Abstract
We have previously shown that CdSe/ZnS core/shell luminescent semiconductor nanocrystals or QDs (quantum dots) coated with PEG [poly(ethylene glycol)]-appended DHLA (dihydrolipoic acid) can bind AcWG(Pal)VKIKKP9GGH6 (Palm1) through the histidine residues. The coating on the QD provides colloidal stability and this peptide complex uniquely allows the QDs to be taken up by cultured cells and readily exit the endosome into the soma. We now show that use of a polyampholyte coating [in which the neutral PEG is replaced by the negatively heterocharged CL4 (compact ligand)], results in the specific targeting of the palmitoylated peptide to neurons in mature rat hippocampal slice cultures. There was no noticeable uptake by astrocytes, oligodendrocytes or microglia (identified by immunocytochemistry), demonstrating neuronal specificity to the overall negatively charged CL4 coating. In addition, EM (electron microscopy) images confirm the endosomal egress ability of the Palm1 peptide by showing a much more disperse cytosolic distribution of the CL4 QDs conjugated to Palm1 compared with CL4 QDs alone. This suggests a novel and robust way of delivering neurotherapeutics to neurons.
Keywords: cell targeting, drug delivery, nanoparticle, neuronal uptake, pyramidal neuron, quantum dot
Abbreviations: CL4, compact ligand; CNS, central nervous system; CPP, cell-penetrating peptide; DAPI, 4′,6-diamidino-2-phenylindole; DHLA, dihydrolipoic acid; EM, electron microscopy; ER, endoplasmic reticulum; GFAP, glial fibrillary acidic protein; HEK-293, human embryonic kidney 293 cell; NeuN, neuron-specific nuclear protein; PEG, polyethylene glycol; PPT1, palmitoyl:protein thioesterase 1; QD, quantum dot
INTRODUCTION
Neuropeptides are an attractive system in which to utilize chaperone effects in order to remedy protein misfolding in a variety of disorders (for reviews, see Arakawa et al., 2006; Fan and Ishii 2007; Parenti 2009). We wanted to test this approach utilizing a robust and molecularly well-defined system that could easily visualize the administration and distribution of various peptides to an in vitro hippocampal slice culture system. We decided to use luminescent nanoparticles in order to track peptide delivery, and observed that the nanoparticles themselves could be modified in order to target the peptides specifically to neurons.
The small (10 nm) size and unique spectral properties of CdSe/ZnS core/shell luminescent semiconductor nanocrystals make them attractive delivery vehicles for drugs and the visualization of cellular structures and processes (Mihrimah 2004; Emerich and Thanos 2006; Hild et al., 2008; Delehanty et al., 2009; Algar et al., 2011). There is also some evidence that the coating on the nanoparticle can affect the fate of the particle in that carboxy groups increase leukocyte adherence and migration whereas amino groups and PEG [poly(ethylene glycol)] do not (Rehberg et al., 2010) and an RGD sequence can target QDs (quantum dots) to tumours (Smith et al., 2008). Based on the idea that the surface chemistry of QDs can affect their in vivo fate (Smith et al., 2008) we therefore tested different coatings for drug delivery to the CNS (central nervous system) in a model of brain tissue in vitro, the hippocampal slice culture, where individual cells can be easily identified and microenvironmental conditions are well controlled (Kunkler and Kraig 1997; Mitchell et al., 2010; Grinberg et al., 2011).
There are three general ways of targeting QDs to cells (Delehanty et al., 2009, 2010). Passive delivery primarily relies on intrinsic properties between the QD and cell to mediate uptake, such as surface functionalization and charge, and therefore lacks the specificity necessary for a more targeted approach required by most medical applications (Delehanty et al., 2009). Active delivery, such as electroporation, while invaluable to basic research, is much too invasive for translation to treatment (Delehanty et al., 2009). The third, facilitated delivery, wherein a delivery agent is conjugated to the QD in order to assist in uptake, offers the best possibility for true medical applications to emerge (Delehanty et al., 2010). Two areas are of primary importance in regards to facilitated delivery, namely escaping the endolysosomal system and selective delivery to intracellular compartments (Delehanty et al., 2006).
We have previously shown that PEGylated (Figure 1A) QDs functionalized with a polyarginine ‘Tat-like’ CPP (cell-penetrating peptide) can be specifically delivered to cells via endocytic uptake with no adverse effects on cellular proliferation (Delehanty et al., 2006), but remain sequestered within acidic endolysosomal vesicles for at least 3 days after initial uptake (Delehanty et al., 2010). During this time the CPP mostly remains stably associated with the QD rather than being delivered directly to the cytosol through endosomal escape (Delehanty et al., 2010). To overcome this problem Sugahara et al. (2010) co-administered a tumour-penetrating peptide in order to allow drugs to penetrate into extravascular tumour tissue without the drug being chemically conjugated to the peptide. However, although co-injection with the peptide improved the therapeutic index of drugs, there was no evidence of endosomal cargo escape. We overcame this delivery and subsequent release problem by using an amphiphilic peptide (Palm1, AcWG(Pal)VKIKKP9GGH6, Figures 1B and 1C) designed to both mediate cell penetration and vesicle membrane interaction (Delehanty et al., 2010). This peptide exhibited rapid QD uptake by endocytosis followed by a slower, efficient endosomal release peaking at 48 h in a number of different cell lines. Importantly, this QD-peptide bioconjugate elicited minimal cytotoxicity in the cell lines tested.
