Abstract
Understanding and predicting the consequences of warming for complex ecosystems and indeed individual species remains a major ecological challenge. Here, we investigated the effect of increased seawater temperatures on the metabolic and consumption rates of five distinct marine species. The experimental species reflected different trophic positions within a typical benthic East Atlantic food web, and included a herbivorous gastropod, a scavenging decapod, a predatory echinoderm, a decapod and a benthic-feeding fish. We examined the metabolism–body mass and consumption–body mass scaling for each species, and assessed changes in their consumption efficiencies. Our results indicate that body mass and temperature effects on metabolism were inconsistent across species and that some species were unable to meet metabolic demand at higher temperatures, thus highlighting the vulnerability of individual species to warming. While body size explains a large proportion of the variation in species' physiological responses to warming, it is clear that idiosyncratic species responses, irrespective of body size, complicate predictions of population and ecosystem level response to future scenarios of climate change.
Keywords: metabolic rate, body size, consumption rate, global warming, metabolic theory, climate change
1. Introduction
It is estimated that by the end of this century, global temperature will have increased by 1.8–4.0°C [1]. As temperature regulates the basic physiological processes that underpin survival, including metabolism, consumption, reproduction and growth, any thermal impact may have catastrophic effects on individual species, and therefore on whole communities. Food webs are complex, dynamic networks of species that directly and indirectly interact with each other, and it is the complexity of these multi-species networks that makes understanding and predicting the consequences of perturbations a major challenge [2].
Studies investigating the impact of warming on biological processes have varied in focus and scale, from individual species [3–5] to functionally similar species [6,7], and from large-scale literature-based multiple species comparisons [8–10] to measures of entire ecosystem metabolism [11]. Metabolic theory provides a useful platform to investigate the effects of temperature and body size on species' physiological processes [9,12–14]. Metabolism is an enzyme-catalysed process in which food and energy are taken up by an organism from its environment, transformed via catabolism and anabolism into complex molecules and subsequently used to fuel growth, reproduction and other biological processes. In essence, it is the key biological rate on which all other processes depend. Metabolic theory predicts that the metabolic rate of a species scales with body mass by a ¾ power-law [14] and scales exponentially with temperature [9,13]. This theory also proposes similar allometrically scaled relationships for a range of biological processes, including consumption rate, mortality rate and ontogenetic growth [13]. While the power-law scaling of metabolism with body mass is generally accepted, there has been some debate regarding the value of the scaling exponent [15–19]. Support for the predicted consumption relationships has also not been absolute, as some studies have reported hump-shaped relationships between consumption rates and increasing body mass [7,20–23].
Here, we investigate the effect of increased seawater temperatures on the metabolic rates and consumption rates of five species that occupy different trophic positions in subtidal East Atlantic food webs. Using the metabolic theory framework, we determine the body mass scalings of metabolism and consumption for each species, and assess changes in their consumption efficiencies, i.e. the ratio between consumption and metabolism, with increasing temperature. To reflect the diversity of a typical benthic community, the species studied have been chosen for their intrinsic trophic, locomotor and anatomical differences, and include a herbivorous gastropod, a scavenging decapod, a predatory echinoderm, a predatory decapod and a predatory fish. We explicitly test the hypothesis that an increase in temperature will lead to an increase in metabolic rate and that this will translate into increased rates of consumption.
2. Material and methods
The metabolic rates and consumption rates of Marthasterias glacialis (Linnaeus)—a spiny seastar; Gobius niger (Linnaeus)—a black goby; Carcinus maenas (Linnaeus)—a shore crab; Palaemon serratus (Pennant)—a prawn; and Gibbula umbilicalis (da Costa)—a gastropod—were measured in August 2009. All measurements were made at the Lough Hyne research facility, Ireland (51°29′52′ N, 9°17′46′ W). Measurements were carried out at two temperatures, 16.5°C (the water temperature of the Lough on the first day of the experiment) and 18.5°C (a 2°C increase reflecting predicted regional climatic changes in temperature over the next 50–100 years) [24]. Animals were starved for 3 days prior to the experiments. The respiration chambers and feeding arenas were submerged in a large bath of filtered seawater (50 µm), which was continuously circulated at a constant temperature using a thermocirculator (TECO TC20).
(a). Metabolic rate measurements
Using an intermittent flow-through system [25], we measured the standard metabolic rates of seven individuals of each species, with individuals of each species varying in size. The aim of this procedure was to produce a species-specific relationship between body mass and standard metabolic rate for each species. Animals were weighed before being placed into respiration chambers and were left to acclimatize for 12 h. The chambers were Perspex cylinders with a movable lid, allowing for the adjustment of chamber volume to accommodate animals of varying size. Each measurement began with close to 100 per cent oxygen saturation, after which the chamber was closed and the oxygen level was monitored using an oxygen meter (PreSens Fibox 3) until oxygen concentrations declined to 70 per cent oxygen saturation. During this time, water was continuously circulated within the closed system using a peristaltic pump (ISMATEC MCP—ISM404B). This procedure was repeated three times for each individual to provide a single average estimate of metabolic rate (oxygen consumption rate) for that animal. All oxygen saturation (% air saturation)–time regressions recorded r2 values were greater than 0.98.
