Abstract
Climate change has complex structural impacts on coastal ecosystems. Global warming is linked to a widespread decline in body size, whereas increased flood frequency can amplify nutrient enrichment through enhanced run-off. Altered population body-size structure represents a disruption in top-down control, whereas eutrophication embodies a change in bottom-up forcing. These processes are typically studied in isolation and little is known about their potential interactive effects. Here, we present the results of an in situ experiment examining the combined effects of top-down and bottom-up forces on the structure of a coastal marine community. Reduced average body mass of the top predator (the shore crab, Carcinus maenas) and nutrient enrichment combined additively to alter mean community body mass. Nutrient enrichment increased species richness and overall density of organisms. Reduced top-predator body mass increased community biomass. Additionally, we found evidence for an allometrically induced trophic cascade. Here, the reduction in top-predator body mass enabled greater biomass of intermediate fish predators within the mesocosms. This, in turn, suppressed key micrograzers, which led to an overall increase in microalgal biomass. This response highlights the possibility for climate-induced trophic cascades, driven by altered size structure of populations, rather than species extinction.
Keywords: multiple stressors, trait-mediated, functional trait, European green crab, Lough Hyne, biodiversity
1. Introduction
Ecosystems are being affected by climate change in many ways. The physical environment is being altered by changes in means and variation in temperature, UV radiation and precipitation [1], with increased frequency of extreme events likely to have some of the greatest effects [2]. The fact that organisms are responding with changes in distribution and phenology has now been well documented [3]. More recently, it has emerged that there is also a trend for reduced body size in response to climate change [4–6]. Community interactions, food webs and ecosystem processes are very likely to be disrupted by these changes, but relevant experimental evidence is limited. Although there has been considerable research on responses to aspects of climate change by individual species, much less work has been carried out on the combined effects of multiple aspects of climate change at higher levels of biological organization, capturing the complex changes in abiotic and biotic variables that will arise [7,8].
In freshwater, estuarine and coastal marine ecosystems, another key impact of climate change will be the increased frequency of extreme precipitation events [9]. Resultant flooding can bring about an intensified input of terrestrially derived contaminants such as biocides and nutrients, which imposes stress on aquatic ecosystems. This can lead to marked changes in productivity, modifying ecosystems via impacts on diversity, community structure and stability [10–12]. Eutrophication has even been linked to decreasing mean body size in marine communities [13], which should decrease community stability [14,15] and severely modify the strength of species interactions [16]. In a theoretical study, Binzer et al. [17] show that increases in consumer body mass and warming synergistically buffer the consequences of nutrient enrichment. Thus, not only is climate change itself multifaceted, but it influences systems that are also being affected by a wide range of local stressors [18]. In developing strategies to minimize and offset the impacts of climate change, it is vital that we improve understanding of how multiple global and local stressors combine to influence ecosystems [19]. In this way, management interventions can be targeted to localized pressures which, when magnified by climate change, would have greatest impact and whose reduction would therefore be most beneficial. Given the degree of complexity involved, it is vital that generalities are identified that can guide decisions where specific data are not available.
Ecosystems can be bottom-up controlled through nutrient availability or top-down controlled through predation and consumer effects [20]. The impacts of individual species loss can therefore cascade through food webs, secondarily affecting species further up or down the food chain [16,21,22] and sometimes inducing major regime shifts [23]. Such cascades have been found in a variety of ecosystems [24] and tend to be stronger if larger species from higher trophic levels are lost [16,21]. Thus, the interaction of altered top-down forcing, driven by the loss of large top predators, and bottom-up processes, driven by energy supplements to the basal resources, may play an important role in determining community structure and dynamics [25].
Climatic stressors do not lead to instantaneous species extinctions but take effect gradually and indirectly, as reviewed by Brose et al. [26]. Metabolic rates are very sensitive to increased temperature [27] and as a consequence warming also modifies feeding and interaction strengths [28–31], imposes demographic changes [3], and alters population size structure [4–6,32] and linkages to other populations [33]. Investigating the effects of gradual changes in predator population size structure, in contrast to their complete removal, is therefore needed to provide a higher resolution of insight into top-down mechanisms.
Bottom-up and top-down forces have typically been studied only in isolation, but there have been attempts to disentangle their effects [20,34,35] and some interactions between them have been shown [36,37]. An improved understanding of interactions between bottom-up and top-down processes could provide a basis for generalizations in assessing impacts of different elements of climate change combined with local stressors. Thus, in the current study, we aim to disentangle the individual and combined (additive, synergistic or antagonistic) effects of nutrient availability (bottom-up) and the size structure of predatory crab populations (top-down) in a marine ecosystem on mean community body mass, abundance and biomass (all three parameters averaged across all species except the manipulated crabs), species richness and the biomass and abundance of individual taxa from all trophic levels in the community.
2. Methods
The study was conducted at Lough Hyne, a highly sheltered marine nature reserve in southwest Ireland (N 51°29′52′ W 9°17′46′), from 29 July to 16 September 2011. Owing to its biological and physical conditions, Lough Hyne is well suited to experimental work and is broadly representative of temperate, shallow-water Atlantic communities (see O'Gorman & Emmerson [38] and references therein). An experiment was established, with cages measuring 42 × 41 × 10 cm and a mesh width of approximately 7 mm. The cages were loaded with 5 kg of 1–2 cm gravel at the outset and situated in the shallow subtidal of a bay with weak current on the south shoreline of Lough Hyne. Two blocks were set up at a depth of 1 m and two blocks at a depth of 1.5 m at low tide (tidal range being approximately 1 m). The distance between any two cages was at least 2.5 m to reduce the likelihood of confounding factors. Two experimental factors were manipulated in a full-factorial design: (i) ‘bottom-up treatments’ in which fertilizer pellets were added to cages to yield an enriched nutrient level compared to cages with ambient levels; (ii) ‘top-down treatments’ in which the body mass of the top predator was altered by placing populations with small, medium and large average body mass into the cages, plus a control without predators. Each treatment was replicated four times in randomized blocks, yielding a total of 32 cages.
