Abstract
Body size is a major factor constraining the trophic structure and functioning of ecological communities. Food webs are known to respond to changes in basal resource abundance, and climate change can initiate compounding bottom-up effects on food-web structure through altered resource availability and quality. However, the effects of climate and co-occurring global changes, such as nitrogen deposition, on the density and size relationships between resources and consumers are unknown, particularly in host–parasitoid food webs, where size structuring is less apparent. We use a Bayesian modelling approach to explore the role of consumer and resource density and body size on host–parasitoid food webs assembled from a field experiment with factorial warming and nitrogen treatments. We show that the treatments increased resource (host) availability and quality (size), leading to measureable changes in parasitoid feeding behaviour. Parasitoids interacted less evenly within their host range and increasingly focused on abundant and high-quality (i.e. larger) hosts. In summary, we present evidence that climate-mediated bottom-up effects can significantly alter food-web structure through both density- and trait-mediated effects.
Keywords: herbivore, global warming, predator-prey, temperature, top-down control
1. Introduction
Body size is a fundamental trait that characterizes species and individuals. Many other characteristics of species, such as growth rates, bioenergetic needs, dispersal, longevity and population densities, are strongly related to body size [1]. There is growing recognition that body size can constrain who will interact with (e.g. eat) whom, and consequently that allometric relationships play an important role in population dynamics and in determining food-web structure [1–3].
Recent studies have suggested that general rules related to morphological, metabolic or foraging constraints—many of which are closely correlated with body size—can capture the complexity of feeding interaction networks [4,5]. Animal consumers are often considerably larger than their prey, and size structuring is most apparent when organisms are gape-limited [6]. Yet, at the other end of the spectrum, parasites and pathogens are usually much smaller than their resources [7]. Nevertheless, rules relating to size structuring have been proposed to be potentially applicable to predator–prey interactions in general [8], irrespective of size differences.
Insect parasitoids complete their larval development feeding on a single host individual, and therefore represent a distinct feeding class. They are often similar in size to their insect hosts [9], and thus lie between the extremes described earlier. Therefore, if there are general rules that apply to predator–prey dynamics, these must adequately describe the role of body-size relationships in host–parasitoid food webs. Understanding the factors governing host–parasitoid interactions is important both because of the ubiquity of these interactions in nature, and because of the widespread use of parasitoids in biological pest control [10].
Previous research has shown some size structuring of host–parasitoid interactions, with parasitoid individuals scaling to the body lengths of their individual hosts [9]. However, it has also been shown that plant resource quality can mediate interactions involving plants, herbivores and their parasitoids [11], by producing larger herbivore hosts, which can provide a better quality resource [12] and thus be preferred [13]. Consequently, recent studies have shown that bottom-up changes to host and parasitoid body size contributed, alongside density-mediated effects, to overall changes in food-web structure [14,15]. In addition to size-related host preferences, parasitoid body size may affect dispersal and search ability, whereas host body size can be inversely correlated with abundance [3], and these two factors may affect encounter rates and food-web structure [15].
Climate warming is known to alter herbivore population growth [16] in addition to affecting individual body size [17] and metabolic process rates [18], and these changes may combine to affect food-web interactions through a variety of pathways [19]. Similarly, nitrogen deposition is known to alter plant quality, with cascading effects on herbivore individual fitness and population growth [13]. Despite the important implications of these changes for interaction dynamics, empirical evidence on the effects of raising temperatures on host–parasitoid systems, particularly with regard to their size structuring, is currently lacking. Moreover, it is important to understand how co-occurring global change drivers [20], such as nitrogen deposition, may combine with temperature to alter basal resources and food-web structure [21].
In this study, we use an artificial field warming experiment with factorial temperature and nitrogen treatments to test their effects on prey and consumer density and body-size relationships using a grassland caterpillar-parasitoid system. Elevated temperature and nitrogen could be expected to increase herbivore density and also lead to higher body size of some hosts [20]. Such changes would augment community-wide species differences in host availability and quality (size). Thus, we hypothesized that parasitoid interactions would shift towards more abundant hosts—reflecting a density effect—and with larger hosts being increasingly favoured. Provided that the activation energy of attack is low enough, consumers in warmer environments are predicted to spend more time searching and less time handling their prey [18]. Therefore, connectance would be expected to increase with warming [18], but feeding links would become quantitatively oriented towards fewer, stronger pairwise interactions, thereby altering the interaction structure and decreasing the quantitative complexity of the food web as a whole.
2. Material and methods
(a). Study site and experimental set-up
We set up an artificial warming experiment adjacent to the University of Canterbury field station at Cass in the Waimakariri River catchment, South Island of New Zealand.
The experiment comprised a 2 × 2 factorial design, with warming and nitrogen as treatments with two levels each (control and elevated) and five true replicates per treatment combination, totalling 20 plots of 3.5 m length and width (12.25 m2).
We planted well-established individuals of four species of tussock grasses that were common to the area and typical of New Zealand's subalpine grasslands [22]. We used consistent composition and layout for each plot, and designed their relative abundance to mimic their natural occurrence (50 × Poa cita, 50 × Festuca novae-zelandiae, 12 × Chionochloa rigida and 12 × Chionochloa flavecens per plot). This resulted in each plot being planted with 124 individual plants, amounting to 2480 tussocks in total. Overall plant composition at the time of sampling did not differ significantly between treatments (data not shown), reflecting the relatively young age of the experiment and the dominance of the standardized planting over other vegetation that is slowly colonizing the plots. Nevertheless, to exclude any bottom-up effects of newly colonized plant species, we sampled only planted tussock plants for herbivores. In contrast, plant biomass was greater in the plots under high nitrogen, both at low and elevated temperature (see the electronic supplementary material, appendix A, for plant biomass data).
