Abstract
Failure of organisms to adapt to sudden environmental changes may lead to extinction. The type of mating system, by affecting fertility and the strength of sexual selection, may have a major impact on a population's chances to adapt and survive. Here, we use experimental evolution in bulb mites (Rhizoglyphus robini) to examine the effects of the mating system on population performance under environmental change. We demonstrate that populations in which monogamy was enforced suffered a dramatic fitness decline when evolving at an increased temperature, whereas the negative effects of change in a thermal environment were alleviated in polygamous populations. Strikingly, within 17 generations, all monogamous populations experiencing higher temperature went extinct, whereas all polygamous populations survived. Our results show that the mating system may have dramatic effects on the risk of extinction under environmental change.
Keywords: extinction vortex, environmental stress, sexual selection, fertility, adaptation
1. Introduction
Rapid environmental changes, including those caused by human activity, can result in a mismatch between an organism's physiology and its new environment, potentially leading to population decline and extinction [1,2]. The chances of avoiding extinction will depend on the ability of a population to adapt. However, adaptation may be difficult if environmental stress has a severe effect on demography. Population bottlenecks, caused by unfavourable environmental conditions, may deplete genetic variation necessary for the adaptation process. Moreover, such bottlenecks increase inbreeding, leading to reduced survival and reproductive capacity of individuals associated with inbreeding depression (reviewed by Keller & Waller [3]), hence amplifying negative effects of environmental stress on demography. These synergistic interactions between environmental and genetic factors of extinction, termed ‘extinction vortex’ [4], are further exacerbated by the tendency for inbreeding depression to increase with environmental stress [5].
The type of mating system may affect a population's chances to survive environmental change in several ways. On the one hand, it will affect the opportunity for sexual selection [6]—a process that may enhance the rate of adaptation, provided that better-adapted males win reproductive competitions over maladapted rivals (reviewed by Candolin & Heuschele [7]). Thus, sexual selection should increase the chances of fixation of favourable alleles [8]. Furthermore, sexual selection may increase the effectiveness of selection against deleterious mutations [9–12], which are the main cause of inbreeding depression [13]. Finally, if environmental change adversely affects male fertility, polyandrous matings may benefit females by assuring egg fertilization [14–16]. In a population facing an environmental challenge, this should increase demographic stability, as most or all females capable of producing progeny will be fertilized. Thus, polyandry might prevent populations from entering an extinction vortex.
On the other hand, polygamy decreases effective population size by increasing variance in male reproductive success [17,18]. In consequence, it may increase inbreeding and the risk of beneficial alleles being lost as a result of genetic drift [19,20]. Furthermore, adaptations to sexual competition may trade off with other fitness components [21–23], possibly hindering ecological adaptation and increasing extinction risk [24] (but see [25,26]). Finally, sexual selection may lead to sexual conflict, which in turn may be detrimental to female fitness [27].
Thus, the net effect of mating system on survival prospects of a population subject to environmental stress is not easy to predict and should be addressed with an experimental approach. Experiments investigating the role of mating systems during adaptation to novel environments have given inconsistent results [28–30]. However, as discussed above, influencing the rate of adaptation is one of a number of ways by which mating systems may affect the risk of population extinction under environmental challenge. Crucially, the effects of mating system on population performance under environmental stress severe enough to potentially cause extinction have not, to our knowledge, been studied.
Here, we investigate the impact of mating system on long-term response to increased temperature in replicate lines of the bulb mite Rhizoglyphus robini (Acari: Acaridae), drawn from a laboratory-adapted base population. The study species is characterized by high promiscuity: both sexes mate many times a day, with average time to remating estimated at 80 min. [31]. Females mated to multiple males produce more fecund daughters than those mated to a single male, as a result of genetic correlation between female fecundity and male sperm competitiveness [32]. Apart from sperm competition, sexual selection in this species involves fierce male fights over access to females [33]. Thus, sexual selection acting both pre- and post-copulation may improve fitness of bulb mite populations. Indeed, a polygamous mating system has been shown to help purge inbreeding depression and decrease extinction rate, compared with a monogamous system, in bottlenecked bulb mite populations [34]. On the other hand, sexual conflict has been shown to negatively affect reproductive success of bulb mite females, and the harmful effects of males on females were selected against under enforced monogamy [35]. These harmful effects of polygamy may actually increase the risk of the population being caught into an extinction vortex.
