Abstract
Background
Human papillomavirus (HPV) is known to cause of oropharyngeal squamous cell carcinoma (SqCC). HPV positive SqCCs overexpress p16 and are associated with better survival. Several markers of cell cycles and apoptosis have been reported as a prognostic value. We examined the prognostic value of HPV status, p16, cyclin D1, and Bcl-2 in patients with tonsillar SqCC.
Methods
Tissue microarrays were constructed in 56 cases of tonsillar SqCC for which we performed an immunohistochemistry and an in situ hybridization (ISH) of the HPV.
Results
Of the 56 cases, 31 (55.3%) were positive for p16 and 20 (35.7%) were positive for HPV ISH. The expressions of p16, cyclin D1, and Bcl-2 were not correlated with the clinicopathologic variables including smoking status, differentiation and pT- and pN-stages. The HPV ISH positive group showed a better overall survival than the HPV negative group (p=0.04), and the p16 positive group showed a better disease free survival (DFS) than the negative group (p=0.016). Cox regression analysis showed that only p16 positivity was an independent prognostic factor for DFS (p=0.03; hazard ratio, 10.1).
Conclusions
Our results indicate that both p16 expression and HPV status are useful indicators for risk stratification in patients with tonsillar SqCC.
Keywords: Human papillomavirus; Palatine tonsil; Carcinoma, squamous cell carcinoma; p16; Bcl-2; Cyclin D1
Human papillomavirus (HPV)-related tumors represent an increasing fraction of newly diagnosed with squamous cell carcinoma (SqCC) of the head and neck.1 In fact, HPV-positive SqCC is now recognized as a unique type of tumor with different demographics, a characteristic molecular profile and a distinctly better prognosis than its HPV-negative counterpart.2,3 Epidemiologic studies have shown that HPV-positive SqCC is more frequently seen in younger patients with a higher socioeconomic status who have higher numbers of sex partners and higher exposure to oral sex.4 In addition, it has also been reported that HPV-positive SqCC is less strongly associated with the use of alcohol and tobacco a compared with its HPV-negative counterpart.4 At the molecular level, HPV-positive SqCC harbors less frequent p53 mutations than its HPV-negative counterpart.5 Besides, it is almost always associated with the over-expression of p16, which is uncommon in its HPV-negative counterpart.6
It is also likely that HPV plays a role in the deregulation of the cell cycle in cases of HPV-positive tonsillar SqCC.7,8 The emerging paradigm is that loss of normal cell cycle control is central to malignant transformation and that at least one of the four key regulators of the cell cycle (p16INK4a, cyclin D, cyclin-dependent kinase 4, and retinoblastoma [RB]) is dysregulated in the majority of human cancers.9 In cells that harbor mutations in any one of these other genes, the function of RB is disrupted even if the RB gene itself is not mutated.10 The transforming proteins of several oncogenic human DNA viruses seem to act, in part, by neutralizing the growth-inhibitory activities of RB. In these cases, RB protein is functionally inactivated by binding to a viral protein and it no longer acts as a cell cycle inhibitor. HPV E7 protein all binds to the hypophosphorylated form of RB. This occurs in the same RB pocket that normally sequesters E2F transcription factors. The binding to HPV proteins has a strong preference for certain viral types, such as HPV type 16 that confers high risk for the development of cervical carcinomas. Thus, the RB protein, unable to bind the E2F transcription factors, is functionally inactivated, and the transcription factors are free to cause cell cycle progression.11 In line with these studies, a correlation between HPV, pRb negativity and over-expression of p16INK4a in cases of oropharyngeal carcinoma has also been reported.7,8
Apoptosis, a programmed cell death, is involved in tissue homeostasis and tumorigenesis. Bcl-2 is another anti-apoptotic protein, which is localized in intracellular membranes, mostly in the outer mitochondrial membrane, nuclear membrane, and the endoplasmic reticulum. At these sites, Bcl-2 controls ion channels, caspase status, and cytochrome c localization, and has an anti-apoptotic function.12 Changes in the expression of Bcl-2 have been examined in various types of cancer because of their contributions to the development of cancer. To our knowledge, however, there are no reports about the correlation between the expression of Bcl-2 and its clinical significance in cases of tonsillar SqCC. Moreover, apoptosis-related proteins have recently been highlighted with the development of apoptosis-targeted anti-cancer drugs.13 It is therefore necessary to examine whether changes in the expression of apoptosis-related proteins have a prognostic value in the targeted anti-cancer therapy.
Given the above background, we conducted this study to examine the relationship between the HPV status and the expressions of p16 and cyclin D1 (two key cell cycle proteins) and that between it and the expression of Bcl-2 (an apoptosis-related protein) in 56 cases of tonsillar SqCC. To do this, we analyzed the presence or absence of HPV DNA on in situ hybridization (ISH) and evaluated the immunohistochemical findings to quantify the expression of the above prognostic markers. In addition, we also examined whether any of these targeted molecular markers had a significant correlation with clinicopathologic parameters and they had a prognostic value.
