Abstract
There is a clear crisis in the maintenance of biodiversity. It has been generated by a multitude of factors, notably habitat loss, now compounded by the effects of climate change. Predicted changes in climate include increased severity and frequency of extreme climatic events. To manage landscapes, an understanding of the processes that allow recovery from these extreme events is required. Understanding these landscape-scale processes of community assembly and disassembly is hindered by the large scales at which they operate. Model systems provide a means of studying landscape scale processes at tractable scales. Here, we assess the combined effects of temperature and habitat-patch isolation on assembly of naturally diverse moss microarthropod communities after a high-temperature event. We show that community assembly depends on temperature and on degree of habitat isolation. Heated communities were heavily dominated in abundance by two species, one of them relatively large. The resulting size-structure is unlike that seen in the field. Community composition in habitat fragments appears also to have been influenced by the source pool of recolonizing fauna. Our results highlight the value of dispersal in disturbed landscapes and the potential for habitat connectivity to buffer communities from the effects of climate change.
Keywords: climate, climatic extremes, global warming, habitat fragmentation, microcosm, assembly
1. Introduction
There is a global crisis in the maintenance of biodiversity [1,2]. This crisis affects all levels of biological organization and has been generated by a multitude of factors, including habitat loss, degradation and fragmentation, the impacts of which are now being compounded by changes in climate [3,4]. Predicted changes in climate encompass not only changes in mean temperature and rainfall, but also an increase in the frequency and magnitude of extreme climatic events (e.g. heatwaves, droughts) [5,6]. These events can be even the main structuring influences of ecological communities [7,8].
The capacity of ecological communities to recover from extreme events, and the rate at which they do so, is closely linked to the process of community assembly. Contrasting models of assembly of ecological communities have been proposed, which attribute different degrees of importance to dispersal, resource availability, competition and environmental conditions, among other factors, as determinants of community structure in time and space [9–13]. As extreme climatic events become more frequent and severe, the need to understand when and to what extent different factors determine the trajectory of assembling communities becomes more pressing [5,14,15]. This is especially so given (i) the growing limitations on dispersal that are being imposed by increasing habitat fragmentation, (ii) the background of changing environmental conditions under which extreme climatic events are occurring and (iii) the potential for dispersal limitation and harsh environmental conditions to negatively reinforce each other [16,17]. The planning of effective ecological restoration programmes under a changing climate demands that we understand better the process of community assembly at the landscape level [12].
Experimental studies on ecological communities are notoriously difficult to implement. The large spatial and temporal scales at which communities operate can significantly hinder progress in understanding key processes such as the assembly and disassembly of ecological communities [18]. However, model systems can provide tractable ways to approach complex questions, and have provided many useful insights [19,20]. In particular, moss microarthropod communities present a level of experimental tractability superior to many other model systems, while still possessing diverse communities with multiple trophic levels [21]. These communities have been used extensively for the study of dispersal in fragmented landscapes [22–24].
Studies on moss landscapes have provided much-needed data confirming that habitat corridors between otherwise isolated habitat patches can prevent or slow down the process of disassembly of complex communities, and that increased dispersal among habitat patches is most likely responsible for this result [22,23]. This ‘rescue effect’ of dispersal corresponds well with theoretical expectations based on island biogeography and metapopulation theory [25–27]. Where isolation has not been found to be an important negative factor affecting community structure, it has been suggested that dispersal may be more important under harsh climatic conditions [28]. Under these conditions, maintenance of population sizes of vulnerable species may be more reliant on dispersal within landscapes [28]. These expectations have been supported empirically by subjecting moss landscapes with different levels of habitat fragmentation to intensive drought treatments [29]. In the face of this stressor, microarthropod communities from isolated habitats were found to be less resilient than those in more connected habitats, implying a role for dispersal in the recovery of impacted communities.
In the present study, we assessed the effect of habitat fragmentation and increased temperature on moss microarthropod community assembly following an extreme high-temperature event. Given the numerous stressors to which moss communities will be subjected, we hypothesize that a few high-tolerance species will dominate in abundance in the most stressed communities (warmed and highly isolated). We further hypothesize that the outcomes of the community assembly process (i.e. community composition and relative species abundances) will be affected by temperature, with warming producing communities unlike those found in winter conditions in the field. Finally, we predict that the severity of the effect of isolation will depend on environmental conditions; i.e. isolation and temperature will interact in shaping moss-microarthropod communities.
2. Methods
(a). Collection of samples
Moss patches (Dicranoloma sp.) were collected in the Yarra Ranges National Park, Victoria, Australia (37°29′13″ S, 145°49′59″ E, 800 m, permit 10004595 of the Department of Sustainability and Environment, State Government of Victoria). The site is situated in a cool temperate rainforest dominated by Mountain Ash (Eucalyptus regnans) and Myrtle Beech (Nothofagus cunninghamii) trees. A site near the town of Monbulk, Victoria (37°, 52′57″ S, 145°23′07″ E, 327 m), approximately 45 km from the collection site was used for the experimental set-up. This site is situated in a small clearing of cool temperate rainforest of similar composition to the collection site, and is at a similar altitude with a similar temperature regime (data not shown).