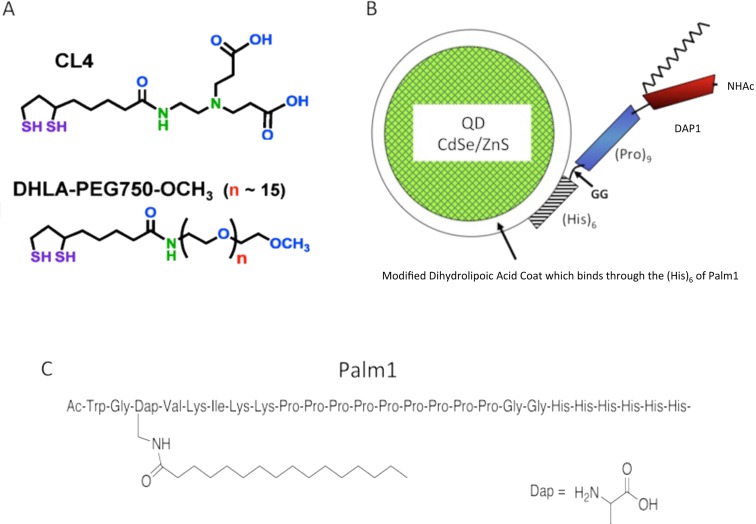
Figure 1. Stuctural characteristics of Palm1-coated QDs.
(A) Structure of CL4 (above) shown in relation to PEG (below). (B) Schematic representation of Palm1 peptide conjugated to the QD via histidine binding to the ZnS surface. (C) Structure of Palm1 peptide that contains the endosome egress signal DAP1 (G-palmitoyldiaminoproprionate-VKIKK) (Dawson et al. 2010).
In order to extend the usefulness of such QDs we varied the coating and peptide/QD ratio and tested them for cell-type specificity and subcellular distribution in a differentiating rat hippocampal slice culture system. Previous studies using QDs have largely failed to show selective cell targeting, although two recent studies have shown selective targeting of streptavidin-coated QDs to microglia (Minami et al., 2012) and atheronol-B analogue-coated QDs to macrophages (Prapainop et al., 2012). More promising has been a report that nanoparticles coated with polysorbate 80 could transport an analgesic peptide across the blood–brain barrier after intranasal administration (Ruan et al., 2012). Our goal was to target the QD–peptide to neurons while minimizing distribution elsewhere, using a hippocampal slice culture model system. We find that a negatively heterocharged polyampholyte coating, designated CL4 (compact ligand) (Susumu et al., 2011), exhibits robust targeting to neurons in these slices with little to no uptake by astrocytes, microglia or oligodendrocytes. Additionally, we confirm evidence of endosomal escape due to the Palm1 peptide, shown previously only in HEK-293 cells (human embryonic kidney 293 cells), in a much more complex hippocampal slice culture system.
EXPERIMENTAL
Quantum dots
CdSe/ZnS core–shell QDs with emission maxima centred at 625 nm were synthesized (Invitrogen) and made hydrophilic by exchanging the native hydrophobic capping shell with DHLA (dihydrolipoic acid)-based ligands. For peptide-mediated delivery, we attached neutral PEG-appended DHLA ligands as described previously (Susumu et al., 2007; Mei et al., 2008). For the cell-specific delivery, we used a more compact, negatively charged polyampholyte CL4 ligand.
Peptides
The palmitoylated peptide (Palm1) sequence used was AcWG(Pal)VKIKKP9GGH6 where ‘Pal’ corresponds to a palmitate group that is covalently attached to a non-hydrolysable thiol-resembling diaminopropionic acid residue functionality synthesized into the peptide backbone (Sapsford et al., 2007). All peptides were synthesized using Boc (t-butoxycarbonyl)-solid phase peptide synthesis, purified by HPLC, and purity verified by ESI-MS (electrospray ionization MS) (Dawson et al., 2010). All peptide sequences are written in the conventional N-to-C terminal orientation.
Peptide-mediated delivery
QD–Palm1 bioconjugates were formed by diluting a stock solution of preformed peptide–QD complexes (1 μM QD assembled with 12.5, 25 or 75 Palm1 peptides per QD in 0.1 M borate buffer, pH 8.9) into a complete growth medium to a final QD concentration of 50–100 nM. These peptide/QD ratios were determined experimentally for the peptide to be the ratio that yielded the optimal degree of uptake. The self-assembled bioconjugates were then incubated with cells as described below.
