Abstract
Background
Pigment epithelium-derived factor (PEDF) is an anti-angiogenic factor. The purpose of this study is to examine the involvement of PEDF in the angiogenesis and biological behavior of bladder transitional cell carcinoma (TCC).
Methods
We examined the expression of PEDF in 99 bladder TCCs and ten non-neoplastic tissues, and evaluated microvessel density (MVD).
Results
The positive immunoreactivity for PEDF was seen in normal urothelium in 60% (6/10) and TCC in 13% (13/99). The PEDF expression had a significant correlation with MVD, i.e., a low MVD in 42% (5/12), a middle MVD in 11% (8/76) and a high MVD 0% (0/11) of tumors. The PEDF expression was not significantly correlated with the differentiation and invasion of TCC, but the degree of MVD was significantly higher in both high grade TCC and the pT2 tumors.
Conclusions
The degree of PEDF expression is significantly higher in normal bladder urothelium than bladder TCC; it is inversely correlated with the angiogenesis; and it is not related to the differentiation and progression of TCC. It can therefore be concluded that bladder TCC would initially occur if there is a lack of the PEDF expression.
Keywords: Bladder transitional cell carcinomas, Angiogenesis, Pigment epithelium-derived factor
Unbalanced expression of pro- and anti-angiogenic factors occurs in pathologic conditions leading to excessive angiogenesis and these include hypoxia and tumor growth. Angiogenesis is an essential event for the growth, persistence and metastasis of solid tumors. In addition, it has also been studied in bladder transitional cell carcinomas (TCC).1,2 The quantification of angiogenesis is made, using microvessel density (MVD) as an indicator that is presumed to be a valuable prognostic indicator. Antibodies against CD34, which is predominantly found in endothelial cells, have proven to be particularly reliable in assessing MVD.3 Pigment epithelium-derived factor (PEDF) is, a glycoprotein with a molecular weight of 50-kDa, and it was first identified and isolated from the conditioned media of primary human fetal retinal pigment epithelial cells.4 It was later found to have a potent anti-angiogenic activity.5 It has been reported that PEDF has an inhibitory effect on tumor growth in a variety of cancers.6-9 Recent studies have shown that PEDF expression is decreased and it is inversely correlated with the expression of vascular endothelial growth factor (VEGF) in bladder TCC.10
Given the above background, we conducted this study to examine the expression of PEDF in bladder TCC using an immunohistochemical staining. To do this, we analyzed the degree of the expression of PEDF in association with clinicopathological parameters and MVD. Thus, we attempted to clarify the involvement of PEDF in angiogenesis and the biological behavior of bladder TCC.
MATERIALS AND METHODS
Tissue samples and the patient population
We used 99 paraffin-embedded bladder TCCs and 10 normal bladder tissues that had been collected at the Department of Pathology at Dongguk University Gyeongju Hospital. The cancer tissues were obtained from a transurethral resection of the bladder TCC. In addition, the normal bladder epithelial tissues were obtained from cases of chronic cystitis. The tumor was graded in accordance with the World Health Organization/International Society of Urological Pathology (WHO-ISUP) classification, and the pathological T stage (pT, depth of invasion) was also determined.11 The age distribution of the patients ranged between 30 and 87 years old, and the male to female ratio was 6.1:1.