We established selection lines in which we either enforced monogamy or allowed promiscuity. We applied two temperature regimes—lines were maintained either at the increased temperature or at the temperature to which our base colony was adapted. This way, we manipulated the opportunity for sexual selection and conflict (mating system treatment) both among environmentally challenged and control lines (temperature treatment). Moreover, owing to the increased variance in male reproductive success under polygamy on the one hand, and negative effects of thermal stress on mite fertility on the other (see §3b), the effective population sizes (Ne) were likely to differ between treatments. Specifically, we expect that among the lines evolving at control temperature, polygamous ones had lower Ne owing to increased variance in male reproductive success [18]. Conversely, temperature-induced infertility should have a stronger effect on Ne of monogamous lines, because infertility of one individual in a pair would automatically prevent reproduction of the other. Such differences in Ne will be inherently linked to differences between mating systems in nature and are likely to be an important part of the net effect of mating system on the probability of population extinction. We show that, overall, a polygamous mating system helps populations to adapt to increased temperature and prevents their extinction, which severely affect monogamous populations.
2. Material and methods
(a). General procedures
Base populations and larger groups of mites were kept in plastic containers (2.5 cm diameter and 2 cm high). Individually isolated mites and pairs were kept in 0.8 cm diameter glass tubes (2 cm high) with plaster of Paris bases soaked with water. Humidity of greater than 90 per cent was maintained and powdered yeast was supplied ad libitum as food.
(b). Base population
The mites used in the experiment originated from a stock culture combined of two populations adapted to 24°C, one for 12 years (approx. 280 generations) and the other for 3 years (approx. 60 generations). Each of these two populations had been derived from a colony of about 200 individuals found on onions in a garden near Kraków, Poland, in 1998 and 2008, respectively, and had been maintained in the laboratory in large numbers (more than 1000 individuals, subdivided into periodically mixed sub-populations). The two populations had been mixed approximately 10 generations prior to the commencement of our experiment. Such a procedure is expected to increase additive standing genetic variation, which is crucial in experimental evolution.
(c). Selection lines
To test whether mating system affects evolution at increased temperature, we established selection lines in which we either enforced monogamy (M lines, 20 pairs per line) or allowed sexual selection (polygamy, P lines, 20 mites of each sex interacting freely). We applied two temperature regimes (‘selection temperatures’)—lines were maintained either at standard 24°C (controls, C lines) or at 28°C (high temperature, HT lines). Higher temperatures are rarely encountered by bulb mites in nature—soil temperature at the depths where bulb mites are found may occasionally reach 28°C only around midday in summer (M. Klimek 2012, Field Research Station in Łazy, personal communication)—and had not been encountered by our laboratory populations throughout their history of adaptation to 24°C (see §2b). Our pilot study showed that stock population females developing at 28°C had drastically decreased fecundity (mean number of eggs per female ± s.e.: 47.53 ± 5.89) compared with those developing at 24°C (163.87 ± 15.85; t62 = 8.55, p < 0.001), and their progeny (also developing at 28°C) had lower embryonic viability (78 ± 5.8%) than the 24°C controls (98 ± 1.3%; t48 = 3.29, p = 0.002). Thus, increased temperature has considerable negative effects on reproductive fitness in the bulb mite.
In the M-HT treatment, we established six replicate lines, and the remaining three treatments were represented by five replicates each. In P lines, 20 males and 20 females were placed into one container for 5 days, so that intra- as well as inter-sexual selection could operate, whereas in M lines individuals were kept in randomly assigned pairs for 5 days, so that sexual selection was prevented. After this time, all females from each line were moved to a common container to lay eggs. The size of the containers ensured low density of ovipositing females and developing larvae in all the lines, and food was provided ad libitum. When tritonymphs (last larval stage) emerged, about 90 of them were isolated to individual vials. Emerging adults were then sexed, and 20 virgin males and 20 virgin females were put together either in pairs (M lines) or in groups (P lines) in order to obtain the next generation. Such a procedure enables natural selection on survival and fertility in both M and P lines, as larvae with either higher survival or those produced by more fecund females will be over-represented in the group used to start the next generation.
(d). Line extinction
Whenever it was not possible to obtain 20 adults of each sex despite isolating all available tritonymphs, all emerged adults were used to establish the next generation. A line was considered extinct if (i) the total number of individuals surviving to adulthood was less than 5, (ii) no eggs were produced by females or (iii) all eggs failed to hatch.
(e). Female fecundity and fertility assay
The assay was performed after 14 generations of selection. At this stage, only three M-HT lines were available for this experiment, as the other three had gone extinct at generations 11, 12 and 14. Fecundity of females from each of the surviving lines was measured at both temperatures (24°C and 28°C).