Uptake by rat hippocampal slice cultures
Hippocampal cultures were prepared as described previously (Kunkler and Kraig 1997; Mitchell et al., 2010). All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Chicago and conducted in accordance with the guidelines of the National Institutes of Health. Briefly, Wistar rat pups (P9–P10, where P is postnatal day) were anaesthetized with a progressive exposure to 100% CO2 and decapitated, the brains removed and placed into HBSS (Hanks balanced salt solution, 3°C) supplemented with D-glucose (6.5 mg/ml). Hippocampi were then isolated and sectioned (350 μm thick) perpendicular to their septotemporal axis. Slices displaying an intact pyramidal neuron cell layer and dentate gyrus were transferred to uncoated 30 mm Millicell-CMTM tissue culture inserts (Millipore) in six-well culture dishes. Cultures were initially maintained in medium containing 23% horse serum (no. 26050–088; Invitrogen) for 18 days in vitro before being transferred to serum-free media for an additional 3 days in vitro before experimentation (Mitchell et al., 2010). Slice cultures were used between 21 and 35 days in vitro. The constituents of horse-serum-based and serum-free media are defined elsewhere (Mitchell et al., 2010).
Hippocampal brain slice cultures mature in vitro and closely resemble the anatomical and physiological characteristics of their in vivo counterparts (e.g., see Kunkler and Kraig 1997, 1998, 2004; Hulse et al., 2004; 2004; Kunkler et al., 2005; Grinberg et al., 2011, 2012; Pusic et al., 2011).
Antibodies and staining
Hippocampal slices were fixed to gel-coated slides using Prolong Gold Antifade (Invitrogen, no. P36931) containing DAPI (4′,6-diamidino-2-phenylindole), a nuclear stain which binds strongly to A–T-rich regions in DNA. Neuronal staining was carried out through Nissl staining with NeuroTrace 500/525 green (Invitrogen, no. N21480), which binds to negatively charged nucleic acids in the ER (endoplasmic reticulum) of neurons (Quinn et al., 1995). We used a monoclonal RIP (receptor-interacting protein) antibody (Millipore, no. MAB1580), which has been shown to specifically recognize 2′,3′-cyclic nucleotide 3′-PDE (phosphodiesterase) in oligodendrocytes (Watanabe et al., 2006). To stain for astrocytes, we used a monoclonal antibody to GFAP (glial fibrillary acidic protein, Sigma, no. G3893), largely expressed in astrocytes, and shown to be a robust method of detection (Courel et al., 1986). Microglia were stained using isolectin b4 (Invitrogen, no. I21411), isolated from the African legume Griffonia simplicifolia, shown to be a robust stain for microglia (Streit 1990). For toxicity studies, cultures were imaged after incubating for 20 m in serum-free medium containing Sytox Green (500 nM, no. S7020; Invitrogen), a dead cell marker, at 24 h intervals. No evidence of neural cell death above control was observed. Culture cytoarchitecture was visualized by staining with NeuN (neuron-specific nuclear protein) at 1:1000 dilution (no. MAB377; Chemicon International). Toxicity images were compared with the positive controls incubated with NMDA (N-methyl-D-aspartate) (20 μM) in serum-free media for 60 min inducing excitotoxicity (Hulse et al., 2008).
Microscopy and image analysis
The intracellular distribution of QDs was analysed by fluorescence microscopy using a Marianas fully automated Yokogawa-type spinning disc confocal microscope equipped with ×40 and ×100 oil-immersion lenses.
Electron microscopy
All EM (electron microscopy) pictures were prepared and imaged at the University of Chicago Electron Microscopy Core. Samples were fixed with 2% glutaraldehyde and 4% PFA (paraformaldehyde) in 0.1 M sodium cacodylate buffer for 2 h and then washed with sodium cacodylate buffer thrice for 5 m. Samples were postfixed for 60 min in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer. Samples were then polymerized with Polymerize Spurr and placed at 60°C for 1–2 days. A series of 90 nm sections were cut using a Reichert-Jung Ultracut E microtome and stained with uranyl acetate and lead citrate. All images were taken at 300 KV with an FEI Tecnai F30 and visualized using a Gatan CCD (charge-coupled-device) digital micrograph.
RESULTS
Selective targeting to neurons in hippocampal slice culture
We administered 10–12 μl of colloidal QD–peptide conjugate to 1.2 ml of hippocampal slice culture medium (final concentration of 50–100 nM) and incubated at 36°C for 24 h. Afterwards, slices were fixed and prepared for imaging as described above. After 24 h in culture, CL4-coated QD–peptide conjugates showed patterns of distribution primarily within neurons along the CA3 cell layer (Figure 2). In contrast, we observed little to no delivery to GFAP-positive astrocytes, isolectin-positive microglia or CNPase (2',3'-cyclic-nucleotide 3'-phosphodiesterase)-positive oligodendrocytes (Figure 3). Additionally, we observed no QD-induced cytotoxicity in the cultures after 72 h of CL4–Palm1 exposure and staining with Sytox (Figure 4). Neurons were identified by morphology and NeuN staining. This is in agreement with Minami et al. (2012), who observed no cytotoxic effects after the direct injection into the mouse hippocampus for up to 28 days.