(b). Experimental set-up
The experimental set-up was constructed as follows. A 4 cm layer of sand was placed on a 5 × 5 m tarpaulin, with substrate warmers (Adloheat Horticultural Products Pty Ltd., Berwick, Victoria, Australia) kept 2 cm below the sand's surface. Each substrate warmer consisted of a high-resistance heating wire arranged 10 cm apart on plastic mesh, and produced an even heat at the sand surface (data not shown). Warmers were placed under all experimental landscapes but only turned on for the warmed ones. A 1 m-wide barrier of plastic was laid on the ground surrounding the set-up and a 4 cm wide barrier of Tanglefoot Pest Barrier (Contech Enterprises Inc., Victoria, British Columbia, Canada) was applied to its edge to exclude crawling insects. Shade cloth (90% interception) was used to create a roof (1.5 m tall) and walls for the set-up, mimicking conditions under a shaded forest canopy. Air and rainwater could penetrate this cloth.
Moss patches were configured into a series of ‘landscapes’ on top of the sand-covered tarpaulin (figure 1). A circular moss patch (30 cm in diameter, hereafter ‘mainland’) was taken directly from the field and placed in the centre of each landscape. Smaller circular patches (10 cm in diameter, hereafter ‘satellites’) were placed at three different distances from the mainland: 0 cm (herein ‘d1’), 1 cm (‘d2’) and 15 cm (‘d3’). Before placement, all satellite patches were defaunated (24 h Tullgren-funnel extraction) [30]. The procedure extracts microarthropods by heat and humidity gradients created by a light source.
Figure 1.
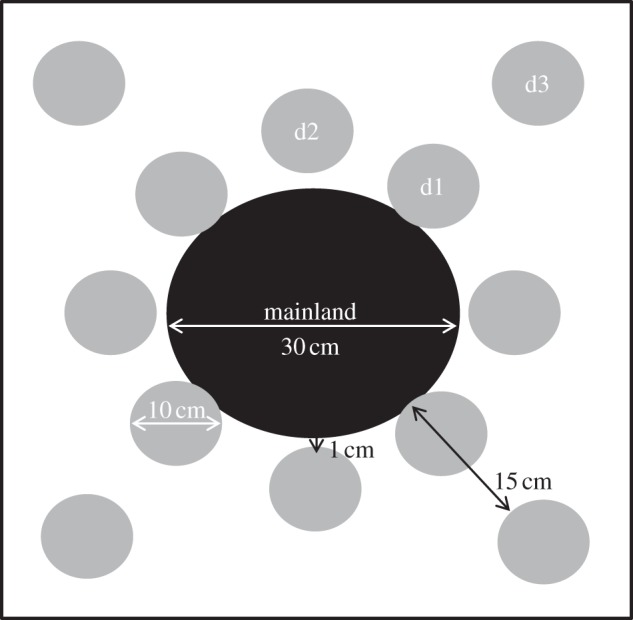
Configuration of an experimental moss landscape. A circular moss patch (‘mainland’) was taken directly from the field and placed in the centre of each landscape. Smaller circular patches (‘satellites’) were placed at three different distances from the mainland: 0 cm (‘d1’), 1 cm (‘d2’) and 15 cm (‘d3’). All satellite patches were subjected to an extreme high temperature event (24 h Tullgren-funnel extraction) before being placed in the set-up. A total of seven landscapes were constructed.
A total of seven landscapes were constructed. Space was allocated to landscapes on three 1 × 5 m strips on the sand-covered tarpaulin. Strips corresponded to the three independently controlled substrate warmers underneath the sand, two of which were not turned on. Five were left at ambient temperature (landscapes 1–5). Average temperature during the day in the moss collection site is 8°C in winter and 20°C in summer; mean difference between minimum and maximum temperatures within a day ranged from 8°C to 15°C (Bureau of Meteorology, station code: 86050). Landscapes 6 and 7 were subjected to a heating treatment, adding 6°C to the ambient temperature at the site (measured automatically at 2 min intervals). This treatment maintained natural patterns of variability in temperature. The experiment ran for three months, starting in winter (June 2009). At the end of the experiment, all moss patches were subjected to Tullgren funnel extraction (at 45°C) for 24 h. Pilot studies showed that this time and temperature were sufficient to extract greater than 80 per cent of individuals and the majority of taxa, but still leave the moss intact and capable of recovery. Fauna was stored in 70 per cent ethanol, until sorting. Microarthropods (less than 2 mm) were sorted to morphospecies on the basis of morphological features. One to seven individuals of abundant morphospecies were sent for expert taxonomic identification (see acknowledgement section).
Morphospecies abundance data per moss patch is stored in the Dryad data repository [31].
(c). Statistical analyses
The experiment was analysed as a split-plot: replicate landscapes (random factor, ntot = 7) were assigned to climate treatments (fixed factor, two levels: ambient (n = 5), heated (n = 2)). Fragmentation (fixed factor, categorical with three levels: d1, d2 and d3 and continuous with log10 (+1) transformation of distance (0, 1 and 15 cm)) was replicated within the landscapes (four replicates per fragmentation treatment within landscapes).