(a). Bottom-up treatments
Nutrient enrichment was achieved by placing a mesh bag filled with 500 g of plant fertilizer pellets (Scotts Miracle-Gro Osmocote slow release all purpose plant food: NPK(Mg), (17 : 9 : 11(: 2)%) in each cage. This quantity was chosen based on the successful application of nutrient enrichment in previous marine research [39]. Identical mesh bags, containing 500 g of gravel, were placed in the cages with ambient nutrient levels to avoid procedural confounding. The nutrient enrichment was validated by water samples taken two weeks after the start of the experiment. Herein, the total nitrogen content of ambient (n = 16) and enriched (n = 16) cages differed significantly (two-tailed t-test: t = 3.632, d.f. = 15.4, p = 0.002). The ambient cages also did not differ from background samples (n = 4), taken from several locations further along the shore (two-tailed t-test, t = −0.456, d.f. = 4.4, p = 0.671).
(b). Top-down treatments
The European green crab or shore crab, Carcinus maenas, which is native to Lough Hyne, was chosen as the top predator in the experiment. C. maenas exhibits very high abundance and a wide range in individual body size. It is an aggressively competing omnivore with a wide tolerance for many environmental factors [40]. This flexibility may reflect a special aptitude in adjusting individual growth, reproduction rate and population size structure gradually to temperature and other external stressors, as demonstrated for the similarly dominant Portunid blue crab, Callinectes sapidus [41]. Originating from Europe and North Africa, C. maenas has invaded rocky shores all over the world and threatens to outcompete many native species [40]. Hence, its ecological impacts on other species are of the utmost interest. Thus, C. maenas is a suitable experimental organism to simulate changes in population size structure as caused by warming.
Individual body mass of C. maenas was calculated using a carapace width–fresh weight relationship from the study site [42]. Both male and female individuals were used in the experiment, although crabs parasitized by the rhizocephalan Sacculina carcini were excluded. The top-down manipulation comprised four levels, one control without crabs and three body-size classes: small (S), medium (M) and large (L), initiated with average body masses, MC, of MCS = 6.00 g (range from 1.86 to 16.68 g), MCM = 12.93 g (3.60–39.01 g), MCL = 24.48 g (8.18–72.29 g). These were achieved by using an allometric design (see Schneider et al. [43] and electronic supplementary material). The established densities of C. maenas, NC, were assumed to depend on the average population body mass following an allometric power law (see the electronic supplementary material, figure S1). This yielded differing densities in the three treatments (NCS = 10, NCM = 7 and NCL = 6) which represent a trade-off between fixing the biomass or abundance of the crabs over all treatments. The average top-predator body mass increased slightly during the experiment owing to individual growth and higher crab mortality of smaller crabs. The latter was most likely caused by cannibalism. Out of 184 crabs introduced to the experiment, 112 crabs survived until the end. The final body-size distribution of crabs for each cage still largely reflected the initial distribution (see the electronic supplementary material, figure S2).
Note that while the body-size classes of C. maenas used in this experiment probably represent different ontogenetic stages in their growth, gut content analysis carried out on the crabs at the end of the experiment suggests that the taxonomic composition of the diet was largely similar across the size classes (see electronic supplementary material, table S1). Instead, the variation in C. maenas body size determines the optimal foraging niche, with different taxonomic groups representing a greater proportion of the diet for different size classes of crabs (most likely related to strength of the chelae, mobility and intimidating presence).
(c). Measuring response variables
To sample small invertebrate species, each cage was supplied with a settlement pad attached to its ground mesh (red plastic pot scourer, polyamide, approx. 7.5 × 6 × 3.5 cm). Pot scourers are commonly used to sample mobile benthic invertebrates (see O'Gorman et al. [44] and references therein). At the end of the experiment, contents of the settlement pads were flushed through a fine laboratory test sieve (250 μm). Larger animals were collected from each cage by hand and stored in ethanol. All animals were identified and counted. Sessile species were counted within an area of 10 × 10 cm on the inside roof of the cages (Janua pagenstecheri and Pomatoceros triqueter) or on the entire inside roof (Ascidiella aspersa). In total, 33 843 individuals from 119 taxa were extracted from the cages. Ninety-three taxa were identified to species level (see the electronic supplementary material, table S2 for details). Up to 25 individuals per species and cage were measured for body mass estimates using vernier callipers or a glass micrometer (50 mm in 0.1 mm divisions). Individual lengths were converted to body masses using length–dry weight relationships [42]. Where species-specific length–weight relationships were not available, relationships of the closest relative species or a similarly shaped species were assumed. Dry weights were transformed to fresh weights by multiplying by a factor of four [45]. For C. maenas, Gobiusculus flavescens, Marthasterias glacialis, Palaemon serratus and Pomatoschistus pictus, lengths were directly calculated to fresh weight owing to available length–fresh weight relationships. To unify the different methods of sampling (pot scourers, direct sampling and counting), population densities were scaled to number of individuals per square metre. Population biomass (g m−²) was calculated by multiplying the average species body mass by population density. The chlorophyll content of microalgae was quantified on glass microscope slides (5.5 × 2.6 cm) hanging from the lid of the cage. Here, standardized acetone extraction was applied [46] and the concentration of chlorophyll in the extract was measured.
(d). Statistical analyses
Statistical data processing was performed with R v. 2.14.0 [47]. The effect of the top-predator body mass and fertilization was tested on the following response parameters: number of species, S, within each cage (subsequently termed species richness); total community biomass density, B (g m−²); total community individual density, N (ind. m−2) and average community body mass, M (g ind.−1) of all individuals in the cage. Furthermore, we examined the change in biomass and abundance of every functional species (i.e. taxonomic species or grouping of species that carry out similar functional roles in the system) in the experiment in response to the bottom-up and top-down treatments. Finally, microalgal chlorophyll was tested as a proxy for primary production. The aim here was to determine whether a trophic cascade permeated through the animal community to alter the standing stock of microalgae in the system. Linear mixed effects models [48,49] were used to describe this set of response parameters, accounting for experimental block as a random factor. The average body mass of the predator population, MC′, the presence or absence of fertilizer in the cage, Nut, and the interaction of both were used as explanatory parameters (fixed effects). For the continuous parameter MC′, the mean of the top-predator body mass at the beginning and at the end of the experiment were averaged for each replicate. The binary parameter Nut was given a value of 0 for ambient nutrient level and 1 for enriched nutrient level. For species densities and biomasses containing zeros in some replicates, a value of one was added to all replicates. All response parameters were log10-transformed to meet the assumptions of normality and homogeneity of variance.