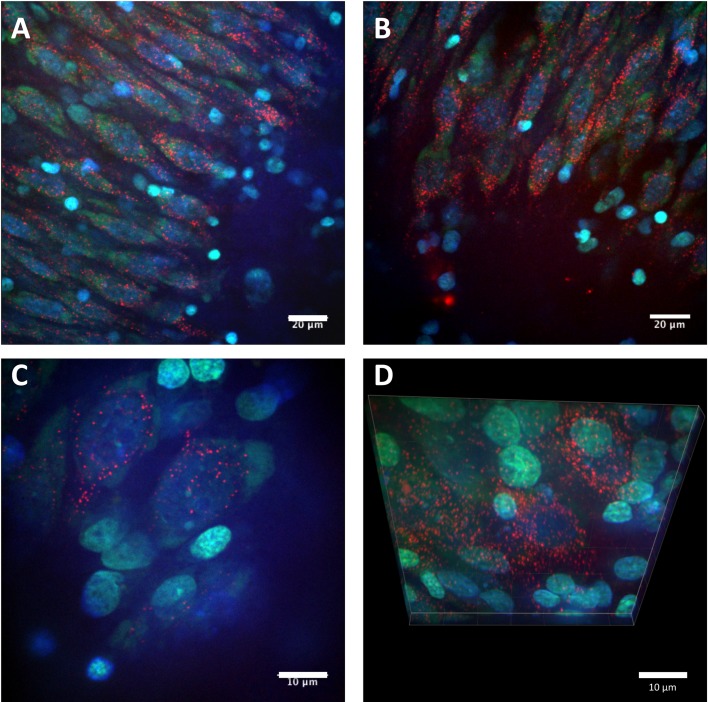
Figure 2. Confocal images of cell uptake of the CL4 QD–Palm1 complex.
(A–D) Representative images of cell bodies located in the CA3 cell layer of the rat hippocampus. QDs (red) containing the CL4 coat were conjugated to Palm1 and applied to the hippocampal slice culture preparation for 24 h. Cells were fixed and imaged with confocal microscopy (Nissl is green, DAPI is blue). The QDs showed neuronal targeting and intracellular localization, exhibiting a punctate, perinuclear distribution. Scale bars in A, B are 20 μm. C, D are 10 μm.
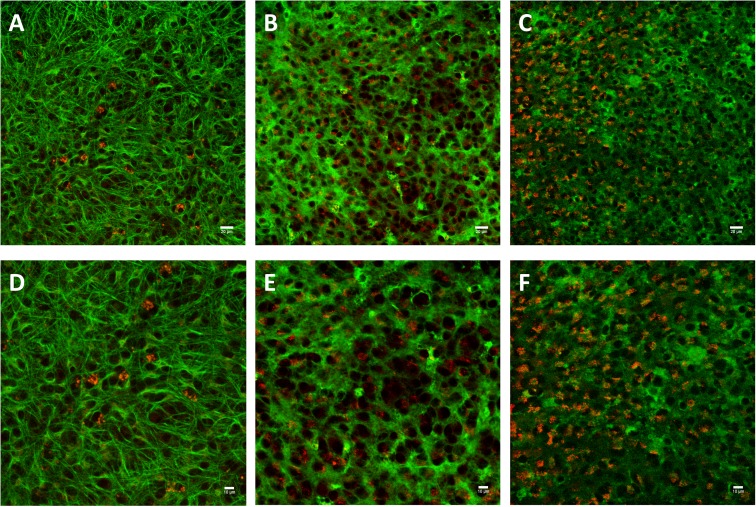
Figure 3. Confocal images show that the CL4 QD–Palm1 complex is neuron specific.
We utilized cell-specific staining (green, described in the Experimental section) to determine if the QDs (red) were distributing to other common organelles found in our slice culture system and found no trafficking to astrocytes (A,D), microglia (B,E) or oligodendrocytes (C,F). This confirms that our QD–Palm1 complex is targeting neurons specifically in a complex in vitro system. Scale bars in (A–C) are 20 μm, (D–F) are 10 μm.
Figure 4. Lack of QD-induced cytotoxicity in rat hippocampal slices after QD–Palm1 treatment for up to 72 h.
CL4 QD–Palm1 (red) after 48 h (A) and 72 h (B) in culture (Nissl is green, DAPI is blue). (C–F) Sytox staining. We observed no QD-induced cytotoxicity to the neurons after 72 h in culture. (C) NeuN stain showing cytoarchitechture. (D) Negative control. (E) CL4 QD–Palm1 after 72 h. (F) Positive control. Scale bars in (A,B) are 20 μm and in (C) are 200 μm.
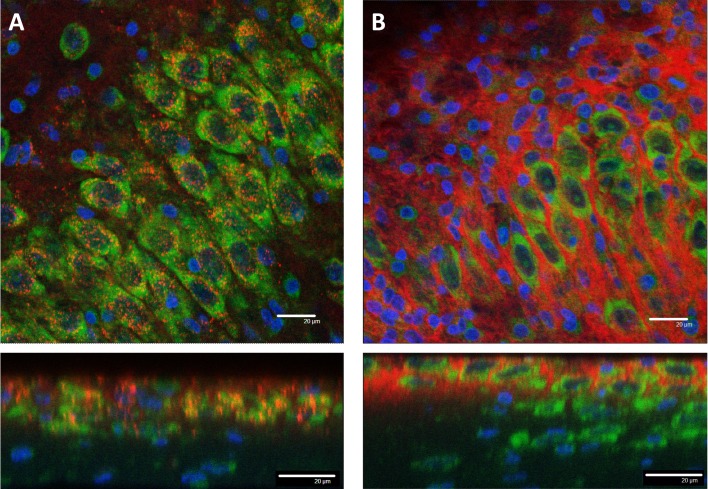
We then showed that CL4-coated QDs lacking the Palm1 peptide showed the same neuronal specificity as those described above (Figure 5A). This was in marked contrast to QDs coated with PEG, also without the Palm1 peptide, which failed to show either the specific cell-type targeting or cell uptake (Figures 5B–5D). Surprisingly, the CL4 coat alone confers unique neuronal targeting properties to these QDs with no need for a targeting peptide in the in vitro slice model, thus providing researchers with additional variables for tuning the QD–peptide delivery system to target cells of interest, possibly in a variety of disease models and applications. However, the lack of the Palm-1 means that the QDs do not readily egress into other subcellular compartments.
