Abstract
Background
Immunohistochemical staining for p16INK4a and Ki-67 has been used to improve the accuracy in making a diagnosis of the uterine cervix cancer on biopsy. This study was conducted to examine the usefulness of these markers in the pathological diagnosis based on cervical biopsy.
Methods
We selected a consecutive series of 111 colposcopically directed cervical punch biopsies. Using these biopsy samples, we performed an immunohistochemical staining for p16INK4a and Ki-67 to establish a diagnosis. The slides were circulated among four pathologists in a sequential order: the hematoxylin and eosin (H&E) slide, H&E slide and p16INK4a-stained slide, and H&E slide, p16INK4a- and Ki-67-stained slides.
Results
The overall rates of the concordance in the first, the second, and the third diagnoses were 77.5%, 82.0%, and 82.0%, respectively. The rate of the concordance in the diagnosis of cervical intraepithelial neoplasm (CIN) 2/3 was increased from 62.2% to 73.0%. But there was a variability in the rate of the revision of the diagnosis between the pathologists. With the application of criteria for interpreting the expressions of p16INK4a and Ki-67, benign and CIN 1 lesions showed a p16INK4a expression score of 0 or 1. But CIN 2 and CIN 3 lesions showed a p16INK4a expression score of 2 and 3, respectively.
Conclusions
The immunostain for p16INK4a and Ki-67 might be useful in reducing an inter-observer variability. But criteria for interpreting both markers should be strictly applied.
Keywords: Uterine cervix, p16INK4a, Ki-67
Histologic assessment of the uterine cervix is a standard method in the management of women with abnormal cytologic smears who are suspected to have premalignant or malignant conditions.1 But there can be a misinterpretation of histologic changes in association with various situations such as atrophy, immature metaplasia, transitional metaplasia, repair or inflammatory atypia, inadequate sample size and tissue artifact, which may eventually cause an interobserver variation and a poor intraobserver reproducibility.1-3 To date, various techniques have been introduced to improve diagnostic accuracy.4,5 Of these, it has been proven that p16INK4a expression is specific to cervical intraepithelial neoplasm (CIN) 1 lesions with a high-risk human papillomavirus (HR-HPV), CIN 2/3 and invasive squamous lesions and both non-invasive and invasive glandular lesions. In addition, it has also been widely used in a daily practice of surgical pathology.2-13 Nevertheless, it has been reported to be disadvantageous in that there is an inconsistency in the methods and criteria for interpretation of p16INK4a expression.2,13 To improve the diagnostic accuracy, other markers including Ki-67 have been used along with p16INK4a in a histological assessment of squamous and glandular dysplasia and carcinoma of the uterine cervix.2,5,9 There is a discrepancy, however, in the interpretation of these proliferative markers between the authors.
Given the above background, we conducted this study to examine the usefulness of the immunohistochemical stain for p16INK4a and Ki-67 in the pathological evaluation of uterine cervical biopsy samples and the re-assessment based on criteria for interpreting their expression.
MATERIALS AND METHODS
Case selection
We selected a consecutive series of 111 colposcopically directed cervical punch biopsies from the Department of Pathology, Korea University Anam Hospital. Using these biopsy samples, we performed an immunohistochemical staining for p16INK4a and Ki-67 to establish a diagnosis during a period ranging from September 2009 to June 2010. Exclusion criteria were invasive squamous cell carcinoma and glandular lesions.
Immunohistochemical staining
The immunohistochemical stain for p16INK4a was performed using CINtec Histology Kit (mtm laboratories AG, Heidelberg, Germany) and for Ki-67 using mouse monoclonal antibody, clone MIB-1 from Dako Corp. (Carpinteria, CA, USA). DAKO Link48 immunostainer (Dako Corp.) was used for an immunohistochemical staining. The sections were de-paraffinized and sequentially treated for antigen epitope retrieval and endogenous peroxidase blocking. Following a 30-minute incubation with primary antibodies, the section for p16INK4a was treated with visualization polymer reagent conjugated with horseradish peroxidase and goat anti-Mouse Fab' antibody fragment. For the section for Ki-67, Dako EnVision TM+/HRP kits (Dako Corp.) was used for 30 minutes. Following a 5-minute application of substrate-3,3'-diaminobenzidine chromogen solution, the section was counterstained with Harris hematoxylin. This was followed by the dehydration and mounting for a microscopic examination.
To validate the staining procedure for each staining run, we used a positive control slide containing tissue section from the cervical biopsy of invasive squamous cell carcinoma with known diffuse immunoreactivity for p16INK4a. For a negative control, we used the buffer solution rather than the primary antibody.
Evaluation of the slides
Four surgical pathologists reviewed the hematoxylin and eosin (H&E)-stained slides, and then made a diagnosis based on the histological features. These diagnoses include benign lesions such as immature squamous metaplasia, transitional metaplasia or borderline condyloma-like lesion, CIN 1, CIN 2, and CIN 3. Then, each pathologist performed the second-round review of the H&E slide and the slide stained for p16INK4a, and the third-round review of H&E slide and those for p16INK4a and Ki-67. This was followed by the interpretation of the slides by each pathologist based on his or her own criteria. Then, the diagnosis was revised.