MATERIALS AND METHODS
Selection of study patients
The current study was approved by the Institutional Review Board (IRB) of the Catholic University of Korea, Seoul St. Mary's Hospital. Data were collected in all the cases, but four cases, for which the HPV analysis of the specimens of tonsillar SqCC was performed as a part of routine clinical and laboratory tests during a period ranging from 1994 to 2010. Clinicopathologic parameters were analyzed through a retrospective review of the medical records and pathologic reports at our medical institution. Our series of patients (n=56) comprised 51 (91.1%) men and five (8.9%) women with a mean age of 57.3 years. Besides, there were 18 (32.1%) never smokers and 38 (67.9%) light-and-heavy smokers. Furthermore, there were seven cases (12.5%) of well-differentiated tumor, 44 cases (78.6%) of moderately-differentiated tumor and five cases (8.9%) of poorly-differentiated tumor. Outcomes were determined from the date of surgery until death or July 31, 2011, which resulted in the period of a follow-up ranging from 1 to 137 months. Cases lost to follow-up and deaths caused by problems other than tonsillar SqCC were censored from survival analysis. Of the 56 patients for whom follow-up data were available, 14 (25.0%) had a loco-regional recurrence and seven (12.5%) had a distant metastasis. There were no patients who received preoperative therapy. Besides, there were 42 patients who received adjuvant chemotherapy (CTx) or radiation therapy. Furthermore, postoperatively, there were one patient (1.8%) who received only CTx, 25 patients (44.6%) who received the chemoradiation therapy (RTx) and 16 patients (28.6%) who received the radiation therapy.
Tissue microarray construction and immunohistochemistry
Tissue microarrays were constructed from paraffin-embedded blocks after a review of the glass slides in 56 cases of tonsillar SqCC. One core of tumor tissue was obtained using a punch and it was re-embedded in recipient blocks using a tissue array-generating device (Beecher Instruments, Sun Praire, WI, USA) with 2.0-mm cylinders. Each microarray block contained 17 cases of tonsillar SqCC. The paraffin-embedded tissue was sectioned at a thickness of 4 µm and mounted on precoated glass slides. The sections were deparaffinized, rehydrated and rinsed in a distilled water. Immunohistochemistry assays for p16INK4A (1:200, anti-p16 mouse monoclonal antibody, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Bcl-2 (1:20, Dako, Glostrup, Denmark), cyclin D1 (1:50, Thermo Science, Runcorn, UK) were performed using the Ventana NX automated immunohistochemistry system (Ventana Medical Systems, Tucson, AZ, USA).
p16 expression was scored as positive if the nucleus and cytoplasm were stained both strongly and diffusely in ≥70% of the tumor specimens. Specimens of cervical carcinoma were served as a positive control for p16 staining. Bcl-2 expression was scored based on criteria of Jäckel et al.14 Based on the intensity scores (0 point, absent; 1 point, weak; 2 points, moderate; 3 points, strong) and prevalence scores (0 point, the proportion of stained tumor <25%; 1 point, 25-75%; 2 points, >75%) were added, if the total scores were >3 points, this was considered positive. Stromal lymphocytes in tonsils were served as positive controls for Bcl-2 staining. Cyclin D1 was scored using the same methods as performed for Bcl-2.15
ISH for HPV
The Ventana INFORM HPV ISH assay uses a nonamplified high-risk HPV III probe which consists of a cocktail directed against HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66. The ISH assays were performed on 3-µm sections with the Benchmark Automated Slide Stainer. The automated system used herein is composed of the Ventana ISH Protease 3 (8 minutes) to degrade proteins surrounding the target DNA, the above-mentioned high-risk HPV probe and the Ventana iVIEW Blue detection kit. The detection system uses a biotinylated antifluorescein antibody to detect the hybridized probe followed by streptavidin to bind biotin then a chromogen reaction with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) for detection. The HPV ISH test was scored as positive if there was any blue reaction product that co-localized with the nuclei of malignant cells.16 Diffuse nuclear and cytoplasmic staining and punctuated nuclear staining were scored as positive. Diffuse staining of tumor and stromal tissues, considered to represent non-specific chromogen precipitate, which was scored as negative.
Statistical analysis
All statistical analyses, including Pearson chi square test, Fisher's exact test and student t-test, were performed to identify correlations between the grouped immunodetection data and multiple clinical variables using IBM SPSS ver. 18.0 (Chicago, IL, USA). Survival analysis was performed using the Kaplan-Meier method and the resulting curves were compared using a log-rank test. Multivariate disease free survival (DFS) analysis was performed using the COX proportional hazard regression model. A p<0.05 was considered statistically significant.