Figure 5. CL4 alone mediates intracellular uptake.
Confocal images of CL4 QDs (A) or PEG QDs (B) without peptide. (A) CL4-coated QDs showed intracellular delivery to neuronal cell bodies in the CA3 cell layer of a hippocampal slice culture after 24 h application. (B) PEG coated QDs were not delivered intracellularly and remained on the periphery of cell bodies in the CA3 cell layer after 24 h. QD is red, Nissl is green, DAPI is blue. Scale bars are 20 μm.
QD delivery was not impeded by slice culture thickness or application method
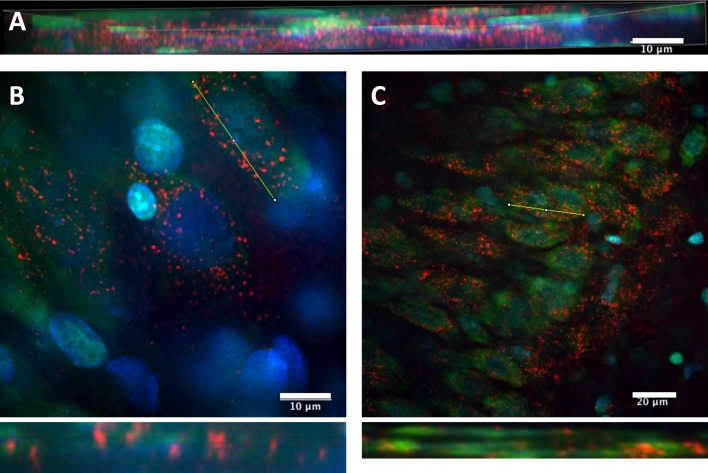
In our brain slice culture system, hippocampal slices sit upon a membrane suspended above the culture media. The QDs are applied directly to the media and allowed to naturally diffuse into the tissue from the bottom up. To eliminate concerns that the QDs might not distribute past the surface of the tissue, we compiled a series of z-stacks that showed that the CL4 QDs did penetrate the surface and were distributed throughout the tissue (Figure 6A). Additionally, z-stack images also show that the QDs penetrated through the cell membrane to the intracellular milieu, as opposed to merely sticking to the surface of the soma (Figures 6B and 6C).
Figure 6. Slice penetration of CL4–QD complex.
(A) Representative z-stack confocal image (representing around 30 separate images stacked together) of QD-peptide distribution within a portion of a slice. This confirms that our application method results in distribution past the surface of our slice preparations. (B,C) Re-sliced z-stacks of several neurons showing intracellular distribution of QDs within single neurons. Yellow bar indicates site of re-slicing, re-sliced z-plane is below. QDs are red, Nissl is green, DAPI is blue. Scale bars (A,B) are 10 μm, (C) is 20 μm.