Analysis of the results
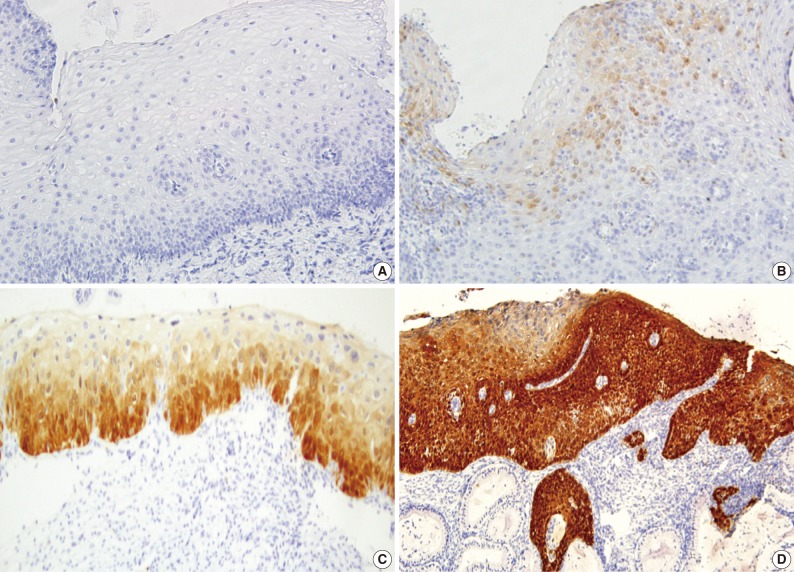
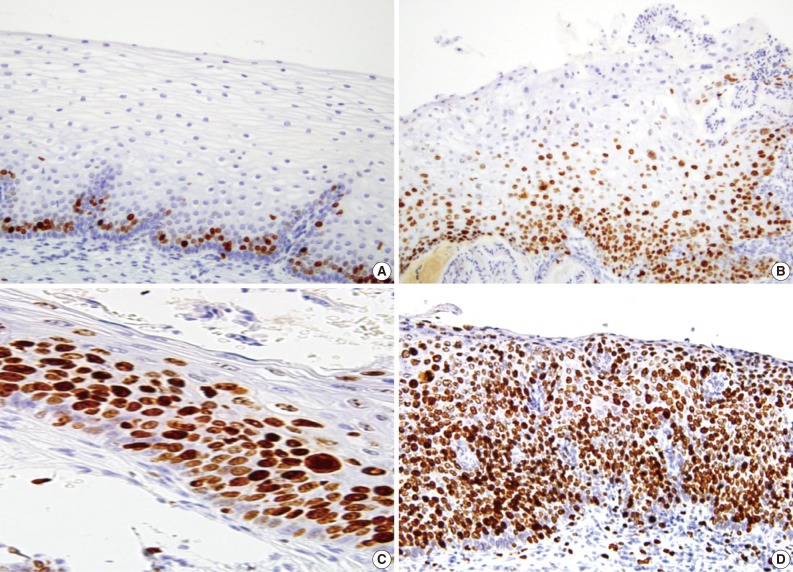
A majority of diagnoses were made based on concordance in the diagnosis between the pathologists; a diagnosis was established when at least three of four pathologists agreed on the diagnosis. Thus, majority diagnosis was defined. If there was a lack of the majority diagnosis, a final consensus diagnosis was reached through a simultaneous meeting of all the four pathologists. This was followed by the interpretation of the immunohistochemical findings for p16INK4a and Ki-67 based on the interpretation criteria. The expression of p16INK4a was scored as 0 (negative), 1 (focal and weak cytoplasmic staining with or without nuclear staining in superficial or intermediate layers), 2 (intermediate between 1 and 3), and 3 (diffuse and strong nuclear staining in lower 2/3 or whole layers with or without cytoplasmic staining) (Fig. 1). The expression of Ki-67 was evaluated based on the distribution and proportion of positive cells in the squamous epithelium. Ki-67 was occasionally seen in suprabasal layers of the normal squamous epithelium (score 0), while its expression was extended to upper layers of the squamous epithelium with the progression of dysplasia from CIN 1 (score 1) to CIN 3 (score 3) (Fig. 2).
Fig. 1.
The expression of p16INK4a is scored as 0 (A), 1 (B), 2 (C), and 3 (D).
Fig. 2.
The expression of Ki-67 is scored as 0 (A), 1 (B), 2 (C), and 3 (D).
Statistical analysis
Statistical analysis was performed using SPSS ver. 12.0 (SPSS, Inc., Chicago, IL, USA). An intra-observer variability of each pathologist was evaluated by calculating the kappa value for the pairs of 'first and second' and 'first and third' diagnoses. The p16INK4a immunostain scores were classified into 0/1 and 2/3. Then, these values were compared with the histologic groups: benign/CIN 1 and CIN 2/3. A p-value of <0.05 was considered statistically significant.