Fifteen previously mated females from each selection line were placed in a single container for 3 days of oviposition. After this time, the females were discarded. Half of the eggs were placed at 24°C, and the other half at 28°C (‘test temperatures’). Emerging tritonymphs were isolated to individual vials in order to obtain virgin individuals for the fecundity assay. After reaching maturity, females (10–15 per line per test temperature, 429 in total) were placed individually in vials, and each of them was mated to a randomly assigned male from the same line. After 24 h, the male was discarded and replaced by another male from the same line. The second male was replaced 24 h later with a third male, which stayed with the female for the remaining 72 h until the completion of fecundity assay. The purpose of sequential mating with three males was to ensure that individual male effects on female fecundity were minimized: bulb mites do not lay unfertilized eggs, so male sterility would prevent a female from laying eggs if she did not have a chance to remate (however, mating with one fertile partner is sufficient to ensure egg fertility for about one week).
After 5 days of constant access to males, the number of eggs laid by each female was scored. The number of females that had not laid any eggs was also recorded for each line.
Bulb mite females oviposit continuously for about two to three weeks at a nearly constant rate; hence, the number of eggs laid during the first 5 days of oviposition is a good estimate of lifetime fecundity [36].
Fecundity of fertile females was analysed in Statistica, using a nested ANOVA with mating system, selection temperature and test temperature as fixed factors, and line as a random factor nested in the mating system × selection temperature interaction.
Proportion of fertile females was analysed in R [37], using a generalized linear model with binomial errors (numbers of fertile and infertile females in each line constituted a response variable, whereas mating system, selection temperature and test temperature were explanatory variables).
(f). Trans-generation effects
Although mites used for the fecundity and fertility assay were reared in common environments since the egg stage, their parents developed and mated at their own selection temperatures. The reason for this was that progressing extinction of M-HT lines prompted us to perform the assays without a delay, to make sure that the three surviving M-HT lines were still available. In order to assess the potential trans-generation effects of temperature and mating system on female fecundity and fertility at the stressful environment, we performed a separate experiment.
We placed groups of 50–100 previously mated stock females in common containers for oviposition. We then assigned each container with developing eggs either to the 24°C or to the 28°C treatment. Emerging tritonymphs were isolated individually to obtain virgin adults for the parental generation. In the polygamy treatment, 10 males and 10 females were placed into a single container, such that sexual selection could operate, whereas in the monogamy treatment, males and females were kept in pairs. In both treatments, mites interacted for 5 days. After this period, females from each treatment were placed in fresh containers to lay eggs. Two days later, all eggs were placed to develop at 28°C. Emerging tritonymphs were isolated individually to obtain virgin adults for the fecundity and fertility assay. In this assay, virgin females were placed individually in vials at 28°C, and each was sequentially mated to two randomly assigned males from the same treatment (males were replaced after 24 h; the second male stayed with the female until the completion of the assay). After 5 days of constant access to males, eggs laid by each female were counted. A proportion of fertile females and their fecundity were analysed using logit model and ANOVA, respectively. Significant influence of parental temperature and mating system conditions on female fecundity and fertility would indicate that trans-generation effects of these environmental variables could have also affected female reproductive performance in our main experiment (see §2e).
(g). Data accessibility
The data (Excel) are deposited in DRYAD (doi:10.5061/dryad.3s2q0).
3. Results
(a). Female fecundity
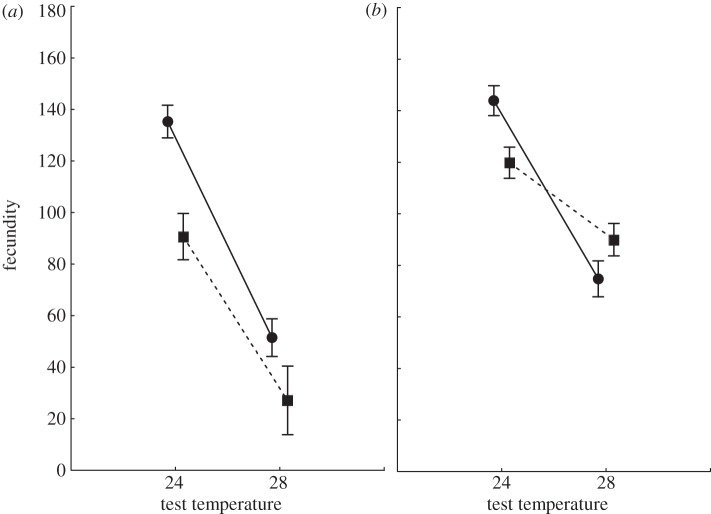
We found significant selection temperature × test temperature and mating system × test temperature interaction effects on the number of eggs laid by fertile females (table 1). Given significant interactions, we further analysed the effects of selection temperature and test temperature separately for both the M and P treatments to look for the evidence of thermal adaptation under different mating systems. M-HT lines were less fecund than M-C lines at both temperatures (F1,76 = 6.8, p = 0.032), and both types of M lines had decreased fecundity at 28°C (F1,113 = 76.3, p < 0.001; interaction: F1,113 = 1.4, p = 0.232; figure 1a). In contrast, in polygamous lines, there was a significant selection temperature × test temperature interaction: compared with C lines, HT lines were more fecund at 28°C but less fecund at 24°C (selection temperature: F1,82 = 0.3, p = 0.591; test temperature: F1,185 = 55.2, p < 0.001; interaction: F1,185 = 8.7, p = 0.004; figure 1b). The significant interaction indicates that adaptation to increased temperature had occurred in polygamous lines.