Evenness in the distribution of abundances of species in each sample was calculated as Shannon's diversity divided by the natural logarithm of species richness. Evenness, total abundance (log-transformed) and species richness were used jointly to test for similarity in general structure among communities. A similarity matrix was constructed with these data, and the CLUSTER/SIMPROF routine in PRIMER-E [32] was used to assess for agglomerative structure in the dataset. As evenness was found to drive clustering, the Akaike Information Criterion (AIC) was used to compare the fit of generalized linear mixed effects models (with Gaussian errors) with evenness as a predicted variable, and landscape, fragmentation, warming and the warming–fragmentation interaction as predictors. Differences of more than two AIC units were considered to indicate significant differences in model fits. Effect size and significance in the model with lowest AIC and fewest predictors were then calculated via a Markov Chain Monte Carlo procedure using the ‘lme4’ package of R Statistical Software [33,34].
The effects of landscape, climate, fragmentation and climate–fragmentation interaction on community structure were assessed via permutational multivariate analysis of variance (PERMANOVA) for split-plot designs, performed in PRIMER-E with PERMANOVA+ [35] and analysis of similarity (ANOSIM, [36]). Species abundances were log(x + 1) transformed and then standardized by the maximum abundance in the patch. Bray–Curtis dissimilarity was calculated. Data from a seasonal comparison of moss microarthropod communities from the collection site (G. Perdomo, R. M. Thompson & P. Sunnucks 2011, unpublished data) were added to multi-dimensional scaling plots of the experimental samples to provide a comparison with natural communities and indications of the size structure of the communities under natural field conditions. A discriminant analysis was carried out to assess the distinctiveness of community composition at different fragmentation levels, using scores of the communities along axes generated via canonical analysis of principal coordinates. These analyses were conducted in PRIMER-E with PERMANOVA+.
3. Results
A total of 12 891 individuals were counted, comprising 176 morphospecies of mites and 11 of collembolans. Oribatid mites (Acari, Sarcoptiformes) were the most speciose group in the samples, with a total of 17 families identified (see the electronic supplementary material). Four families of mesostigmatid mites (Acari, Mesostigmata) and six of springtails (Collembola) were identified.
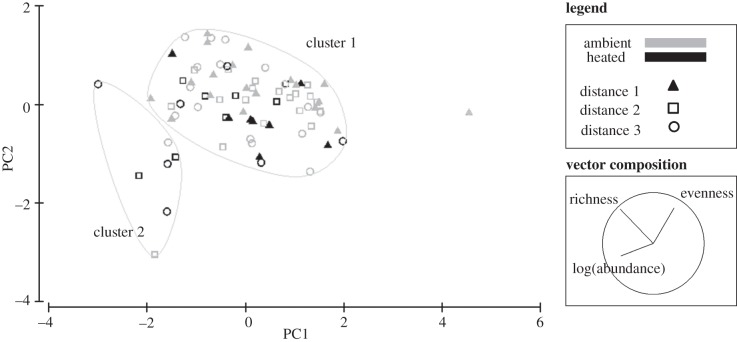
When comparing moss-patch microarthropod communities with respect to species richness, total abundance and evenness, two major clusters of communities were found (figure 2). The majority of the communities grouped into one of these clusters, characterized by higher evenness and lower abundance than the second cluster. Total abundance and community evenness were found to be roughly inversely correlated in the principal component space, and patch species richness was not a driver of difference between the clusters or treatments (vector composition, figure 2). The second cluster was made up of seven moss patches, characterized by considerably lower values of evenness and higher total abundances. None of the patches left adjacent to the faunal source formed part of this cluster.
Figure 2.
Comparison of moss microarthropod community structure from fragmented landscapes subjected to experimental heating. Dissimilarity with respect to species richness, total abundance (log-transformed) and evenness was used in a principal components analysis. Variables were normalized to provide equal weighting in the analyses. Euclidean distance was used. Large grey circles represent clusters. The inset on the bottom right shows original variable vectors, with direction of change indicated by the line; the length of the line represents the variable's relative contribution to the construction of the principal components axes.
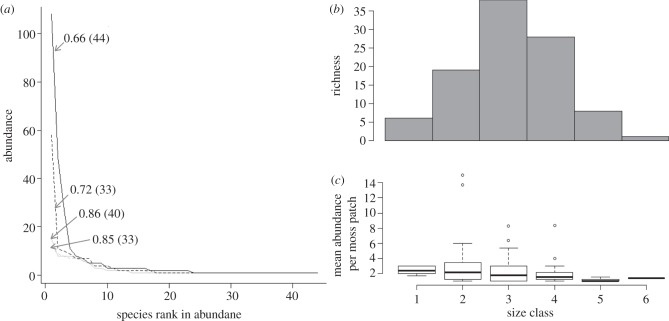
Landscape identity and warming affected evenness (lowest AIC with fewest predictor variables, table 1), with heating decreasing it by an average of 0.07 units (mean MCMC estimate, s.d. = 0.07, p < 0.01). In one of the heated landscapes, fragmentation had a significant effect, with the highest degree of isolation producing lower evenness values (ANOVA, F2,8 = 5.05, p < 0.05). Skewed species abundance distributions (low evenness) were caused by unusually high relative abundances of springtails—in particular, Isotoma sp. (body-size class 4), and, to a lesser extent, Sphaeridia sp. (body-size class 3, figure 3). Although we have found 28 morphospecies of size class 4 in the field, it is uncommon for their abundances to be high or even to exceed three individuals per moss patch (figure 3b,c). Abundances of Isotoma sp. reached up to 147 individuals per moss patch in the heated communities.