RESULTS
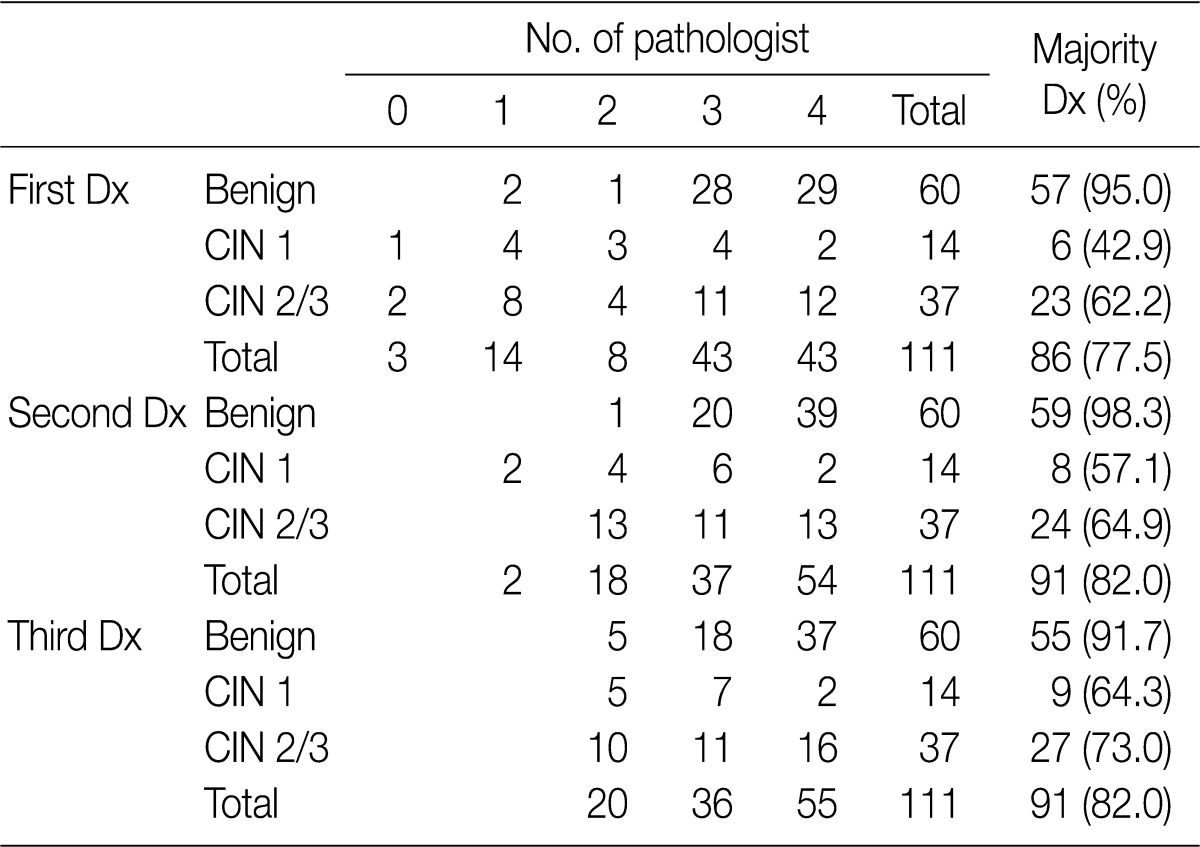
Following an analysis of the agreement that was reached in more than three pathologists according to the category (benign, CIN 1 and CIN 2/3), the overall rates of the concordance in the first (H&E slide only), the second (H&E slide and p16INK4a-stained slide), and the third (H&E slide, p16INK4a- and Ki-67-stained slides) diagnoses were 77.5%, 82.0%, and 82.0%, respectively. The rate of the concordance in the diagnosis of CIN 2/3 was increased from 62.2% to 73.0%. For the first-round diagnosis made based on the H&E slide only, none or one pathologist agreed on the same diagnosis as a consensus diagnosis in 17 cases (15.3%). But there were at least two pathologists who agreed on both the second- and third-round diagnosis. The second-round diagnosis was a consensus one in two cases (1.8%) (Table 1).
Table 1.
The majority diagnosis based on the first-round diagnosis (hematoxylin and eosin [H&E] slide only), the second-round diagnosis (H&E slide and p16INK4a-stained slide) and the third-round diagnosis (H&E p16INK4a- and Ki-67-stained slides) according to the histologic category
Values are presented as number (%).
Dx, diagnosis; CIN, cervical intraepithelial neoplasm.
Following a review of the immunostained slides for p16INK4a and Ki-67, the pathologist 1, 2, 3, and 4 revised the diagnosis at concordance rates of 17.1% and 4.5%; 15.3% and 13.5%; 4.5% and 6.3%; and 29.7% and 10.8%, respectively, in the corresponding order. In revising the diagnosis, the pathologist 3 had the highest concordance rate (kappa value, 0.925 and 0.900) and the pathologist 4 did at the lowest concordance rate (kappa value, 0.589 and 0.850). In addition, the pathologist 1 and 2 had the concordance rates of 0.710 and 0.929 and 0.733 and 0.774, respectively, in the corresponding order. There were significant differences in the kappa values between the four pathologists (p<0.05).
In 29 cases, we reviewed the H&E slides and the immunostained slides for p16INK4a and Ki-67. Then, the consensus diagnosis was reached. There were eight cases showing an immature squamous metaplasia or a borderine condyloma-like change with a p16INK4a expression score of 2 or an increased Ki-67 labeling. Of these cases, five cases and the remaining three finally had a revised diagnosis of benign cellular change and CIN 1, respectively (Fig. 3). There were two cases of senile atrophy with CIN 2 or CIN 3 lesions with a p16INK4a expression score of 3 and an increased Ki-67 labeling. Typically, atrophic squamous epithelium showed a rare Ki-67 expression (Fig. 4). The remaining 19 cases showed a broad-spectrum of squamous epithelial changes from squamous metaplasia to CIN 3 on the same biopsy. But these cases had a p16INK4a and Ki-67 expression score of 2 or 3, which led to the diagnosis CIN 2 or 3 (Fig. 5).
Fig. 3.