RESULTS
Correlation between expressions of HPV ISH with its related proteins and clinicopathological parameters
Our series of patients (n=56) were composed of 20 cases (35.7%) of HPV ISH positive tumors. Besides, the expressions of p16, cyclin D1 and Bcl-2 were detected in 31 cases (55.4%), 26 cases (46.4%) and 13 cases (23.2%), respectively (Fig. 1). Details of patient characteristics and marker status are summarized in Table 1. There was a correlation between HPV ISH and p16 expression, which was seen in 30 patients (60%; p=0.015, Fisher exact test). Of them, 15 patients (53.6%) had an HPV ISH-positive tumor and all of these cases were positive for p16 expression. There was no correlation between HPV ISH and cyclin D1 expression (p=0.857). But there was an inverse correlation between p16 and cyclin D1 expression (p=0.010, r=-0.38). In addition, Bcl-2 expression was correlated with both HPV ISH (p=0.005) and p16 expression (p=0.036) and it was inversely correlated with cyclin D1 expression (p=0.018, r=-0.401). The mean age was 55.0±6.529 years in the HPV ISH-positive group and 58.25±8.602 years in the HPV ISH-negative group. These results indicate that the mean age was slightly younger in the HPV ISH-positive group as compared with the HPV ISH-negative group. But these differences reached no statistical significance. In addition, no markers including HPV ISH, p16, cyclin D1, and Bcl-2 were significantly correlated with any of the clinicopathological variables including sex, age, smoking status, degree of differentiation, T stage, N stage, and treatment status.
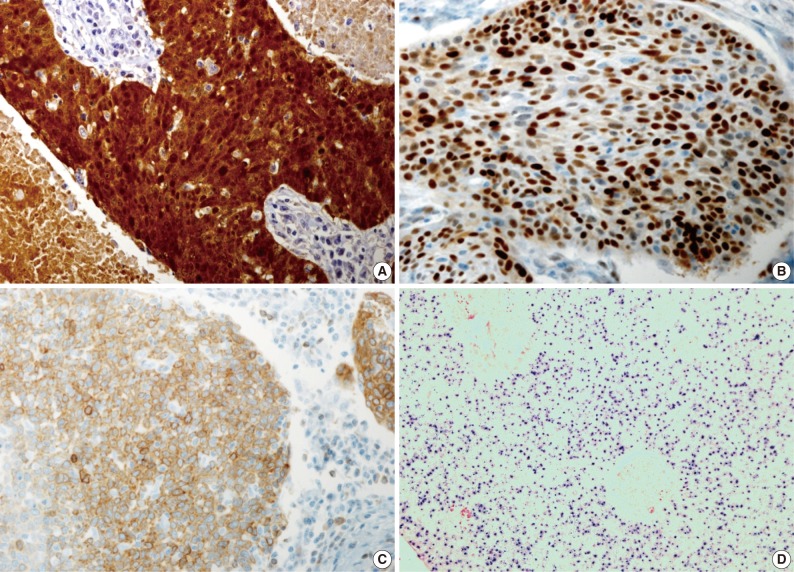
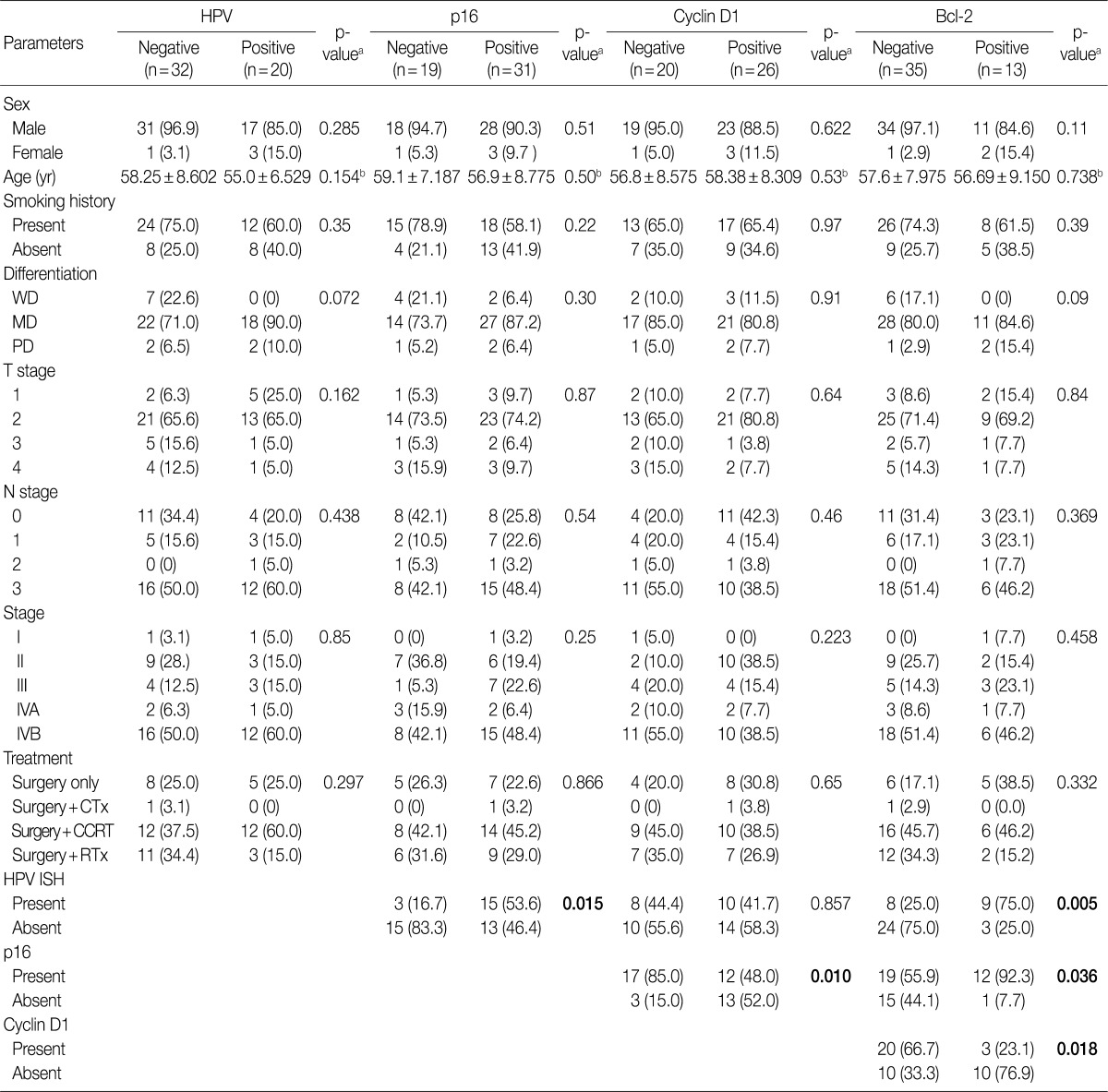
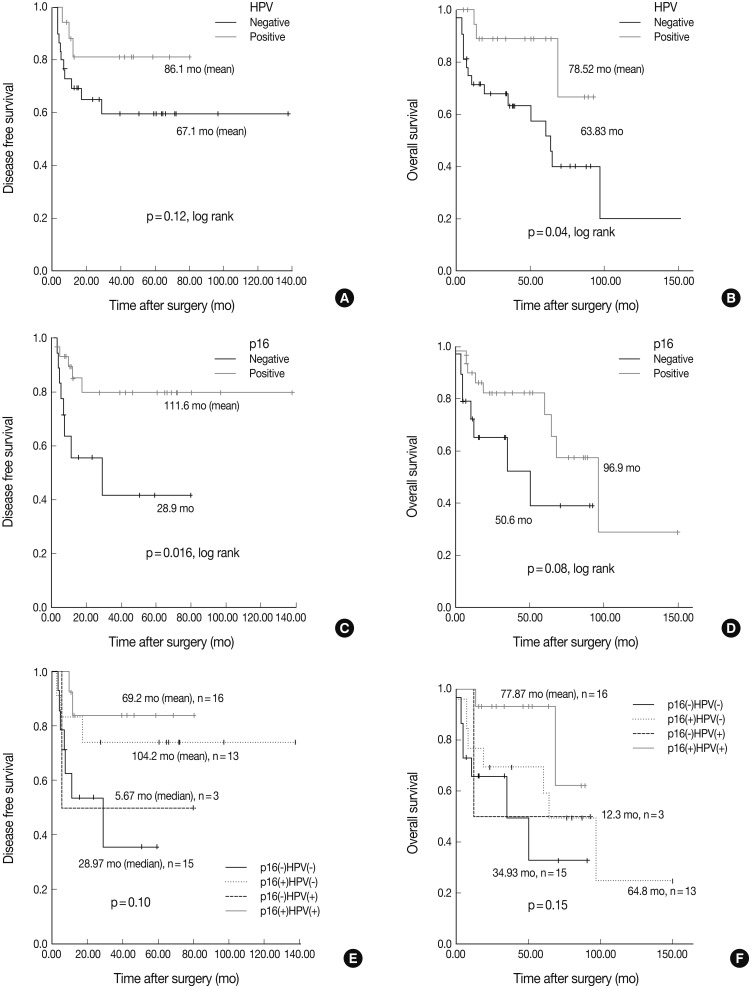