Table 1.
Effects of mating system, selection temperature and test temperature on female fecundity analysed using a nested ANOVA (mating system, selection temperature and test temperature are fixed factors, line ID is a random factor in the model; line ID × test temperature interaction was removed from the model based on p = 0.299).
| effect | d.f. | F | p |
|---|---|---|---|
| mating system | 1,18.5 | 17.149 | <0.001 |
| selection temperature | 1,18.5 | 6.707 | 0.018 |
| test temperature | 1,298 | 120.733 | <0.001 |
| mating system × selection temperature | 1,18.7 | 4.069 | 0.058 |
| line (mating system × selection temperature) | 14,298 | 1.933 | 0.023 |
| mating system × test temperature | 1,298 | 4.665 | 0.032 |
| selection temperature × test temperature | 1,298 | 7.057 | 0.008 |
| mating system × selection temperature × test temperature | 1,298 | 0.715 | 0.398 |
| error | 298 | — | — |
Figure 1.
Mean (±s.e.) number of eggs laid by females in a 5-day fecundity assay in (a) monogamous and (b) polygamous lines at 24°C and 28°C after 14 generations of experimental evolution. Circles denote control lines, squares denote high-temperature lines.
(b). Female fertility
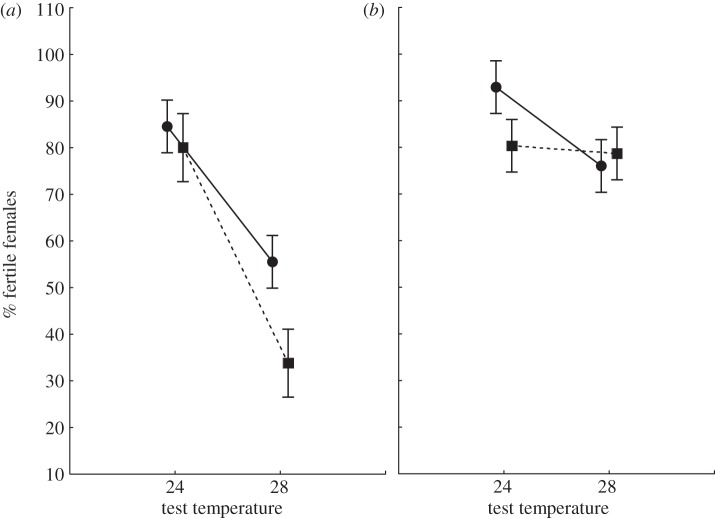
A number of females assayed failed to lay eggs. There was a significant mating system × selection temperature × test temperature interaction effect on the proportion of fertile females (p = 0.033; table 2 and figure 2). Both M-C and M-HT lines had lower fertility rates at 28°C (p < 0.001; figure 2a). In polygamous lines, however, there was a significant selection temperature × test temperature interaction: polyandrous control (P-C) lines, but not P-HT lines, showed decreased fertility at 28°C (p = 0.048; figure 2b).
Table 2.
Effects of mating system, selection temperature and test temperature on female fertility analysed using a generalized linear model with binomial errors.
| effect | d.f. | deviance | residual d.f. | residual deviance | p |
|---|---|---|---|---|---|
| null | 35 | 95.25 | |||
| mating system | 1 | 18.87 | 34 | 76.39 | 0 |
| selection temperature | 1 | 3.78 | 33 | 72.60 | 0.052 |
| test temperature | 1 | 25.25 | 32 | 47.35 | 0 |
| mating system × selection temperature | 1 | 0.61 | 31 | 46.74 | 0.435 |
| mating system × test temperature | 1 | 4.61 | 30 | 42.14 | 0.032 |
| selection temperature × test temperature | 1 | 0.44 | 29 | 41.70 | 0.509 |
| mating system × selection temperature × test temperature | 1 | 4.57 | 28 | 37.14 | 0.033 |
Figure 2.
Mean (±s.e.) proportion of fertile females in (a) monogamous and (b) polygamous lines after 14 generations of experimental evolution. Circles denote control lines, squares denote high-temperature lines.