The oxygen consumption rate in the chambers containing focal species, O2cons, was calculated as
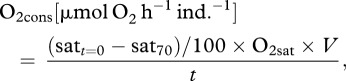 |
2.1 |
where satt=0 is the percentage oxygen saturation at the beginning of the experiment; sat70 is at 70 per cent oxygen saturation; O2sat (µmol O2 l–1) is the amount of oxygen in 1 l of oxygen-saturated (100%) seawater at the given temperature and atmospheric pressure; V is the volume of water (l) in the chamber and tubing used for the experiment (corrected for animal volume); and t is the time taken to reach sat70 (h).
To account for microbial respiration in the chambers, three control measurements (no animal present in chamber) were taken during each species' run of measurements, the average of which was used to correct the experimental measurement for that species. Assuming that 1 ml O2 equals 20.1 J [26], we converted the measured oxygen values (ml O2 s−1) to energetic equivalents (J s–1). To account for the significant amount of metabolically inert body mass for the crab, prawn, seastar and gastropod species, soft tissue body masses were derived from measured wet weights using published relationships for these species [27–29].
(b). Consumption rate measurements
Given the diversity of consumer species, we used a range of different prey resources for the feeding trials. Mussels (Mytilus sp.) were used as prey in the seastar and crab trials, small prawns (P. serratus) for the black goby trial, and algal matter for the gastropod trial (algal biofilms were collected using glass slides, which were colonized under natural conditions in Lough Hyne for a number of weeks prior to the experiment). Because prawns are scavenging omnivores, we used squid mantle pieces (0.1 g) as a food source for this species (following a series of preliminary feeding trials).
A 24 h feeding trial was carried out for each consumer species and included 10 individuals of varying body sizes. Each trial consisted of twelve arenas (including two control arenas), measuring 27 × 20 × 10 cm for large consumer individuals and 24 × 17.5 × 8.5 cm for medium and small individuals. All arenas included one consumer individual and its associated prey. The control arenas contained only prey species. The arenas were filled with water from a large, constant-temperature water bath, then sealed and submerged in the bath. Each arena was continuously aerated throughout the trial.
For the seastar and crab feeding trials, 20 mussels were placed in each arena. The shell-free wet weight of the consumed prey was calculated as mentioned in [27]. For the black goby trial, 16 prawns were added to each arena. Smaller arenas (12 × 12 × 6.5 cm) were used for the gastropod trials, with two glass slides placed in each. The glass slides occupied a significant portion of each arena, and the gastropods did not completely remove the biofilm from the slides during the trials. The percentage algal matter consumed when compared with control slides was recorded. Using an unpublished relationship between algal dry mass (g) and percentage cover of algae (algal biomass = −0.0003 + 0.0151 × percentage cover, p < 0.01, r2 = 0.72), we calculated algal consumption by gastropods. The consumption of algae on the glass slide was scaled to the inner surface area of the arena assuming that gastropods graze continuously as they move. For the prawn feeding trial, 20 squid pieces were weighed and placed in each arena. The average mass of a squid piece was calculated for each arena and the number of pieces consumed per arena was recorded. Consumer and prey body masses were recorded pre- and post-experiment, and the average was used in the analyses. Prey items in the control arenas were counted and their biomass measured after the experiments. Consumption rates were calculated per unit volume of the water-filled arena (J s−1 l−1) to discount for any variation due to arena size and by converting the mass of prey consumed to energetic equivalents, given that the energy content per gram of prey wet mass and prey dry mass is 7000 and 22 000 J, respectively [26]. Consumption rates were then compared on a per mass basis, with specific reference to each individual's body mass.
(c). Statistical analysis
Following metabolic theory, the effect of body mass on an individual's metabolic rate is described by
| 2.2 |
where Ij is the metabolic rate for individual j (J s−1); i0 is a normalization constant independent of body size and temperature; Mj is the body mass (g) individual j; b is an allometric scaling exponent. Similarly, the effect of body mass on an individual's rate of consumption is described by
| 2.3 |
where here, Cij is the per capita consumption rate per unit volume of the water-filled arena (J s−1 l−1), and c0 is a normalization constant.
Finally, we calculated the consumption efficiency, yij [21,30], using the equation
| 2.4 |
where wij is the assimilation efficiency (which is independent of body size and temperature) [26], and equals 0.45 for herbivores and 0.85 for carnivores [31]; Cij is consumption rate (J s−1); and λ is a constant converting basal metabolic rate to field metabolic rate and equals 3 [16]. Units of energy and time, but not volume, were used for consumption in equation (2.4), resulting in a dimensionless value for consumption efficiency. We were unable to parametrize λ for ectotherms, and in particular for the species used here. Consequently, we used a sensitivity analysis to examine the effect of varying λ. This analysis indicated that varying λ had no qualitative effect on our results. Values of yij less than the critical threshold of 1 indicated that those individuals had not consumed sufficient food to meet their metabolic demand. The effect of body mass on per capita consumption efficiency was calculated by
| 2.5 |
for both temperatures, and analysed in the same way as the metabolic and consumption rates above.