Endosomal release
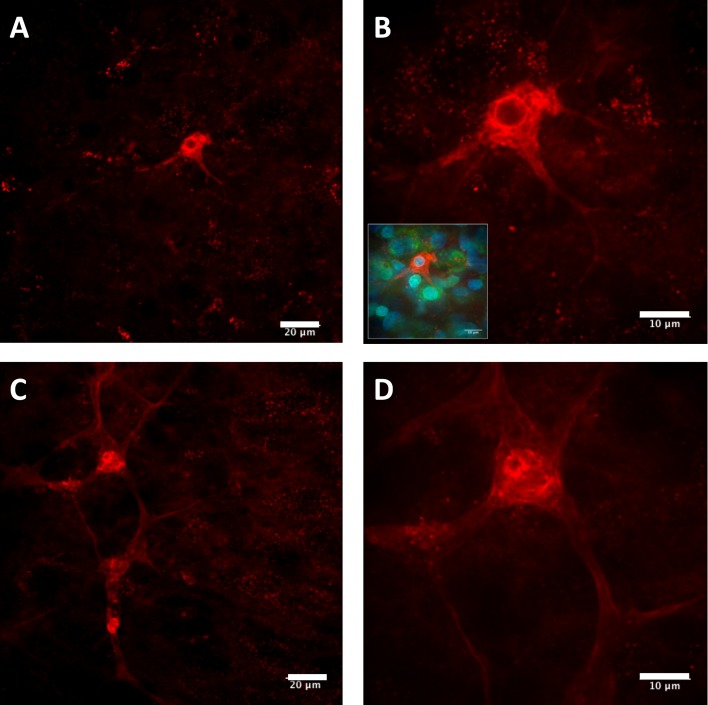
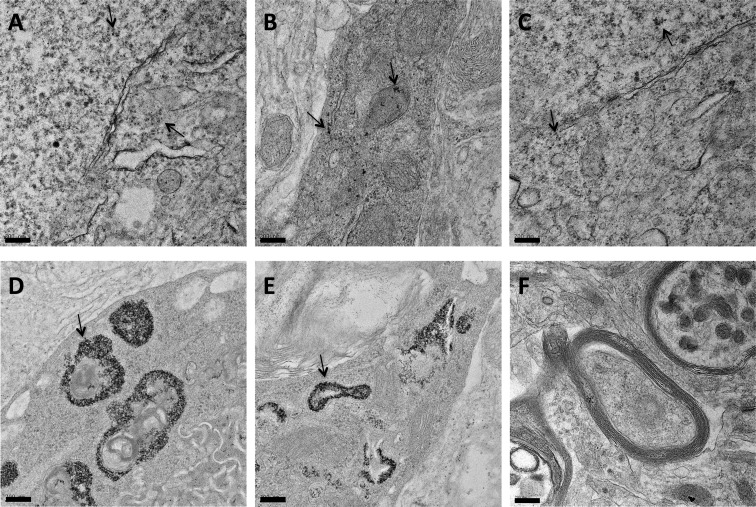
In addition to observing a punctate, perinuclear distribution of QDs in the CA3 cell layer of the hippocampus following application of the CL4-coated QD–peptide complex, pyramidal neurons outside of this region showed a more diffuse distribution of QD–peptide conjugates, indicating successful escape from the endolysosomal system and subsequent release into the cytosol that was not restricted to the pyramidal cell body but included the neuronal processes as well (Figure 7). This diffuse fluorescence was only observed with the CL4-coated QDs conjugated to the Palm1 peptide, and not the CL4-coated QDs alone, leading us to follow these data up with EM. Previously, we had shown endolysosomal escape with this peptide using only isolated HEK-293T/17 cell cultures (Delehanty et al., 2010). Here, we show this peptide exhibits a similar effect in neurons in a hippocampal slice culture system that contains many non-neuronal cell types that were not affected, thus demonstrating a remarkable specificity for this coat and efficacy for this peptide in a much more complex model system. EM confirmed that the CL4 QDs were being transported to the cytosol, were not cytotoxic and were not forming large aggregates when conjugated to Palm1 (Figures 8A–8C). However, CL4 QDs without peptide, while still targeting neurons, remained sequestered within intracellular compartments (Figures 8D and 8E), validating our fluorescence data above.
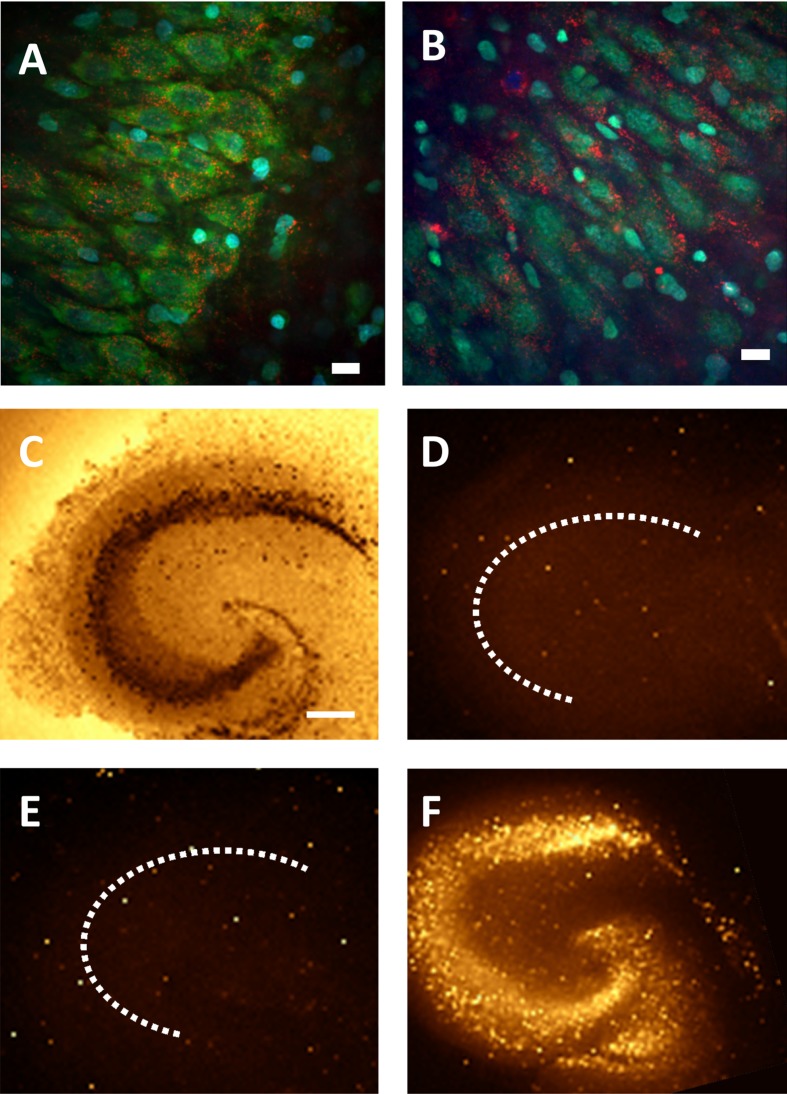
Figure 7. Representative pyramidal neurons showing uptake and diffusion of the CL4 QD-peptide complex into the soma and continuing through the neuronal processes.