For the warming treatment, we used underground heating cables to maintain a difference of 3°C above the ambient temperature. Underground cables have the disadvantage that they may not significantly warm the above-ground components of the ecosystem, such that any warming effects observed here are likely to be plant-mediated. However, all warming methods have limitations; for example, it is unclear whether infrared radiation would affect insect host-plant choice, and open-top chambers could limit dispersal into and among the plots. The nitrogen treatment consisted of a fertilization regime amounting to a total of 50 kg ha−1 yr−1. Both the temperature and nitrogen treatments are within the global change projections for the next century [23]. We started sampling a full year after establishment of the experiment, and continued sampling at monthly intervals for a total of 11 rounds. Sampling entailed visually searching for caterpillars on tussock plants and teasing apart the dense vegetation to find any hidden larvae. See the electronic supplementary material, appendix B for details on the experimental set-up and sampling.
(b). Insect identification and body-size measures
To allow collection of parasitoids, we individually reared all Lepidoptera larvae to maturity (resulting in either the emergence of the adult moth or a parasitoid if the caterpillar was parasitized). All emerged parasitoids were identified to species level where possible, and to morphospecies for organisms lacking a recognized classification. We sought the expertise of two taxonomists to help with the identification: John S. Dugdale confirmed the lepidopteran ID, helped with developing a larval key and identified all the Tachinid flies. Jo Berry validated hymenopteran morphospecies and formally identified all known species.
We excluded from analyses all caterpillars that died during rearing. Successful rearing allowed the identification of 983 herbivores (27 Lepidoptera species) and 333 interactions with 21 parasitoid species (10 Hymenoptera and 11 Diptera). We weighed all caterpillars directly after collection and any parasitoids at emergence. For practical reasons, we used the average adult weight of each species in our models (see the electronic supplementary material, appendix C).
(c). Data analysis
(i). Community composition
As a first step to identify changes to community structure, we tested the effect of temperature and nitrogen on herbivore and parasitoid community composition. A substantial shift in herbivore composition would influence the ability of parasitoids to interact with particular hosts, and could therefore affect the role of body size (e.g. if the herbivore community showed a remarkably different size distribution under different treatments) and host abundance. Conversely, shifts in parasitoid composition (e.g. if a subset of parasitoid species became dominant under the elevated treatments) could also generate changes in the architecture of interactions, and potentially override changes in host selection and size structuring within species.
To account for such community changes, we tested herbivore and parasitoid community composition (pooled data over time) using permutational distance multivariate ANOVA carried out with the Primer v. 6 software and the PERMANOVA package [24,25]. While ANOVA/MANOVA assumes normal distributions and, implicitly, Euclidean distance between samples, PERMANOVA works with any distance measure that is appropriate to the data, and uses permutations to avoid the assumption of normally distributed data. We used two different distance measures, one accounting for species composition and abundance (modified Gower base 10) and one focusing on species presence–absence (Jaccard dissimilarity). In these analyses, we used herbivore or parasitoid composition as response variables, predicted by warming, nitrogen addition and their interaction as fixed factors.
(ii). General linear models and generalized linear mixed models
We carried out univariate analyses using R v. 2.12.0 [26]. In addition to the composition tests mentioned earlier, we tested how the total and relative abundance of species changed under elevated temperature and nitrogen. We tested total abundance of both hosts and parasitoids as total insect counts per plot (pooled over time), and this was predicted by warming and nitrogen in a generalized linear model with a Poisson error structure and log link function. To test abundance changes within species, we used generalized linear mixed effects models [27] in the lme4 package [28] in R. These models were the same as those for abundance above, but included species identity as a random effect to test for changes within each species. Together, these two tests allowed us to discern whether there was an overall difference in herbivore abundance and, if so, whether these differences were caused by a similar response across species, or if the total abundance was driven by a subset of species showing a particularly strong increase.
To verify the overall size structuring in our system, we tested how parasitoid size responded to herbivore size in a linear mixed model, which included interaction, herbivore and parasitoid identities as crossed (i.e. non-nested) random effects to account for species- and pairwise-interaction-specific variation that may mask the overall correlation between host and parasitoid size [27].
(iii). Body size and parasitism change metric
To test whether increases in body size of host larvae under the treatments led to higher attack rates (i.e. preferential choice by parasitoids), we examined whether any changes in body size were associated with a change in parasitism as follows: (i) for each species, we calculated average body size in the control (C) and under each treatment combination (T), and calculated a size change metric S = (T − C)/C; (ii) for each species, we then obtained total parasitism rates for control plots (P) and each treatment (Q); (iii) we used the same change metric in (i) to calculate a comparable change in parasitism rate for each host, e.g. R = (Q − P)/P; and (iv) used a linear regression of R against S. If R increases with S, it would suggest that those hosts experiencing the largest relative increases in body size also attracted the greatest increase in the number of attacks by parasitoids.
(iv). Model construction
Our Bayesian approach to modelling interaction counts (defined as the number of parasitism events between pairs of parasitoid and host species) uses models common to regression analysis, and for simplicity, we assume that interaction counts are Poisson-distributed. As with most large food webs, the data display overdispersion with large numbers of zero values (missing interactions), and so a zero-inflated Poisson (ZIP) model is appropriate [29]. In a ZIP model, two generalized linear models are used to explain the data: a logit part for the binary presence–absence of an interaction, and a Poisson part for its magnitude (i.e. frequency). The Poisson part gives an indication of the preference (or strength) for an interaction once the encounter has taken place. More formally, the number of interactions between host i and parasitoid j is given by a set of explanatory variables xij:
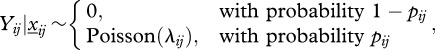 |
2.1 |
where λij is the expected value of a Poisson distribution. The probability of an interaction being present is modelled using a logistic regression:
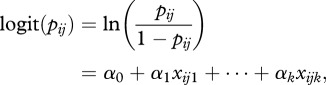 |
2.2 |
with regression parameters αk. The expected value for the Poisson distribution is given by
| 2.3 |
with regression parameters βk. Any combination of explanatory variables (ecological covariates) can be specified independently for the logit and Poisson parts. In this study, the logistic part always contained an intercept and parameter associated with host density; the Poisson part contained an intercept and one of eight combinations of parameters associated with six ecological covariates: host density, parasitoid density, host body size, parasitoid body size, site nitrogen treatment and site temperature treatment. The eight models are listed in table 1, and details and sample R code for running the ZIP are included in the electronic supplementary material, appendices D and E.