3. Results
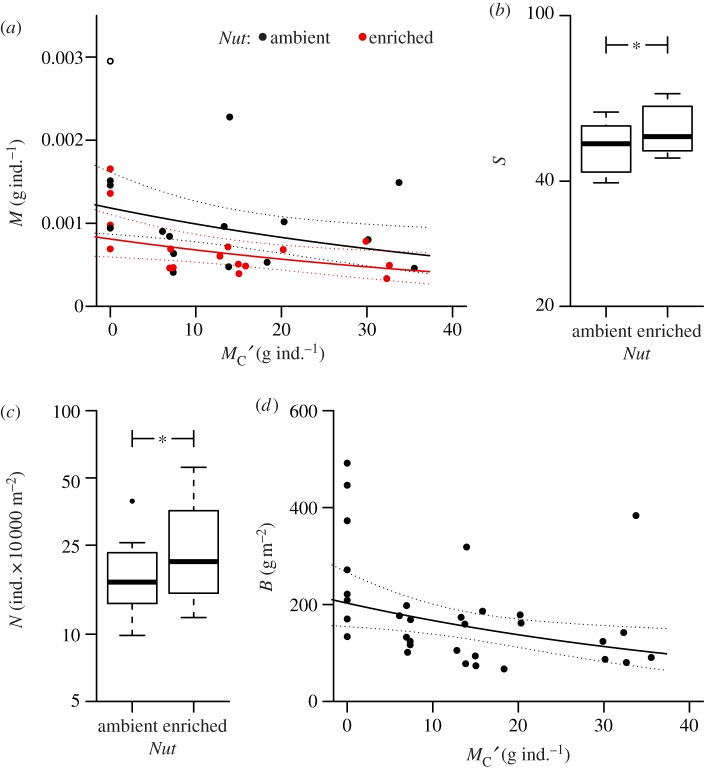
The linear models did not indicate any significant interaction between nutrient enrichment and average top-predator body mass in affecting the response parameters (table 1, Nut:MC′; p > 0.05, Wald F-test). The average community body mass, M, was additively affected by top-down and bottom-up treatments. It responded positively to decreasing top-predator body mass (table 1, x-axis in figure 1a, p = 0.026) and negatively to nutrient enrichment (table 1, black versus red points in figure 1a, p = 0.033). Nutrient enrichment caused a significant increase in species richness, S, (table 1 and figure 1b, p = 0.023) and the overall density of individuals, N, (table 1 and figure 1c, p = 0.019). There was no significant effect of top-predator body mass on either species richness or density. The reduction in top-predator body mass caused a significant increase in the overall biomass density of the community, B, (table 1 and figure 1d, p = 0.023). There was no significant effect of nutrient enrichment on overall biomass.
Table 1.
Wald F-tests for linear mixed effect models to describe response parameters. Top-predator body mass, MC′, as continuous and nitrogen fertilizer, Nut, as binary fixed effects (inserted sequentially). Treatment block is taken into account as a random effect. The community response variables are: average community body mass, M; species richness, S; overall density of individuals, N; and overall biomass density, B. Population response variables are: the biomass density of Perciformes, the individual density of meiofaunal micrograzers, and the chlorophyll concentration on glass slides. p-values are the likelihood for a parameter to equal zero. Nut:MC′ is the test for an interactive effect of both explanatory parameters. Numerator degrees of freedom, d.f. = 1; denominator d.f. = 25; F- and p-values for Wald tests. *p < 0.05; **p < 0.01; ***p < 0.001.
| F-value | p-value | F-value | p-value | F-value | p-value | F-value | p-value | |
|---|---|---|---|---|---|---|---|---|
| log10
M (g ind.−1) |
log10
S |
log10
N (ind. m−2) |
log10
B (g m−2) |
|||||
| (intercept) | 7217.654 | <0.001*** | 17013.970 | <0.001*** | 11541.500 | <0.001*** | 3201.263 | <0.001*** |
| Nut | 5.112 | 0.033* | 5.880 | 0.023* | 6.256 | 0.019* | 0.479 | 0.495 |
| MC′ | 5.593 | 0.026* | 0.089 | 0.768 | 0.162 | 0.690 | 5.903 | 0.023* |
| Nut:MC′ | 0.251 | 0.621 | 0.438 | 0.514 | 0.043 | 0.837 | 0.122 | 0.730 |
| log10 B Perciformes +1 (g m−2) | log10 N grazer (ind. m−2) | log10 chlorophyll (mg m−2) | ||||||
| (intercept) | 543.221 | <0.001*** | 17061.320 | <0.001*** | 2194.009 | <0.001*** | ||
| Nut | 2.661 | 0.115 | 6.939 | 0.014* | 1.246 | 0.275 | ||
| MC′ | 9.433 | 0.005** | 5.493 | 0.027* | 4.286 | 0.049* | ||
| Nut:MC′ | 0.030 | 0.864 | 0.001 | 0.975 | 0.987 | 0.330 | ||
Figure 1.
The average body mass of the top predator (Carcinus maenas), MC′, and nutrient addition, Nut (highlighted in red), had (a) additive effects on average individual body mass M of all other species; log10 M = −2.927(±0.066 s.e.) − 0.008(±0.003)MC′−0.165(±0.072)Nut. Addition of nutrients had a significant effect on (b) species richness, S; log10 S = 1.683(±0.016) + 0.041(±0.017)Nut, and (c) total individual density, N; log10 N = 5.251(±0.054) + 0.112 (±0.043)Nut; *p < 0.05 (F-test on simplified mixed effect model with only Nut as explanatory variable). Predator body mass affected (d) total biomass, B; log10 B = 2.307(±0.058) − 0.008(±0.003)MC′. (a) and (d) linear models on log10-transformed response variables with 95% confidence bands. Response variables were corrected for the random effect of experimental block.