(c). Line extinction
By generation 17, three more M-HT lines went extinct (following the three lines extinct prior to the fecundity and fertility assay), resulting in 100 per cent of M-HT lines being extinct, compared with no extinctions in the remaining treatments. Thus, mating system significantly affected extinction probability in HT lines (Fisher's exact test, p = 0.002).
(d). Trans-generation effects
Poor performance and extinction of M-HT lines could have resulted from their genetic deterioration or from the detrimental effects of thermal stress carried over to the next generation via parental effects. In an additional experiment testing for such effects, we found that the proportions of fertile matings did not differ between progenies of parents reared at different temperatures and mating systems: they were 0.84, 0.85, 0.71 and 0.84 for polygamous 28°C, monogamous 28°C, polygamous 24°C and monogamous 24°C treatments, respectively (parental rearing temperature: Wald's t = 0.64, p = 0.425; mating system: Wald's t = 0.85, p = 0.355; interaction: Wald's t = 0.53, p = 0.466). Similarly, we have found no significant effect of parents' rearing temperature on daughter fecundity (F1,86 = 0.1, p = 0.78; means ± s.e.: polygamy at 28°C, 56.52 ± 5.29; monogamy at 28°C, 46.00 ± 4.06; polygamy at 24°C, 54.00 ± 7.24; monogamy at 28°C, 45.54 ± 4.78). The interaction of parents' rearing temperature × mating system was also non-significant (F1,86 = 0.04, p = 0.849). The effect of parents' mating system was marginally non-significant (F1,86 = 3.1, p = 0.081).
4. Discussion
Our data consistently show that mating system strongly affected population performance under a novel selection pressure. At the beginning of the experiment, both monogamous and polygamous populations in the HT treatment faced a high risk of entering an extinction vortex [4,38] where stress-reduced female fecundity and embryonic viability potentially decrease the effective population size, hampering adaptation and increasing inbreeding, generation after generation. Indeed, our data provide a spectacular example of an extinction vortex in progress: by generation 14, half of the monogamous high-temperature (M-HT) populations were extinct, and over 60 per cent of females in the surviving M-HT lines were sterile (figure 2), which was a much higher percentage compared with 15 per cent at the second generation of monogamy exposed to 28°C, as estimated in our trans-generation effects experiment. An additional experiment (see the electronic supplementary material) showed that both sexes in M-HT lines were equally likely to be sterile.
This decline in fertility can be explained by progressing inbreeding, as predicted by the extinction vortex scenario. Indeed, the next extinctions of M-HT lines followed closely, and none of the M-HT lines survived to generation 18. By contrast, all polygamous high-temperature (P-HT) populations survived, and their higher fecundity at 28°C compared with the P-C lines indicates that they have been adapting to elevated temperature.
Neither the higher extinction rate of the M-HT lines, nor their lower fertility and fecundity could be attributed to trans-generation effects of increased temperature. An additional experiment, designed to address this possibility, showed that fertility of progeny reared at 28°C and produced by parents exposed to 28°C was over 80 per cent, irrespective of the mating system. Such low infertility levels could not on their own lead to extinction. Importantly, parental environment effects on fertility and fecundity were not significant and also did not interact with mating system. A marginally non-significantly higher fecundity of daughters of polyandrous females replicated earlier results, which demonstrated that this effect was due to genetic, rather than maternal, effects of polyandry [32,36].
Previous work showed that in very small populations (n = 10 individuals), polygamy helps to purge inbreeding depression, decreasing the chances of extinction due to exposure of deleterious mutations under inbreeding: 27 per cent of polygamous versus 49 per cent of monogamous populations went extinct over eight generations [34]. Here, the populations were larger (N = 40), and hence the expected effective population sizes at the onset of the experiment (i.e. before temperature- and inbreeding-induced sterility began to reduce them) were Ne = N = 40 for monogamy and Ne = 8N/(5.3 + 2 + 4) = 28.3 for polygamy (assuming random amount of variance in female reproductive success and variance in male reproductive success equal to 5.3, as estimated by Radwan et al. [39]). Earlier experimental evolution studies have shown that populations with Ne of similar [40] or even lower size [41] can respond to manipulation of mating system. On the basis of effective population sizes in the present study, the inbreeding coefficient after 10 generations (i.e. just before extinctions occurred) under unchanged environment could be estimated at 0.12 and 0.17 for monogamy and polygamy, respectively. This level of inbreeding was much lower than that at population size N = 10 [34] (0.5 and 0.7 at generation 10 for monogamy and polygamy, respectively) and did not on its own cause extinctions in either monogamous (M-C) or polygamous (P-C) control populations. However, under environmental challenge, the dramatic effects of mating system on population survival were revealed.