Table 1.
DIC values for eight ZIP models across three data-grouping resolutions. The logistic part always contained an intercept and parameter associated with host density; the Poisson part contained an intercept and one of eight combinations of parameters associated with six ecological covariates: host density (HD), parasitoid density (PD), host body size (HBS), parasitoid body size (PBS), site nitrogen content (N) and site temperature (T) (see the electronic supplementary material, appendix D). Models with lower DIC are preferred to models with higher DIC, and numerical comparisons should only be made within columns (best-fit model in bold). Twenty food web replicates were evenly divided into four treatment classifications: control nitrogen and control temperature; control nitrogen and elevated temperature; elevated nitrogen and control temperature; and elevated nitrogen and elevated temperature. The food-web data were studied at three resolutions—coarse, medium and fine—according to how the food web replicates were grouped (see main text). Some models were not applicable to certain groupings (denoted by X). Simulations were run in R using the R2jags package that interfaces with jags 2.2.0 with 50 000 iterations after a burn-in of 50 000 iterations, three chains, thinning = 20. Convergence was assessed using the Gelman–Rubin diagnostic function [30] provided in Jags v. 2.2.0.
| model | coarse |
medium |
fine |
||||||
|---|---|---|---|---|---|---|---|---|---|
| all | control (N) | N | control (T) | T | control | temperature | nitrogen | temp. + N | |
| HD PD | 944.8 | 434.1 | 526.1 | 498.9 | 466.5 | 201.7 | 244.6 | 298.1 | 231.5 |
| HD PD N | 943.3 | X | X | 498.9 | 469.1 | 203.6 | 246.0 | 299.5 | 233.0 |
| HD PD T | 940.8 | 428.1 | 526.5 | X | X | 205.0 | 246.1 | 299.6 | 228.0 |
| HD PD N T | 938.8 | X | X | X | X | 205.2 | 247.3 | 299.2 | 230.7 |
| HD PD HBS PBS | 935.7 | 432.0 | 519.6 | 498.5 | 464.7 | 204.5 | 243.8 | 300.8 | 223.6 |
| HD PD HBS PBS N | 935.1 | X | X | 496.5 | 472.6 | 207.1 | 244.7 | 303.0 | 224.5 |
| HD PD HBS PBS T | 932.2 | 443.6 | 521.5 | X | X | 206.9 | 246.3 | 302.2 | 218.5 |
| HD PD HBS PBS N T | 932.0 | X | X | X | X | 207.8 | 248.0 | 303.7 | 219.6 |
In order to remain conservative in our analysis, we set uninformative priors for each of the regression parameters (αk and βk): specifically, normal distributions with extremely large variance [31,32]. As is commonly performed, Markov chain Monte Carlo (MCMC) runs were used to sample from the full posterior distributions for all parameters [32,33]. All simulations were run in R using the R2jags package [34] that interfaces with Jags v. 2.2.0 with the following settings: 50 000 iterations after a burn-in of 50 000 iterations, three chains, thinning = 20. Convergence was assessed using the Gelman–Rubin diagnostic function [35] provided in Jags v. 2.2.0.
We used the deviance information criterion (DIC) as measure of model fit, because it is easily calculated from the samples generated by an MCMC simulation [36]. DIC is a generalization of the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) and, as with AIC and BIC, it penalizes more complex models [37].
Assessed within a given model, each posterior distribution indicates the extent to which its associated covariate explains the presence of an interaction (logit part) or contributes to the magnitude of the interaction (Poisson part). If the credible interval of a posterior distribution contains zero, then its associated covariate explains little of the empirical data. The width of the distribution denotes the confidence one can have in a parameter estimate (mean, median or mode) given the empirical data: a wide distribution indicates greater uncertainty in its value. Very often, if the same analysis is run using a frequentist approach and a Bayesian approach, the mean (or median, or mode depending on the prior used) of the resulting posterior distribution is very close to the single frequentist estimator [31,32].
ZIP model posterior distributions were used to measure the influence of the various ecological covariates on pairwise interaction count under different experimental treatments involving temperature and nitrogen content. The 20 food-web replicates were evenly divided into four treatment classifications: control nitrogen and control temperature; control nitrogen and temperature treatment; nitrogen treatment and control temperature; and nitrogen treatment and temperature treatment. The food-web data were studied at three resolutions: coarse, medium and fine. At the coarse resolution, all replicate webs were grouped together; at the medium resolution, replicate webs were grouped by nitrogen treatment and grouped by temperature treatment; and at the fine resolution, replicate webs were grouped by nitrogen and temperature, leading to four treatment combinations. No single resolution is inherently better or more informative than another: the resolutions account for a trade-off between more data but less environmental-discriminatory power. At the coarse resolution, we obtained well-resolved behaviour for each ecological covariate over all the data. Yet as a consequence, we cannot comment on how the influence of host or parasitoid density might change under the treatments. To do so, one must partition the data, as we have carried out for the fine resolution, but this comes at the expense of incorporating fewer data into the analytical models.
The basic effect of an ecological covariate on interaction count can be determined from the sign and magnitude of its associated parameter estimate (the corresponding posterior distribution) within a grouping. The effect of a treatment on interaction count can be determined by comparing parameter estimates between groupings at the same resolution. We incorporated average host body size for each species into the model in two ways: (i) across all individuals in all treatments; and (ii) across all individuals within each treatment combination (control, nitrogen only, warming only, and nitrogen and warming). The use of these two approaches allowed us to compare the effect of treatment-induced changes in host body size brought about by the altered relative abundance of smaller and larger species (i) with the effect of changes in body size of individuals within species (ii).