Immunohistochemistry and assessment
Urinary bladder sections of 4 µm thickness were made and they were spread on poly-L-lysine coated slides. The paraffin sections were immersed in three changes of xylene and they were hydrated using a graded series of alcohol solutions. Antigen retrieval was routinely performed by immersing the sections in a 0.01 M citrate buffer (pH 6.0) in an autoclave for 15 minutes. The endogenous peroxidase activity was blocked with a 3% hydrogen peroxide for 15 minutes. This was followed by the incubation of the sections with primary antibody for two hours at room temperature, where the primary antibodies include mouse monoclonal anti-PEDF antibody (1:200, Merck Millipore, Billerica, MA, USA) and anti-CD34 antibody (1:200, Dako, Santa Barbara, CA, USA). Immunohistochemical staining was done with an EnVision kit (Dako) and the color was developed with 3, 3'-diaminobenzidine tetrahydrochloride (Zymed Laboratories, Inc., South San Francisco, CA, USA) as a chromogen. The sections were counterstained with Meyer's hematoxylin for three minutes and then mounted. Mouse IgG isotype rather than the primary antibody was used as a negative control. The immunoreactivity for PEDF was evaluated based on the extensity and intensity. The extensity was graded according to a 4-point scale based on the percentage of stained tumor cells: 0 (the percentage of stained tumor cells, 0-10%), 1 (the percentage of stained tumor cells, 11-20%), 2 (the percentage of stained tumor cells, 21-30%), and 3 (the percentage of stained tumor cells, >30%). In addition, intensity was also graded based on a 3-point scale: 1 (mild), 2 (moderate), and 3 (strong). Based on the sum of extensity and intensity, our cases were divided into two groups: the negative group (1-2) and the positive group (3-6). The MVD was calculated with the identification and quantification of the cross-sections of CD34-positive lumens in five random fields per tumor at a magnification rate of ×400, as reported by Weidner.12 To identify the correlation between the expression of PEDF and MVD, our cases were divided into three categories based on the MVD and these include low (MVD<11), middle (11<MVD<40), and high (MVD>40). We have attempted to perform an immunohistochemical staining for VEGF and transforming growth factor-β in several cases of colon cancers or bladder TCC. But there was no consistency in the pattern of staining. Therefore, we did not perform it in bladder TCCs.
Statistical analysis
Statistical analysis was done with Chi-square test, Fisher's exact test, t-test and one-way ANOVA. Statistical significance was set at a p<0.05. All data was expressed as mean±standard error.
RESULTS
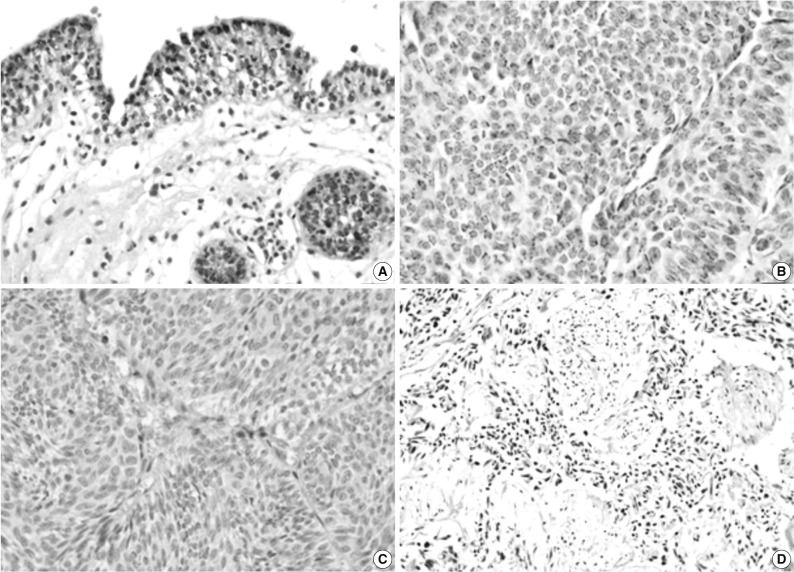
Our cases comprised 39 cases of stage pTa, 31 cases of pT1, and 29 cases of pT2. There were 44 cases of low grade tumor, and 55 cases of high grade tumor. The expression of PEDF had a granular pattern in the cytoplasm of normal urothelium and tumor cells (Fig. 1). The positive immunoreactivity for PEDF was seen in normal urothelium at a proportion of 60% (6/10) and TCC at a rate of 13% (13/99) (Fig. 1). The degree of PEDF expression was significantly higher in normal bladder mucosa as compared with TCC (p=0.025). The positive rate of PEDF expression was seen in 14% (6/44) of total cases of a low grade tumor and in 13% (7/55) of those of a high grade one. In addition, it was also seen in 13% (5/39) of the pTa tumors, 16% (5/31) of the pT1 ones, and 10% (3/29) of the pT2 ones. The expression of PEDF was not significantly correlated with tumor grade and tumor invasion (p>0.05). In addition, it was not also significantly correlated with gender and age (data not shown).