Table 1.
Model fit comparison using Akaike Information Criterion (AIC) for microarthropod community evenness as predicted by the experimental treatments. Generalized linear mixed effects models were used, with evenness (Shannon's diversity divided by the natural logarithm of species richness) as a predicted variable, and landscape (L, random factor), fragmentation (F, fixed), climate (C, fixed) and the climate–fragmentation interaction (C : F, fixed) as predictors. Analyses were run using fragmentation as a categorical variable (levels: d1, d2, d3) and as a continuous variable (log10(distance from mainland+1)).
| model | categorical |
continuous |
||
|---|---|---|---|---|
| d.f. | AIC | d.f. | AIC | |
| C + L | 4 | −120.96 | 4 | −120.96 |
| C + F + L | 6 | −120.80 | 5 | −122.08 |
| F + L | 5 | −119.08 | 4 | −120.21 |
| L | 3 | −118.74 | 3 | −118.74 |
| C + F + C : F + L | 8 | −117.84 | 6 | −121.12 |
Figure 3.
(a) Effect of warming on species abundance distributions. Rank-abundance curves from two experimental moss patches are shown. Patches were chosen to illustrate the differences in the shape of the rank-abundance distribution with warming. Warmed patches are shown in black, patches left at ambient temperature are shown grey. All patches were subjected to the same fragmentation treatment (d3). Evenness values for each community are indicated with the arrow, species richness is shown in parenthesis. The species with an abundance of 108 individuals (Isotoma sp., heated patch) corresponds to size class 4 in panels b and c. (b,c) Size-structure in the moss microarthropod community. Size of the microarthropods (from field samples) was measured using body length. The range of sizes was separated into six classes, after log10 transformation. Each class spans 0.2 units. Class 1 starts at 2.2. Panel (b) shows species richness per size class (plots indicate means with boxes showing inter-quartile ranges and whiskers indicating 95% confidence intervals); panel (c) shows mean abundance of the species (per moss patch) in the size classes.
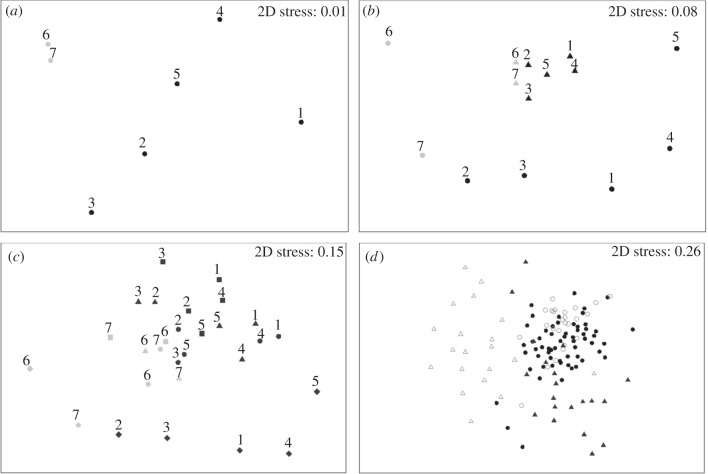
Warming had a significant effect on community structure of the moss landscapes (PERMANOVA using landscape centroids, p = 0.01, figure 4a). Communities left at ambient temperatures were more similar to natural winter communities than were the heated ones (figure 4d). Significant effects of landscape identity on satellite community structure were detected (figure 4b,c, PERMANOVA, p < 0.01), with satellites resembling their respective mainlands (figure 4b, ANOSIM with landscape as a predictor variable and the similarity between mainlands and the centroids of satellite patches as the predicted variable, Global R = 0.34, p < 0.05).
Figure 4.
Multi-dimensional scaling ordinations of moss microarthropod community structure. Moss landscapes (figure 1) were subjected to different climate treatments with replicate levels of fragmentation. Satellite patches were subjected to an initial drought event. Landscapes left at ambient temperature are represented in black; those subjected to heating are shown in grey. Numbers represent different landscapes. (a) Landscape centroids. (b) Centroids of satellite and mainland patches per landscape. Mainlands are represented by circles, satellites by triangles. (c) Centroids of mainlands and isolation treatments. Mainlands are represented by diamonds, distance 1 by circles, distance 2 by triangles and distance 3 by squares. (d) Individual moss patches in the experiment (circles) and from a study of seasonal variability in the collection site (triangles, summer in grey, winter in black).