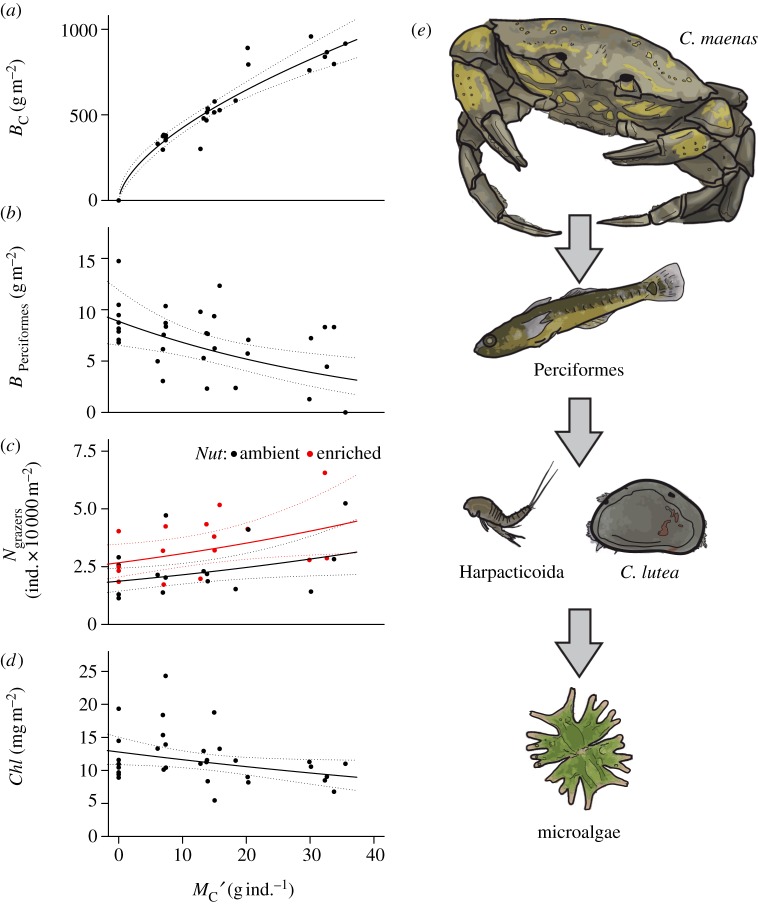
A number of key functional species were significantly affected by decreasing top-predator body mass, which is strictly coupled to a decrease in biomass (figure 2a). The biomass density of the order Perciformes increased significantly with decreasing top-predator body mass (table 1 and figure 2b, p = 0.005). Here, the Perciformes comprise the painted goby, P. pictus, and two-spot goby, G. flavescens. The two most prominent meiofaunal micrograzers in the system, harpacticoid copepods and the dominant ostracod, C. lutea, showed a reduction in individual density with decreasing top-predator body mass (table 1; x-axis in figure 2c, p = 0.027), while being additively increased by nutrient enrichment (table 1; black versus red points in figure 2c, p = 0.014). Chlorophyll concentration also increased significantly with decreasing top-predator body mass (table 1 and figure 2d, p = 0.049). These three organism groups represent a trophic cascade following altered top-predator body mass (figure 2e). The decreasing biomass of the apex predator, C. maenas, released the intermediate predator order, Perciformes, which increased in biomass. These fish predators then fed down on the harpacticoid copepods and the ostracod C. lutea, suppressing their population density. Finally, the decline of these micrograzers resulted in the increased standing stock of microalgae (represented by an increased concentration of chlorophyll) at the bottom of the food web.
Figure 2.
Allometric trophic cascade. With an increase in average body mass of the top predator (Carcinus maenas), MC′, (a) its biomass, BC, increased following an allometric power law, BC = 2.02 MC′0.60. In consequence, (b) the group of Perciformes decreased in biomass; log10 (BPerciformes +1) = 0.994(±0.057 s.e.) − 0.01(±0.003) MC′, (c) the predominant mobile grazers, Cythere lutea and the crustacean order of Harpacticoida, increased in population density (and also as a consequence of nutrient addition); log10 Ngrazers = 4.269(±0.056) + 0.006(±0.003)MC′ + 0.155(±0.058)Nut, and (d) the chlorophyll concentration of microalgal biofilm on glass slides decreased; log10 Chl = 1.107(±0.035) − 0.004(±0.002)MC′. Response variables were corrected for the random effect of experimental block. Lines show back-transformed, log-linear models with 95% confidence bands. (e) Hypothesized trophic cascade.
4. Discussion
We conducted a field experiment investigating the effects of nutrient enrichment and altered top-predator population size structure, two major stressors resulting from a combination of climatic and anthropogenic impacts. We found evidence for bottom-up and top-down forces driving different parts of the benthic community. The species richness and overall density of individual organisms increased while mean community body mass decreased with nutrient enrichment. Community biomass and mean community body mass increased with decreasing top-predator body mass. The data do not support an interaction of bottom-up and top-down forces for any response parameter. Mean community body mass and micrograzer abundance responded to both nutrient enrichment and altered top-predator body mass, revealing the additive nature of both stressors. Interestingly, a four-level trophic cascade driven by altered top-predator body mass suggests that shifts in population size structure, as induced by climate change, can have severe consequences across trophic levels. Our study documents a gradual effect of altered population size structure on community structure that takes effect prior to rigorous extinction. We anticipate that this simulates the indirect effects on communities after gradual warming and echoes the effects of warming described by Brose et al. [26] and Shurin et al. [37].