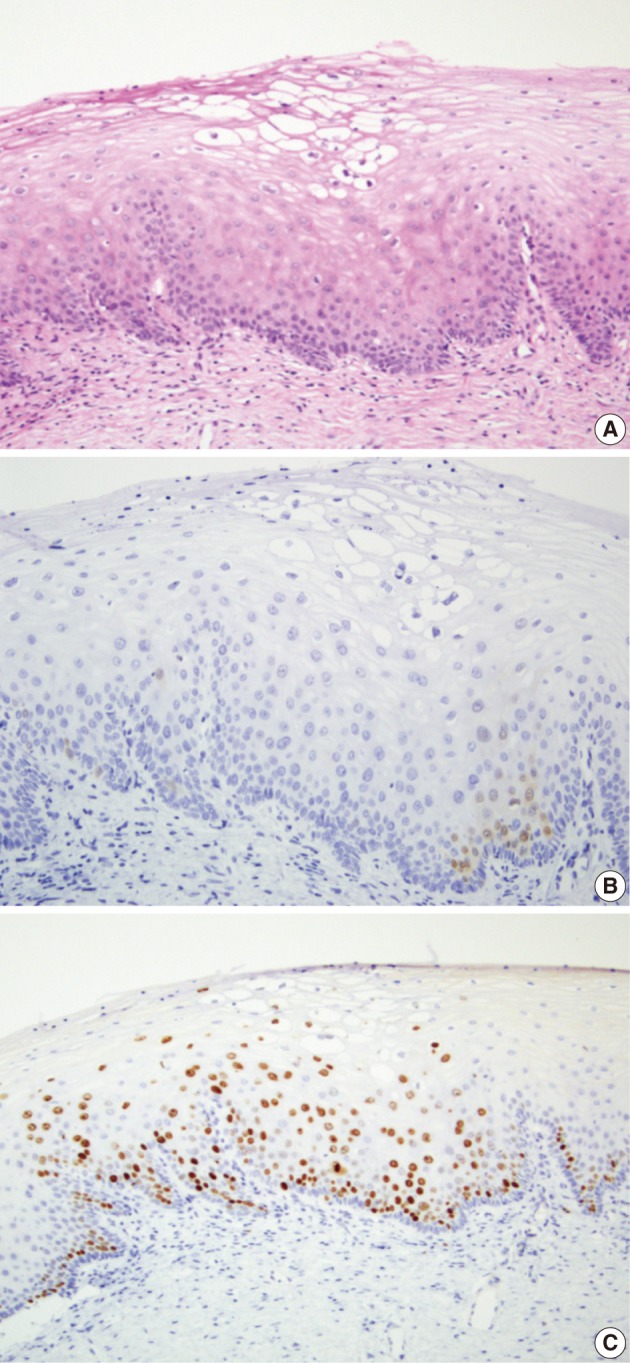
Squamous epithelium with an area of koilocytotic atypia (A) shows a focal nuclear expression (score 1) of p16INK4a in suprabasal layer (B) and an increased expression of (score 1) Ki-67 (C). The consensus diagnosis is cervical intraepithelial neoplasm 1.
Fig. 4.
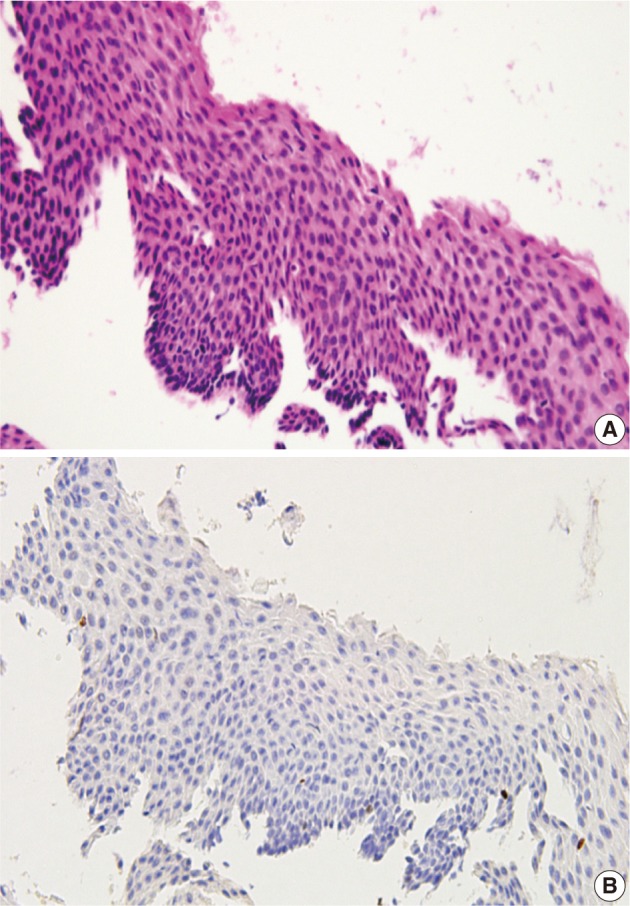
Atrophic squamous epithelium mimicking cervical intraepithelial neoplasm 3 (A) shows a rare (score 0) Ki-67 expression (B).
Fig. 5.
