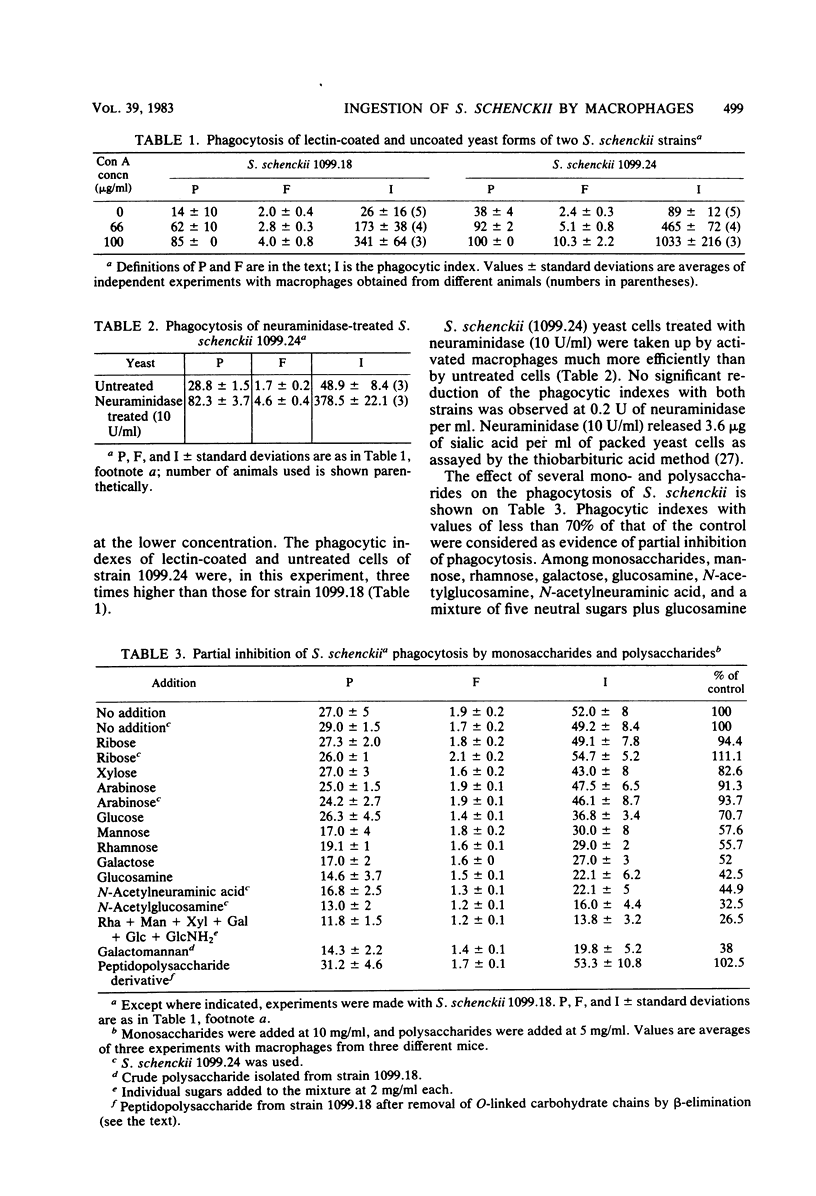
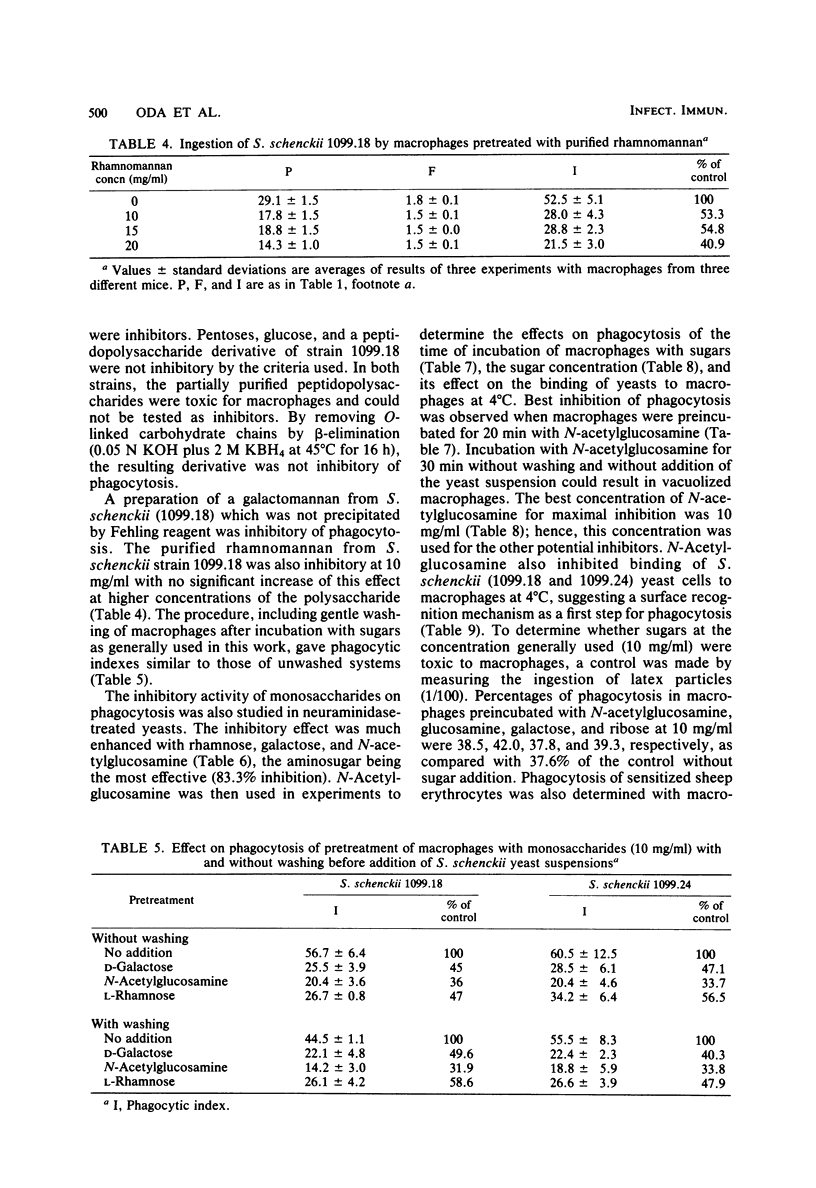
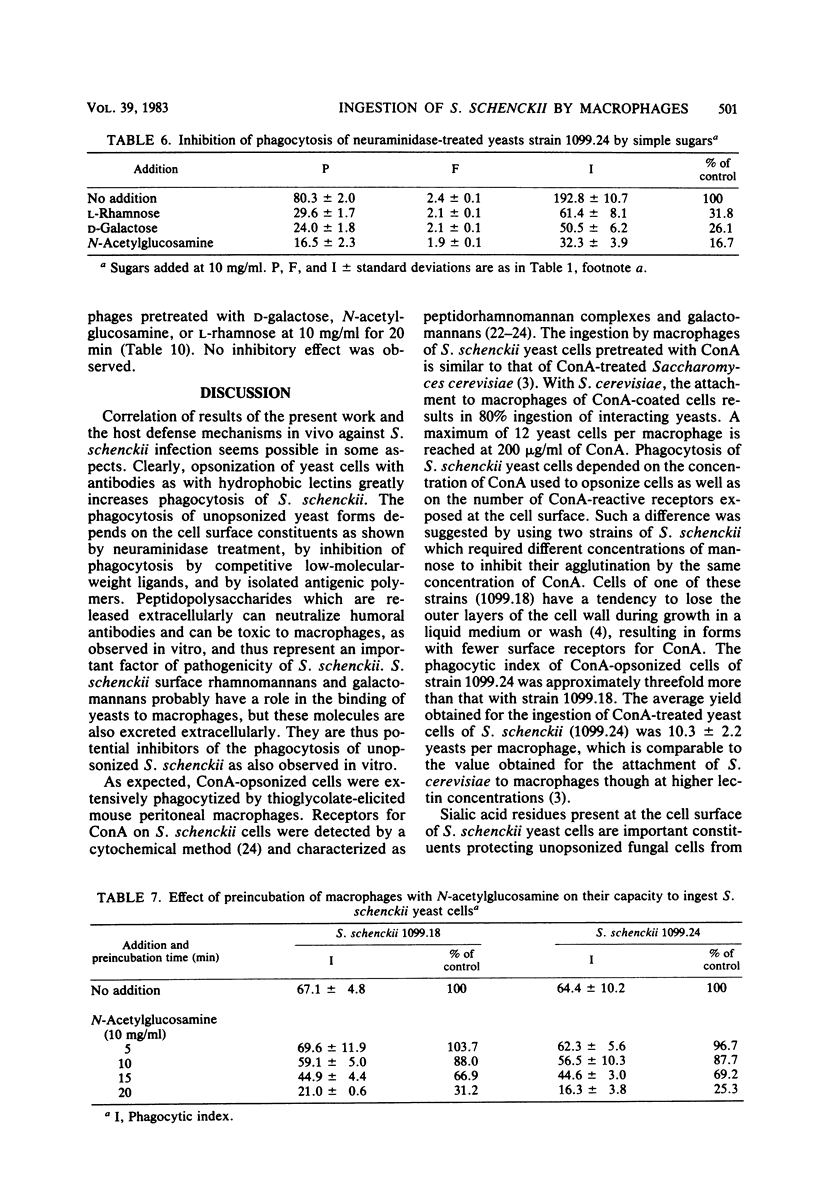
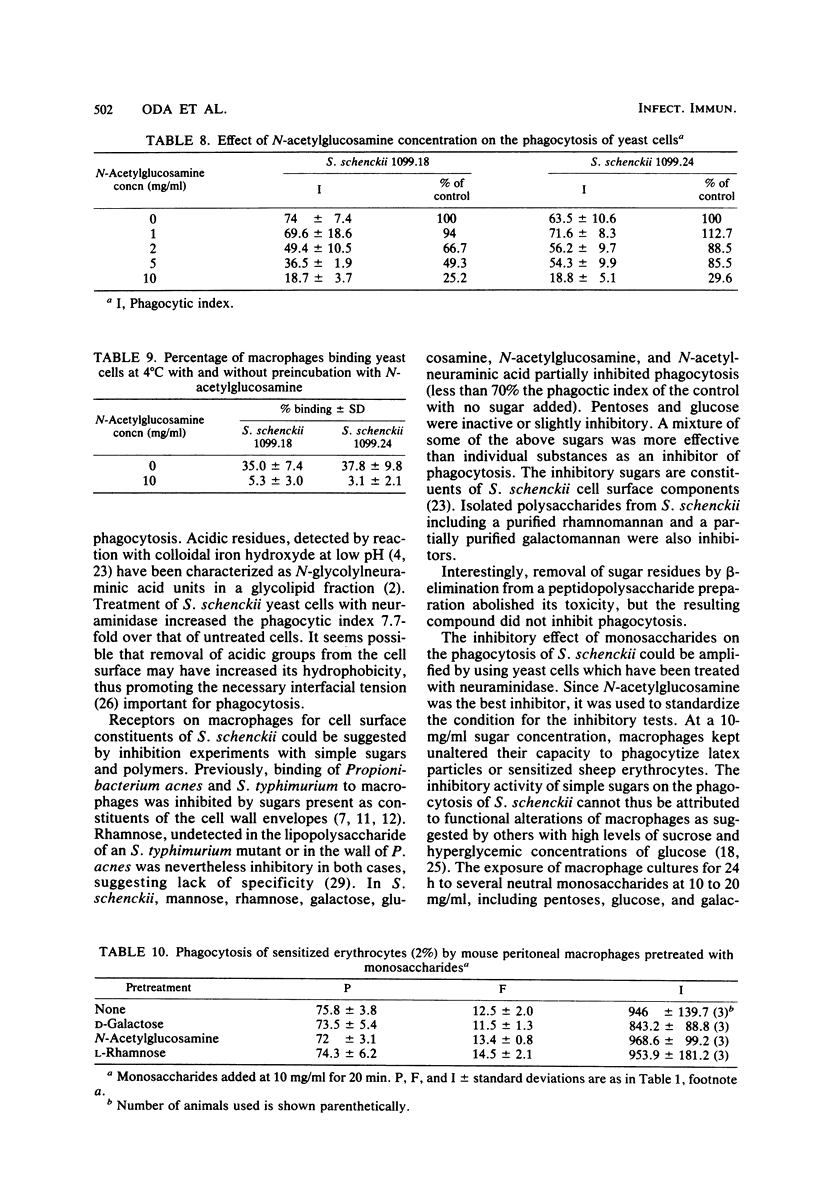
Abstract
The ingestion by thioglycolate-elicited mouse peritoneal macrophages of yeast forms of two strains of Sporothrix schenckii was studied. Yeast forms opsonized with concanavalin A (ConA) were extensively phagocytized, and the phagocytic indexes depended on the concentration of ConA and apparently on the number of lectin receptors at the yeast surface as well. Neuraminidase treatment of S. schenckii increased the ingestion of unopsonized yeasts 7.7-fold. The addition of monosaccharides and derivatives partially inhibited phagocytosis. Mannose, rhamnose, and galactose, which are major constituents of S. schenckii surface antigens, reduced the phagocytic indexes by 40 to 50%. Glucosamine, N-acetylglucosamine, and N-acetylneuraminic acid were equally effective as inhibitors of phagocytosis. A mixture of five neutral sugars and glucosamine inhibited phagocytosis by 73%. The inhibitory effect of simple sugars could be amplified by using neuraminidase-treated yeast cells. Pentoses and glucose were inactive or slightly inhibitory. A purified rhamnomannan inhibited phagocytosis of the homologous strain, whereas partially purified peptidopolysaccharides were toxic to peritoneal macrophages. A partially purified galactomannan from S. schenckii was inhibitory (62% inhibition), and a peptidopolysaccharide fraction in which