Abstract
Background
To elucidate the clinicopathologic features and their implications on the immunohistochemistry in cases of molecular apocrine breast cancer (MABC).
Methods
Immunohistochemical (IHC) staining for estrogen receptor (ER), human epidermal growth factor receptor 2 (HER-2), cytokeratin (CK) 5/6, epidermal growth factor receptor (EGFR), androgen receptor (AR), gamma-glutamyltrasferase 1 (GGT1) and Ki-67 was performed on tissue microarray breast cancer samples from 204 patients. Phenotypes of breast cancer were divided based on the IHC status of ER, AR and GGT1 into the following: luminal type, ER positive and AR and/or GGT1 positive; basal type, ER, AR, and GGT1 negative; non-basal type, ER positive and AR and GGT1 negative; and MABC type, ER negative and AR and/or GGT1 positive.
Results
In our series of patients (n=204), there were 26 cases of MABC. Besides, there were 18, 60, and 100 cases of luminal type, basal type and non-basal type, respectively. The MABC demonstrated apocrine histology and a higher prevalence of HER-2 positivity than other phenotypes. With the basal type, the MABC manifested a more frequent expression of CK5/6 and EGFR and a higher Ki-67 index than other phenotypes (p<0.001). There were no significant differences in patient prognosis between the phenotypes of breast cancer.
Conclusions
MABC are distinguishable from other phenotypes based on the apocrine histology and a higher expression rate of HER-2.
Keywords: Breast neoplasms, Molecular apocrine, Immunohistochemistry
Breast cancer is one of the representative diseases for which the molecular classification is based on the profile of gene expression in addition to the conventional histological grade. Its molecular classifications include luminal A, luminal B, human epidermal growth factor receptor 2 (HER-2), basal-like and normal-like types.1,2 Based on a gene clustering analysis, Farmer et al.3 separately described the molecular apocrine breast cancer (MABC). The MABC accounts for 8-14% of total cases of breast cancer and it is characterized by the apocrine histology, androgen receptor (AR) positivity, estrogen receptor (ER) negativity and presence outside the basal-like subtypes. Furthermore, HER-2 amplification is frequently found. The MABC is thought to be associated with a poor long-term survival. Although it is defined by a gene clustering analysis, there are specific surrogate immunohistochemical markers such as AR and gamma-glutamyltrasferase 1 (GGT1).3,4 MABC is one of the subtypes of breast cancer based on its molecular classification, little is known about its clinicopathologic characteristics as compared with other molecular subtypes such as luminal type, HER-2 type or basal-like type.
Given the above background, we conducted this study to elucidate the clinicopathologic features and their implications on the immunohistochemistry (IHC) in cases of MABC.
MATERIALS AND METHODS
Patient selection and clinicopathologic analysis
The current study enrolled patients who had been diagnosed with invasive ductal carcinoma at Severance Hospital during the year of 2001. Exclusion criteria include patients who underwent preoperative neoadjuvant treatment as hormonal therapy, chemotherapy, and radiotherapy. The current study was approved by the Institutional Review Board (IRB) of Severance Hospital. A total of 204 patients were enrolled in the current study, who submitted a written informed consent. Representative hematoxylin and eosin (H&E)-stained slides from each patient were retrospectively reviewed by two pathologists (YJC and JSK). Histologic grade was estimated using the Nottingham grading system.5 Apocrine histology was evaluated from H&E-stained slides, which was defined by such histopathologic findings as abundant granular eosinophilic cytoplasm, cytoplasmic vacuolization and vesicular nuclei with prominent nucleoli in more than 10% of tumor cells.6 Tumor staging was done based on the 7th American Joint Committee on Cancer (AJCC) criteria.7 Disease-free survival (DFS) was calculated from the date of the first curative surgery to that of the first loco-regional or systemic relapse, or death without any type of relapse. Overall survival (OS) was estimated from the date of the first curative operation to that of the last follow-up or death from any cause. Clinical parameters include age at initial diagnosis, lymph node status, local recurrence, systemic recurrence, and patient survival.