The per capita metabolic rate and consumption rate for each species was calculated at 16.5°C and 18.5°C. While different approaches have been used recently to analyse mass–abundance [32] and mass–metabolism relationships [33], our aim was to explicitly test for intraspecific variation in the mass–metabolism relationships with change in temperature, and we therefore analysed the metabolism and consumption rate data using ANCOVA models, treating species, temperature and body mass as main effects. Species identity was treated as a fixed effect as it represented an important source of variation that we wished to quantify. The model used was
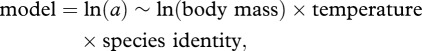 |
2.5 |
where a was the response variable (i.e. metabolic rate, consumption rate or consumption efficiency), body mass was a co-variate, and temperature and species identity were factors with two and five levels, respectively. The data used in these analyses are available at http://www.datadryad.org (doi:10.5061/dryad.951j9). All analyses were conducted using the R statistical package (v. 2.15.0; http://www.r-project.org/).
3. Results
(a). Metabolism
The metabolism analysis resulted in a significant three-way interaction term, indicating that the metabolism–body mass relationships of the five marine species varied as temperature increased (ANCOVA, mass × species identity × temperature, p < 0.01, see electronic supplementary material, table S1). When the data were pooled for each temperature, irrespective of species identity, the resulting pre- and post-warming slopes were 0.25 and 0.33, respectively, which were both significantly different from the hypothetical metabolism–mass scaling coefficient of 0.75 (t-test, p < 0.001, figure 1). As there is some debate regarding the value of the scaling exponent [17,33–36], further t-tests were carried out to compare the metabolism–body mass slopes with exponents of 0.67 and 1 (table 1). At 16.5°C, the seastar, crab and black goby metabolism–body mass regressions recorded slopes similar to 0.75, with slopes of 0.52, 0.35 and 0.64, respectively. There was no significant warming effect on the metabolism–mass relationship for seastars, and while body mass accounted for variation in the metabolic rate of black gobies at 16.5°C (r2 = 0.92, table 2), warming resulted in a breakdown of this relationship (r2 = 0.003, table 2).
Figure 1.
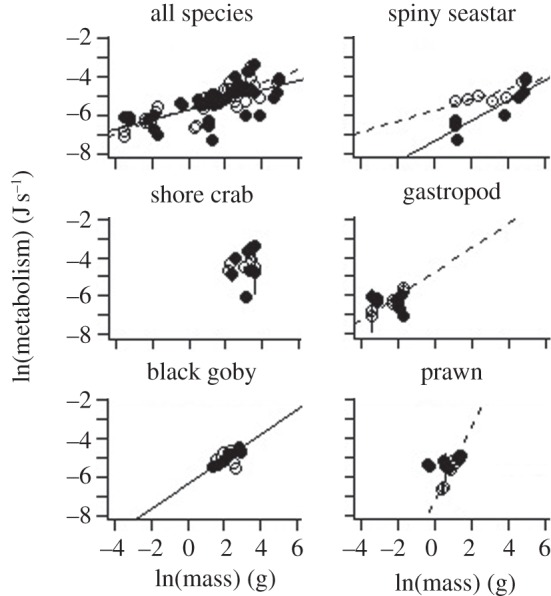
Metabolism (J s−1)–body mass (g, soft tissue wet mass) regressions for each species at 16.5°C (solid circles and solid lines) and 18.5°C (open circles and dashed lines). For clarity, standard error estimates for metabolism have been added to the species-specific plots, rather than to the pooled species plot. See table 2 for intercept and slope values.
Table 1.
Results of t-tests comparing the slopes, b, of the metabolism-, consumption- and consumption efficiency–body mass regressions with predicted slopes of 0.67, 0.75 and 1.
| metabolism |
consumption |
consumption efficiency |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | 0.67 | 0.75 | 1 | b | 0.67 | 0.75 | 1 | b | 0.67 | 0.75 | 1 | |
| 16.5°C | ||||||||||||
| seastar | 0.52 | n.s. | n.s. | p<0.05 | −0.02 | n.s. | n.s. | n.s. | −0.46 | n.s. | n.s. | n.s. |
| crab | 0.35 | n.s. | n.s. | n.s. | 1.11 | n.s. | n.s. | n.s. | 0.99 | n.s. | n.s. | n.s. |
| gastropod | −0.25 | p<0.01 | p<0.01 | p<0.01 | −0.18 | p<0.001 | p<0.001 | p<0.001 | 0.08 | p<0.01 | p<0.001 | p<0.001 |
| black goby | 0.64 | n.s. | n.s. | p<0.01 | −0.93 | n.s. | n.s. | n.s. | −0.54 | n.s. | n.s. | n.s. |
| prawn | 0.22 | p<0.01 | p<0.05 | p<0.001 | 0.71 | n.s. | n.s. | n.s. | 0.48 | n.s. | n.s. | n.s. |
| all species | 0.25 | p<0.001 | p<0.001 | p<0.001 | 0.20 | p<0.01 | p<0.001 | p<0.001 | 0.14 | p<0.001 | p<0.001 | p<0.001 |
| 18.5°C | ||||||||||||
| seastar | 0.28 | p<0.01 | p<0.01 | p<0.001 | — | — | — | — | — | — | — | — |
| crab | 0.35 | n.s. | n.s. | n.s. | 0.67 | n.s. | n.s. | n.s. | 0.55 | n.s. | n.s. | p<0.05 |
| gastropod | 0.63 | n.s. | n.s. | p<0.05 | 0.90 | n.s. | n.s. | n.s. | 0.27 | n.s. | n.s. | n.s. |
| black goby | −0.04 | n.s. | n.s. | p<0.05 | 0.24 | n.s. | n.s. | p<0.05 | 0.54 | n.s. | n.s. | n.s. |
| prawn | 1.88 | p<0.01 | p<0.01 | p<0.05 | 0.83 | n.s. | n.s. | n.s. | –1.05 | p<0.01 | p<0.01 | p<0.01 |
| all species | 0.33 | p<0.001 | p<0.001 | p<0.001 | 0.43 | n.s. | p<0.05 | p<0.001 | 0.62 | n.s. | n.s. | p<0.05 |
Table 2.