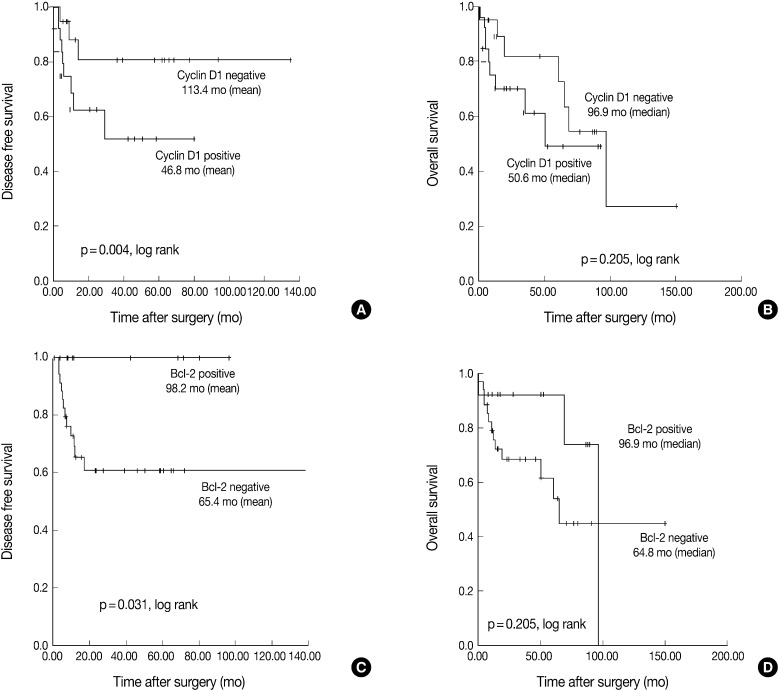
Fig. 1.
Representative photomicrographs of an tonsillar squamous cell carcinoma stained with antibodies against (A) p16, (B) cyclin D1, (C) Bcl-2, and (D) human papillomavirus (HPV) in situ hybridization. All of staining results are interpreted as positive.
Table 1.
Cliniocopathologic characteristics of patients and HPV in situ with related proteins
Values are presented as number (%).
HPV, human papillomavirus; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; CTx, chemotherapy; CCRT, Chemoradiaton; RTx, radiation therapy; ISH, in situ hybridization.
aBy chi-square test; bBy student t-test.
Survival analysis
The Kaplan-Meier analysis identified p16 positive expression (p=0.016, log-rank) (Fig. 2C), cyclin D1 negative expression (p=0.004, log-rank) (Fig. 3A) and Bcl-2 positive expression (p=0.031, log-rank) (Fig. 3C) as predictors of longer DFS. HPV ISH positivity (p=0.04, log-rank) (Fig. 2B) was a predictor of longer overall survival (OS). In the current study, a multivariate survival analysis was also performed to evaluate the DFS. This showed that only p16 positivity was a statistically significant, independent and good prognostic factor (p=0.006; hazard ratio, 10.1; 95% confidence interval, 1.939 to 52.806) among the other variables affecting patient survival (Table 2).
Fig. 2.
Survival curves obtained using the Kaplan-Meier method by the log-rank test. (A, C) Disease free survival for human papillomavirus (HPV) and p16. (B, D) Overall survival for HPV and p16. (E, F) Survival curves for a subgroup analysis.
Fig. 3.
Survival curves obtained using the Kaplan-Meier method by the log-rank test. (A, C) Disease free survival for cyclin D1 and Bcl-2. (B, D) Overall survival for cyclin D1 and Bcl-2.