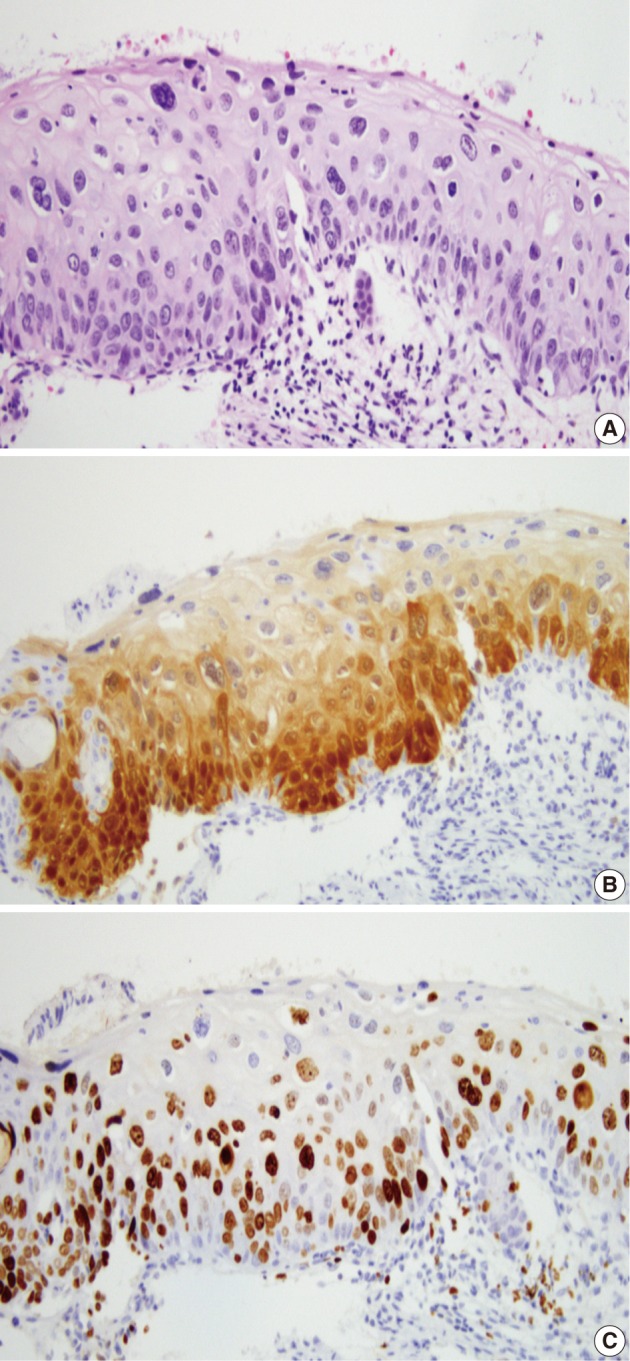
The cases interpreted as cervical intraepithelial neoplasm (CIN) 2 by two pathologists and CIN 1 and CIN 3 by one pathologist each (A) show a p16INK4a expression score of 2 (B) and a Ki-67 expression score of 2 (C). The consensus diagnosis is CIN 2.
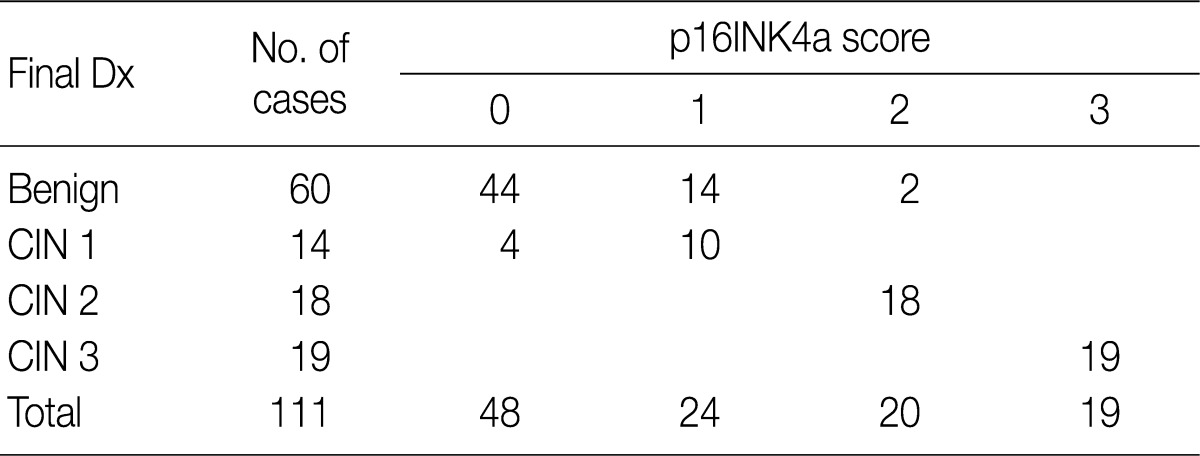
Following a review of the H&E-stained slides and immunostained ones for p16INK4a and Ki-67, there were 60 cases whose diagnosis was classified as a benign lesion. In addition, there were 44 cases showing no expression of p16INK4a, 14 cases with a p16INK4a expression score of 1 and two cases with a p16INK4a expression score of 2. Fourteen cases were diagnosed as CIN 1 lesions, which comprised four cases with a p16INK4a expression score of 1 and ten cases with a p16INK4a expression score of 2 (Fig. 6). All the 18 cases of CIN 2 lesions showed a p16INK4a expression score of 2, but 19 cases of CIN 3 lesions showed a p16INK4a expression score of 3 (Table 2).
Fig. 6.