3. Results
(a). Community composition
Herbivore and parasitoid species composition did not differ significantly under the temperature- and nitrogen-deposition treatments. Specifically, we found no treatment effect on overall composition, including either the relative abundance of species (F1,19 < 1.60, p > 0.1 for both herbivores and parasitoids) or only the presence–absence (composition) of species (F1,19 < 1.24, p > 0.1 in both cases). In contrast, we found that total herbivore abundance increased strongly under warming (parameter estimate = 0.46, Z = 4.56, p < 0.0001) and nitrogen treatments (parameter estimate = 0.36, Z = 3.28, p = 0.001), with a sub-additive effect of the treatments (warming × nitrogen interaction term = −0.39, Z = −2.86, p = 0.004, note: parameter estimates for Poisson models are log-linked). We found a similar result when using abundance data per species instead of total abundance. Temperature and nitrogen had a strong positive effect (parameter estimate = 0.44, Z = 4.33, p < 0.0001 and 0.29, Z = 2.74, p = 0.006, respectively), and their effect was sub-additive (interaction term = −0.38, Z = −2.78, p = 0.005).
(b). Body-size and density effects
Overall, we found that host and parasitoid body masses were strongly correlated with each other (parameter estimate < 0.02 t = 2.82, p = 0.005), which suggests that some general size structuring is present in the relationship between host and parasitoid species within the community. We explored this further using the Bayesian analysis.
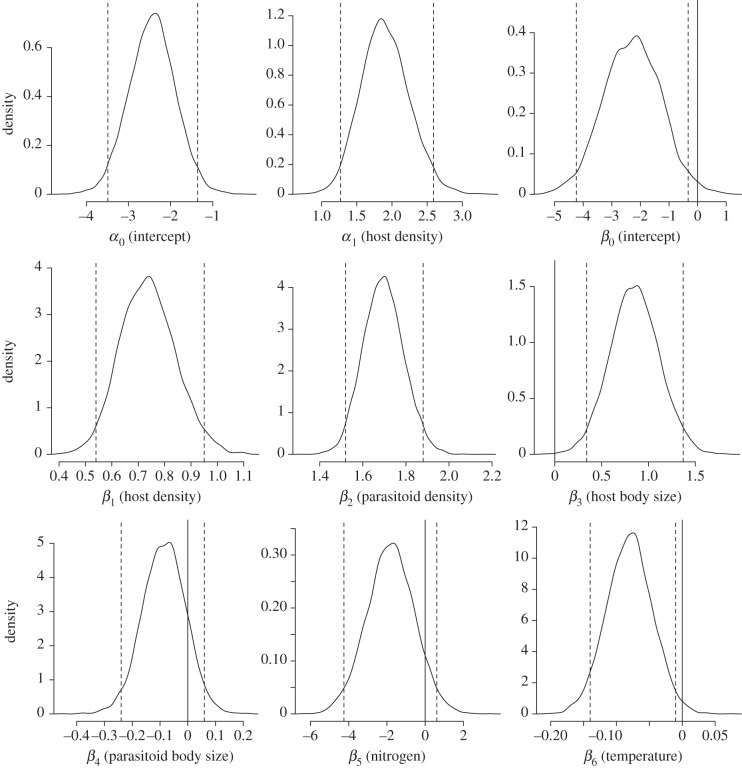
At the coarse resolution with all data grouped together, pairwise interaction count (the number of parasitism events between pairs of parasitoid and host species) depended on both host and parasitoid density and body size. Furthermore, we found that elevated temperature or nitrogen had a negative effect on host–parasitoid interaction counts (table 1 and figure 1).
Figure 1.
Posterior distributions for the most complex ZIP model at the coarse resolution. α0 and β0 are intercepts in the logistic and Poisson parts, respectively. The other parameters are associated with ecological covariates and follow the nomenclature given in equations (2.1)–(2.3). Positive parameter values indicate a positive contribution to interaction count. Vertical dashed lines indicate Bayesian credible intervals (95% level), and distributions including zero (vertical solid line) are considered less significant.
At the medium resolution, elevated temperature in isolation had a negative effect on pairwise interaction count; however, the temperature treatment did not have any further effect in combination with nitrogen. Similarly, the nitrogen treatment had a negative effect on interaction counts at control temperature, but not at elevated temperature (figure 2). Additionally, we found that both host density and parasitoid density better explained the incidence of interaction counts under elevated temperature or nitrogen. We also found that increasing temperature or nitrogen led to larger host species being attacked more often.
Figure 2.
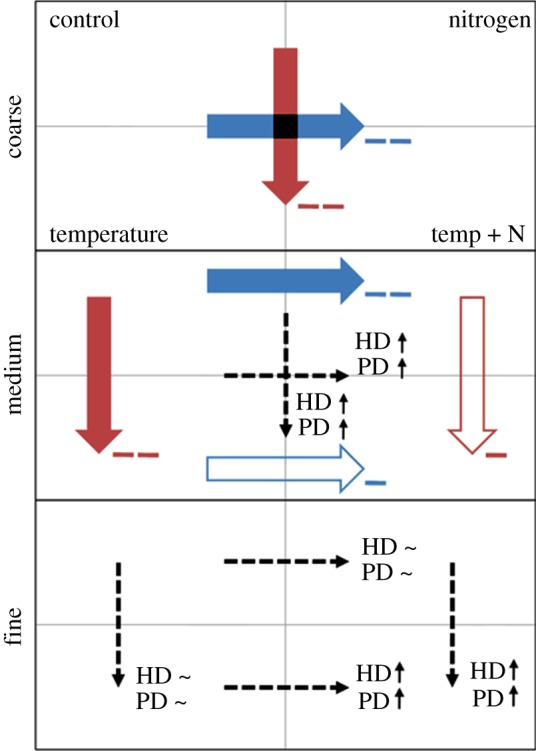
Effect of temperature (red arrows) and nitrogen (blue arrows) treatments on interaction count at coarse, medium and fine resolutions. Subpanels represent treatment combinations (control, top left; nitrogen, top right; temperature, bottom left; and temperature and nitrogen, bottom right). Thick arrows reference within-grouping behaviour of posterior distribution sign in response to nitrogen and temperature; for example, at medium resolution the effect of the nitrogen treatment is more strongly negative (− −) on interaction count when temperatures are low compared with when temperatures are high (−). Dashed arrows reference between-grouping changes in host density (HD) and parasitoid density (PD) posterior distribution magnitude (up-arrows indicate increasing influence, tilde indicate non-significant influence); for example, at fine resolution when temperatures are low, the influence on interaction count attributable to host and parasitoid density does not change as nitrogen levels are increased; however, when temperatures are higher, the influence of host and parasitoid density are increasingly positive as nitrogen levels rise.