Regression coefficients for metabolism-, consumption- and consumption efficiency–body mass relationships.
| metabolism |
consumption |
consumption efficiency |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| intercept | slope |
r2 | intercept | slope |
r2 | intercept | slope |
r2 | ||||
| 16.5°C | ||||||||||||
| seastar | −7.36 | 0.52 | p < 0.01 | 0.78 | −2.00 | −0.02 | n.s. | 0.0004 | 4.84 | −0.46 | n.s. | 0.13 |
| shore crab | −5.57 | 0.35 | n.s. | 0.04 | −5.76 | 1.11 | p < 0.05 | 0.46 | −1.17 | 0.99 | p < 0.05 | 0.44 |
| gastropod | −6.99 | −0.25 | n.s. | 0.27 | −2.90 | −0.18 | n.s. | 0.22 | 1.79 | 0.08 | n.s. | 0.05 |
| black goby | −6.38 | 0.64 | p < 0.001 | 0.92 | −2.94 | −0.93 | n.s. | 0.04 | 0.72 | −0.54 | n.s. | 0.01 |
| prawn | −5.41 | 0.22 | n.s. | 0.44 | −4.43 | 0.71 | n.s. | 0.30 | 0.69 | 0.48 | n.s. | 0.17 |
| all species | −5.69 | 0.25 | p < 0.001 | 0.42 | −3.68 | 0.20 | n.s. | 0.04 | 1.16 | 0.14 | n.s. | 0.02 |
| 18.5°C | ||||||||||||
| seastar | −5.76 | 0.28 | p < 0.05 | 0.69 | — | — | — | — | — | — | — | — |
| shore crab | −5.24 | 0.35 | n.s. | 0.22 | −4.13 | 0.67 | p < 0.01 | 0.61 | 0.17 | 0.55 | p < 0.05 | 0.50 |
| gastropod | −4.80 | 0.63 | p < 0.01 | 0.81 | −3.33 | 0.90 | p < 0.05 | 0.40 | −0.84 | 0.27 | n.s. | 0.06 |
| black goby | −4.88 | −0.04 | n.s. | 0.003 | −5.38 | 0.24 | n.s. | 0.08 | −1.25 | 0.54 | n.s. | 0.26 |
| prawn | −7.27 | 1.88 | p < 0.001 | 0.94 | −5.03 | 0.83 | n.s. | 0.33 | 1.96 | −1.05 | n.s. | 0.44 |
| all species | −5.65 | 0.33 | p < 0.001 | 0.71 | −4.18 | 0.43 | p < 0.01 | 0.21 | −0.54 | 0.62 | p < 0.001 | 0.51 |
(b). Consumption
ANCOVA applied to the consumption rate data revealed one significant interaction term (consumer mass × temperature, see electronic supplementary material, table S1) suggesting that, irrespective of species identity, warming significantly increased the slope of the consumption–consumer mass relationship. (t-test, p < 0.05, figure 2). The non-significant three-way (mass × temperature × species identity, see electronic supplementary material, table S1) interaction term suggests that there is a consistent warming response across species, with respect to their consumption–body mass relationships. The resultant common slopes were 0.20 and 0.43, for 16.5°C and 18.5°C, respectively; however, the relationships were weak (table 2). It was interesting to note that at 16.5°C, seastar consumption rates declined at larger body masses, and no prey was consumed by this species at the increased temperature of 18.5°C.
Figure 2.
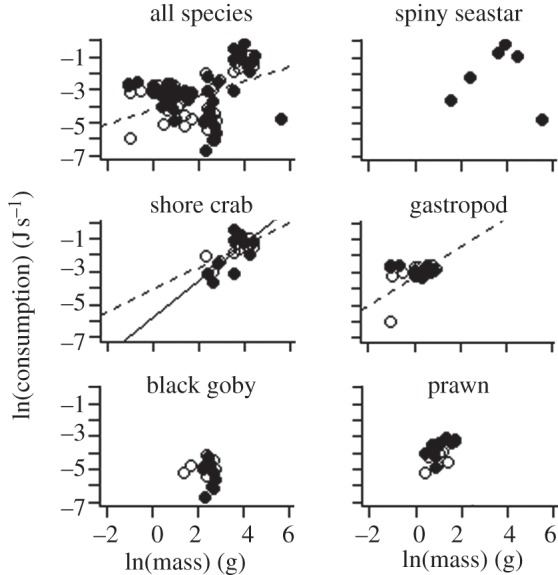
Consumption (J s−1 l−1)–body mass (g, wet mass) regressions for each species at 16.5°C (solid circles and solid lines) and 18.5°C (open circles and dashed lines). The seastars did not consume any prey at 18.5°C and are therefore not included in this figure. See table 2 for intercept and slope values.