Tissue microarrays
Representative invasive tumor areas were selected from the whole H&E-stained tumor slides. Corresponding spots were marked on the surface of the paraffin block. The selected area was punched out with a tissue extractor. In addition, a 3-mm tissue core was placed into a 6×5 recipient block. More than two tissue cores were extracted to minimize the extraction bias. Each tissue core was assigned with a unique tissue microarray location number that was linked to a database containing other clinicopathologic data.
Immunohistochemistry (IHC)
The antibodies and dilution used for IHC are shown in Table 1. Formalin-fixed, paraffin-embedded tissue sections from the tissue microarray were prepared for the IHC. The 3-mm sections were deparaffinized in xylene and rehydrated through a graded alcohol series to distilled water. The slides were subjected to antigen retrieval by a microwave irradiation and then incubated with primary antibodies. Binding was detected with biotinylated anti-mouse immunoglobulin, followed by peroxidase-labeled streptavidin with 3,3'-diaminobenzidine chromogen as the substrate. Optimal primary antibody incubation times and concentrations were determined by serial dilution for each immunohistochemical assay using a tissue block fixed and embedded exactly as for the experiments. Slides were counterstained with Harris hematoxylin, for which two pathologists interpreted the staining using a multi-view microscope.
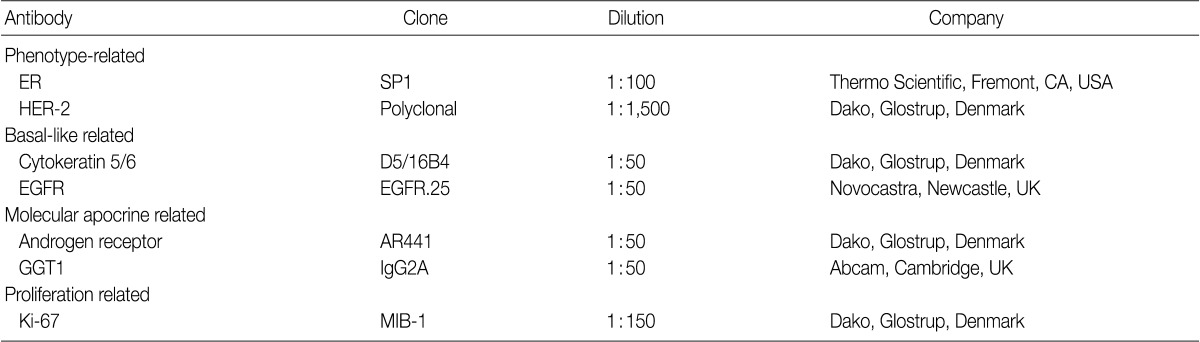
Table 1.
Clone, dilution, and source of antibodies
ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; EGFR, epidermal growth factor receptor; GGT1, gamma-glutamyltrasferase 1.
Interpretation of IHC
All immunohistochemical markers were assessed on light microscopy. Slides were scored according to the percentage of labeled tumor cells exhibiting nuclear (ER and AR), cytoplasmic (GGT1 and cytokeratin [CK] 5/6) and membranous (HER-2 and epidermal growth factor receptor [EGFR]) staining. The ER IHC was considered positive when more than 1% of the invasive tumor cells showed ER expression.8 The HER-2 staining was scored according to the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines based on the grading system: 0, no immunostaining; 1+, weak incomplete membranous staining in any percentage of the tumor cells; 2+, complete membranous staining, either non-uniform or weak in at least 10% of tumor cells; and 3+, uniform intense membranous staining in >30% of tumor cells. Specimens with 0 to 1+ were regarded as negative and those with 3+ were considered positive.9 Results for CK5/6, EGFR, AR, and GGT1 were considered positive when more than 1% of the tumor cells were stained.