Fig. 1.
Immunohistochemical staining of pigment epithelium-derived factor (PEDF) in normal urothelium (A) and bladder transitional cell carcinoma (TCC) (B-D). The expression of PEDF has a granular pattern in the cytoplasm of normal and neoplastic urothelial cells (B), and some TCCs do not show PEDF expression (C, D).
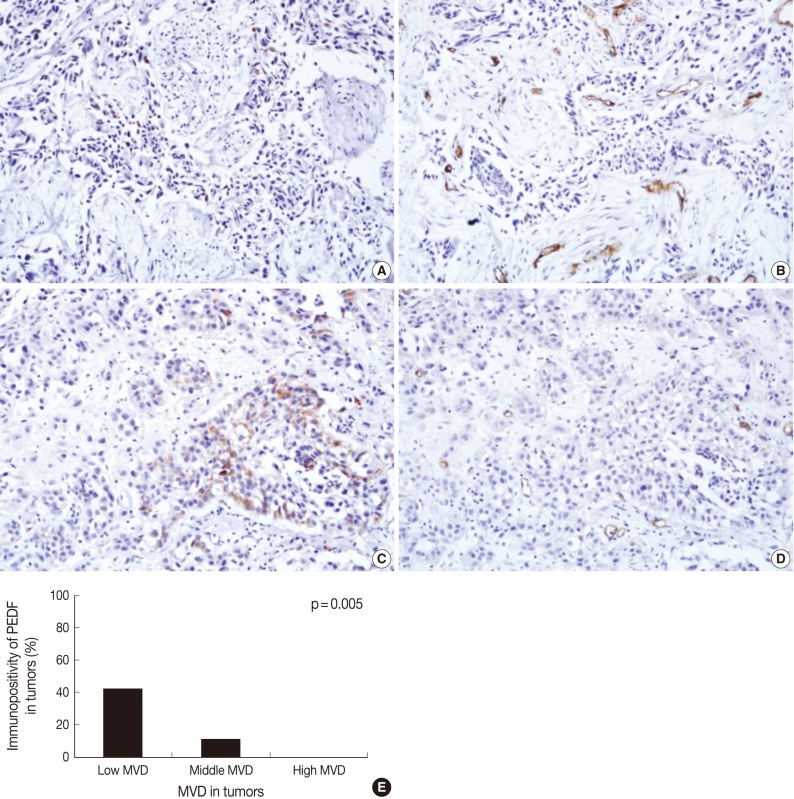
Overall, the mean MVD was 24.0±1.3 in our clinical series (range, 4 to 57; standard deviation, 12.6). In addition, it was 19.2±2.0 in low grade tumors and 27.6±1.5 in high grade ones. Furthermore, it was 19.0±2.0 in the pTa tumors, 24.0±2.0 in the pT1 ones and 30.0±2.1 in the pT2 ones. These results indicate that the mean MVD was significantly higher in high grade TCC as compared with their low grade counterparts (p=0.001). In addition, it was significantly higher in the pT2 tumors as compared with both the pTa and pT1 ones (p<0.05). There were 12 cases (12%) of the low degree of MVD, 76 cases (77%) of the middle degree, and 11 cases (11%) of the high degree. As shown in Fig. 2, the expression of PEDF had a positive correlation with low MVD at a proportion of 42% (5/12), a middle MVD at a proportion of 11% (8/76), and a high MVD at a proportion of 0% (0/11). There was a significant reciprocal correlation between PEDF expression and MVD (p=0.005).
Fig. 2.
Immunohistochemical staining of pigment epithelium-derived factor (PEDF) (A, C) and CD34 (B, D) in bladder transitional cell carcinoma (TCC), and the relationship between PEDF expression and microvessel density (MVD) (E). The degree of MVD is significantly higher in TCCs without PEDF expression (A, B) than TCC with PEDF expression (C, D). Bladder TCC shows a significant reciprocal correlation between PEDF expression and MVD (p=0.005) (E).