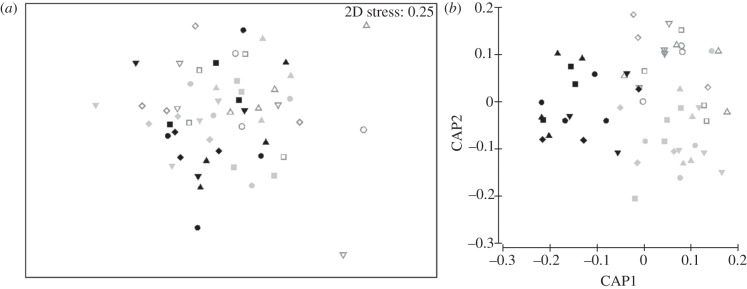
Although no effect of fragmentation or heating-by-fragmentation interaction on community structure was detectable from the analysis of the full dataset (PERMANOVA, p = 0.298 and p = 0.195, respectively), analysis of the subset of landscapes left at ambient temperature revealed a significant effect of fragmentation (figure 5, PERMANOVA, p < 0.05). In the absence of information about the identity of a patch, the use of data on its community structure allowed identification of the patch's isolation level, with an accuracy of 53, 58 and 70 per cent for patches from distances 1, 2 and 3, respectively (ambient treatment, discriminant analysis, figure 5). These rates are above what could be obtained by chance alone (33%). No effects of landscape identity or fragmentation were detected in the two heated landscapes when analysed independently of the landscapes left at ambient temperature (PERMANOVA, p = 0.11 and p = 0.16, respectively).
Figure 5.
Effects of patch isolation on moss microarthropod community structure in fragmented landscape left at ambient temperature. Symbols represent landscape identity. Distance 1 is represented in light grey empty symbols, distance 2 by dark grey symbols and distance 3 by black ones. (a) Multi-dimensional scaling ordination (Bray–Curtis distance). (b) Principal axes generated through canonical analysis of principal coordinates (CAP).
4. Discussion
Landscape-scale disturbance of ecosystems is widespread, with habitat fragmentation and climate change representing two of the major threats to biodiversity today [2,3]. This experiment mimicked a likely future climate scenario—an extreme high-temperature event followed by assembly against a background of warming. Our results clearly show that temperature can determine community composition after an extreme event. Although the role of climate as a filter in the process of assembly of ecological communities has been suggested theoritically, and been reported in long-term datasets [37,38], its relevance and ubiquity continue to be debated [12]. Our results, and those from similarly structured soil microarthropod communities subjected to experimental warming [39], and seasonal changes [40,41] clearly indicate that environmental conditions can shape microarthropod biodiversity patterns in time and space.
The similarity we found among satellite communities within landscapes (i.e. the significant landscape effect on community structure), and between satellites and their respective mainlands, suggests a role for dispersal from undisturbed regions in structuring communities after an extreme thermal/moisture event. This is consistent with theoretical expectations from island biogeography and metapopulation theory [25,26], and large-scale experiments on community assembly on islands [42,43]. Although a role for dispersal in preventing community disassembly has been reported extensively using the moss microarthropod system [22–24], our work and that of Starzomski & Srivastava [29] shows that it can also be relevant in community assembly. Furthermore, our work underscores the value of undisturbed communities in the recovery of regions impacted by an extreme climatic event [44,45].
The potential for climate change to interact with habitat fragmentation has long been identified, although little community-level experimental research has been carried out to date [16,17]. Temperature-induced increases in movement rates may yield higher extinction rates where dispersal represents a risk [46]. Although we found no statistical support for a warming-fragmentation interaction in our experiments, it is worth noting that the most extreme communities with regard to uneven species abundance distributions were the ones subjected to both warming and the highest degree of isolation. The generally higher unevenness of species abundances in heated landscapes suggests that temperature may have altered species interactions. Heated landscapes had unusually high abundances of a relatively mobile prey species with short life cycles (springtails). We suggest that dispersal capacity may make such species more tolerant of habitat fragmentation, and that altered trophic interactions may be further responsible for the changes in community composition observed here.
High relative abundance of species with large body-sizes is uncommon in moss microarthropod communities, as it is in many ecological communities [47]. Recent studies have proposed that warming is selecting for smaller body sizes, although this pattern is weakest for invertebrates [48]. Warming effects have also been found to depend on traits such as diet generalism, and to be idiosyncratic [49–51]. Habitat fragmentation may be expected to strongly select for species with good dispersal capabilities, which may thus select for larger body sizes [52]. The potential for trade-offs between warming and fragmentation forces affecting community size-structure is poorly understood and warrants further attention. Interestingly, our results show that warming can increase abundance of large species, and that fragmentation may further favour these large and highly mobile species in isolated habitats. It seems that a suite of traits of the springtails allowed persistence in the face of an extreme climatic event, habitat fragmentation and warming.
This study represents one of the first to combine realistic climate change treatments with habitat fragmentation. However, there are limitations. Taxonomic information on many taxa was limited, and we used morphospecies in this study. The morphospecies used have high taxonomic validity at family and likely genus level, but we cannot explore patterns of species richness per se. The length of the experiment also imposed constraints, as we do not know how long the impacts of the extreme event or the warming treatment might persist. However, many components of the community have very rapid generation times (days to weeks), and it is likely that population-level adaptation to conditions occurred within the term of this experiment. The potential for evolutionary change over longer time periods cannot be dismissed, and given the relatively long periods over which warming will occur in reality, these may alter the community outcomes.