the O-linked carbohydrate chains had been removed neither was toxic to macrophages nor inhibited phagocytosis. Pretreatment of macrophages with simple sugars under conditions inhibiting ingestion or binding of S. schenckii did not affect phagocytosis of latex particles or sensitized sheep erythrocytes. The presence of receptors at the peritoneal macrophages which bind S. schenckii cell surface components is suggested.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. M., Cook G. M., Poole A. R. Action of concanavalin A on the attachment stage of phagocytosis by macrophages. Exp Cell Res. 1971 Oct;68(2):466–467. doi: 10.1016/0014-4827(71)90178-9. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit Z., Goldman R. Concanavalin A-mediated attachment and ingestion of yeast cells by macrophages. Exp Cell Res. 1976 May;99(2):221–236. doi: 10.1016/0014-4827(76)90578-4. [DOI] [PubMed] [Google Scholar]
- Benchimol M., de Souza W., Travassos L. R. Distribution of anionic groups at the cell surface of different Sporothrix schenckii cell types. Infect Immun. 1979 Jun;24(3):912–919. doi: 10.1128/iai.24.3.912-919.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Ehrenreich B. A. The uptake, storage, and intracellular hydrolysis of carbohydrates by macrophages. J Exp Med. 1969 Jan 1;129(1):201–225. doi: 10.1084/jem.129.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimer N. B., Ogmundsdóttir H. M., Blackwell C. C., Sutherland I. W., Graham L., Weir D. M. The role of cell wall carbohydrates in binding of microorganisms to mouse peritoneal exudate macrophages. Acta Pathol Microbiol Scand B. 1978 Apr;86(2):53–57. doi: 10.1111/j.1699-0463.1978.