DISCUSSION
Angiogenesis is the complex biological process that is involved in the development and formation of new blood vessels and it plays a critical role in the growth and metastasis of tumor. Tumors release both angiogenic factors and angiogenic inhibitors; the latter include angiostatin, thrombospondin, endostatin, and PEDF.5,13-15 PEDF directly inhibits the proliferation and migration of endothelial cells and it also induces apoptosis in activated endothelium. In addition, it also has an anti-angiogenic effect based on the derangement of VEGF signaling pathway.16 It has been reported that PEDF is a more powerful inhibitor than any other known anti-angiogenic factors, whose potency is more than twice higher than angiostatin and more than seven times higher than endostatin.5 There is a significant correlation between the PEDF expression and a low MVD in such malignancies as pancreatic ductal cancer, breast cancer or non-small cell lung cancer.7,17,18 It has recently shown that there is an inverse correlation between the expression of PEDF and VEGF in bladder TCC.10 Our results also showed that there was an inverse correlation between PEDF expression and MVD.
PEDF exerts an anti-migratory activity in a number of tumor cell lines, whose mechanism can be mediated with the downregulation of matrix metalloproteinase-9 that binds to collagen type I and III and by inducing the release of chemoattractants with anti-migratory effect.16,19 In addition, PEDF promotes the differentiation of tumors of neuronal origin.20 Study has therefore been conducted to examine the relationship of PEDF expression with tumor differentiation and pathological T stage. In the current study, however, we could not identify any significant correlations between them. According to a recent study, there was a correlation between the decreased PEDF expression and a higher grade of the tumor but it was not correlated with a higher stage of the bladder TCC.10 In pancreatic ductal cancer, a lower degree of the PEDF expression was associated with a liver metastasis and a shorter survival, but its expression was not correlated with the depth of invasion and histopathological grading.17 The relationship between PEDF expression and clinicopathlogic parameters in breast cancer was also seen and its pattern was consistent with that seen in pancreatic ductal cancer.18 As a whole, PEDF has effect on the migration and differentiation of tumor cells in a cell-type dependent manner.
Our results showed that the degree of PEDF expression was significantly higher in normal bladder urothelium than bladder TCC. This was also seen in a recent study.10 PEDF triggers apoptosis in several tumor cell lines.8,21 Our results could not clarify the mechanisms by which PEDF is involved in apoptosis in normal urothelium. Our results indicate, however, that PEDF expression in normal urothelium may protect epithelial cells by sensitizing cells to stress-induced apoptosis and it can also promote apoptosis indirectly by disrupting angiogenesis. This eventually leads to a hypoxic stress. It can therefore be inferred the bladder TCC would initially occur if there is a lack of the PEDF expression. Further studies are warranted to examine the mechanisms by which the expression of PEDF is involved in the occurrence of bladder TCC.
To summarize, our results showed that the degree of PEDF expression is significantly higher in normal bladder urothelium than bladder TCC; it is inversely correlated with the angiogenesis; and it is not related to the differentiation and progression of TCC. It can therefore be concluded that bladder TCC would initially occur if there is lack of the PEDF expression.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jiménez B, Volpert OV. Mechanistic insights on the inhibition of tumor angiogenesis. J Mol Med (Berl) 2001;78:663–672. doi: 10.1007/s001090000178. [DOI] [PubMed] [Google Scholar]