At the fine resolution, within treatment combinations, nitrogen or temperature treatments had no detectable effect on interaction count, nor did they significantly alter the effect of host or parasitoid density on interaction count. However, at simultaneously elevated levels of both treatments, the influence of both host density and parasitoid density on interaction count became increasingly positive (table 2 and figure 2).
Table 2.
Parameter estimates (mean value of posterior distributions) for zero-inflated Poisson model explaining interaction counts at fine resolution. Parameters are consistent with the definition given in equations (2.1)–(2.3), and those in bold are statistically significant. Values in parentheses are s.d. of the parameter estimate; values in brackets are 95% credible intervals. Bayesian posteriors were calculated as in table 1. The model includes seven parameters (α0 and α1 are omitted for clarity) and is run separately for each combination of treatments.
| parameter | control | temp. | nitrogen | temp. + N |
|---|---|---|---|---|
| intercept: β0 | −6.81 (1.51) [−9.99, −4.01] | −6.74 (1.40) [−9.56, −4.05] | −6.11 (1.09) [-8.31, −4.07] | −4.77 (1.32) [−7.48, −2.28] |
| host density: β1 | 1.21 (0.15) [0.91, 1.53] | 1.17 (0.14) [0.91, 1.46] | 1.26 (0.17) [0.92, 1.61] | 1.43 (0.14) [1.15, 1.72] |
| parasitoid density: β2 | 1.64 (0.22) [1.24, 2.09] | 1.57 (0.17) [1.25, 1.94] | 1.54 (0.16) [1.23, 1.88] | 1.79 (0.18) [1.45, 2.17] |
| host body size: β3 | 0.36 (0.48) [−0.53, 1.36] | 0.61 (0.36) [−0.07, 1.36] | 0.31 (0.27) [−0.21, 0.87] | 1.27 (0.40) [0.52, 2.11] |
| parasitoid body size: β4 | −0.21 (0.18) [−0.58, 0.14] | −0.21 (0.17) [−0.55, 0.10] | −0.06 (0.12) [−0.32, 0.18] | 0.02 (0.16) [−0.30, 0.33] |
Although the trend for parasitoids to prefer larger host species was not significant when each treatment was applied individually, it became significant when both treatments were applied together (table 2 and figure 3). Results were qualitatively similar whether we used host body-size averages calculated across all treatments (the entire dataset, figure 3a), or averaged separately within each treatment combination (figure 3b). In both cases, the model indicated a strong effect of per-unit change in herbivore size on interaction count; thus, the interaction shift towards larger hosts was determined by changes in parasitoid choice rather than an effect owing to changes in the size composition of hosts.
Figure 3.
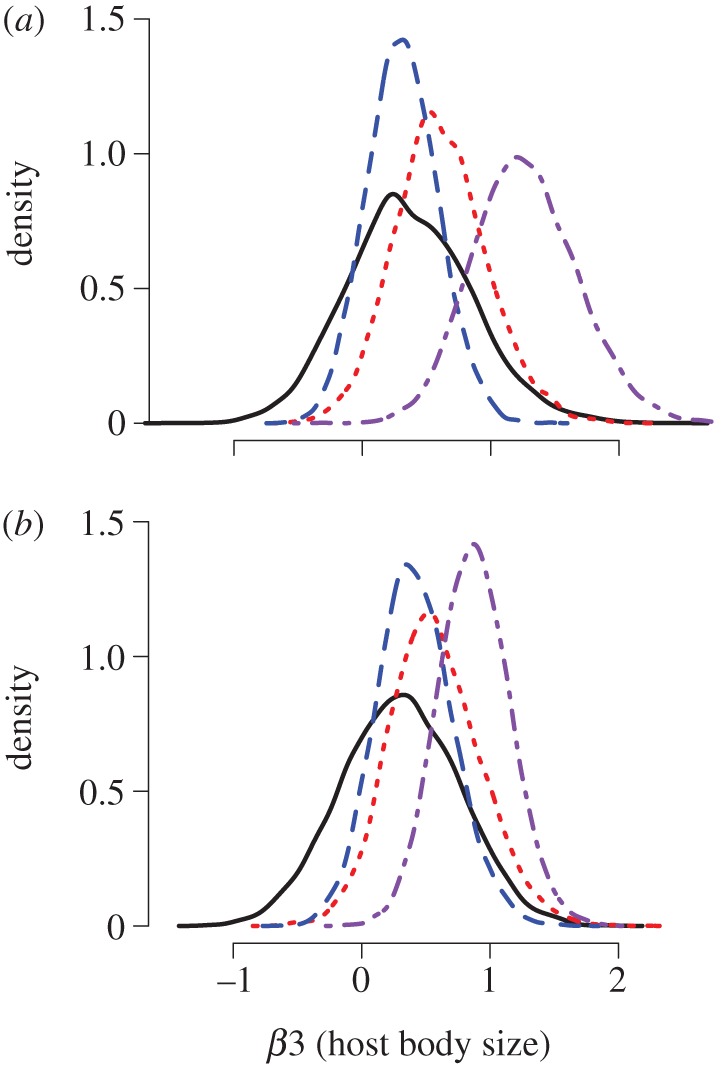
Influence of host body size on interaction count at fine resolution. (a) Host body size uses average values across all treatments, and (b) host body size uses four treatment-specific sets of point estimates. Both figures show posterior distributions of the parameter associated with host body size under four treatments: control (solid line, black); control nitrogen and elevated temperature (dotted line, red); elevated nitrogen and control temperature (dashed line, blue); and elevated nitrogen and elevated temperature (dot-dashed line, purple). Host body size positively influences interaction count most strongly when nitrogen and temperature treatments are applied together. Note that the x-axis scale is parameter value, and does not represent a biological measurement.