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Goldman R., Bursuker I. Differential effects of lectins mediating erythrocyte attachment and ingestion by macrophages. Exp Cell Res. 1976 Dec;103(2):279–294. doi: 10.1016/0014-4827(76)90265-2. [DOI] [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça L., Gorin P. A., Lloyd K. O., Travassos L. R. Polymorphism of Sporothrix schenckii surface polysaccharides as a function of morphological differentiation. Biochemistry. 1976 Jun 1;15(11):2423–2431. doi: 10.1021/bi00656a028. [DOI] [PubMed] [Google Scholar]
- Ogmundsdóttir H. M., Weir D. M., Marmion B. P. Binding of microorganisms to the macrophage plasma membrane; effects of enzymes and periodate. Br J Exp Pathol. 1978 Feb;59(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Ogmundsdóttir H. M., Weir D. M. The characteristics of binding of Corynebacterium parvum to glass-adherent mouse peritoneal exudate cells. Clin Exp Immunol. 1976 Nov;26(2):334–339. [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M. Studies on the immunoglobulins which stimulate the ingestion of glutaraldehyde-treated red cells attached to macrophages. J Immunol. 1967 Dec;99(6):1115–1120. [PubMed] [Google Scholar]
- Rabinovitch M. The dissociation of the attachment and ingestion phases of phagocytosis by macrophages. Exp Cell Res. 1967 Apr;46(1):19–28. doi: 10.1016/0014-4827(67)90405-3. [DOI] [PubMed] [Google Scholar]