The relatively small number of replicate landscapes in this study also limits some of the power of inference, particularly given the effects of landscape identity. Given that satellite communities resembled that of the mainland in their respective landscapes, we suggest that the effects associated with landscape identity are a realistic biological phenomenon of potential significance in ecological management. Distance from faunal sources can determine the degree to which communities recover from catastrophic events [42,43]. Understanding the mechanisms that underlie this is likely to require detailed information on species traits [53], but may allow assessment of community vulnerability under changing frequency and severity of climatic extremes. Detailed understanding of biological traits and their role in community assembly is still in its infancy, although considerable progress has been made recently [54].
In previous studies of the effects of warming, treatments have often been applied that increase temperatures to stable, higher means [55]. This does not mimic natural variation in temperature, and confounds an increase in mean temperature with a reduction in temperature variability. Studies that have maintained variability in temperature in warmed treatments have maintained variability typical of current climatic conditions [39]. Yet globally, increases in mean temperatures are predicted to be associated with increased frequency of extreme events [5], with profound impacts on communities. While there is a small body of literature describing community-level impacts of warming, and a larger group of studies on assembly after extreme events, ours is the first to describe the assembly of a community after an extreme event, against a backdrop of altered climate, and in the context of habitat fragmentation.
Our results show that assembly after a catastrophic event in the moss microarthropod community depends on habitat connectivity, temperature and the source of recolonizing fauna. In a climate future dominated by increased frequency of extreme events, communities are likely to comprise a resistant core of species with high resistance to extreme events, and a shifting cast of species assembling based on dispersal traits. Understanding the rules for community assembly in such a dynamic environment require detailed studies that reconcile ‘extreme event’ and ‘warming’ studies into single experiments, taking into account the highly fragmented nature of most natural ecosystems. Studies of small-spatial-scale communities consisting of taxa with diverse thermal tolerances, dispersal and life-history traits are likely to be highly informative in predicting effects of altered climates on already fragmented natural ecosystems.
Acknowledgments
This research was supported by grants to G.P. from the Holsworth Wildlife Research Fund, the Venezuelan Ministry for Higher Education (Fundayacucho) and Monash University. R.M.T. was supported by an Australian Research Council Future Fellowship. We thank Bruce Halliday, Matthew Colloff, Penelope Greenslade and Jenő Kontschán for invaluable support with taxonomic identification. We thank David and Sharon Taylor for the experimental site. The manuscript was greatly improved, thanks to suggestions from two anonymous reviewers and the handling Editor, Ulrich Brose.
References
- 1.Andelman S., Willig M. 2003. Present patterns and future prospects for biodiversity in the Western Hemisphere. Ecol. Lett. 6, 818–824 10.1046/j.1461-0248.2003.00503.x (doi:10.1046/j.1461-0248.2003.00503.x) [DOI] [Google Scholar]
- 2.Pimm S. L., Raven P. 2000. Biodiversity: extinction by numbers. Nature 403, 843–845 10.1038/35002708 (doi:10.1038/35002708) [DOI] [PubMed] [Google Scholar]
- 3.Parmesan C., Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 10.1038/nature01286 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 4.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 10.1146/annurev.ecolsys.37.091305.110100 (doi:10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 5.Easterling D. R., Meehl G. A., Parmesan C., Changnon S. A., Karl T. R., Mearns L. O. 2000. Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074 10.1126/science.289.5487.2068 (doi:10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 6.Meehl, G. A. et al 2007. Global climate projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignore M., Miller H. L.). Cambridge, UK: Cambridge University Press [Google Scholar]
- 7.Holmgren M., et al. 2006. Extreme climatic events shape arid and semiarid ecosystems. Front. Ecol. Environ. 4, 87–95 10.1890/1540-9295(2006)004[0087:ECESAA]2.0.CO;2 (doi:10.1890/1540-9295(2006)004[0087:ECESAA]2.0.CO;2) [DOI] [Google Scholar]
- 8.Ciais P., et al. 2004. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437, 529–533 10.1038/nature03972 (doi:10.1038/nature03972) [DOI] [PubMed] [Google Scholar]