(c). Consumption efficiency
The consumption efficiency response to increasing temperature was consistent across species (ANCOVA, consumer mass × temperature × species identity, p = 0.23, electronic supplementary material, table S1). The resultant common consumption efficiency-body mass slopes at 16.5°C and 18.5°C were 0.14 and 0.62, respectively (table 2 and figure 3). The 18.5°C slope was similar to the predicted 0.67 and 0.75 scalings but significantly different from 1. Overall, regardless of consumer identity, efficiencies were seen to decrease with increasing temperature, with 26 per cent of the individuals not consuming sufficient food to meet metabolic demand at 16.5°C, compared with 58 per cent at 18.5°C.
Figure 3.
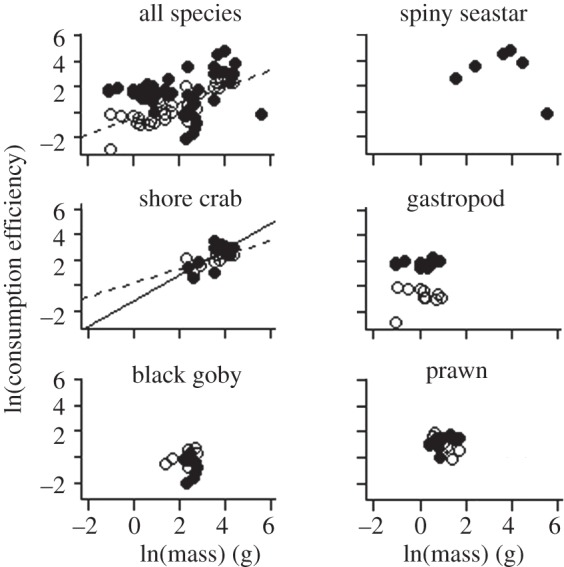
Consumption efficiency–body mass (g, wet mass) regressions for each species at 16.5°C (solid circles and solid lines) and 18.5°C (open circles and dashed lines). The seastars did not consume any prey at 18.5°C and are therefore not included in this figure. See table 2 for intercept and slope values.
4. Discussion
Metabolic theory predicts that species metabolism scales with body mass as a ¾ power-law [14]. Similarly, the same theory can be applied to various biological rates, including consumption. Several studies, however, offer alternative predictions [33–36]. Glazier [37] and Killen et al. [38] suggest that metabolic scalings not only are influenced by temperature and body mass, but that these scalings can be affected by the activity level and ecology of organisms. Glazier [37] has suggested that depending on the activity level of an animal, the scaling exponent can vary from 0.67 to 1; therefore, as metabolic (activity) level increases, the scaling exponent decreases from 1 to 0.67 and then starts to return to 1. This U-shaped relationship reflects an increase in metabolic rate from low resting rates to active rates. The animals investigated in our study vary greatly in their ecological lifestyle, largely influenced by their functional feeding strategies, and although standard (resting) metabolic rates were measured for this study, the intrinsic ecological adaptations of these animals may account for some of the variation found in these results.
(a). Metabolism
Surprisingly, our results show some marked deviations from the 0.67, 0.75 and 1 scaling predictions, which require further explanation. Species body-mass–metabolism relationships did not respond to increasing temperature, as predicted. Although the scaling exponents of three of the five species (seastar, crab and black goby) measured at the lower temperature were consistent with theory, the prawn and gastropod species had considerably lower exponents that varied significantly from the predicted 0.67, 0.75 and 1 slopes. The subsequent breakdown of the black goby metabolic rate–body mass relationship and the unpredictable warming response of seastars suggest that, in this system, these species may have been close to their upper thermal tolerances at our lower temperature. Our experiments were conducted in August to coincide with a period when temperatures are close to their seasonal maximum in the study system and when small increases in temperature would have large physiological effects.
The seastar was the largest species in the experiment, and yet when corrected for metabolically inert body mass, it did not have the highest estimates of metabolic rate. This result may seem surprising, but in previous studies, echinoderms have been known to exhibit very low metabolic rates [39,40]. The echinoderm oxygen transport system is relatively inefficient, and only a small proportion of the body accounts for 90 per cent of its oxygen consumption. The echinoderm body also consists of a largely metabolically inert calcified skeleton and perivisceral fluid, which when combined with their relatively low activity levels accounts for their low metabolic capacity [41].
The present study used a relatively narrow range of body masses in contrast to large-scale synthetic studies that have quantified mass–metabolism relationships [13], and this might represent a limitation of the present study. It is therefore perhaps not surprising that we find our overarching body-mass–metabolism relationship deviates from a ¾ scaling. However, we believe that the experimental species in this study adequately represent a typical shallow sub-tidal benthic community, and represent a body size range that would be typically found at fine spatial scales. Additionally, several published studies have investigated body-mass–metabolism scaling, using equivalent or smaller orders of magnitude of body mass (see [10,21,42] for examples).