(A) Pyramidal neuron off of the CA3 cell layer showing diffuse distribution of QD-peptide after 24 h. (B) Same neuron as in (A) at higher magnification, inset shows Nissl and DAPI stains of same image (QDs are red, Nissl is green, DAPI is blue). (C) Pyramidal neuron adjacent to the CA3 cell layer showing diffuse distribution of QD-peptide well into the processes after 24 h. (D) Same neuron as in (C) at higher magnification. Scale bars in (A,C) are 20 μm and (B,D) are 10 μm.
Figure 8. EM showing CL4–QD delivery with or without peptide to the cytosol of hippocampal neurons.
(A–C) CL4 QD conjugated to the Palm1 peptide is delivered intracellularly and largely dispersed throughout the cytosol (Black dots). (D,E) CL4 QD without peptide is delivered to the cell but largely remains sequestered around intracellular organelles. (F) Negative control. Arrows point to QDs. Scale bars are 200 nm.
DISCUSSION
For the first time, we show evidence that QDs can be selectively targeted to neurons in a structured tissue preparation, thus providing an example of a versatile delivery system that could be used to target drugs to neurons in the mammalian CNS. CL4 QD–Palm1 application showed neuron-specific, punctate delivery to the perinuclear region of neuronal cell bodies in the CA3 cell layer of hippocampal slice cultures with no toxicity or morphological changes (up to 72 h in culture). Additionally, pyramidal neurons outside the CA3 cell layer showed robust and diffuse QD uptake in both the soma and their associated processes. Cytosolic delivery, dependent on the Palm1 peptide, was further visualized through EM. No intracellular delivery to associated cells was observed across multiple experiments leading to the conclusion that the negatively heterocharged (polyampholyte) nature of the CL4 coating appears to be critical in mediating uptake in a neuronal specific manner, since the neutral charged PEG coating did not have this property.
Non-hydrolysable peptides such as GDap(Pal)VKIKK (DAP1) (Dawson et al., 2002) are competitive inhibitors of PPT1 (palmitoyl:protein thioesterase 1) in the low micromolar range (Dawson et al., 2010). They are taken up readily by cells by virtue of their basic amino acid sequences and function to some extent as chaperones in lymphoblasts from Battens disease patients with missense mutations which lead to misfolded PPT1 protein and subsequent destruction by the ER stress response and ubiquitination (Dawson et al., 2010). Our initial goal was to utilize semiconductor QDs as a fluorescent tag for visualization of Palm1 delivery into the CNS. Unexpectedly, the presence of the palmitate-peptide structure was shown to confer the function of endosomal escape on the peptide when conjugated to (Pro)9GG(His)6(Palm1) and bound to PEG-coated QD in HEK-293 cell culture (Delehanty et al., 2010). Our study extends dramatically this previous finding by not only showing that endosomal escape is possible in a much more complex culture system, by utilizing structured tissue in the form of a hippocampal slice culture, but also showing that, when the peptide is paired with the CL4 coat, the QD–Palm1 complex specifically targets neurons and then escapes into the cytosol. We attribute this neuronal specificity to the zwitterionic nature of the CL4 coat, since it is not observable with neutrally charged PEG. Further, the peptide appears to remain attached to the QD (by virtue of the binding of the polyhistidine sequence to the Zn in the QD) based on previous FRET (fluorescence resonance energy transfer) studies in cultured cells (Delehanty et al., 2010).
Our findings show that semiconductor QDs coated with CL4 can be used to target neurons while avoiding non-neuronal cells with little to no toxicity in a hippocampal slice culture model. Additionally, a sequence derived from the Palm1 peptide, while potentially acting as a chaperone of misfolded PPT1 in Battens disease patients (Dawson et al., 2010), can additionally be used as an effector of endosomal escape for the CL4 QDs. Concern over the possible toxicity and biodistribution of CdSe/ZnS core–shell QDs has recently been addressed in Sprague–Dawley rats (Hauck et al., 2012) and rhesus monkeys (Ye et al., 2012) and no appreciable toxicity was observed after several months, even after breakdown of the QDs. Both used 1 mg/kg cadmium and biochemical markers were in the normal range after 90 days, with two treated monkeys showing no ill effects after 1 year. Together, these findings suggest a neurotherapeutic application for peptide conjugated QDs, which display minimal photobleaching, are non-toxic and can deliver biologically active compounds specifically to most subcellular compartments of neurons. Thus, QD-delivery systems can be an effective therapeutic vehicle for a variety of CNS disorders.
ACKNOWLEDGEMENTS
We thank Miraim Domowicz for helpful discussions as well as Vitas Bindokas and Christine Labno in the Microscopy Core for help and advice with the interpretation of images. We thank Yimei Chen, Technical Director of the electron microscopy facility at University of Chicago, for EM images and analysis and Sylvia A Dawson for the artwork.