- 9.Diamond J. M. 1975. Assembly of species communities. In Ecology and evolution of communities (eds Cody M. L., Diamond J. M.), pp. 342–444 Cambridge, MA: Harvard University Press [Google Scholar]
- 10.Hubbel S. P. 2001. The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- 11.Tilman D. 1982. Resource competition and community structure. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 12.Choi Y. D. 2004. Theories for ecological restoration in changing environment: toward ‘futuristic’ restoration. Ecol. Res. 19, 75–81 10.1111/j.1440-1703.2003.00594.x (doi:10.1111/j.1440-1703.2003.00594.x) [DOI] [Google Scholar]
- 13.Thompson R., Townsend C. 2006. A truce with neutral theory: local deterministic factors, species traits and dispersal limitation together determine patterns of diversity in stream invertebrates. J. Anim. Ecol. 75, 476–484 10.1111/j.1365-2656.2006.01068.x (doi:10.1111/j.1365-2656.2006.01068.x) [DOI] [PubMed] [Google Scholar]
- 14.Smith M. D. 2011. An ecological perspective on extreme climatic events: a synthetic definition and framework to guide future research. J. Ecol. 99, 656–663 10.1111/j.1365-2745.2011.01798.x (doi:10.1111/j.1365-2745.2011.01798.x) [DOI] [Google Scholar]
- 15.Scheffer M., Carpenter S. R. 2003. Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol. Evol. 18, 648–656 10.1016/j.tree.2003.09.002 (doi:10.1016/j.tree.2003.09.002) [DOI] [Google Scholar]
- 16.Leimu R., Vergeer P., Angeloni F., Ouborg N. J. 2010. Habitat fragmentation, climate change, and inbreeding in plants. Ann. NY Acad. Sci. 1195, 84–98 10.1111/j.1749-6632.2010.05450.x (doi:10.1111/j.1749-6632.2010.05450.x) [DOI] [PubMed] [Google Scholar]
- 17.Opdam P., Wascher D. 2004. Climate change meets habitat fragmentation: linking landscape and biogeographical scale levels in research and conservation. Biol. Conserv. 117, 285–297 10.1016/j.biocon.2003.12.008 (doi:10.1016/j.biocon.2003.12.008) [DOI] [Google Scholar]
- 18.Lawton J. H. 1999. Are there general laws in ecology? Oikos 84 177–192 10.2307/3546712 (doi:10.2307/3546712) [DOI] [Google Scholar]
- 19.Reiss J., Forster J., Cássio F., Pascoal C., Stewart R., Hirst A. G. 2010. Chapter 2: when microscopic organisms inform general ecological theory. In Advances in ecological research (ed. Guy W.), pp. 45–85 New York, NY: Academic Press [Google Scholar]
- 20.Srivastava D. S., et al. 2004. Are natural microcosms useful model systems for ecology? Trends Ecol. Evol. 19, 379–384 10.1016/j.tree.2004.04.010 (doi:10.1016/j.tree.2004.04.010) [DOI] [PubMed] [Google Scholar]
- 21.Lindo Z., Gonzalez A. 2010. The Bryosphere: an integral and influential component of the earth's biosphere. Ecosystems 13, 612–627 10.1007/s10021-010-9336-3 (doi:10.1007/s10021-010-9336-3) [DOI] [Google Scholar]
- 22.Gilbert F., Gonzalez A., Evans-Freke I. 1998. Corridors maintain species richness in the fragmented landscapes of a microecosystem. Proc. R. Soc. Lond. B 265, 577–582 10.1098/rspb.1998.0333 (doi:10.1098/rspb.1998.0333) [DOI] [Google Scholar]
- 23.Gonzalez A., Lawton J. H., Gilbert F. S., Blackburn T. M., Evans-Freke I. 1998. Metapopulation dynamics, abundance, and distribution in a microecosystem. Science 281, 2045–2047 10.1126/science.281.5385.2045 (doi:10.1126/science.281.5385.2045) [DOI] [PubMed] [Google Scholar]
- 24.Staddon P., Lindo Z., Crittenden P. D., Gilbert F., Gonzalez A. 2010. Connectivity, non-random extinction and ecosystem function in experimental metacommunities. Ecol. Lett. 13, 543–552 10.1111/j.1461-0248.2010.01450.x (doi:10.1111/j.1461-0248.2010.01450.x) [DOI] [PubMed] [Google Scholar]
- 25.MacArthur R., Wilson E. O. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- 26.Hanksi I. 1999. Metapopulation ecology. Oxford, UK: Oxford University Press [Google Scholar]
- 27.Brown J. H., Kodric-Brown A. 1977. Turnover rates in insular biogeography: effect of immigration on extinction. Ecology 58, 445–449 10.2307/1935620 (doi:10.2307/1935620) [DOI] [Google Scholar]
- 28.Hoyle M., Gilbert F. 2004. Species richness of moss landscapes unaffected by short-term fragmentation. Oikos 105, 359–367 10.1111/j.0030-1299.2004.12832.x (doi:10.1111/j.0030-1299.2004.12832.x) [DOI] [Google Scholar]
- 29.Starzomski B. M., Srivastava D. S. 2007. Landscape geometry determines community response to disturbance. Oikos 116, 690–699 10.1111/j.0030-1299.2007.15547.x (doi:10.1111/j.0030-1299.2007.15547.x) [DOI] [Google Scholar]
- 30.Tullgren A. 1918. Ein sehr einfacher Ausleseapparat für terricole Tierfaunen. Z. Angew. Entomol. 4, 149–150 [Google Scholar]
- 31.Perdomo G., Sunnucks P., Thompson R. M. 2012. Dryad data repository. (doi:10.5061/dryad.59gp4)
- 32.Clarke K., Gorley R. 2006. PRIMER v6: User Manual/Tutorial. Plymouth, MA: PRIMER-E [Google Scholar]
- 33.R Development Core Team 2009. R: a language and environment for statistical computing. In Computing (ed. R Foundation for Statistical Computing). Austria, Vienna [Google Scholar]