(c). Parasitism rates
We found that the increased host body size in the treatments relative to the control was associated with an increase in parasitism rates for each species (linear model: parameter estimate = 0.64, t = 2.20, p = 0.047), supporting the results from the Bayesian analysis. Therefore, to further investigate the role of host body size, we looked at parasitism at the individual-species level. In the control treatment, we found that the two most abundant host species, Persectania aversa and Tmetolophota unica, attracted the most parasitism events (they accounted for 76% of the available host abundance and together yielded 86% of parasitoid interactions)—a pattern that primarily reflects density effects. Under elevated temperature and nitrogen, these two species increased in abundance, although to a comparatively lesser extent than other host species. However, their share of parasitism became disproportionately high: under simultaneously elevated temperature and nitrogen, these two species combined attracted almost the same fraction of interactions despite making up 13 percentage points less of the available resources (63% of the available host abundance and together garnering 84% of parasitoid interactions). Interestingly, this increase in proportional parasitism was accompanied by a 52 per cent (P. aversa) and 41 per cent (T. unica) increase in average size.
4. Discussion
We demonstrated that climate warming and nitrogen deposition can have significant effects on a size structured host–parasitoid food web. We found a shift towards interactions involving more abundant host species, which was associated with elevated temperature or nitrogen and evidenced by an increasing influence of both host and parasitoid density on the presence and frequency of interactions in those treatments. These results are consistent with the increased unevenness of host–parasitoid interactions observed when certain hosts become more abundant (and disproportionately attacked) following land-use intensification [38].
Parasitoids also favoured larger hosts under elevated temperature and nitrogen treatments, and displayed less variability in their use of larger hosts than under control conditions. Nitrogen and temperature are known to generally favour herbivore individual and population growth [16,39], and it is conceivable that the increase in host availability and quality allowed parasitoids to be more selective in their host use, favouring those that grew larger and were more abundant. In this issue, Henri et al. [30] found a hyperparasitoid niche to be strongly biased towards larger host species, and they concluded that climate driven changes in foraging behaviour could impact the structure of the indirect interaction network of individual hosts. Our results support and expand these findings to the community level.
Warmer temperatures or increased nitrogen levels led parasitoids to become more selective of larger-bodied or abundant hosts. At the warmest temperatures or highest nitrogen levels within the treatment set, a greater number of host species met this requirement. Thus, although the parasitoids became more selective in their host use, under certain conditions their feeding behaviour aggregated such that they fed more generally—interacting prominently with the more available set of abundant and larger (more attractive) host species.
We found that herbivore abundance was strongly affected by the treatments, and previous work on the same system has shown that changes in plant biomass and composition can play a primary role in mediating herbivore responses to global environmental changes [40]. Grasslands are known to respond rapidly and strongly to changes in abiotic conditions, and previous studies have shown that plant biomass and richness measures can have contrasting effects on insect herbivores [41]. However, in our experiment, initial plant richness and composition were standardized (and did not change significantly during the course of this study), suggesting that changes in herbivores were mainly due to changes in plant biomass (see the electronic supplementary material, appendix A, for details on plant biomass). In contrast, we found that, overall, parasitoids did not respond as strongly as herbivores to any of the treatments.
Community composition of both herbivores and natural enemies (parasitoids) was not significantly affected by elevated temperatures or nitrogen deposition. However, total host abundance was higher under all treatments relative to the control, and this effect was consistent across species. Thus, this system proved well-suited for disentangling the effects of host body size and density on community structure, without the confounding issue of communities differing significantly in their species composition (potentially shifting communities towards a subset of heavier or lighter species and thereby altering the available set of pairwise interactions), which would render the role of body size more difficult to interpret.
Consistent with our hypothesis, temperature and nitrogen impacted host–parasitoid food-web structure by altering the response of parasitoid species to host density and size structure. In particular, we observed a shift of interactions towards abundant and heavier host species, perhaps reflecting the elevated starvation risk of high-level consumers under elevated temperature and nutrient enrichment [42]. Optimal foraging theory suggests that food-web interactions depend on the body sizes of predators and prey [43], and we found that this size-dependence (and the likely foraging benefit of specializing on certain hosts) is altered by global environmental changes. Thus, our observations primarily suggest that food webs will increasingly become characterized by fewer, stronger links between relatively abundant species, likely resulting in a decrease in web complexity, with unclear consequences for stability [44].
While host body size was coupled with changes in species abundance under all treatments, its effect on interaction structure emerged clearly only under the combined effect of temperature and nitrogen. This host-selective behaviour could bear important implications for the occurrence and evolution of host–parasitoid interactions under climate change: if parasitoids become consistently able to choose ‘ideal’ hosts in a warmer world, it is likely that this would lead to increased parasitoid fitness in the generations to come, which could counteract the negative effect of the treatments on parasitism rates, and potentially generate strong selection pressures against preferred host species. In the face of global change, bottom-up effects of temperature and nitrogen on plant and insect resource quality, which we found to affect body-size preferences, are likely to have strong impacts on ectotherm community structure and the ongoing coevolution of parasitoids and their hosts.
Acknowledgments
We thank Rebecca Jackson, Michael Bartlett and Kirsty Trotter for their help in the field and in the laboratory. We are extremely grateful to J. Berry and J. S. Dugdale for help with insect identification. Jenny Ladley, Andrew Winther and John Hunt helped with the set up of the artificial warming experiment. Stefano Allesina helped with Bayesian model development. C.D.S. is supported by a University of Canterbury Doctoral Scholarship and a Hellaby Trust Fellowship. P.P.A.S. is supported by NSF SMA no. 1042164, and J.M.T. is funded by a Rutherford Discovery Fellowship, administered by the Royal Society of New Zealand. This research was funded by the Marsden Fund (UOC-0705) and the Miss E. L. Hellaby Indigenous Grassland Research Trust.