Fluorescence in situ hybridization (FISH) analysis
Cases of HER-2 with a staining grade of 2+ on the IHC were further tested on the FISH analysis to detect the HER-2 gene amplification. Prior to this, invasive tumors were examined on the H&E stained slides. This was followed by the FISH analysis of the invasive tumor cells using a PathVysion HER-2 DNA Probe Kit (Vysis, Downers Grove, IL, USA) according to the manufacturer's instructions. The HER-2 gene copy number was counted using an epifluorescence microscope (Olympus, Tokyo, Japan) on each slide. At least 60 nuclei of the tumor cells were examined to count the number of HER-2 and chromosome 17 signals. According to the ASCO/CAP guidelines,9 if the absolute HER-2 gene copy number was <4 or the HER-2 gene/chromosome 17 copy number ratio (HER-2/Chr17 ratio) was <1.8, this was considered a lack of the HER-2 amplification. But if the absolute HER-2 copy number was 4-6 or the HER-2/Chr17 ratio was 1.8-2.2, this was considered to show an equivocal amplification of HER-2. Finally, if the absolute HER-2 copy number was >6 or the HER-2/Chr17 ratio was >2.2, this was considered HER-2 amplified.
Phenotypic classification of the breast cancer
In the current study, we divided the phenotypes of breast cancer into the subtypes based on the molecular classification and these include the luminal A type, ER positive and HER-2 negative; luminal B type, ER positive and HER-2 overexpressed; HER-2 overexpressed type, ER negative and HER-2 overexpressed; and