One way by which polygamy could positively affect population survival under stress is by alleviating harmful effects of inbreeding, and thus mitigating the risk of entering extinction vortex. Sexual selection has been demonstrated to increase the efficacy of purging inbreeding depression [34], whereas environmental stress tends to magnify deleterious effects of inbreeding [5]. Therefore, sexual selection could allow more efficient selection against alleles’ increasing sensitivity to stress when homozygous. Additionally, stress-induced increase in proportion of sterile individuals will have a smaller impact on the effective population size under polygamy, because such a mating system augments the chances that all fertile individuals actually reproduce. This effect could, to some extent, counterbalance the lower Ne of polygamous populations resulting from their higher variance in male reproductive success.
The difference in extinction probability between M and P high-temperature lines may also be due to the effects of sexual selection [7,12], where males carrying alleles beneficial in the novel environment achieve the highest reproductive success, therefore increasing the rate of adaptation [42] (but see [43]). Our results suggest that in contrast to monogamous lines, polygamous lines adapted to increased temperature, as indicated by significant test temperature × selection temperature interactions (figures 1 and 2). Adaptation must have increased the resilience of polygamous populations to thermal stress, thus preventing them from entering the extinction vortex. To date, the only study that looked at thermal adaptation in the context of sexual selection failed to find any effects of mating system [30]. However, the level of polygamy imposed in that experiment was the lowest possible (two mating partners), as was the number of replicate lines. Furthermore, the level of stress imposed by increased temperature in that study was probably lower than in ours, as no cases of extinction were reported.
In conclusion, we have provided the first (to our knowledge) empirical test of the effects of mating system on population extinction risk in the face of environmental challenge. We show that these effects may indeed be dramatic: in our experiment, all monogamous lines went extinct within only 17 generations of thermal stress, whereas the populations where sexual selection was operating have survived and adapted. These results may have important implications for assessing the vulnerability of animal species endangered by human-induced environmental changes.
Acknowledgements
We thank Joe Tomkins (University of Western Australia), Łukasz Michalczyk (University of East Anglia), Göran Arnqvist (University of Uppsala), William Rice (University of California, Santa Barbara), Eoin Duffy (Jagiellonian University) and two anonymous reviewers for their comments on earlier versions of the manuscript, Magda Jarzębowska for assistance in laboratory work, and Mariusz Klimek for information on soil temperatures. The project was supported by grant no. N303 529438 from the Polish Ministry of Science and Higher Education, and by the Foundation for Polish Science (professor subsidy no. 9/2008 to J.R).
References
- 1.Burger R., Lynch M. 1995. Evolution and extinction in a changing environment: a quantitative-genetic analysis. Evolution 49, 151–163 10.2307/2410301 (doi:10.2307/2410301) [DOI] [PubMed] [Google Scholar]
- 2.Chown S. L., Hoffmann A. A., Kristensen T. N., Angilletta M. J., Jr, Stenseth N. C., Pertoldi C. 2010. Adapting to climate change: a perspective from evolutionary physiology. Clim. Res. 43, 3–15 10.3354/cr00879 (doi:10.3354/cr00879) [DOI] [Google Scholar]
- 3.Keller L. F., Waller M. 2002. Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241 10.1016/S0169-5347(02)02489-8 (doi:10.1016/S0169-5347(02)02489-8) [DOI] [Google Scholar]
- 4.Gilpin M. E., Soulé M. E. 1986. Minimum viable populations: processes of extinction. In Conservation biology: the science of scarcity and diversity (ed. Soulé M. E.), pp. 19–34 Sunderland, MA: Sinauer Associates [Google Scholar]