Table 2.
Prognostic factor for disease free survivala
CI, confidence interval; HPV, human papillomavirus; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated.
aBy cox proportional hazard regression.
Under the hypothesis that the above predictors might be independently correlated to the outcomes, we evaluated them in combination with each other. Fig. 2A-D shows the relationship of individual outcomes according to HPV and p16 status, and Fig. 2E and F does the Kaplan-Meier curves for DFS and OS based on combinations of HPV and p16 status. The groups were divided into the p16 negative/HPV ISH negative group (n=16), the p16 positive/HPV ISH negative group (n=13), the p16 negative HPV ISH positive group (n=3) and the p16 positive/HPV positive group (n=5). Of the HPV ISH positive groups (p16-/HPV ISH+ and p16+/HPV ISH+), the p16 positive subgroup had a mean DFS of 69.2 months and the p16 negative subgroup had a mean DFS of 5.67 months. These results suggest that the p16 positivity might be a better prognostic indicator. But this difference reached no statistical significance (p=0.10, log-rank) (Fig. 2E). OS also showed a similar pattern as the results obtained from the DFS (p=0.15, log-rank) (Fig. 2F). In addition, to estimate the magnitudes of the hazards associated with HPV ISH and p16, we performed Cox proportional hazard regression analysis for the bivariate combination of HPV ISH with p16 (Table 3). Cases of p16 negative/HPV ISH negative alone had 5.28 hazard ratios for recurrence (p=0.039; 95% confidence interval, 1.092 to 25.56) and 5.342 hazard ratios for death (p=0.037; 95% confidence interval, 1.105 to 25.81) compared to p16 positive/HPV ISH positive cases of SqCC.