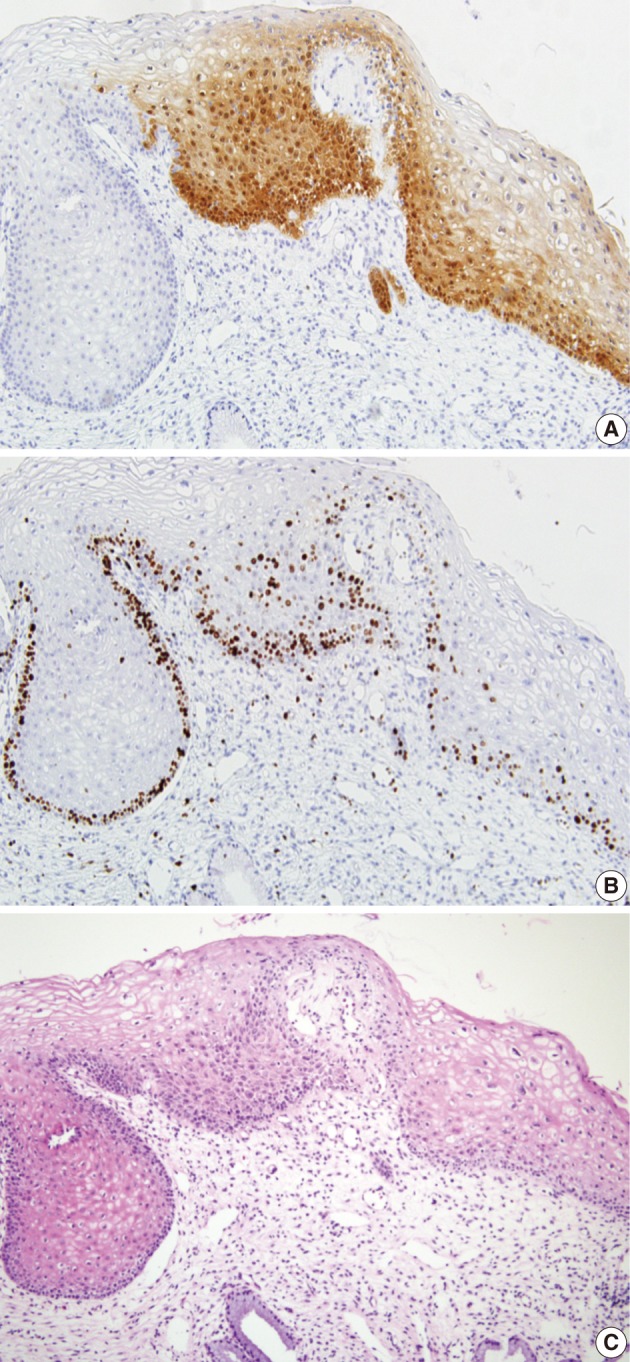
The squamous lesion shows a p16INK4a expression score of 2 (A) but a Ki-67 expression score of 1 (B). The consensus diagnosis is cervical intraepithelial neoplasm 1 (C).
Table 2.
The p16INK4a-immunostain score according to the final histologic category
Dx, diagnosis; CIN, cervical intraepithelial neoplasm.
DISCUSSION
p16INK4a is a cyclin-dependent kinase (CDK)-4 inhibitor that blocks the cell cycle by inhibiting CDK-4 with the inactivation of pRb protein. Its expression in normal adult tissue is limited to the proliferative endometrium, breast ducts, gastric antral cells, esophageal squamous epithelium, salivary glands, and some neuroendocrine cells.14 The expression of p16INK4a has been shown to disappear in some types of tumor including familial melanoma, but it is overexpressed in other types of tumor such as gastric adenocarcinoma, malignant lymphoma and lung cancer.15=19 Although p16INK4a is not expressed in the normal epithelium, it is overexpressed in almost all cases of epithelial neoplasia of uterine cervix. Therefore, an immunohistochemistry of p16INK4a has been used as an indicator for making a diagnosis of pre-invasive and invasive epithelial neoplasia of uterine cervix.2-5,7-13,20 In uterine cervix, E7 protein of HR-HPV inactivates Rb protein, which normally inhibits transcription of p16INK4a, resulting in the accumulation of p16INK4a protein.21 It can therefore be inferred that HR-HPV-infected epithelial cells of the uterine cervix have a positive immunohistochemistry for p16INK4a. This can therefore be used as a surrogate indicator for the presence of HR-HPV-induced squamous and glandular lesions of the uterine cervix. A substantial number of studies have been conducted to examine the diagnostic value of p16INK4a immunostain in improving the accuracy of histological diagnosis using the tissue samples of the uterine cervix. This showed that it is useful in many aspects; the immunostain for p16INK4a assists in detecting very small lesions with fragmentation which can be missed or overlooked on the H&E stain.3,7,8 In addition, it is also helpful for distinguishing HPV-related lesions from benign mimickers including severe atrophy, reactive changes with inflammation or immature metaplasia.2,7,9 Our results revealed these findings in cases which were misdiagnosed as CIN 3 on the H&E slide but proved to be severe atrophy following the second-round review of the slide stained for p16INK4a. We conducted the current study to examine the usefulness of p16INK4a and Ki-67 immunostaining in reducing an inter-observer variation in the histopathological interpretation of cervical biopsy between the pathologists. The pathologist 4 revised the diagnosis the most frequently based on the p16INK4a- and Ki-67-immunostains but the pathologist 3 did the least frequently. Klaes et al.3 reported that the concordance rate was improved to 96%. But Horn et al.8 reported that it was improved to 74%. In the current study with the additional use of p16INK4a immunostains, the concordance rate was increased from 77.5% to 82.0%. The current three-tier CIN classification system in the interpretation of the H&E slides is well known for its poor reproducibility.22 In addition, it is also difficult to make a differentiation between benign mimickers and CIN 1.3,22 Our results also showed that no majority diagnosis was made for some cases because there was a variability in the interpretation based on the current three-tier CIN system between the pathologists.
