.1365-2656.2007.01219.x) [DOI] [PubMed] [Google Scholar]
- 37.Hadley G. L., Rotella J. J., Garrott R. A., Nichols J. D. 2006. Variation in probability of first reproduction of Weddell seals. J. Anim. Ecol. 75, 1058–1070 10.1111/j.1365-2656.2006.01118.x (doi:10.1111/j.1365-2656.2006.01118.x) [DOI] [PubMed] [Google Scholar]
- 38.Rotella J. J., Link W. A., Nichols J. D., Hadley G. L., Garrott R. A., Proffitt K. M. 2009. An evaluation of density-dependent and density-independent influences on population growth rates in Weddell seals. Ecology 90, 975–984 10.1890/08-0971.1 (doi:10.1890/08-0971.1) [DOI] [PubMed] [Google Scholar]
- 39.Schwarz C. J., Arnason A. N. 1996. A general methodology for the analysis of capture–recapture experiments in open populations. Biometrics 52, 860–873 10.2307/2533048 (doi:10.2307/2533048) [DOI] [Google Scholar]
- 40.Williams B. K., Nichols J. D., Conroy M. J. 2002. Analysis and management of animal populations. New York, NY: Academic Press [Google Scholar]
- 41.Burnham K. P., Anderson D. R., White G. C., Brownie C., Pollock K. H. 1987. Design and analysis methods for fish survival experiments based on release-recapture, pp. 260–278 Bethesda, MD: American Fisheries Society [Google Scholar]
- 42.Burnham K. P., White G. C. 2002. Evaluation of some random effects methodology applicable to bird ringing data. J. Appl. Stat. 29, 245–264 10.1080/02664760120108755 (doi:10.1080/02664760120108755) [DOI] [Google Scholar]
- 43.Seber G. A. F. 1982. The estimation of animal abundance and related parameters. London, UK: Griffen [Google Scholar]
- 44.Rotella J. J., Link W. A., Chambert T., Stauffer G. E., Garrott R. A. 2012. Evaluating the demographic buffering hypothesis with vital rates estimated for Weddell seals from 30 years of mark–recapture data. J. Anim. Ecol. 81, 162–173 10.1111/j.1365-2656.2011.01902.x (doi:10.1111/j.1365-2656.2011.01902.x) [DOI] [PubMed] [Google Scholar]
- 45.White G. C., Burnham K. P., Anderson D. A. 2001. Advanced features of program MARK. In Proceedings of the second international wildlife management congress (eds Field R., Warren R. J., Okarma H., Sievert P. R.). Bethesda, MD: The Wildlife Society 368–377 [Google Scholar]
- 46.R Development Core Team. 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 47.Caswell H. 2001. Matrix population models: construction, analysis, and interpretation, 2nd edn. Sunderland, MA: Sinauer Associates [Google Scholar]
- 48.Boness D. J., Bowen W. D. 1996. The evolution of maternal care in pinnipeds. BioScience 46, 645–654 10.2307/1312894 (doi:10.2307/1312894) [DOI] [Google Scholar]
- 49.Wheatley K., Bradshaw C., Harcourt R., Hindell M. 2008. Feast or famine: evidence for mixed capital–income breeding strategies in Weddell seals. Oecologia 155, 11–20 10.1007/s00442-007-0888-7 (doi:10.1007/s00442-007-0888-7) [DOI] [PubMed] [Google Scholar]
- 50.Smith W. O., Ainley D. G., Cattaneo-Vietti R. 2007. Trophic interactions within the Ross Sea continental shelf ecosystem. Phil. Trans. R. Soc. B 362, 95–111 10.1098/rstb.2006.1956 (doi:10.1098/rstb.2006.1956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheffer M., Carpenter S. R. 2003. Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol. Evol. 18, 648–656 10.1016/j.tree.2003.09.002 (doi:10.1016/j.tree.2003.09.002) [DOI] [Google Scholar]
- 52.Holmgren M., et al. 2006. Extreme climatic events shape arid and semiarid ecosystems. Front. Ecol. Environ. 4, 87–95 10.1890/1540-9295(2006)004[0087:ECESAA]2.0.CO;2 (doi:10.1890/1540-9295(2006)004[0087:ECESAA]2.0.CO;2) [DOI] [Google Scholar]