triple-negative breast cancer (TNBC) type, ER and HER-2 negative. TNBC types were subdivided into the basal-like subtype (CK5/6 and/or EGFR positive) and non-basal-like subtype (CK5/6 and EGFR negative). Separate from molecular subtypes, we classified the phenotypes of breast cancer, based on the expressions of ER and MABC markers,3 into the luminal type, ER positive and AR and/or GGT1 positive; basal type, ER, AR and GGT1 negative; non-basal type, ER positive and AR and GGT1 negative; and MABC type, ER negative and AR and/or GGT1 positive.
Statistical analysis
Statistical analysis was done using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). Continuous and categorical variables were analyzed using Student's t-test and Fisher's exact test, respectively. Statistical significance was set at p<0.05. Kaplan-Meier analysis and log-rank statistics were used to evaluate both the time to tumor recurrence and overall survival. Multivariate regression analysis was performed using a Cox proportional hazards model.
RESULTS
Clinicopathologic characteristics according to the molecular subtypes of breast cancer
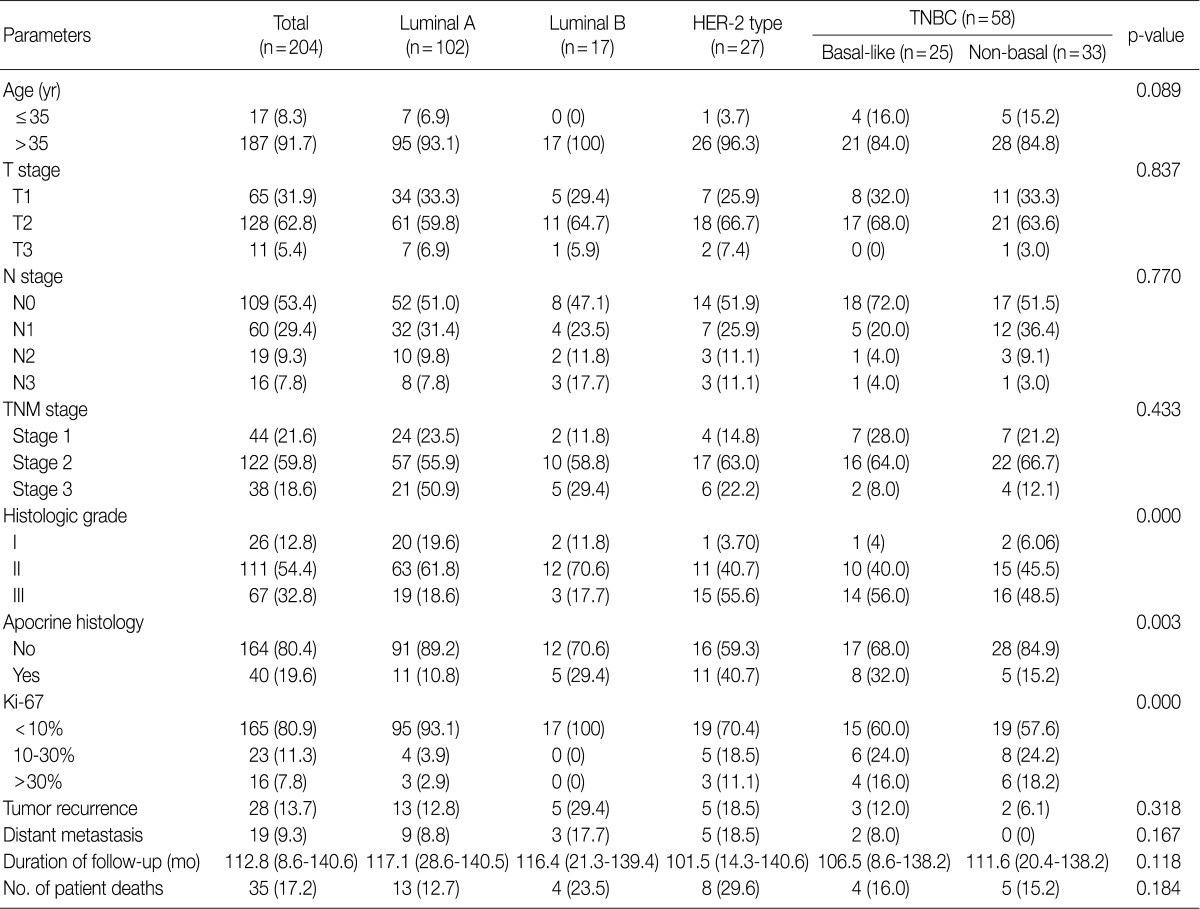
Our series of patients (n=204) were composed of 102 cases (50.0%) of luminal A type, 17 cases (8.3%) of luminal B type, 27 cases (13.2%) of HER-2 type and 58 cases (28.4%) of TNBC type. Besides, cases of TNBC type comprised of 25 cases of basal-like type (25/58, 43.1%) and 33 cases of non-basal like type (33/58, 56.9%). Clinicopathologic features