Technical and interpretational methods of p16INK4a immunostain are another cause of problem. Various p16INK4a antibody clones have been used by authors. In addition, a lack of the standard staining protocol has been pointed as potential factors of inconsistent result of p16INK4a immunostain.2,13 Lack of standardized criteria for interpretation of p16INK4a immunostain was also problematic. Staining intensity, number of stained cells and distribution of stained cells were arbitrarily selected by authors.2-5,7-13,20,23,24 Moreover, there was also a variability in the cut-off point for grading the percentage of the stained cells depending on the authors. Because p16INK4a is a nuclear protein, its immunostaining property is expected to show nuclear stain. But cytoplasmic stain with or without nuclear stain can be seen through posttranscriptional modification or overproduction of p16INK4a protein.13,20
Based on our grading system, we scored the expression of p16INK4a as 0 (negative), 1 (focal and weak cytoplasmic staining with or without nuclear staining in superficial or intermediate layers), 2 (intermediate between 1 and 3), and 3 (diffuse and strong nuclear staining in lower 2/3 or whole layers with or without cytoplasmic staining). This showed that cases with score 3 were categorized into CIN 2 and 3 but most of the non-neoplastic cervix were negative for p16INK4a. Our results showed that the first-round diagnosis was modified if the lesion showed a diffuse stain of p16INK4a in full layers. Conversely, a complete absence of p16INK4a immunostaining could rule out an associated high-grade squamous intraepithelial lesion.25,26 A small number of weakly stained cells were solely found or clustered in upper layers of the CIN 1 lesions. But some lesions showed a strong expression in lower part of the epithelium in diffuse pattern. The latter cases were thought to be associated with a HR-HPV infection.21 This deserves more meticulous follow-up studies because there is a possibility that the above lesions might progress to a higher degree of CIN.2,3 In the current study, the first diagnosis of CIN 1 was revised the most frequently following the review of the p16INK4a stain. It is presumed, however, that there are potential risks of misdiagnosis in regard to the weakly or scattered stained cells. This is because weak positive stain can be seen in non-HPV related uterine cervix such as in normal epithelium with inflammation or metaplasia.4,10,11,24 Staining property of non-epithelial cells including fibroblasts, endothelial cells and inflammatory cells has also been reported.4,6,24 Our results showed that there were 14 cases with a p16INK4a expression score of 1 and two cases with a p16INK4a expression score of 2. Following the re-assessment of the histologic sections in combination with the Ki-67 labeling, however, the above cases were interpreted as an immature squamous metaplasia or a borderline condyloma-like lesion.
Despite a diagnostic value of p16INK4a in significantly improving the accuracy in making a diagnosis of the epithelial lesions of the uterine cervix, there are various exceptions that it should be used in combination with other markers in establishing a diagnosis in a routine clinical setting.12 Ki-67 has been used in combination with p16INK4a because it can be of help for making a differentiation of a high-grade CIN from benign mimickers. This is particularly the case with atrophy and a low-grade CIN whose p16INK4a immunostains are ambiguous or equivocal.2,5 But there is a variability in the interpretation of Ki-67-immunostained slides depending on the authors. The normal squamous epithelium of uterine cervix shows occasionally positive cells in suprabasal layers,27 and its expression is increased from the lower to upper layers with a progression of the dysplasia from CIN 1 to CIN 3. We evaluated the degree of expression of Ki-67 in squamous epithelium, in lower 1/3, half or more than 2/3, corresponding to the CIN 1, CIN 2 or CIN 3, respectively. Its expression was helpful in not only interpreting the atrophic squamous epithelium which was misinterpreted as CIN 3 but also grading the CIN lesions in combination with p16INK4a. Again, Ki-67 expression is also increased in the inflammatory and reactive cases or normal epithelium in the luteal phase.5,10
In conclusion, the concomitant use of Ki-67 and p16INK4a immunostains might be more effective. In addition, criteria for interpreting both markers should be strictly applied to the evaluation of cervical lesions, which would be essential for reducing an inter-observer variability.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ ASCCP-Sponsored Consensus Conference. 2001 Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120–2129. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 2.Kalof AN, Cooper K. p16INK4a immunoexpression: surrogate marker of high-risk HPV and high-grade cervical intraepithelial neoplasia. Adv Anat Pathol. 2006;13:190–194. doi: 10.1097/00125480-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Klaes R, Benner A, Friedrich T, et al. p16INK4a immunohistochemistry improves interobserver agreement in the diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol. 2002;26:1389–1399. doi: 10.1097/00000478-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Tringler B, Gup CJ, Singh M, et al. Evaluation of p16INK4a and pRb expression in cervical squamous and glandular neoplasia. Hum Pathol. 2004;35:689–696. doi: 10.1016/j.humpath.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Keating JT, Ince T, Crum CP. Surrogate biomarkers of HPV infection in cervical neoplasia screening and diagnosis. Adv Anat Pathol. 2001;8:83–92. doi: 10.1097/00125480-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153:1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Neill CJ, McCluggage WG. p16 expression in the female genital tract and its value in diagnosis. Adv Anat Pathol. 2006;13:8–15. doi: 10.1097/01.pap.0000201828.92719.f3. [DOI] [PubMed] [Google Scholar]