- 2.Bochner BH, Cote RJ, Weidner N, et al. Angiogenesis in bladder cancer: relationship between microvessel density and tumor prognosis. J Natl Cancer Inst. 1995;87:1603–1612. doi: 10.1093/jnci/87.21.1603. [DOI] [PubMed] [Google Scholar]
- 3.Teo NB, Shoker BS, Jarvis C, Martin L, Sloane JP, Holcombe C. Angiogenesis and invasive recurrence in ductal carcinoma in situ of the breast. Eur J Cancer. 2003;39:38–44. doi: 10.1016/s0959-8049(02)00248-4. [DOI] [PubMed] [Google Scholar]
- 4.Tombran-Tink J, Johnson LV. Neuronal differentiation of retinoblastoma cells induced by medium conditioned by human RPE cells. Invest Ophthalmol Vis Sci. 1989;30:1700–1707. [PubMed] [Google Scholar]
- 5.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 6.Hase R, Miyamoto M, Uehara H, et al. Pigment epithelium-derived factor gene therapy inhibits human pancreatic cancer in mice. Clin Cancer Res. 2005;11(24 Pt 1):8737–8744. doi: 10.1158/1078-0432.CCR-05-1323. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Chen J, Ke Y, Mansel RE, Jiang WG. Expression of pigment epithelial derived factor is reduced in non-small cell lung cancer and is linked to clinical outcome. Int J Mol Med. 2006;17:937–944. [PubMed] [Google Scholar]
- 8.Guan M, Pang CP, Yam HF, Cheung KF, Liu WW, Lu Y. Inhibition of glioma invasion by overexpression of pigment epithelium-derived factor. Cancer Gene Ther. 2004;11:325–332. doi: 10.1038/sj.cgt.7700675. [DOI] [PubMed] [Google Scholar]
- 9.Guan M, Jiang H, Xu C, Xu R, Chen Z, Lu Y. Adenovirus-mediated PEDF expression inhibits prostate cancer cell growth and results in augmented expression of PAI-2. Cancer Biol Ther. 2007;6:419–425. doi: 10.4161/cbt.6.3.3757. [DOI] [PubMed] [Google Scholar]
- 10.Feng CC, Ding Q, Zhang YF, et al. Pigment epithelium-derived factor expression is down-regulated in bladder tumors and correlates with vascular endothelial growth factor and matrix metalloproteinase-9. Int Urol Nephrol. 2011;43:383–390. doi: 10.1007/s11255-010-9834-4. [DOI] [PubMed] [Google Scholar]
- 11.Epstein JI, Amin MB, Reuter VR, Mostofi FK Bladder Consensus Conference Committee. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Am J Surg Pathol. 1998;22:1435–1448. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169–180. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 13.O'Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 14.Good DJ, Polverini PJ, Rastinejad F, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Garcia NI, Volpert OV, Jimenez B. Pigment epithelium-derived factor as a multifunctional antitumor factor. J Mol Med (Berl) 2007;85:15–22. doi: 10.1007/s00109-006-0111-z. [DOI] [PubMed] [Google Scholar]
- 17.Uehara H, Miyamoto M, Kato K, et al. Expression of pigment epithelium-derived factor decreases liver metastasis and correlates with favorable prognosis for patients with ductal pancreatic adenocarcinoma. Cancer Res. 2004;64:3533–3537. doi: 10.1158/0008-5472.CAN-03-3725. [DOI] [PubMed] [Google Scholar]
- 18.Zhou D, Cheng SQ, Ji HF, et al. Evaluation of protein pigment epithelium-derived factor (PEDF) and microvessel density (MVD) as prognostic indicators in breast cancer. J Cancer Res Clin Oncol. 2010;136:1719–1727. doi: 10.1007/s00432-010-0830-y. [DOI] [PubMed] [Google Scholar]
- 19.Notari L, Miller A, Martínez A, et al. Pigment epithelium-derived factor is a substrate for matrix metalloproteinase type 2 and type 9: implications for downregulation in hypoxia. Invest Ophthalmol Vis Sci. 2005;46:2736–2747. doi: 10.1167/iovs.04-1489. [DOI] [PubMed] [Google Scholar]
- 20.Crawford SE, Stellmach V, Ranalli M, et al. Pigment epithelium-derived factor (PEDF) in neuroblastoma: a multifunctional mediator of Schwann cell antitumor activity. J Cell Sci. 2001;114(Pt 24):4421–4428. doi: 10.1242/jcs.114.24.4421. [DOI] [PubMed] [Google Scholar]
- 21.Doll JA, Stellmach VM, Bouck NP, et al. Pigment epithelium-derived factor regulates the vasculature and mass of the prostate and pancreas. Nat Med. 2003;9:774–780. doi: 10.1038/nm870. [DOI] [PubMed] [Google Scholar]