are shown in Table 2. Both the HER-2 type and TNBC type had a higher histologic grade (p<0.001) and a higher Ki-67 index (p<0.001) than other types. The apocrine histology was observed in 19.6% (40/204) of total cases, which was notably seen in cases of HER-2 type and those of basal-like TNBC type (p=0.003).
Table 2.
Clinicopathologic characteristics of patients according to the molecular subtype of breast cancer
Values are presented as number (%) or mean (range).
HER-2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer; TNM, tumor, node and metastasis.
Clinicopathologic characteristics according to breast cancer phenotype
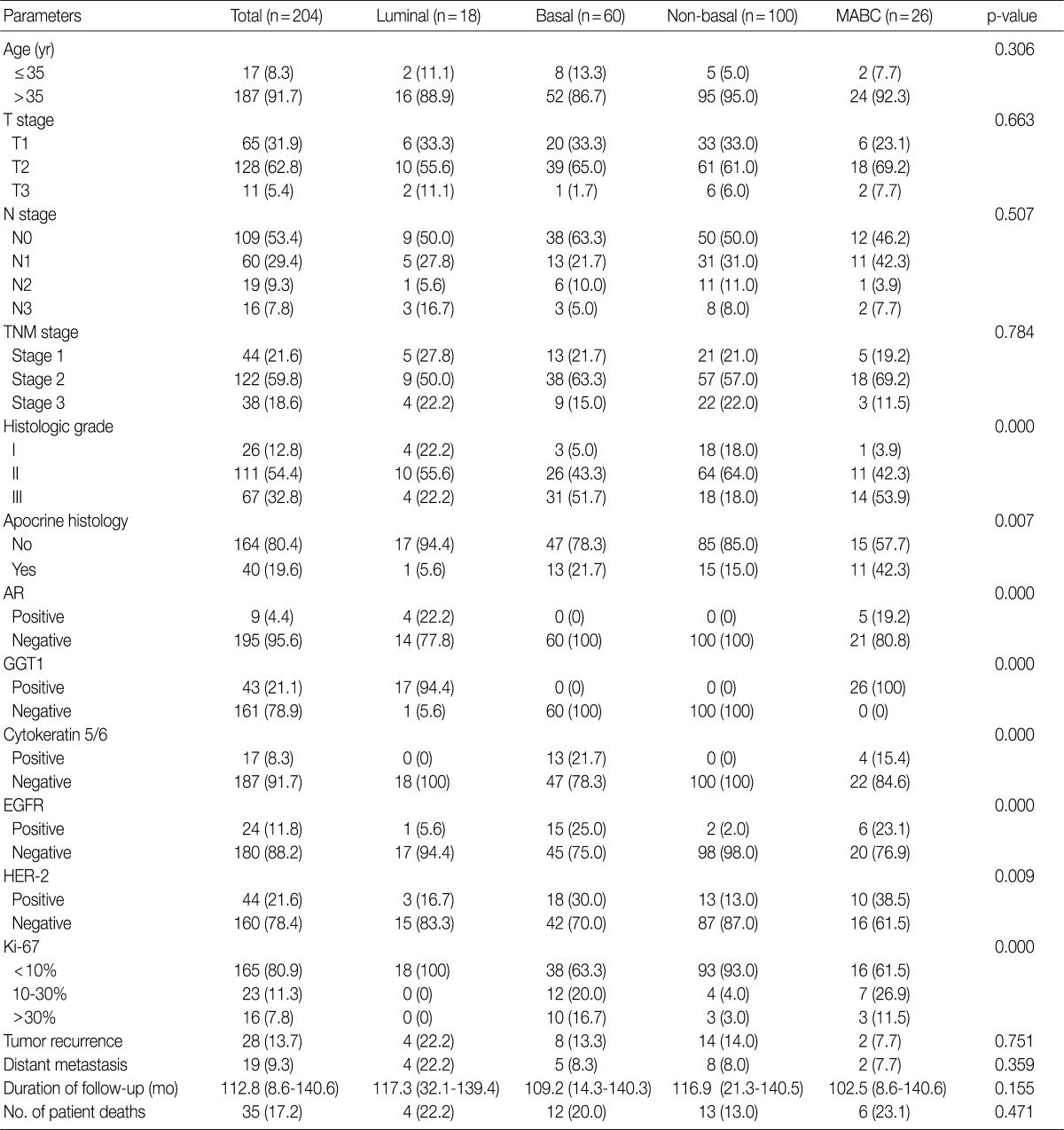
Clinicopathologic features of patients according to breast cancer phenotypes are shown in Table 3 and Fig. 1. The apocrine histology was the most notable feature found in cases of MABC (p=0.007). Compared to other phenotypes, both basal type and MABC demonstrated a higher histologic grade (p<0.001), CK-5/6 positivity (p<0.001), EGFR positivity (p<0.001), HER-2 positivity (p=0.009), and a higher Ki-67 index (p<0.001).
Table 3.
Clinicopathologic characteristics of patients according to breast cancer phenotype
Values are presented as number (%) or mean (range).
MABC, molecular apocrine breast cancer; TNM, tumor, node and metastasis; AR, androgen receptor; GGT1, gamma-glutamyltrasferase 1; EGFR, epidermal growth factor receptor; HER-2, human epidermal growth factor receptor 2.
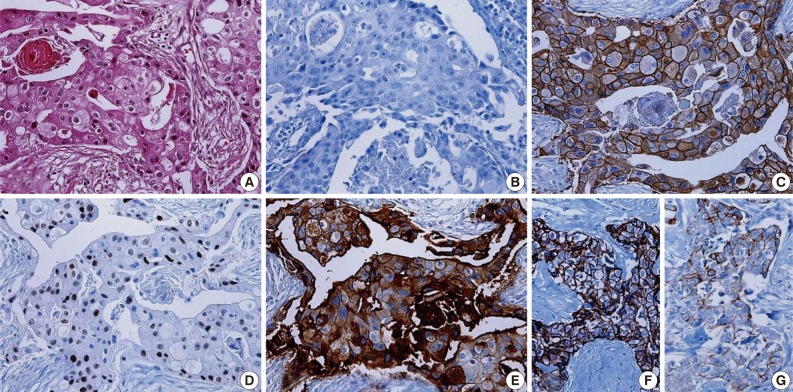
Fig. 1.