Previous research on trophic cascades has focused on the potential for extinction or changes in the population abundance of top predators to alter the subsequent trophic levels (reviewed by Heithaus et al. [50]). Here, we demonstrate that changes in the body-size structure of an apex predator population have the capacity to bring about a cascade of alterations in the biomass or abundance of other key groups (figure 2). A reduction in the mean body mass of C. maenas increased the biomass of the next lower trophic level, the intermediate predators comprising two small gobies of the order Perciformes in this experiment. Given that Perciformes do not form a large part of the diet of the crabs in this experiment (see the electronic supplementary material, table S1), this effect is most likely to be mediated by behavioural interactions. For example, competition and fear of predation often lead to effects that are even stronger than direct consumption [51]. Additionally, the Perciformes have previously been shown to avoid predators and aggregate in areas where risk of predation is reduced [44]. As the biomass of the Perciformes increased, they suppressed the abundance of key meiofaunal micrograzers. Harpacticoid copepods and phytal ostracods such as C. lutea are recognized as the most important consumers of microalgae in marine systems [52–54]. They often feed above the benthos through filter feeding [55] and grazing of epiphytic biofilms [56,57]. Both taxa are also observed prey of Perciformes in the Lough Hyne system [38]. Finally, as micrograzer abundance was suppressed, the standing stock of microalgal biomass increased, as observed through the higher concentration of chlorophyll. Crucially, this allometrically induced trophic cascade simulates the expected response of natural predator communities to the effects of global warming [4–6,26,37]. We caution that, as in most studies on trophic cascades, the described cascading effect on biomass densities is phenomenological only and not corroborated by observations of direct feeding or indirect interactions. However, direct feeding data of the top predator obtained by stomach content analyses (see electronic supplementary material, table S1) support our interpretation. Overall, our results suggest that warming-induced reductions in top-predator body mass may cascade to lower trophic levels, profoundly affecting ecosystem functions.
Average community body mass scaled negatively with nutrient enrichment and positively with decreasing top-predator body mass, exhibiting a cumulative effect of bottom-up and top-down forces (figure 1a). The top-down effect on average community body mass is most likely driven by the ability of the apex predator to suppress the next trophic level below, either through direct consumption or trait-mediated indirect interactions [44,51]. Given that body size is correlated with trophic height [26,58,59], this next trophic level should consist of predators intermediate in size to the apex crabs and the primary consumers in the system. As the size structure of the apex predator shrinks, it exerts increasingly less control over the intermediate predators, thus promoting a community dominated by larger intermediate predators, as observed by the trophic cascade in the system (figure 2b). Allometric diet breadth theory [60] suggests that the intermediate predators may in turn shift the size distribution of the primary consumers towards smaller body mass. But given the large disparity in size between intermediate predators such as the fish in this study and the invertebrate primary consumers (e.g. copepods, ostracods, snails and bivalves), the cumulative effect is an overall increase in mean community body mass with decreasing top-predator body mass (figure 1a) and a coincident increase in total community biomass (figure 1d), which is consistent with prior studies [4,37]. Further experimentation would be required, however, to determine whether this is just a short-term effect that may be ameliorated over time or change during seasons [37].
The negative bottom-up effect of nutrient enrichment on mean community body mass is less intuitive. The key to understanding the underlying mechanism centres on the increased species richness and density of organisms in the system (figure 1b,c). Consistent with meta-analyses [25], bottom-up supply caused the arrival of more grazer species and an increase in the abundance of micrograzers (figure 2c). It is likely that the influx of nutrients to the system promoted increased primary production (similar to Harding & Perry [61]). Although the standing stock of chlorophyll in the cages remained constant in response to nutrient enrichment, the increased diversity and abundance of grazers most likely channelled the additional energy rapidly through the system via intense grazing pressure [62]. Indeed, harpacticoid copepods and ostracods have been shown to exponentially increase grazing rates in response to increased microalgal availability [52]. The increased number and abundance of these small primary consumers in response to nutrient addition thus lowered the mean body size of the community. This prevented an overall increase in community biomass with nutrient enrichment, as might be expected from previous research [13]. Perhaps with a longer duration of study or a system unconstrained by cage structures, the energy supplement from the grazer community would sustain more higher trophic-level (i.e. larger) animals, thus increasing mean community body size and total biomass.
5. Conclusion
Our data highlight how global change might alter community dynamics in marine ecosystems by providing new insights into mechanisms of bottom-up and top-down control and their relationship and relative importance under climate change scenarios. Nutrient enrichment seems to control the food web via a diversity–abundance channel, whereas top-predator body mass affects overall biomass and community structure. The allometrically induced trophic cascade described here highlights how changes to the body-size structure of top predators can lead to severe consequences for the whole food web below, thus illustrating the concept of thermal top-down cascades. In a similar way, changes in prey population size structure are likely to trigger bottom-up cascading effects on the predators of the focal species. Alterations of this kind are highly likely to be mediated by climatic changes in the coming years. It is therefore of great interest to look beyond removal experiments and further investigate the impacts of altered population size structure. This will lead to a new quality of predictions about the gradual consequences of growing numbers of climate- and human-induced stressors to ecosystems. The interrelation of bottom-up and top-down forces is further influenced and complicated by additional stressors. Therefore, it is of major importance to investigate ecosystem responses to multiple stressors, including natural and anthropogenic sources of disturbance. Recent findings suggest that research into multiple stressors must take account of global change [63]. We therefore have to integrate our knowledge and efforts derived from multiple fields of research to counter the impacts of the growing human population and its pressure on ecosystems worldwide.
Acknowledgments
M.J. was funded by the Evangelisches Studienwerk Villigst e.V.; F.D.S. was funded by the Deutsche Bundesstiftung Umwelt (www.dbu.de); T.P.C. was supported by Science Foundation Ireland's Research Frontiers Programme (08/RFP/EOB1057); U.B. is funded by the German Research Foundation (BR 2315/13); E.J.OG. was funded by IRCSET during the study and is currently funded by NERC grant no. NE/I009280/1. Thanks to Tânia Salvaterra for help with the setup of the experiment and to Jennifer Coughlan for assistance in the lab. M.J., F.D.S., T.P.C., U.B. and E.J.OG. conceived and designed the experiment and wrote the paper. M.J., F.D.S. and E.J.OG. conducted the experiment. M.J., F.D.S., U.B. and E.J.OG. analysed the data.