Table 3.
Multivariate analysisa (compared to p16 positive and HPV ISH positive SqCC)
HPV, human papillomavirus; ISH, in situ hybridization; SqCC, squamous cell carcinoma; HR, hazard ratio; CI, confidence interval; (+), positive; (-), negative.
aBy cox proportional hazard regression.
We also performed survival analysis depending on the treatment methods, but excluded patients who received neoadjuvant therapy. Patients were divided into four groups: the group 1 comprising only patients who underwent surgery, the group 2 comprising those who underwent surgery and CTx, the group 3 comprising those who underwent surgery and chemoradiation and the group 4 comprising those who underwent surgery and RTx. The analysis was then stratified by pStage. Data was available for only pStage II and IVB. In pStage II, the group 3 showed a good DFS (p=0.001, log-rank) and a good OS (p=0.011, log-rank). In pStage IVB, the group 4 showed a good DFS (p=0.049, log-rank) and a good OS (p=0.002, log-rank).
DISCUSSION
Clinical trials have shown that the HPV status is a key prognostic indicator in patients with oropharyngeal carcinoma.17 It would therefore be mandatory to standardize or to determine the best test methods for high-risk HPV. Besides, it is also necessary to combine multiple tests, which would be essential for making an accurate diagnosis. According to studies, the prevalence of HPV infections in cases of tonsillar SqCC has been reported to be about 37-80% in western countries.2,3 A variety of detection methods are currently available and these include p16 immunohistochemistry (IHC), high-risk HPV ISH, p16 IHC/HPV ISH combination, DNA quantitative polymerase chain reaction (qPCR), p16 IHC/DNA qPCR combination, DNA/RNA qPCR and p16 IHC/RNA qPCR combination. Each detection method has its own merits and demerits. In addition, the sensitivity and specificity of each method for defining the HPV status vary to such a considerable extent that the clinical usefulness of some detection methods have been questioned.18 Schache et al.19 evaluated the sensitivity and specificity of various diagnostic modalities for HPV in cases of oropharyngeal SqCC, for which these authors compared them based on the viral mRNA expression using the qPCR techniques on fresh-frozen-derived total DNA samples. This showed that the sensitivity was 88% of HPV ISH, 94% of p16 IHC and 97% of DNA qPCR. Besides, the specificity was 88%, 82%, and 87% in the corresponding order. These results indicate that the false negativity of HPV ISH was relatively higher. Lewis et al.16 found more HPV positive cases by the DNA PCR from the HPV ISH negative cases. Although this RNA based HPV detection may prove valuable in a research setting, it would be logistically difficult to introduce into a routine pathology service where diagnostic algorithms are based on the assessment of formalin-fixed, paraffin-embedded tissue. It would therefore be mandatory to perform an internal validation of the sensitivity and specificity between RNA qPCR and HPV ISH, which is essential for evaluating the accuracy of HPV ISH. In comparison, our results showed a relatively lower prevalence of HPV infection (35.7%) by the HPV ISH. Despite a lower prevalence of HPV infection, however, we did not validate the HPV ISH, which is one of limitations of the current study. In addition, we cautiously suggested that another reason for a lower prevalence of HPV infection might be due to the conservative sexual behavior based on Confucian ethics in Korean people.
It is not entirely clear why there is an improved outcome for patients with HPV positive tonsillar SqCC. Some studies have shown that HPV 16-positive head-and-neck tumors are more likely to carry wild-type tumor protein p53 (TP53) and express p16,3,20 which supports the notion that the improved prognosis may be actually attributed to the HPV infection. In contrast to tobacco-associated head-and-neck SqCC, the presence of functional TP53 may render the tumor susceptible to radiation-induced apoptosis.21 However, the mechanism by which HPV infection attributes to the improved outcome is less than simple, as studies have shown that not all HPV-positive head-and-neck tumors are transcriptionally active.20
The expression of p16 as an optimal biomarker for tonsillar SqCC might be limited because p16 can be overexpressed by other mechanisms.2 Indeed, head and neck SqCCs can be p16 positive but are HPV negative. Robinson et al.22 reviewed the results of the HPV testing by pooling the results of six studies examining 496 tumors using such a classification and these authors found p16 positive/HPV negative results in 5% and p16 negative/HPV positive results in 8% of total cases. By contrast, our results showed that there were such a greater number of p16 positive/HPV ISH negative cases that the proportion of p16 positive/HPV ISH negative cases and p16 negative/HPV ISH positive ones were 27.6% and 6.3%, respectively. These results might originate from a lower sensitivity of the HPV ISH. Considering the proportion of p16 negative/HPV ISH positive cases was similar to other studies, however, a lower sensitivity of the HPV ISH was not solely responsible for the problem. In addition, 30% of p16 positive tumors have been reported to be HPV negative.23 If so, what is the mechanism of p16 overexpression in the absence of HPV E7 expression? To answer this, it has been hypothesized not only that the tumors develop completely independently from the HPV but also that they have innate p16 overexpression. The second, current HPV-specific tests may not be recognizing HPV. For example, there may be unidentified HPV genotypes that do not contain the consensus DNA sequences that are detectable by the ISH or PCR.