Histologic and immunologic features of molecular apocrine breast cancer (MABC). It shows abundant granular eosinophilic cytoplasm, cytoplasmic vacuolization and vesicular nuclei with prominent nucleoli on histology (A). It is negative for estrogen receptor (B), but is also frequently positive for human epidermal growth factor receptor 2 (C), androgen receptor (D) and gamma-glutamyltrasferase 1 (E). The expression of basal markers such as epidermal growth factor receptor (F) and cytokeratin 5/6 (G) is also occasionally seen in cases of MABC.
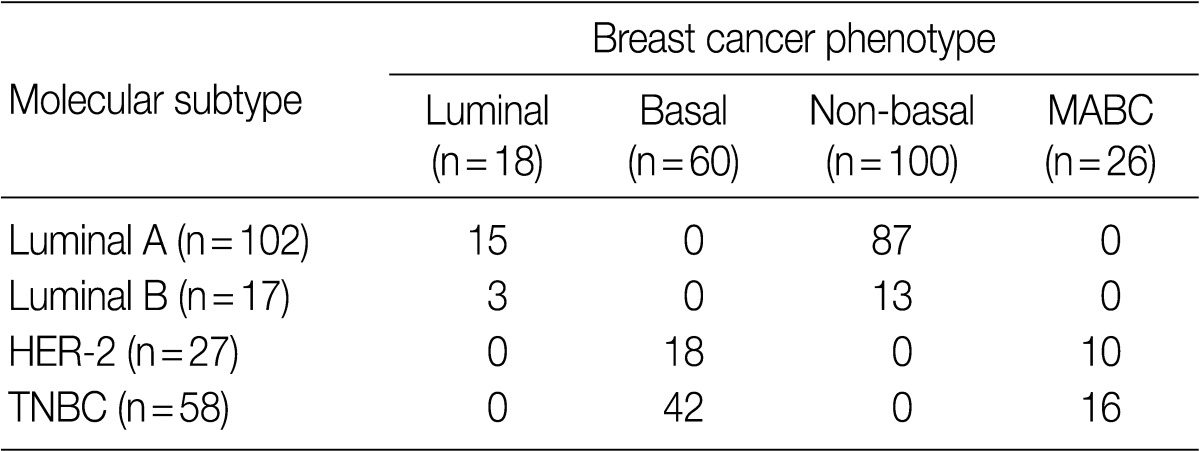
Correlation between the molecular subtype and the phenotype in cases of breast cancer
Following an analysis of the correlation between the molecular subtype and the phenotype of breast cancer, the MABC was found to comprise 38.4% (10/26) of HER-2 type and 61.5% (16/26) of TNBC type (Table 4). Both luminal type and non-basal type corresponded to the luminal A molecular subtype and a majority of basal type were TNBC type (p=0.001).
Table 4.