- 5.Fox C. W., Reed D. H. 2011. Inbreeding depression increases with environmental stress: an experimental study and meta-analysis. Evolution 61, 246–258 10.1111/j.1558-5646.2010.01108.x (doi:10.1111/j.1558-5646.2010.01108.x) [DOI] [PubMed] [Google Scholar]
- 6.Shuster S. M., Wade M. J. 2003. Mating systems and strategies. Princeton, NJ: Princeton University Press [Google Scholar]
- 7.Candolin U., Heuschele J. 2008. Is sexual selection beneficial during adaptation to environmental change? Trends Ecol. Evol. 23, 446–452 10.1016/j.tree.2008.04.008 (doi:10.1016/j.tree.2008.04.008) [DOI] [PubMed] [Google Scholar]
- 8.Lorch P. D., Proulx S., Rowe L., Day T. 2003. Condition-dependent sexual selection can accelerate adaptation. Evol. Ecol. Res. 5, 867–881 [Google Scholar]
- 9.Siller S. 2001. Sexual selection and the maintenance of sex. Nature 411, 689–692 10.1038/35079578 (doi:10.1038/35079578) [DOI] [PubMed] [Google Scholar]
- 10.Agrawal A. F. 2001. Sexual selection and the maintenance of sexual reproduction. Nature 411, 692–695 10.1038/35079590 (doi:10.1038/35079590) [DOI] [PubMed] [Google Scholar]
- 11.Radwan J. 2004. Effectiveness of sexual selection in removing mutations induced with ionizing radiation. Ecol. Lett. 7, 1149–1154 10.1111/j.1461-0248.2004.00681.x (doi:10.1111/j.1461-0248.2004.00681.x) [DOI] [Google Scholar]
- 12.Whitlock M. C., Agrawal A. F. 2009. Purging the genome with sexual selection: reducing mutation load through selection on males. Evolution 63, 569–582 10.1111/j.1558-5646.2008.00558.x (doi:10.1111/j.1558-5646.2008.00558.x) [DOI] [PubMed] [Google Scholar]
- 13.Charlesworths D., Willis J. H. 2009. The genetics of inbreeding depression. Nat. Rev. Genet. 10, 783–796 10.1038/nrg2664 (doi:10.1038/nrg2664) [DOI] [PubMed] [Google Scholar]
- 14.Arnqvist G., Nilsson T. 2000. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 60, 145–164 10.1006/anbe.2000.1446 (doi:10.1006/anbe.2000.1446) [DOI] [PubMed] [Google Scholar]
- 15.Michalczyk Ł., Martin O. Y., Millard A. L., Emerson B. E., Gage M. J. G. 2010. Inbreeding depresses sperm competitiveness, but not fertilization or mating success in male Tribolium castaneum. Proc. R. Soc. B 277, 3483–3491 10.1098/rspb.2010.0514 (doi:10.1098/rspb.2010.0514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalczyk Ł., Millard A. L., Martin O. Y., Lumley A. J., Emerson B. C., Chapman T., Gage M. J. G. 2011. Inbreeding promotes female promiscuity. Science 333, 1739–1742 10.1126/science.1207314 (doi:10.1126/science.1207314) [DOI] [PubMed] [Google Scholar]
- 17.Falconer D. S. 1989. Introduction to quantitative genetics, 4th edn. Harlow, UK: Longman [Google Scholar]
- 18.Snook R. R., Brüstle L., Slate J. 2009. A test and review of the role of effective population size on experimental sexual selection patterns. Evolution 63, 1923–1933 10.1111/j.1558-5646.2009.00682.x (doi:10.1111/j.1558-5646.2009.00682.x) [DOI] [PubMed] [Google Scholar]
- 19.Kimura M., Ohta T. 1971. Theoretical aspects of population genetics. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 20.Hedrick P. W. 2005. Genetics of populations, 3rd edn Sudbury, MA: Jones and Bartlett [Google Scholar]
- 21.Brooks N. 2000. Negative genetic correlation between male sexual attractiveness and survival. Nature 406, 67–70 10.1038/35017552 (doi:10.1038/35017552) [DOI] [PubMed] [Google Scholar]
- 22.Radwan J. 2008. Maintenance of genetic variation in sexual ornaments: a review of the mechanisms. Genetica 134, 113–127 10.1007/s10709-007-9203-0 (doi:10.1007/s10709-007-9203-0) [DOI] [PubMed] [Google Scholar]
- 23.Bilde T., Foged D., Schilling N., Arnqvist G. 2009. Postmating sexual selection favours males that sire offspring with low fitness. Science 324, 1705–1706 10.1126/science.1171675 (doi:10.1126/science.1171675) [DOI] [PubMed] [Google Scholar]