References
- 1.Core Writing Team, Pachauri R. K., Reisinger A. (eds) 2007. Climate change 2007: synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: IPCC [Google Scholar]
- 2.Gaines S., Denny M. 1993. The largest, smallest, highest, lowest, longest, and shortest: extremes in ecology. Ecology 74, 1677–1692 10.2307/1939926 (doi:10.2307/1939926) [DOI] [Google Scholar]
- 3.Parmesan C., Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 10.1038/nature01286 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 4.Daufresne M., Lengfellner K., Sommer U. 2009. Global warming benefits the small in aquatic ecosystems. Proc. Natl Acad. Sci. USA 106, 12 788–12 793 10.1073/pnas.0902080106 (doi:10.1073/pnas.0902080106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner J. L., Peters A., Kearney M. R., Joseph L., Heinsohn R. 2011. Declining body size: a third universal response to warming? Trends Ecol. Evol. 26, 285–291 10.1016/j.tree.2011.03.005 (doi:10.1016/j.tree.2011.03.005) [DOI] [PubMed] [Google Scholar]
- 6.Sheridan J. A., Bickford D. 2011. Shrinking body size as an ecological response to climate change. Nat. Clim. Chang. 1, 401–406 10.1038/NCLIMATE1259 (doi:10.1038/NCLIMATE1259) [DOI] [Google Scholar]
- 7.Harley C., Hughes A., Hultgren K., Miner B., Sorte C., Thornber C., Rodriguez L., Tomanek L., Williams S. 2006. The impacts of climate change in coastal marine systems. Ecol. Lett. 9, 228–241 10.1111/j.1461-0248.2006.00917.x (doi:10.1111/j.1461-0248.2006.00917.x) [DOI] [PubMed] [Google Scholar]
- 8.Montoya J. M., Raffaelli D. 2010. Climate change, biotic interactions and ecosystem services. Phil. Trans. R. Soc. B 365, 2013–2018 10.1098/rstb.2010.0114 (doi:10.1098/rstb.2010.0114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Pol M., et al. 2010. Do changes in the frequency, magnitude and timing of extreme climatic events threaten the population viability of coastal birds? J. Appl. Ecol. 47, 720–730 10.1111/j.1365-2664.2010.01842.x (doi:10.1111/j.1365-2664.2010.01842.x) [DOI] [Google Scholar]
- 10.Bonsdorff E., Blomqvist E., Mattila J., Norkko A. 1997. Coastal eutrophication: causes, consequences and perspectives in the Archipelago areas of the northern Baltic Sea. Estuar. Coast. Shelf Sci. 44, 63–72 10.1016/S0272-7714(97)80008-X (doi:10.1016/S0272-7714(97)80008-X) [DOI] [Google Scholar]
- 11.Smith V. H., Tilman G. D., Nekola J. C. 1999. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 100, 179–196 10.1016/S0269-7491(99)00091-3 (doi:10.1016/S0269-7491(99)00091-3) [DOI] [PubMed] [Google Scholar]
- 12.Petchey O., McPhearson P., Casey T., Morin P. 1999. Environmental warming alters food-web structure and ecosystem function. Nature 402, 69–72 10.1038/47023 (doi:10.1038/47023) [DOI] [Google Scholar]
- 13.Beukema J. J. 1991. Changes in composition of bottom fauna of a tidal-flat area during a period of eutrophication. Mar. Biol. 111, 293–301 10.1007/BF01319712 (doi:10.1007/BF01319712) [DOI] [Google Scholar]
- 14.Brose U., et al. 2006. Consumer–resource body–size relationships in natural food webs. Ecology 87, 2411–2417 10.1890/0012-9658(2006)87[2411:CBRINF]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[2411:CBRINF]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 15.Otto S. B., Rall B. C., Brose U. 2007. Allometric degree distributions facilitate food-web stability. Nature 450, 1226–1229 10.1038/nature06359 (doi:10.1038/nature06359) [DOI] [PubMed] [Google Scholar]
- 16.Berlow E. L., Dunne J. A., Martinez N. D., Stark P. B., Williams R. J., Brose U. 2009. Simple prediction of interaction strengths in complex food webs. Proc. Natl Acad. Sci. USA 106, 187–191 10.1073/pnas.0806823106 (doi:10.1073/pnas.0806823106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binzer A., Guill C., Brose U., Rall B. C. 2012. The dynamics of food chains under climate change and nutrient enrichment. Phil. Trans. R. Soc. B 367, 2935–2944 10.1098/rstb.2012.0230 (doi:10.1098/rstb.2012.0230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halpern B. S., McLeod K. L., Rosenberg A. A., Crowder L. B. 2008. Managing for cumulative impacts in ecosystem-based management through ocean zoning. Ocean Coastal Manage. 51, 203–211 10.1016/j.ocecoaman.2007.08.002 (doi:10.1016/j.ocecoaman.2007.08.002) [DOI] [Google Scholar]
- 19.Crain C. M., Kroeker K., Halpern B. S. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315 10.1111/j.1461-0248.2008.01253.x (doi:10.1111/j.1461-0248.2008.01253.x) [DOI] [PubMed] [Google Scholar]
- 20.Power M. E. 1992. Top-down and bottom-up forces in food webs: do plants have primacy? Ecology 73, 733–746 10.2307/1940153 (doi:10.2307/1940153) [DOI] [Google Scholar]
- 21.Estes J., Tinker M., Williams T., Doak D. 1998. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282, 473–476 10.1126/science.282.5388.473 (doi:10.1126/science.282.5388.473) [DOI] [PubMed] [Google Scholar]
- 22.Byrnes J., Stachowicz J., Hultgren K., Hughes A., Olyarnik S., Thornber C. 2006. Predator diversity strengthens trophic cascades in kelp forests by modifying herbivore behaviour. Ecol. Lett. 9, 61–71 10.1111/j.1461-0248.2005.00842.x (doi:10.1111/j.1461-0248.2005.00842.x) [DOI] [PubMed] [Google Scholar]