- 34.Bates D., Maechler M., Bolker B. 2011. lme4: Linear mixed-effects models using S4 classes. R Statistical Software version 0.999375–42 [Google Scholar]
- 35.Anderson M. J., Gorley R., Clarke K. 2008. PERMANOVA+ for PRIMER: guide to software and statistical methods. Plymouth, UK [Google Scholar]
- 36.Clarke K. 1993. Non-parametric multivariate analysis of changes in community structure. Aust. J. Ecol. 18, 117–143 10.1111/j.1442-9993.1993.tb00438.x (doi:10.1111/j.1442-9993.1993.tb00438.x) [DOI] [Google Scholar]
- 37.MacDougall A. S., Wilson S. D., Bakker J. D. 2008. Climatic variability alters the outcome of long-term community assembly. J. Ecol. 96, 346–354 10.1111/j.1365-2745.2007.01333.x (doi:10.1111/j.1365-2745.2007.01333.x) [DOI] [Google Scholar]
- 38.Keddy P. A. 1992. Assembly and response rules: two goals for predictive community ecology. J. Vegetation Sci. 3, 157–164 10.2307/3235676 (doi:10.2307/3235676) [DOI] [Google Scholar]
- 39.Briones M. J. I., Ostle N. J., McNamara N. P., Poskitt J. 2009. Functional shifts of grassland soil communities in response to soil warming. Soil Biol. Biochem. 41, 315–322 10.1016/j.soilbio.2008.11.003 (doi:10.1016/j.soilbio.2008.11.003) [DOI] [Google Scholar]
- 40.Wallwork J. A. 1959. The distribution and dynamics of some forest soil mites. Ecology 40, 557–563 10.2307/1929808 (doi:10.2307/1929808) [DOI] [Google Scholar]
- 41.Luxton M. 1981. Studies on the oribatid mites of a Danish beech wood soil. 6. Seasonal population changes. Pedobiologia 21, 387–409 [Google Scholar]
- 42.Simberloff D. S. 1969. Experimental zoogeography of islands: a model for insular colonization. Ecology 50, 296–314 10.2307/1934857 (doi:10.2307/1934857) [DOI] [Google Scholar]
- 43.Simberloff D. S., Wilson E. O. 1970. Experimental zoogeography of islands. A two-year record of colonization. Ecology 51, 934–937 10.2307/1933995 (doi:10.2307/1933995) [DOI] [Google Scholar]
- 44.Taberlet P., Cheddadi R. 2002. Quaternary refugia and persistence of biodiversity. Science 297, 2009–2010 10.1126/science.297.5589.2009 (doi:10.1126/science.297.5589.2009) [DOI] [PubMed] [Google Scholar]
- 45.Shoo L. P., Storlie C., Vanderwal J., Little J., Williams S. E. 2011. Targeted protection and restoration to conserve tropical biodiversity in a warming world. Global Change Biol. 17, 186–193 10.1111/j.1365-2486.2010.02218.x (doi:10.1111/j.1365-2486.2010.02218.x) [DOI] [Google Scholar]
- 46.Eklöf A., Kaneryd L., Münger P. 2012. Climate change in metacommunities: dispersal gives double-sided effects on persistence. Phil. Trans. R. Soc. B 367, 2945–2954 10.1098/rstb.2012.234 (doi:10.1098/rstb.2012.234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawton J. H. 1990. Species richness and population-dynamics of animal assemblages: patterns in body size-abundance space. Phil. Trans. R. Soc. Lond. B 330, 283–291 10.1098/rstb.1990.0199 (doi:10.1098/rstb.1990.0199) [DOI] [Google Scholar]
- 48.Sheridan J., Bickford D. 2011. Shrinking body size as an ecological response to climate change. Nat. Climate Change 1, 401–406 10.1038/nclimate1259 (doi:10.1038/nclimate1259) [DOI] [Google Scholar]
- 49.Twomey M., Brodte E., Jacob U., Brose U., Crowe T., Emmerson M. C. 2012. Idiosyncratic species effects confound size-based predictions of responses to climate change. Phil. Trans. R. Soc. B 367, 2971–2978 10.1098/rstb.2012.0244 (doi:10.1098/rstb.2012.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lurgi M., López B. C., Montoya J. M. 2012. Climate change impacts on body size and food web structure on mountain ecosystems. Phil. Trans. R. Soc. B 367, 3050–3057 10.1098/rstb.2012.0239 (doi:10.1098/rstb.2012.0239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brose U., Dunne J. A., Montoya J. M., Petchey O. L., Schneider F. D., Jacob U. 2012. Climate change in size-structured ecosystems. Phil. Trans. R. Soc. B 367, 2903–2912 10.1098/rstb.2012.0232 (doi:10.1098/rstb.2012.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bowler D. E., Benton T. G. 2005. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. 80, 205–225 10.1017/S1464793104006645 (doi:10.1017/S1464793104006645) [DOI] [PubMed] [Google Scholar]
- 53.Binzer A., Brose U., Curtsdotter A., Eklöf A., Rall B. C., Riede J. O., de Castro F. 2011. The susceptibility of species to extinctions in model communities. Basic Appl. Ecol. 12, 590–599 10.1016/j.baae.2011.09.002 (doi:10.1016/j.baae.2011.09.002) [DOI] [Google Scholar]
- 54.May R. M. 2009. Food-web assembly and collapse: mathematical models and implications for conservation. Phil. Trans. R. Soc. B 364, 1643–1646 10.1098/rstb.2008.0280 (doi:10.1098/rstb.2008.0280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petchey O. L. 1999. Environmental warming alters food-web structure and ecosystem function. Nature 402, 69–72 10.1038/47023 (doi:10.1038/47023) [DOI] [Google Scholar]