Our results showed that p16 positive tonsillar SqCC has a very favorable prognosis with no respect to its HPV status, which has also been supported by data from other investigators. A large-scale, multi-national Phase III clinical trial was conducted to evaluate the prognostic value of the expressions of p16 and HPV in cases of oropharyngeal SqCC. This also showed that the expression of p16 was identified as an indicator for a good prognosis in a larger cohort of patients as compared with HPV testing alone or the combination of p16 and HPV positivity.24
Our results also showed that there was an inverse correlation between the expression of cyclin D1 and that of p16 but there was no correlation between it and the HPV ISH status (Table 1). In addition, there was also a correlation between the cyclin D1 positivity and a poor DFS. In HPV-associated tumors, the oncoprotein E7 interacts with pRb and thereby induces the degradation of pRb. This eventually leads to the up-regulation of p16INK4A and the down-regulation of cyclin D1. Presumably, the tumor might be developed automatically along with its downstream genes in a cell-cycle-dependent manner following the initiation of its development by HPV E7 protein. This is in agreement with the reports, made by Andl et al.,25 that that E7 might overcome the need for cyclin D1 in the G1 phase of the cell cycle as it interacts with the cyclin D1-binding site on pRb. Indeed, a high level of the expression of cyclin D1 was predominantly seen in cases of HPV-negative tonsillar carcinoma, which strongly indicates that the amplification of cyclin D1 gene might be involved in these cases.25
It has been examined whether Bcl-2 has a prognostic value in cases of oropharyngeal SqCC. Several studies have analyzed Bcl-2, an apoptosis-related protein, in multiple copies or it in combination with other markers. Still, however, the role of Bcl-2 as a prognostic marker remains controversial. Some authors have failed to find any associations between Bcl-2, Bax or Bcl-X expression and prognosis,26 whereas others have found its association with a favorable outcome.27 To date, however, only a few studies have demonstrated that Bcl-2 is an indicator of a poor prognosis.28 To our knowledge, there is only one study of survival analysis that examines the correlations between Bcl-2 and HPV ISH in cases of oropharyngeal SqCC.29 This showed that a higher level of the expression of bcl-2 was correlated with a poor prognosis with no respect to the HPV status. This was in contrast to our results. Accurate mechanisms for a poor prognosis and its correlation with the HPV status remain obscure. Loro et al.30 concluded that a loss of Bcl-2 and Bax in the progression of oropharyngeal SqCC could not be attributed to mutations in the coding regions of these genes, but may result from the transcriptional or post-transcriptional regulation. Molecular (western blot) studies can therefore be applied to SqCC tissue samples to determine whether Bcl-2 and Bcl-X proteins are present in their cleaved forms, thus contributing to an apoptosis. To date, previous studies including ours have examined a relatively smaller number of patients to evaluate the actual role of Bcl-2 in cases of tonsillar SqCC in association with the HPV infections. Further studies are therefore warranted in a larger series of patients.