- 23.Daskalov G. M., Grishin A. N., Rodionov S., Mihneva V. 2007. Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proc. Natl Acad. Sci. USA 104, 10 518–10 523 10.1073/pnas.0701100104 (doi:10.1073/pnas.0701100104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borer E., Seabloom E., Shurin J., Anderson K., Blanchette C., Broitman B., Cooper S., Halpern B. 2005. What determines the strength of a trophic cascade? Ecology 86, 528–537 10.1890/03-0816 (doi:10.1890/03-0816) [DOI] [Google Scholar]
- 25.Borer E. T., Halpern B. S., Seabloom E. W. 2006. Asymmetry in community regulation: effects of predators and productivity. Ecology 87, 2813–2820 10.1890/0012-9658(2006)87[2813:AICREO]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[2813:AICREO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 26.Brose U., Dunne J. A., Montoya J. M., Petchey O. L., Schneider F. D., Jacob U. 2012. Climate change in size-structured ecosystems. Phil. Trans. R. Soc. B. 367, 2903–2912 10.1098/rstb.2012.0232 (doi:10.1098/rstb.2012.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehnes R. B., Rall B. C., Brose U. 2011. Phylogenetic grouping, curvature and metabolic scaling in terrestrial invertebrates. Ecol. Lett. 14, 993–1000 10.1111/j.1461-0248.2011.01660.x (doi:10.1111/j.1461-0248.2011.01660.x) [DOI] [PubMed] [Google Scholar]
- 28.Rall B. C., Vucic-Pestic O., Ehnes R. B., Emmerson M., Brose U. 2010. Temperature, predator–prey interaction strength and population stability. Glob. Change Biol. 16, 2145–2157 10.1111/j.1365-2486.2009.02124.x (doi:10.1111/j.1365-2486.2009.02124.x) [DOI] [Google Scholar]
- 29.Vucic-Pestic O., Ehnes R. B., Rall B. C., Brose U. 2011. Warming up the system: higher predator feeding rates but lower energetic efficiencies. Glob. Change Biol. 17, 1301–1310 10.1111/j.1365-2486.2010.02329.x (doi:10.1111/j.1365-2486.2010.02329.x) [DOI] [Google Scholar]
- 30.Rall B. C., Brose U., Hartvig M., Kalinkat G., Schwarzmüller F., Vucic-Pestic O., Petchey O. L. 2012. Universal temperature and body-mass scaling of feeding rates. Phil. Trans. R. Soc. B 367, 2923–2934 10.1098/rstb.2012.0242 (doi:10.1098/rstb.2012.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Twomey M., Brodte E., Jacob U., Brose U., Crowe T. P., Emmerson M. C. 2012. Idiosyncratic species effects confound size-based predictions of responses to climate change. Phil. Trans. R. Soc. B 367, 2971–2978 10.1098/rstb.2012.0244 (doi:10.1098/rstb.2012.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yvon-Durocher G., Montoya J. M., Trimmer M., Woodward G. 2011. Warming alters the size spectrum and shifts the distribution of biomass in freshwater ecosystems. Glob. Change Biol. 17, 1681–1694 10.1111/j.1365-2486.2010.02321.x (doi:10.1111/j.1365-2486.2010.02321.x) [DOI] [Google Scholar]
- 33.Petchey O. L., Brose U., Rall B. C. 2010. Predicting the effects of temperature on food web connectance. Phil. Trans. R. Soc. B 365, 2081–2091 10.1098/rstb.2010.0011 (doi:10.1098/rstb.2010.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter M. D., Price P. W. 1992. Playing chutes and ladders: heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 73, 724–732 [Google Scholar]
- 35.Greig H. S., Kratina P., Thompson P. L., Palen W. J., Richardson J. S., Shurin J. B. 2012. Warming, eutrophication, and predator loss amplify subsidies between aquatic and terrestrial ecosystems. Glob. Change Biol. 18, 504–514 10.1111/j.1365-2486.2011.02540.x (doi:10.1111/j.1365-2486.2011.02540.x) [DOI] [Google Scholar]
- 36.Worm B., Lotze H., Hillebrand H., Sommer U. 2002. Consumer versus resource control of species diversity and ecosystem functioning. Nature 417, 848–851 10.1038/nature00830 (doi:10.1038/nature00830) [DOI] [PubMed] [Google Scholar]
- 37.Shurin J. B., Clasen J. L., Greig H. S., Kratina P., Thompson P. L. 2012. Warming shifts top-down and bottom-up control of pond food web structure and function. Phil. Trans. R. Soc. B 367, 3008–3017 10.1098/rstb.2012.0243 (doi:10.1098/rstb.2012.0243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Gorman E. J., Emmerson M. C. 2010. Manipulating interaction strengths and the consequences for trivariate patterns in a marine food web. In Advances in ecological research: ecological networks, vol. 42 (ed Woodward G.), pp. 301–419 San Diego, CA: Elsevier Academic Press [Google Scholar]
- 39.Atalah J., Crowe T. P. 2010. Combined effects of nutrient enrichment, sedimentation and grazer loss on rock pool assemblages. J. Exp. Mar. Biol. Ecol. 388, 51–57 10.1016/j.jembe.2010.03.005 (doi:10.1016/j.jembe.2010.03.005) [DOI] [Google Scholar]
- 40.Klassen G., Locke A. 2007. A biological synopsis of the European Green Crab, Carcinus maenas. Can. Manuscr. Rep. Fish. Aquat. Sci. 2818, vii+75 pp [Google Scholar]
- 41.Fisher M. R. 1999. Effect of temperature and salinity on size at maturity of female blue crabs. Trans. Am. Fish. Soc. 128, 499–506 (doi:10.1577/1548-8659(1999)128<0499:EOTASO>2.0.CO;2) [DOI] [Google Scholar]