(b). Consumption
If metabolic demand is to be met, increases in oxygen consumption due to warming should be reflected in subsequent increases in trophic impacts of predators on prey, which our consumption results confirm. With consumption–body mass scalings of 1.11 and 0.71 respectively, the decapods, C. maenas and P. serratus, were the only species to scale consistently with theory. The vulnerability of the seastar to warming is very apparent from the measures of consumption, as none of the individuals consumed prey at 18.5°C. The feeding rate of the seastar, Pisaster ochraceus, has been reported to increase with temperature but plateaus at 16°C, with an optimum rate lying between 15°C and 20°C [4]. Our feeding trials were conducted in August at the ambient seawater temperature of 16.5°C in order to ascertain species physiological rates at the higher limit of the local temperature range. The lack of a feeding response in seastars suggests that this species' population may be close to its maximum temperature tolerance at this latitude, beyond which animals become stressed and can no longer meet their metabolic demand. This evolutionarily ancient species may be physiologically ill-equipped to cope with acute temperature changes. Interestingly, the shape of the consumption rate–body mass relationship of the seastar at 16.5°C is consistent with reported hump-shaped consumption rate–mass relationships [7].
(c). Consumption efficiency
Of the 20 black goby individuals measured during the experiment, only six consumed sufficient prey to meet their metabolic requirements. This species is a ‘sit and wait’ predator, relying largely on its camouflaging coloration [43]. The increased visibility of the predator in the arena may have resulted in greater prey avoidance but this should have had negligible impact, given the high prey densities and the standardized period of starvation imposed on the fish. It is more probable that black gobies require a longer acclimatization period and may have consumed more if the experimental period was prolonged. Arena effects on the consumption rates of the seastar should also have been negligible owing to high mussel densities and varying mussel sizes. The largest seastar in the trials succeeded in consuming prey, suggesting that the size of the arenas were adequate for the largest consumers to feed.
5. Conclusion
In conclusion, our results support the prediction that body mass is positively correlated with metabolism, yet show interspecific mass–metabolism variation that is consistent with scalings among species reported elsewhere [36]. In this study, the effect of body mass and temperature on metabolic rates was inconsistent across species, while species identity was highly significant in explaining the differences in metabolic rate across temperature. These results, together with the inability of some species to meet their metabolic demand at higher temperatures, strongly suggest that while body size explains a large proportion of the variation in species' physiological responses to environmental change, much more needs to be considered when trying to predict the likely responses of species to global warming, e.g. life history, physiology (ectotherms and endotherms) and mode of feeding. Brey [36] questions the validity of universal scaling factors for the mass–metabolism–temperature relationship and instead supports the use of a wide range of species-specific factors, to which the results of this study offer further support. Our results demonstrate that species respond idiosyncratically to warming, around an overarching body-mass-driven trend. Such responses highlight the vulnerability of some species to warming and further complicate predictions regarding population and hence community and ecosystem level responses to future scenarios of climate change. It is clear that further investigation is necessary to understand and characterize deviations from allometric scalings that have been derived across several orders of magnitude of body size. Further examination of species- and taxon-specific scalings across temperate, polar and tropical ecosystems would be necessary to help fine-tune the scale at which taxonomic characteristics are represented yet generalities can be applied.
Acknowledgments
The authors thank R. Anderson, K. Beyer, S. Egerton, L. Harman, O. Heilmayer, N. McSweeney, C. Palmer, A. Twomey, C. Twomey and J. Walsh for their field assistance, and two anonymous reviewers whose comments greatly improved this manuscript. Authors' participation at the SIZEMIC Workshop (Hamburg) was supported by the German Research Foundation (JA 1726/3-1) and the Cluster of Excellence CliSAP (EXC177), University of Hamburg funded through the DFG. Permission to conduct this research was granted by NPWS Permit R13/09, and was funded by the Irish Research Council for Science, Engineering and Technology.
References