Correlation between molecular subtype and tumor phenotype
MABC, molecular apocrine breast cancer; HER-2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
Comparison of clinicopathologic features according to HER-2, basal marker and apocrine histology in cases of MABC
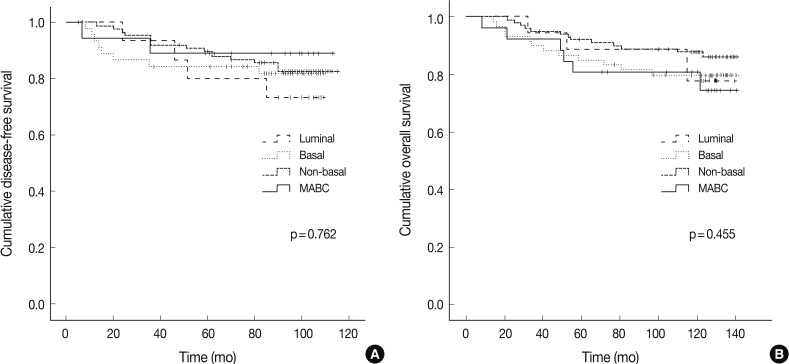
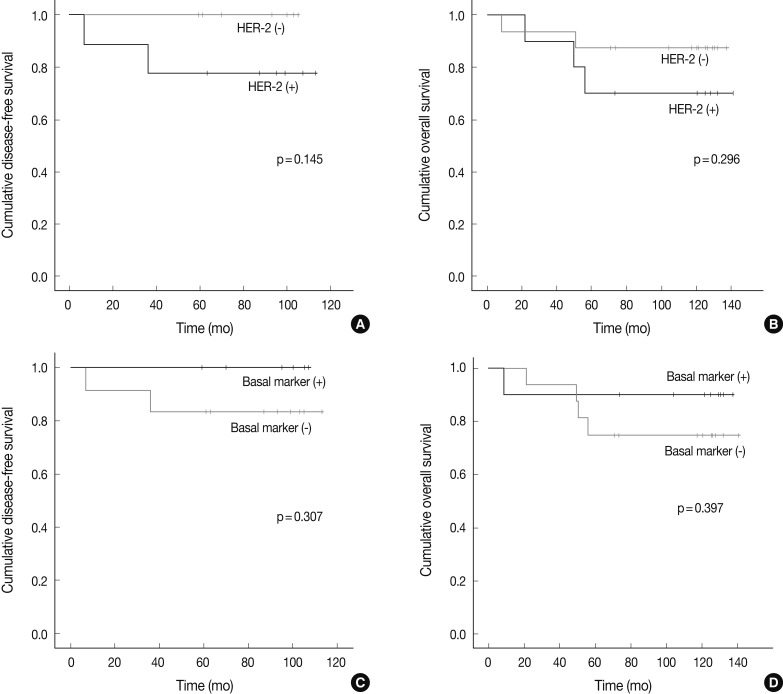
There were no significant differences in the clinicopathologic features according to the HER-2, basal marker and apocrine histology between MABC and other phenotypes of breast cancer. There were no significant differences in the DFS and OS between the phenotypes of breast cancer (Fig. 2). But higher tendency of poor DFS and OS was observed in cases of MABC with positivity to the HER-2 expression and negativity to the basal markers (Fig. 3).
Fig. 2.
Disease-free survival and overall survival depending on the phenotypes of breast cancer. MABC, molecular apocrine breast cancer.
Fig. 3.
The prognostic value of the expression of human epidermal growth factor receptor 2 (HER-2) and basal markers in cases of molecular apocrine breast cancer.
DISCUSSION
We examined the clinicopathologic features and their implications on the IHC in cases of MABC. Our results showed that the MABC accounted for 12.7% (26/204) of total cases, which was similar to a prevalence of 8-14% seen in previous reports.3 Although the MABC has been previously defined by a gene clustering analysis, it was defined based on the immunohistochemical status of the surrogate markers in the current study. It was therefore impossible to simply compare the previous reports with our results. According to previous studies, where the MABC was defined by a gene clustering analysis, all the cases (n=5) showed the positivity for AR and GGT1. GGT1 is considered to be a more specific marker for MABC. This is because expression of GGT1 is found in only 1.5% of non-MABC cases but that of AR is seen in 80% of non-MABC cases.4 Our results showed that the expression of AR and that of GGT were seen in 2.2% (4/178) and 9.6% (17/178) of non-MABC cases, respectively.