References
- 1.Weitz J. S., Levin S. A. 2006. Size and scaling of predator–prey dynamics. Ecol. Lett. 9, 548–557 10.1111/j.1461-0248.2006.00900.x (doi:10.1111/j.1461-0248.2006.00900.x) [DOI] [PubMed] [Google Scholar]
- 2.Emmerson M. C., Raffaelli D. 2004. Predator–prey body size, interaction strength and the stability of a real food web. J. Anim. Ecol. 73, 399–409 10.1111/j.0021-8790.2004.00818.x (doi:10.1111/j.0021-8790.2004.00818.x) [DOI] [Google Scholar]
- 3.Woodward G., Ebenman B., Emmerson M., Montoya J. M., Olesen J. M., Valido A., Warren P. H. 2005. Body size in ecological networks. Trends Ecol. Evol. 20, 402–409 10.1016/j.tree.2005.04.005 (doi:10.1016/j.tree.2005.04.005) [DOI] [PubMed] [Google Scholar]
- 4.Petchey O. L., Beckerman A. P., Riede J. O., Warren P. H. 2008. Size, foraging, and food web structure. Proc. Natl Acad. Sci. USA 105, 4191–4196 10.1073/pnas.0710672105 (doi:10.1073/pnas.0710672105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams R., Martinez N. D. 2000. Simple rules yield complex food webs. Nature 404, 180–183 10.1038/35004572 (doi:10.1038/35004572) [DOI] [PubMed] [Google Scholar]
- 6.Woodward G., Warren P. H. 2007. Body size and predatory interactions infreshwaters: scaling from individuals to communities. In Body size: the structure and function of aquatic ecosystems (eds Hildrew A. G., Raffaelli D., Edmonds-Brown R.), pp. 98–117 Cambridge, UK: Cambridge University Press [Google Scholar]
- 7.Memmott J., Martinez N. D., Cohen J. E. 2000. Predators, parasitoids and pathogens: species richness, trophic generality and body sizes in a natural food web. J. Anim. Ecol. 69, 1–15 10.1046/j.1365-2656.2000.00367.x (doi:10.1046/j.1365-2656.2000.00367.x) [DOI] [Google Scholar]
- 8.Brose U., et al. 2006. Consumer–resource body-size relationships in natural food webs. Ecology 87, 2411–2417 10.1890/0012-9658(2006)87[2411:CBRINF]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[2411:CBRINF]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 9.Cohen J. E., Jonsson T., Muller C. B., Godfray H. C. J., Savage V. M. 2005. Body sizes of hosts and parasitoids in individual feeding relationships. Proc. Natl Acad. Sci. USA 102, 684–689 10.1073/pnas.0408780102 (doi:10.1073/pnas.0408780102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfray H. C. J. 1994. Parasitoid, behavioral and evolutionary ecology. Princeton, NJ: Princeton University Press [Google Scholar]
- 11.Harvey J. A., van Dam N. M., Gols R. 2003. Interactions over four trophic levels: foodplant quality affects development of a hyperparasitoid as mediated through a herbivore and its primary parasitoid. J. Anim. Ecol. 72, 520–531 10.1046/j.1365-2656.2003.00722.x (doi:10.1046/j.1365-2656.2003.00722.x) [DOI] [Google Scholar]
- 12.Mackauer M., Michaud J. P., Volkl W. 1996. Host choice by aphidiid parasitoids (Hymenoptera: Aphidiidae): host recognition, host quality, and host value. Can. Entomol. 128, 959–980 10.4039/Ent128959-6 (doi:10.4039/Ent128959-6) [DOI] [Google Scholar]
- 13.Haddad N. M., Haarstad J., Tilman D. 2000. The effects of long-term nitrogen loading on grassland insect communities. Oecologia 124, 73–84 10.1007/s004420050026 (doi:10.1007/s004420050026) [DOI] [PubMed] [Google Scholar]
- 14.Bukovinszky T., van Veen F. J. F., Jongema Y., Dicke M. 2008. Direct and indirect effects of resource quality on food web structure. Science 319, 804–807 10.1126/science.1148310 (doi:10.1126/science.1148310) [DOI] [PubMed] [Google Scholar]
- 15.Laliberté E., Tylianakis J. M. 2010. Deforestation homogenizes tropical parasitoid–host networks. Ecology 91, 1740–1747 10.1890/09-1328.1 (doi:10.1890/09-1328.1) [DOI] [PubMed] [Google Scholar]
- 16.Bale J. S., et al. 2002. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob. Change Biol. 8, 1–16 10.1046/j.1365-2486.2002.00451.x (doi:10.1046/j.1365-2486.2002.00451.x) [DOI] [Google Scholar]
- 17.Awmack C. S., Harrington R., Lindroth R. L. 2004. Aphid individual performance may not predict population responses to elevated CO2 or O3. Glob. Change Biol. 10, 1414–1423 10.1111/j.1365-2486.2004.00800.x (doi:10.1111/j.1365-2486.2004.00800.x) [DOI] [Google Scholar]
- 18.Petchey O. L., Brose U., Rall B. C. 2010. Predicting the effects of temperature on food web connectance. Phil. Trans. R. Soc. B 365, 2081–2091 10.1098/rstb.2010.0011 (doi:10.1098/rstb.2010.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brose U., Dunne J. A., Montoya J. M., Petchey O. L., Schneider F. D., Jacob U. 2012. Climate change in size-structured ecosystems. Phil. Trans. R. Soc. B 367, 2903–2912 10.1098/rstb.2012.0232 (doi:10.1098/rstb.2012.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tylianakis J. M., Didham R. K., Bascompte J., Wardle D. A. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363 10.1111/j.1461-0248.2008.01250.x (doi:10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 21.Shurin J. B., Clasen J. L., Grieg H. S., Kratina P., Thompson P. L. 2012. Warming shifts top-down and bottom-up control of pond food web structure and function. Phil. Trans. R. Soc. B 367, 3008–3017 10.1098/rstb.2012.0243 (doi:10.1098/rstb.2012.0243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espie P. R., Barratt B. I. P. 2006. Biodiversity of indigenous tussock grassland sites in Otago, Canterbury and the central North Island of New Zealand IV. Vegetation and the effect of disturbance by agricultural development and fire. J. R. Soc. New Zealand 36, 69–82 10.1080/03014223.2006.9517801 (doi:10.1080/03014223.2006.9517801) [DOI] [Google Scholar]