- 1.Climate change 2007. The physical science basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L.) Cambridge, UK and New York, NY: Cambridge University Press [Google Scholar]
- 2.Brose U., Dunne J. A., Montoya J. M., Petchey O. L., Schneider F. D., Jacob U. 2012. Climate change in size-structured ecosystems. Phil. Trans. R. Soc. B 367, 2903–2912 10.1098/rstb.2012.0232 (doi:10.1098/rstb.2012.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allan E. L., Froneman P. W., Hodgson A. N. 2006. Effects of temperature and salinity on the standard metabolic rate (smr) of the Caridean shrimp Palaemon peringueyi . J. Exp. Mar. Biol. Ecol. 337, 103–108 10.1016/j.jembe.2006.06.006 (doi:10.1016/j.jembe.2006.06.006) [DOI] [Google Scholar]
- 4.Gooding R. A., Harley C. D. G., Tang E. 2009. Elevated water temperature and carbon dioxide concentration increase the growth of a keystone echinoderm. Proc. Natl Acad. Sci. USA 106, 9316–9321 10.1073/pnas.0811143106 (doi:10.1073/pnas.0811143106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saska P., Martinkova Z., Honek A. 2010. Temperature and rate of seed consumption by ground beetles (Carabidae). Biol. Control 52, 91–95 10.1016/j.biocontrol.2009.07.016 (doi:10.1016/j.biocontrol.2009.07.016) [DOI] [Google Scholar]
- 6.Powell M. L., Watts S. A. 2006. Effect of temperature acclimation on metabolism and hemocyanin binding affinities in two crayfish, Procambarus clarkii and Procambarus zonangulus . Comp. Biochem. Physiol. A Mol. Integr. Physiol. 144, 211–217 10.1016/j.cbpa.2006.02.032 (doi:10.1016/j.cbpa.2006.02.032) [DOI] [PubMed] [Google Scholar]
- 7.Brose U., Ehnes R. B., Rall B. C., Vucic-Pestic O., Berlow E. L., Scheu S. 2008. Foraging theory predicts predator–prey energy fluxes. J. Anim. Ecol. 77, 1072–1078 10.1111/j.1365-2656.2008.01408.x (doi:10.1111/j.1365-2656.2008.01408.x) [DOI] [PubMed] [Google Scholar]
- 8.Clarke A., Johnston N. M. 1999. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 68, 893–905 10.1046/j.1365-2656.1999.00337.x (doi:10.1046/j.1365-2656.1999.00337.x) [DOI] [PubMed] [Google Scholar]
- 9.Gillooly J. F., Brown J. H., West G. B., Savage V. M., Charnov E. L. 2001. Effects of size and temperature on metabolic rate. Science 293, 2248–2251 10.1126/science.1061967 (doi:10.1126/science.1061967) [DOI] [PubMed] [Google Scholar]
- 10.Meehan T. D. 2006. Mass and temperature dependence of metabolic rate in litter and soil invertebrates. Physiol. Biochem. Zool. 79, 878–884 10.1086/505997 (doi:10.1086/505997) [DOI] [PubMed] [Google Scholar]
- 11.Yvon-Durocher G., Allen A. P. 2012. Linking community size structure and ecosystem functioning using metabolic theory. Phil. Trans. R. Soc. B 367, 2998–3007 10.1098/rstb.2012.0246 (doi:10.1098/rstb.2012.0246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown J. H., Gillooly J. F. 2003. Ecological food webs: high-quality data facilitate theoretical unification. Proc. Natl Acad. Sci. USA 100, 1467–1468 10.1073/pnas.0630310100 (doi:10.1073/pnas.0630310100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 10.1890/03-9000 (doi:10.1890/03-9000) [DOI] [Google Scholar]
- 14.Kleiber M. 1932. Body size and metabolism. Hilgardia 6, 315–353 [Google Scholar]
- 15.Clarke A. 2004. Is there a universal temperature dependence of metabolism? Funct. Ecol. 18, 252–256 10.1111/j.0269-8463.2004.00842.x (doi:10.1111/j.0269-8463.2004.00842.x) [DOI] [Google Scholar]
- 16.Savage V. M., Gillooly J. F., Woodruff W. H., West G. B., Allen A. P., Enquist B. J., Brown J. H. 2004. The predominance of quarter-power scaling in biology. Funct. Ecol. 18, 257–282 10.1111/j.0269-8463.2004.00856.x (doi:10.1111/j.0269-8463.2004.00856.x) [DOI] [Google Scholar]
- 17.Glazier D. S. 2005. Beyond the ‘3/4-power law’: variation in the intra and interspecific scaling of metabolic rate in animals. Biol. Rev. 80, 611–662 10.1017/S1464793105006834 (doi:10.1017/S1464793105006834) [DOI] [PubMed] [Google Scholar]
- 18.Gillooly J. F., Allen A. P., Savage V. M., Charnov E. L., West G. B., Brown J. H. 2006. Response to Clarke and Fraser: effects of temperature on metabolic rate. Funct. Ecol. 20, 400–404 10.1111/j.1365-2435.2006.01110.x (doi:10.1111/j.1365-2435.2006.01110.x) [DOI] [Google Scholar]
- 19.Makarieva A. M., Gorshkov V. G., Li B.-L., Chown S. L., Reich P. B., Gavrilov V. M. 2008. Mean mass-specific metabolic rates are strikingly similar across life's major domains: evidence for life's metabolic optimum. Proc. Natl Acad. Sci. USA 105, 16 994–16 999 10.1073/pnas.0802148105 (doi:10.1073/pnas.0802148105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahlström E., Persson L., Diehl S., Byström P. 2000. Size-dependent foraging efficiency, cannibalism and zooplankton community structure. Oecologia 123, 138–148 10.1007/s004420050999 (doi:10.1007/s004420050999) [DOI] [PubMed] [Google Scholar]
- 21.Rall B. C., Vucic-Pestic O., Ehnes R. B., Emmerson M., Brose U. 2010. Temperature, predator–prey interaction strength and population stability. Global Change Biol. 16, 2145–2157 10.1111/j.1365-2486.2009.02124.x (doi:10.1111/j.1365-2486.2009.02124.x) [DOI] [Google Scholar]