- 24.Doherty P. F., Jr, Sorci G., Royle J. A., Hines J. E., Nichols J. D., Boulinier T. 2003. Sexual selection affects local extinction and turnover in bird communities. Proc. Natl Acad. Sci. USA 100, 5858–5862 10.1073/pnas.0836953100 (doi:10.1073/pnas.0836953100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrow E. H., Pitcher T. E. 2003. Sexual selection and the risk of extinction in birds. Proc. R. Soc. Lond. B 270, 1793–1799 10.1098/rspb.2003.2441 (doi:10.1098/rspb.2003.2441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrow E. H., Fricke C. 2004. Sexual selection and the risk of extinction in mammals. Proc. R. Soc. Lond. B 271, 2395–2401 10.1098/rspb.2004.2888 (doi:10.1098/rspb.2004.2888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michalczyk Ł., Millard A. L., Martin O. Y., Lumley A. J., Emerson B. E., Gage M. J. G. 2011. Experimental evolution exposes female and male responses to sexual selection and conflict in Tribolium castaneum. Evolution 65, 713–724 10.1111/j.1558-5646.2010.01174.x (doi:10.1111/j.1558-5646.2010.01174.x) [DOI] [PubMed] [Google Scholar]
- 28.Fricke C., Arnqvist G. 2007. Rapid adaptation to a novel host in a seed beetle (Callosobruchus maculatus): the role of sexual selection. Evolution 61, 440–454 10.1111/j.1558-5646.2007.00038.x (doi:10.1111/j.1558-5646.2007.00038.x) [DOI] [PubMed] [Google Scholar]
- 29.Rundle H. D., Chenoweth S. F., Blows M. W. 2006. The roles of natural and sexual selection during adaptation to a novel environment. Evolution 60, 2218–2225 [PubMed] [Google Scholar]
- 30.Holland B. 2002. Sexual selection fails to promote adaptation to a new environment. Evolution 56, 721–730 [DOI] [PubMed] [Google Scholar]
- 31.Radwan J., Siva Jothy M. T. 1996. The function of post-insemination mate association in the bulb mite, Rhizoglyphus robini. Anim. Behav. 52, 651–657 10.1006/anbe.1996.0209 (doi:10.1006/anbe.1996.0209) [DOI] [Google Scholar]
- 32.Kozielska M., Krzeminska A., Radwan J. 2004. Good genes and the maternal effects of polyandry on offspring reproductive success in the bulb mite. Proc. R. Soc. Lond. B 271, 165–170 10.1098/rspb.2003.2585 (doi:10.1098/rspb.2003.2585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radwan J., Czyz M., Konior M., Kołodziejczyk M. 2000. Aggressiveness in two male morphs of the bulb mite Rhizoglyphus robini. Ethology 106, 53–62 10.1046/j.1439-0310.2000.00498.x (doi:10.1046/j.1439-0310.2000.00498.x) [DOI] [Google Scholar]
- 34.Jarzębowska M., Radwan J. 2010. Sexual selection counteracts extinction of small populations of bulb mites. Evolution 64, 1283–1289 [DOI] [PubMed] [Google Scholar]
- 35.Tilszer M., Antoszczyk K., Sałek N., Zajac E., Radwan J. 2006. Evolution under relaxed sexual conflict in the bulb mite Rhizoglyphus robini. Evolution 60, 1868–1873 [DOI] [PubMed] [Google Scholar]
- 36.Konior M., Radwan J., Kołodziejczyk M. 2001. Polyandry increases offspring fecundity in the bulb mite. Evolution 55, 1893–1896 [DOI] [PubMed] [Google Scholar]
- 37.R Development Core Team. 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/) [Google Scholar]
- 38.Frankham R. 2005. Genetics and extinction. Biol. Conserv. 126, 131–140 10.1016/j.biocon.2005.05.002 (doi:10.1016/j.biocon.2005.05.002) [DOI] [Google Scholar]
- 39.Radwan J., Urung J., Śnigórska K., Gawrońska K. 2004. Effectiveness of sexual selection in preventing fitness deterioration in bulb mite populations under relaxed natural selection. J. Evol. Biol. 17, 94–99 10.1046/j.1420-9101.2003.00646.x (doi:10.1046/j.1420-9101.2003.00646.x) [DOI] [PubMed] [Google Scholar]
- 40.Martin O. Y., Hosken D. J. 2004. Reproductive consequences of population divergence through sexual conflict. Curr. Biol. 14, 906–910 10.1016/j.cub.2004.04.043 (doi:10.1016/j.cub.2004.04.043) [DOI] [PubMed] [Google Scholar]
- 41.Hosken D. J., Ward P. I. 2001. Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 4, 10–13 10.1046/j.1461-0248.2001.00198.x (doi:10.1046/j.1461-0248.2001.00198.x) [DOI] [Google Scholar]
- 42.Dolgin E. S., Whitlock M. C., Agrawal A. F. 2006. Male Drosophila melanogaster have higher mating success when adapted to their thermal environment. J. Evol. Biol. 19, 1894–1900 10.1111/j.1420-9101.2006.01168.x (doi:10.1111/j.1420-9101.2006.01168.x) [DOI] [PubMed] [Google Scholar]
- 43.Correia L., Yeaman S., Whitlock M. C. 2010. Local adaptation does not always predict high mating success. J. Evol. Biol. 23, 875–878 10.1111/j.1420-9101.2010.01957.x (doi:10.1111/j.1420-9101.2010.01957.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data (Excel) are deposited in DRYAD (doi:10.5061/dryad.3s2q0).