- 8.Horn LC, Reichert A, Oster A, et al. Immunostaining for p16INK4a used as a conjunctive tool improves interobserver agreement of the histologic diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol. 2008;32:502–512. doi: 10.1097/PAS.0b013e31815ac420. [DOI] [PubMed] [Google Scholar]
- 9.McCluggage WG. Immunohistochemistry as a diagnostic aid in cervical pathology. Pathology. 2007;39:97–111. doi: 10.1080/00313020601123961. [DOI] [PubMed] [Google Scholar]
- 10.Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 11.Agoff SN, Lin P, Morihara J, Mao C, Kiviat NB, Koutsky LA. p16(INK4a) expression correlates with degree of cervical neoplasia: a comparison with Ki-67 expression and detection of high-risk HPV types. Mod Pathol. 2003;16:665–673. doi: 10.1097/01.MP.0000077518.78046.0C. [DOI] [PubMed] [Google Scholar]
- 12.Murphy N, Ring M, Heffron CC, et al. p16INK4A, CDC6, and MCM5: predictive biomarkers in cervical preinvasive neoplasia and cervical cancer. J Clin Pathol. 2005;58:525–534. doi: 10.1136/jcp.2004.018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulvany NJ, Allen DG, Wilson SM. Diagnostic utility of p16INK4a: a reappraisal of its use in cervical biopsies. Pathology. 2008;40:335–344. doi: 10.1080/00313020802035907. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen GP, Stemmer-Rachamimov AO, Shaw J, Roy JE, Koh J, Louis DN. Immunohistochemical survey of p16INK4A expression in normal human adult and infant tissues. Lab Invest. 1999;79:1137–1143. [PubMed] [Google Scholar]
- 15.Guenova M, Rassidakis GZ, Gorgoulis VG, et al. p16INK4A is regularly expressed in Hodgkin's disease: comparison with retinoblastoma, p53 and MDM2 protein status, and the presence of Epstein-Barr virus. Mod Pathol. 1999;12:1062–1071. [PubMed] [Google Scholar]
- 16.Vo QN, Geradts J, Boudreau DA, Bravo JC, Schneider BG. CDKN2A promoter methylation in gastric adenocarcinomas: clinical variables. Hum Pathol. 2002;33:1200–1204. doi: 10.1053/hupa.2002.130108. [DOI] [PubMed] [Google Scholar]
- 17.Leversha MA, Fielding P, Watson S, Gosney JR, Field JK. Expression of p53, pRB, and p16 in lung tumours: a validation study on tissue microarrays. J Pathol. 2003;200:610–619. doi: 10.1002/path.1374. [DOI] [PubMed] [Google Scholar]
- 18.Rocco JW, Sidransky D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp Cell Res. 2001;264:42–55. doi: 10.1006/excr.2000.5149. [DOI] [PubMed] [Google Scholar]
- 19.Beasley MB, Lantuejoul S, Abbondanzo S, et al. The P16/cyclin D1/Rb pathway in neuroendocrine tumors of the lung. Hum Pathol. 2003;34:136–142. doi: 10.1053/hupa.2003.8. [DOI] [PubMed] [Google Scholar]
- 20.Murphy N, Ring M, Killalea AG, et al. p16INK4A as a marker for cervical dyskaryosis: CIN and cGIN in cervical biopsies and ThinPrep smears. J Clin Pathol. 2003;56:56–63. doi: 10.1136/jcp.56.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanley MA. Prognostic factors and new therapeutic approaches to cervical cancer. Virus Res. 2002;89:241–248. doi: 10.1016/s0168-1702(02)00192-2. [DOI] [PubMed] [Google Scholar]
- 22.Stoler MH, Schiffman M Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500–1505. doi: 10.1001/jama.285.11.1500. [DOI] [PubMed] [Google Scholar]
- 23.Lee JS, Shin JG, Ko GH, Lee JH, Kim HW. Studies on the expression of the p16(INK4A), p53, and Ki-67 labeling index in inflammatory and neoplastic diseases of the uterine cervix. Korean J Pathol. 2004;38:238–243. [Google Scholar]
- 24.Dray M, Russell P, Dalrymple C, et al. p16(INK4a) as a complementary marker of high-grade intraepithelial lesions of the uterine cervix. I: Experience with squamous lesions in 189 consecutive cervical biopsies. Pathology. 2005;37:112–124. doi: 10.1080/00313020500058607. [DOI] [PubMed] [Google Scholar]
- 25.Wang SS, Trunk M, Schiffman M, et al. Validation of p16INK4a as a marker of oncogenic human papillomavirus infection in cervical biopsies from a population-based cohort in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13:1355–1360. [PubMed] [Google Scholar]
- 26.Hariri J, Øster A. The negative predictive value of p16INK4a to assess the outcome of cervical intraepithelial neoplasia 1 in the uterine cervix. Int J Gynecol Pathol. 2007;26:223–228. doi: 10.1097/01.pgp.0000236942.51840.56. [DOI] [PubMed] [Google Scholar]
- 27.Konishi I, Fujii S, Nonogaki H, Nanbu Y, Iwai T, Mori T. Immunohistochemical analysis of estrogen receptors, progesterone receptors, Ki-67 antigen, and human papillomavirus DNA in normal and neoplastic epithelium of the uterine cervix. Cancer. 1991;68:1340–1350. doi: 10.1002/1097-0142(19910915)68:6<1340::aid-cncr2820680626>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]