In conclusion, our results showed that there are a multiple number of factors associated with the HPV status and these factors might affect the prognosis of tonsillar SqCC. Our results also indicate that both p16 positivity and HPV ISH positivity, the former among the rest, were indicators of a good prognosis and they may have a prognostic value for cases of tonsillar SqCC. In addition, Bcl-2 also may play an important role in a good prognosis in association with the HPV status. With respect to the survival, our results support previous reports that there were favorable outcomes in cases of HPV-positive oropharyngeal SqCC. It would therefore be mandatory to evaluate these markers, which is essential for predicting a prognosis of patients.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 2.Adelstein DJ, Ridge JA, Gillison ML, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9-10, 2008, Washington, D.C. Head Neck. 2009;31:1393–1422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 3.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 4.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 5.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adelstein DJ. Concurrent chemoradiotherapy in the management of squamous cell cancer of the oropharynx: current standards and future directions. Int J Radiat Oncol Biol Phys. 2007;69(2 Suppl):S37–S39. doi: 10.1016/j.ijrobp.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 7.Hafkamp HC, Speel EJ, Haesevoets A, et al. A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5-8. Int J Cancer. 2003;107:394–400. doi: 10.1002/ijc.11389. [DOI] [PubMed] [Google Scholar]
- 8.Klussmann JP, Gültekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162:747–753. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 10.Mendolsohn J, Howley PM, Israel MA, Liotta LA. The molecular basis of cancer. 2nd ed. Philadelphia: W. B. Saunders; 2001. p. 41. [Google Scholar]
- 11.Kumar V, Abbas AK, Fausto N, Aster J. Robbins and Cotran pathologic basis of disease. 8th ed. Philadelphia: Saunders; 2010. p. 290. [Google Scholar]
- 12.Karam JA, Lotan Y, Karakiewicz PI, et al. Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol. 2007;8:128–136. doi: 10.1016/S1470-2045(07)70002-5. [DOI] [PubMed] [Google Scholar]
- 13.Dean E, Jodrell D, Connolly K, et al. Phase I trial of AEG35156 administered as a 7-day and 3-day continuous intravenous infusion in patients with advanced refractory cancer. J Clin Oncol. 2009;27:1660–1666. doi: 10.1200/JCO.2008.19.5677. [DOI] [PubMed] [Google Scholar]
- 14.Jäckel MC, Dorudian MA, Marx D, Brinck U, Schauer A, Steiner W. Spontaneous apoptosis in laryngeal squamous cell carcinoma is independent of bcl-2 and bax protein expression. Cancer. 1999;85:591–599. [PubMed] [Google Scholar]
- 15.Erber R, Klein W, Andl T, et al. Aberrant p21(CIP1/WAF1) protein accumulation in head-and-neck cancer. Int J Cancer. 1997;74:383–389. doi: 10.1002/(sici)1097-0215(19970822)74:4<383::aid-ijc4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JS, Jr, Thorstad WL, Chernock RD, et al. p16 positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–1998. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 18.Braakhuis BJ, Brakenhoff RH, Meijer CJ, Snijders PJ, Leemans CR. Human papillomavirus in head and neck cancer: the need for a standardised assay to assess the full clinical importance. Eur J Cancer. 2009;45:2935–2939. doi: 10.1016/j.ejca.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Schache AG, Liloglou T, Risk JM, et al. Evaluation of human papillomavirus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin Cancer Res. 2011;17:6262–6271. doi: 10.1158/1078-0432.CCR-11-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch FX. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene. 2002;21:1510–1517. doi: 10.1038/sj.onc.1205214. [DOI] [PubMed] [Google Scholar]
- 21.Peltenburg LT. Radiosensitivity of tumor cells: oncogenes and apoptosis. Q J Nucl Med. 2000;44:355–364. [PubMed] [Google Scholar]
- 22.Robinson M, Sloan P, Shaw R. Refining the diagnosis of oropharyngeal squamous cell carcinoma using human papillomavirus testing. Oral Oncol. 2010;46:492–496. doi: 10.1016/j.oraloncology.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 24.Rischin D, Young R, Fisher R, et al. Prognostic significance of HPV and p16 status in patients with oropharyngeal cancer treated on a large international phase III trial. J Clin Oncol. 2009;27(15s):abstr 6004. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andl T, Kahn T, Pfuhl A, et al. Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Cancer Res. 1998;58:5–13. [PubMed] [Google Scholar]
- 26.Sampaio-Góes FC, Oliveira DT, Dorta RG, et al. Expression of PCNA, p53, Bax, and Bcl-X in oral poorly differentiated and basaloid squamous cell carcinoma: relationships with prognosis. Head Neck. 2005;27:982–989. doi: 10.1002/hed.20258. [DOI] [PubMed] [Google Scholar]
- 27.Lo Muzio L, Falaschini S, Farina A, et al. Bcl-2 as prognostic factor in head and neck squamous cell carcinoma. Oncol Res. 2005;15:249–255. doi: 10.3727/096504005776404599. [DOI] [PubMed] [Google Scholar]
- 28.Popović B, Jekić B, Novaković I, et al. Bcl-2 expression in oral squamous cell carcinoma. Ann N Y Acad Sci. 2007;1095:19–25. doi: 10.1196/annals.1397.003. [DOI] [PubMed] [Google Scholar]
- 29.Nichols AC, Finkelstein DM, Faquin WC, et al. Bcl2 and human papillomavirus 16 as predictors of outcome following concurrent chemoradiation for advanced oropharyngeal cancer. Clin Cancer Res. 2010;16:2138–2146. doi: 10.1158/1078-0432.CCR-09-3185. [DOI] [PubMed] [Google Scholar]
- 30.Loro LL, Johannessen AC, Vintermyr OK. Loss of BCL-2 in the progression of oral cancer is not attributable to mutations. J Clin Pathol. 2005;58:1157–1162. doi: 10.1136/jcp.2004.021709. [DOI] [PMC free article] [PubMed] [Google Scholar]