- 23.Millenium Ecosystem Assesment (MEA) 2005. Ecosystems and human wellbeing: scenarios. Washington, DC: Island Press [Google Scholar]
- 24.Clarke R., Gorley R. N. 2006. PRIMER v6: user manual/tutorial. Plymouth, UK: PRIMER-E [Google Scholar]
- 25.Anderson M. J., Gorley R. N., Clarke R. 2008. PERMANOVA+ for PRIMER: guide to software and statistical methods. Plymouth, UK: PRIMER-E [Google Scholar]
- 26.R Development Core Team 2010. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 27.Bolker B. M., Brooks M. E., Clark C. J., Geange S. W., Poulsen J. R., Stevens M. H. H., White J. S. S. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 10.1016/j.tree.2008.10.008 (doi:10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 28.Bates D., Maechler M. 2010. lme4: linear mixed-effects models. R package version 0.999375–37/r1127. See http://R-Forge.R-project.org/projects/lme4/ [Google Scholar]
- 29.Martin T. G., Wintle B. A., Rhodes J. R., Kuhnert P. M., Field S. A., Low-Choy S. J., Tyre A. J., Possingham H. P. 2005. Zero tolerance ecology: improving ecological inference by modelling the source of zero observations. Ecol. Lett. 8, 1235–1246 10.1111/j.1461-0248.2005.00826.x (doi:10.1111/j.1461-0248.2005.00826.x) [DOI] [PubMed] [Google Scholar]
- 30.Henri D. C., Seager D., Weller T., van Veen F. J. F. 2012. Potential for climate effects on the size-structure of host–parasitoid indirect interaction networks. Phil. Trans. R. Soc. B 367, 3018–3024 10.1098/rstb.2012.0236 (doi:10.1098/rstb.2012.0236). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilborn R., Mangel M. 1997. The ecological detective: confronting models with data. Princeton, NJ: Princeton University Press [Google Scholar]
- 32.Clark J. S. 2007. Models for ecological data: an introduction. Princeton, NJ: Princeton University Press [Google Scholar]
- 33.Robert C. P., Casella G. 2004. Monte Carlo statistical methods, 2nd edn New York, NY: Springer [Google Scholar]
- 34.Su Y. S., Yajima M. 2012. A package for running jags from R . 0.03-04 ed. See http://R-Forge.R-project.org/projects/R2jags [Google Scholar]
- 35.Gelman A., Rubin D. B. 1992. Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–511 10.1214/ss/1177011136 (doi:10.1214/ss/1177011136) [DOI] [Google Scholar]
- 36.Spiegelhalter D. J., Best N. G., Carlin B. R., van der Linde A. 2002. Bayesian measures of model complexity and fit. J. R. Stat. Soc. B Stat. Methodol. 64, 583–616 10.1111/1467-9868.00353 (doi:10.1111/1467-9868.00353) [DOI] [Google Scholar]
- 37.Ellison A. M. 2004. Bayesian inference in ecology. Ecol. Lett. 7, 509–520 10.1111/j.1461-0248.2004.00603.x (doi:10.1111/j.1461-0248.2004.00603.x) [DOI] [Google Scholar]
- 38.Tylianakis J. M., Tscharntke T., Lewis O. T. 2007. Habitat modification alters the structure of tropical host–parasitoid food webs. Nature 445, 202–205 10.1038/nature05429 (doi:10.1038/nature05429) [DOI] [PubMed] [Google Scholar]
- 39.Throop H. L., Lerdau M. T. 2004. Effects of nitrogen deposition on insect herbivory: implications for community and ecosystem processes. Ecosystems 7, 109–133 10.1007/s10021-003-0225-x (doi:10.1007/s10021-003-0225-x) [DOI] [Google Scholar]
- 40.de Sassi C., Lewis O. T., Tylianakis J. M. 2012. Plant-mediated and non-additive effects of two global change drivers on an herbivore community. Ecology 93, 1892–1901 10.1890/11-1839.1 (doi:10.1890/11-1839.1) [DOI] [PubMed] [Google Scholar]
- 41.Haddad N. M., Tilman D., Haarstad J., Ritchie M., Knops J. M. H. 2001. Contrasting effects of plant richness and composition on insect communities: a field experiment. Am. Nat. 158, 17–35 10.1086/320866 (doi:10.1086/320866) [DOI] [PubMed] [Google Scholar]
- 42.Binzer A., Guill C., Brose U., Rall B. C. 2012. The dynamics of food chains under climate change and nutrient enrichment. Phil. Trans. R. Soc. B 367, 2935–2944 10.1098/rstb.2012.0230 (doi:10.1098/rstb.2012.0230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beckerman A. P., Petchey O. L., Warren P. H. 2006. Foraging biology predicts food web complexity. Proc. Natl Acad. Sci. USA 103, 13 745–13 749 10.1073/pnas.0603039103 (doi:10.1073/pnas.0603039103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thébault E., Fontaine C. 2010. Stability of ecological communities and the architecture of mutualistic and trophic networks. Science 329, 853–856 10.1126/science.1188321 (doi:10.1126/science.1188321) [DOI] [PubMed] [Google Scholar]