Our results also showed that the MABC, defined based on the IHC findings of the AR and GGT1, demonstrated a significant correlation with the HER-2 expression (p=0.009) and apocrine histology (p=0.007). A variable range of apocrine differentiation was previously found in cases of MABC.3 In the current study, however, the apocrine histology was observed in 42.3% of total cases of MABC. Presumably, this discrepancy might be due to the stricter criteria used in current study for determining the apocrine differentiation/histology. In contrast to the obvious apocrine histology of classical apocrine carcinoma, apocrine histology is not generally observed in cases of MABC. The term "molecular apocrine" has therefore been coined.3 In the same context, our results showed that the apocrine histology was seen in only about half of the total cases. Besides, similarly to previous reports that the expression of HER-2 was seen in 20-50% of total cases of MABC, it was observed in 38.5% of our series of patients.3,4 The molecular mechanism underlying the correlation between HER-2 signaling and MABC remains unclear. One hypothesis is that HER-2 stabilizes AR at the protein level, which is followed by enhancement of androgenic signaling, thus revealing the apocrine phenotype in ER-negative and AR-positive tumor.10
In the current study, the MABC was associated with basal phenotype markers such as CK5/6 and EGFR. According to other studies, the expression of EGFR was seen in 60% of total cases of MABC.4 Moreover, the apocrine differentiation and expression of basal markers including CK5/6 and EGFR are frequently observed in the molecular class HER-2 breast cancer.6 Because Farmer et al.3 showed that MABC significantly overlaps with HER-2 enriched type by means of Stanford gene clustering analysis, MABC is assumed to be closely related to HER-2, apocrine histology and basal markers. Further studies are therefore warranted to understand the underlying contexts of these observations.
Twenty six cases of MABC in this study were classified into HER-2 (n=10) and TNBC (n=16) subtypes. Luminal B and HER-2 subtypes showed HER-2 expression, while the TNBC subtype lacked it. Because HER-2 amplification is typically seen in cases of MABC, the phenomenon that TNBC consisted of 61.5% of MABC seemed to be contradictory. As noted above, MABC is nearly identical to the HER-2 enriched type when it is assessed by Stanford gene clustering analysis.3 However, not every HER-2 enriched type exhibits HER-2 overexpression or HER-2 amplification. According to a gene clustering analysis, 60-65% of the HER-2 enriched type lacks HER-2 overexpression or HER-2 amplification.1,2,4 Therefore, molecular stratification of TNBC indicates that 7-14% of TNBC falls into the HER-2 enriched type/MABC,11 which could explain our findings that TNBC comprised some part of MABC. In the current study, following an analysis of the subgroups of MABC based on the apocrine histology and IHC on HER2, EGFR, CK5/6, despite a lack of the statistical significance, higher tendency of poor DFS and OS was observed in association with the expression of HER-2 and the absence of basal markers. Previous studies have shown that the MABC had a poorer prognosis than other phenotypes.3,12 In the current study, however, there were no significant differences in the prognosis between the MABC and other phenotypes. Presumably, this discrepancy might be due to the difference in a patient population between previous reports and our results. We retrospectively analyzed data of 204 patients who had been diagnosed with invasive ductal carcinoma of the breast at Severance hospital during the year of 2001. These enrolled patients had not received neoadjuvant treatment. But other previous studies have enrolled patients with locally advanced breast cancer who underwent neoadjuvant chemotherapy. In addition, there were also differences in the methods to define the MABC between previous reports and our results. This might also cause a selection bias. Furthermore, previous studies have used a gene profiling analysis in making a definition of MABC. In the current study, however, we used the surrogate IHC markers in defining it.
There are some limitations of the current study and these include a smaller number of enrolled patients and a shorter follow-up period. Further studies are therefore warranted in a large series of patients with a longer follow-up period.
In conclusion, the MABC is a subtype of breast cancer characterized by apocrine histology accompanied by positivity for HER-2 and basal markers such as CK5/6 and EGFR.
Acknowledgments
This research was supported by The Korean Society of Pathologists Grant No. 2011-344.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farmer P, Bonnefoi H, Becette V, et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24:4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 4.Banneau G, Guedj M, MacGrogan G, et al. Molecular apocrine differentiation is a common feature of breast cancer in patients with germline PTEN mutations. Breast Cancer Res. 2010;12:R63. doi: 10.1186/bcr2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 6.Bhargava R, Beriwal S, Striebel JM, Dabbs DJ. Breast cancer molecular class ERBB2: preponderance of tumors with apocrine differentiation and expression of basal phenotype markers CK5, CK5/6, and EGFR. Appl Immunohistochem Mol Morphol. 2010;18:113–118. doi: 10.1097/PAI.0b013e3181b94ff1. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. pp. 347–376. [Google Scholar]
- 8.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 10.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2011;16(Suppl 1):61–70. doi: 10.1634/theoncologist.2011-S1-61. [DOI] [PubMed] [Google Scholar]
- 12.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]