- 22.Vucic-Pestic O., Rall B. C., Kalinkat G., Brose U. 2010. Allometric functional response model: body masses constrain interaction strengths. J. Anim. Ecol. 79, 249–256 10.1111/j.1365-2656.2009.01622.x (doi:10.1111/j.1365-2656.2009.01622.x) [DOI] [PubMed] [Google Scholar]
- 23.Rall B. C., Brose U., Hartvig M., Kalinkat G., Schwarzmüller F., Vucic-Pestic O., Petchey O. L. 2012. Universal temperature and body-mass scaling of feeding rates. Phil. Trans. R. Soc. B 367, 2923–2934 10.1098/rstb.2012.0242 (doi:10.1098/rstb.2012.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweeney J., et al. 2008. Climate change: refining the impacts for Ireland. STRIVE Report. Johnstown Castle, Co. Wexford, Ireland: Environmental Protection Agency
- 25.Gatti S., Brey T., Müller W., Heilmayer O., Holst G. 2002. Oxygen microoptodes: a new tool for oxygen measurements in aquatic animal ecology. Mar. Biol. 140, 1075–1085 10.1007/s00227-002-0786-9 (doi:10.1007/s00227-002-0786-9) [DOI] [Google Scholar]
- 26.Peter R. H. 1983. The ecological implications of body size. Cambridge, UK: Cambridge University Press [Google Scholar]
- 27.Ricciardi A., Bourget E. 1998. Weight-to-weight conversion factors for marine benthic macroinvertebrates. Mar. Ecol. Progr. Series 163, 245–251 10.3354/meps163245 (doi:10.3354/meps163245) [DOI] [Google Scholar]
- 28.Bejerregaard P., Depledge M. H. 2002. Trace metal concentrations and contents in the tissues of the shore crab Carcinus meanas: effects of size and tissue hydration. Mar. Biol. 141, 741–752 10.1007/s00227-002-0859-9 (doi:10.1007/s00227-002-0859-9) [DOI] [Google Scholar]
- 29.McKinney R. A., Glatt S. M., Williams S. R. 2004. Allometric length–weight relationships for benthic prey of aquatic wildlife in coastal marine habitats. Wildl. Biol. 10, 241–249 [Google Scholar]
- 30.Vasseur D. A. M., McCann K. S. 2005. A mechanistic approach for modeling temperature-dependent consumer-resource dynamics. Am. Nat. 166, 184–198 10.1086/431285 (doi:10.1086/431285) [DOI] [PubMed] [Google Scholar]
- 31.Yodzis P., Innes S. 1992. Body size and consumer-resource dynamics. Am. Nat. 139, 1151–1175 10.1086/285380 (doi:10.1086/285380) [DOI] [Google Scholar]
- 32.Isaac N. J. B., Storch D., Carbone C. 2011. Taxonomic variation in size–density relationships challenges the notion of energy equivalence. Biol. Lett. 7, 615–618 10.1098/rsbl.2011.0128 (doi:10.1098/rsbl.2011.0128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehnes R. B., Rall B. C., Brose U. 2011. Phylogenetic grouping, curvature and metabolic scaling in terrestrial invertebrates. Ecol. Lett. 14, 993–1000 10.1111/j.1461-0248.2011.01660.x (doi:10.1111/j.1461-0248.2011.01660.x) [DOI] [PubMed] [Google Scholar]
- 34.Darveau C. A., Suarez R. K., Andrews R. D., Hochachka P. W. 2002. Allometric cascade as a unifying principle of body mass effects on metabolism. Nature 417, 166–170 10.1038/417166a (doi:10.1038/417166a) [DOI] [PubMed] [Google Scholar]
- 35.Glazier D. S. 2006. The 3/4-power law is not universal: evolution of isometric, ontogenetic metabolic scaling in pelagic animals. BioScience 56, 325–332 10.1641/0006-3568(2006)56[325:TPLINU]2.0.CO;2 (doi:10.1641/0006-3568(2006)56[325:TPLINU]2.0.CO;2) [DOI] [Google Scholar]
- 36.Brey T. 2010. An empirical model for estimating aquatic invertebrate respiration. Methods Ecol. Evol. 1, 92–101 10.1111/j.2041-210X.2009.00008.x (doi:10.1111/j.2041-210X.2009.00008.x) [DOI] [Google Scholar]
- 37.Glazier D. S. 2010. A unifying explanation for diverse metabolic scaling in animals and plants. Biol. Rev. 85, 111–138 10.1111/j.1469-185X.2009.00095.x (doi:10.1111/j.1469-185X.2009.00095.x) [DOI] [PubMed] [Google Scholar]
- 38.Killen S. S., Atkinson D., Glazier D. S. 2010. The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol. Lett. 13, 184–193 10.1111/j.1461-0248.2009.01415.x (doi:10.1111/j.1461-0248.2009.01415.x) [DOI] [PubMed] [Google Scholar]
- 39.Binyon J. 1972. Physiology of echinoderms. Oxford, UK: Pergamon Press [Google Scholar]
- 40.Lawrence J. 1986. A functional biology of echinoderms. London, UK: Croom Helm [Google Scholar]
- 41.Webster S. 1975. Oxygen consumption in echinoderms from several geographical locations, with particular reference to the Echinodea. Biol. Bull. 148, 157–164 10.2307/1540656 (doi:10.2307/1540656) [DOI] [PubMed] [Google Scholar]
- 42.Hughes S. J. M., Ruhl H. A., Hawkins L. E., Hauton C., Boorman B., Billett D. S. M. 2011. Deep-sea echinoderm oxygen consumption rates and an interclass comparison of metabolic rates in Asteroidea, Crinoidea, Echinoidea, Holothuroidea and Ophiuroidea. J. Exp. Biol. 214, 2512–2521 10.1242/jeb.055954 (doi:10.1242/jeb.055954) [DOI] [PubMed] [Google Scholar]
- 43.Gibson R. N. 1969. The biology and behaviour of littoral fish. Oceanogr. Mar. Biol. Annu. Rev. 7, 367–410 [Google Scholar]