- 42.O'Gorman E. J., Jacob U., Jonsson T., Emmerson M. C. 2010. Interaction strength, food web topology and the relative importance of species in food webs. J. Anim. Ecol. 79, 682–692 10.1111/j.1365-2656.2009.01658.x (doi:10.1111/j.1365-2656.2009.01658.x) [DOI] [PubMed] [Google Scholar]
- 43.Schneider F. D., Scheu S., Brose U. 2012. Body mass constraints on feeding rates determine the consequences of predator loss. Ecol. Lett. 15, 436–443 10.1111/j.1461-0248.2012.01750.x (doi:10.1111/j.1461-0248.2012.01750.x) [DOI] [PubMed] [Google Scholar]
- 44.O'Gorman E. J., Enright R. A., Emmerson M. C. 2008. Predator diversity enhances secondary production and decreases the likelihood of trophic cascades. Oecologia 158, 557–567 10.1007/s00442-008-1165-0 (doi:10.1007/s00442-008-1165-0) [DOI] [PubMed] [Google Scholar]
- 45.Peters R. H. 1983. The ecological implications of body size. Cambridge, UK: Cambridge University Press [Google Scholar]
- 46.Parsons T. R., Maita Y., Lalli C. M. 1984. A manual of chemical and biological methods for seawater analysis. Oxford, UK: Pergamon Press [Google Scholar]
- 47.R Development Core Team 2011. r: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 48.Pinheiro J., Bates D., DebRoy S., Sarkar D. & R Development Core Team 2011. nlme: linear and nonlinear mixed effects models. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 49.Zuur A. F., Ieno E. N., Walker N., Saveliev A. A., Smith G. M. 2009. Mixed effects models and extensions in ecology with R, 1st edn New York, NY: Springer [Google Scholar]
- 50.Heithaus M. R., Frid A., Wirsing A. J., Worm B. 2008. Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 23, 202–210 10.1016/j.tree.2008.01.003 (doi:10.1016/j.tree.2008.01.003) [DOI] [PubMed] [Google Scholar]
- 51.Werner E., Peacor S. 2003. A review of trait-mediated indirect interactions in ecological communities. Ecology 84, 1083–1100 10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2) [DOI] [Google Scholar]
- 52.Montagna P. A., Blanchard G. F., Dinet A. 1995. Effect of production and biomass of intertidal microphytobenthos on meiofaunal grazing rates. J. Exp. Mar. Biol. Ecol. 185, 149–165 10.1016/0022-0981(94)00138-4 (doi:10.1016/0022-0981(94)00138-4) [DOI] [Google Scholar]
- 53.Carman K., Fleeger J., Pomarico S. 1997. Response of a benthic food web to hydrocarbon contamination. Limnol. Oceanogr. 42, 561–571 10.4319/lo.1997.42.3.0561 (doi:10.4319/lo.1997.42.3.0561) [DOI] [Google Scholar]
- 54.Olafsson E., Modig H., van de Bund W. 1999. Species specific uptake of radio-labelled phyto-detritus by benthic meiofauna from the Baltic Sea. Mar. Ecol. Prog. Ser. 177, 63–72 10.3354/meps177063 (doi:10.3354/meps177063) [DOI] [Google Scholar]
- 55.De Troch M., Steinarsdottir M., Chepurnov V., Olafsson E. 2005. Grazing on diatoms by harpacticoid copepods: species-specific density-dependent uptake and microbial gardening. Aquat. Microb. Ecol. 39, 135–144 10.3354/ame039135 (doi:10.3354/ame039135) [DOI] [Google Scholar]
- 56.Buffan-Dubau E., Carman K. 2000. Diel feeding behavior of meiofauna and their relationships with microalgal resources. Limnol. Oceanogr. 45, 381–395 10.4319/lo.2000.45.2.0381 (doi:10.4319/lo.2000.45.2.0381) [DOI] [Google Scholar]
- 57.De Troch M., Chepurnov V. A., Vincx M., Olafsson E. 2008. The effect of Fucus vesiculosus on the grazing of harpacticoid copepods on diatom biofilms. J. Sea Res. 60, 139–143 10.1016/j.seares.2008.05.005 (doi:10.1016/j.seares.2008.05.005) [DOI] [Google Scholar]
- 58.Digel C., Riede J. O., Brose U. 2011. Body sizes, cumulative and allometric degree distributions across natural food webs. Oikos 120, 503–509 10.1111/j.1600-0706.2010.18862.x (doi:10.1111/j.1600-0706.2010.18862.x) [DOI] [Google Scholar]
- 59.Riede J. O., Brose U., Ebenman B., Jacob U., Thompson R., Townsend C. R., Jonsson T. 2011. Stepping in Elton's footprints: a general scaling model for body masses and trophic levels across ecosystems. Ecol. Lett. 14, 169–178 10.1111/j.1461-0248.2010.01568.x (doi:10.1111/j.1461-0248.2010.01568.x) [DOI] [PubMed] [Google Scholar]
- 60.Petchey O. L., Beckerman A. P., Riede J. O., Warren P. H. 2008. Size, foraging, and food web structure. Proc. Natl Acad. Sci. USA 105, 4191–4196 10.1073/pnas.0710672105 (doi:10.1073/pnas.0710672105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harding L., Perry E. 1997. Long-term increase of phytoplankton biomass in Chesapeake Bay, 1950–1994. Mar. Ecol. Prog. Ser. 157, 39–52 10.3354/meps157039 (doi:10.3354/meps157039) [DOI] [Google Scholar]
- 62.Alpine A., Cloern J. 1992. Trophic interactions and direct physical effects control phytoplankton biomass and production in an estuary. Limnol. Oceanogr. 37, 946–955 10.4319/lo.1992.37.5.0946 (doi:10.4319/lo.1992.37.5.0946) [DOI] [Google Scholar]
- 63.O'Gorman E.J., Fitch J.E., Crowe T.P. 2012. Multiple anthropogenic stressors and the structural properties of food webs. Ecology 93, 441–448 10.1890/11-0982.1 (doi:10.1890/11-0982.1) [DOI] [PubMed] [Google Scholar]