- Rode H. N., Gordon J. Inhibition of the mixed leukocyte culture reaction (MLC) by pre-incubation of the leukocytes with sucrose. J Immunol. 1969 Mar;102(3):786–787. [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis: recognition and ingestion. Semin Hematol. 1975 Jan;12(1):83–116. [PubMed] [Google Scholar]
- Travassos L. R., Gorin P. A., Lloyd K. O. Comparison of the rhamnomannans from the human pathogen Sporothrix schenckii with those from the Ceratocystis species. Infect Immun. 1973 Nov;8(5):685–693. doi: 10.1128/iai.8.5.685-693.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travassos L. R., Lloyd K. O. Sporothrix schenckii and related species of Ceratocystis. Microbiol Rev. 1980 Dec;44(4):683–721. doi: 10.1128/mr.44.4.683-721.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oss C. J. Influence of glucose levels on the in vitro phagocytosis of bacteria by human neutrophils. Infect Immun. 1971 Jul;4(1):54–59. doi: 10.1128/iai.4.1.54-59.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weir D. M., Ogmundsdóttir H. M. Non-specific recognition mechanisms by mononuclear phagocytes. Clin Exp Immunol. 1977 Nov;30(2):323–329. [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. H., Douglas S. D. Dynamics of the macrophage plasma membrane. Annu Rev Microbiol. 1979;33:267–307. doi: 10.1146/annurev.mi.33.100179.001411. [DOI] [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]