Footnotes
This work was supported by USPHS (United States Public Health Service) [grant numbers NS36866-38 (to G.D.) NS-19108 (to R.K.)] and P50 Grant [grant number HD09402 (to G.D. and R.K.)], as well as the Childrens Brain Disease Foundation. Philip Dawson was supported by [GM098871]. I.M. and A.H. were supported by NRL (Naval Research Laboratory), the NRL NSI (Naval Research Laboratory Nanoscience Institute), ONR (Office of Naval Research), DARPA (Defense Advanced Research Project Agency) and DTRA (Defense Threat Research Agency) JSTO (Joint Science and Technology Office) MIPR (Military Interdepartmental Purchase Request) [grant number B112582M].
REFERENCES
- Algar WR, Susumu K, Delehanty JB, Medintz IL. Semiconductor quantum dots in bioanalysis: crossing the valley of death. Anal Chem. 2011;83:8826–8837. doi: 10.1021/ac201331r. [DOI] [PubMed] [Google Scholar]
- Arakawa T, Ejima D, Kita Y, Tsumoto K. Small molecule pharmacological chaperones: from thermodynamic stabilization to pharmaceutical drugs. Biochim Biophys Acta. 2006;1764:1677–1687. doi: 10.1016/j.bbapap.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Courel MN, Girard N, Delpech B, Chauzy C. Specific monoclonal antibodies to glial fibrillary acidic protein (GFAP). J Neuroimmunol. 1986;11:271–276. doi: 10.1016/0165-5728(86)90080-9. [DOI] [PubMed] [Google Scholar]
- Dawson G, Dawson SA, Marinzi C, Dawson PE. Anti-tumor promoting effects of palmitoyl: protein thioesterase inhibitors against a human neurotumor cell line. Cancer Lett. 2002;187:163–168. doi: 10.1016/s0304-3835(02)00403-2. [DOI] [PubMed] [Google Scholar]
- Dawson G, Schroeder C, Dawson PE. Palmitoyl: protein thioesterase (Ppt1) inhibitors can act as pharmacological chaperones in infantile batten disease. Biochem Biophys Res Commun. 2010;395:66–69. doi: 10.1016/j.bbrc.2010.03.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delehanty JB, Medintz IL, Pons T, Brunel FM, Dawson PE, Mattoussi H. Self-assembled quantum dot-peptide bioconjugates for selective intracellular delivery. Bioconjug Chem. 2006;17:920–927. doi: 10.1021/bc060044i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delehanty JB, Mattoussi H, Medintz IL. Delivering quantum dots into cells: strategies, progress and remaining issues. Anal Bioanal Chem. 2009;393:1091–1105. doi: 10.1007/s00216-008-2410-4. [DOI] [PubMed] [Google Scholar]
- Delehanty JB, Bradburne CE, Boeneman K, Susumu K, Farrell D, Mei BC, Blanco-Canosa JB, Dawson G, Dawson PE, Mattoussi H, Medintz IL. Delivering quantum dot-peptide bioconjugates to the cellular cytosol: escaping from the endolysosomal system. Integr Biol (Camb) 2010;2:265–277. doi: 10.1039/c0ib00002g. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Thanos CG. The pinpoint promise of nanoparticle-based drug delivery and molecular diagnosis. Biomol Eng. 2006;23:171–184. doi: 10.1016/j.bioeng.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Fan JQ, Ishii S. Active-site-specific chaperone therapy for Fabry disease. Yin and Yang of enzyme inhibitors. FEBS J. 2007;274:4962–4971. doi: 10.1111/j.1742-4658.2007.06041.x. [DOI] [PubMed] [Google Scholar]
- Grinberg YY, Milton JG, Kraig RP. Spreading depression sends microglia on levy flights. PLoS ONE. 2011;6:e19294. doi: 10.1371/journal.pone.0019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg YY, van Drongelen W, Kraig RP. Insulin-like growth factor-1 lowers spreading depression susceptibility and reduces oxidative stress. J Neurochem. 2012;122:221–229. doi: 10.1111/j.1471-4159.2012.07763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck TS, Anderson RE, Fischer HC, Newbigging S, Chan WC. In vivo quantum-dot toxicity assessment. Small. 2010;6:138–144. doi: 10.1002/smll.200900626. [DOI] [PubMed] [Google Scholar]
- Hild WA, Breunig M, Goepferich A. Quantum dots–nano-sized probes for the exploration of cellular and intracellular targeting. Eur J Pharm Biopharm. 2008;68:153–168. doi: 10.1016/j.ejpb.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Hulse RE, Kunkler PE, Fedynyshyn JP, Kraig RP. Optimization of multiplexed bead-based cytokine immunoassays for rat serum and brain tissue. J Neurosci Methods. 2004;136:87–98. doi: 10.1016/j.jneumeth.2003.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulse RE, Swenson WG, Kunkler PE, White DM, Kraig RP. Monomeric IgG is neuroprotective via enhancing microglial recycling endocytosis and TNFα. J Neurosci. 2008;28:12199–12211. doi: 10.1523/JNEUROSCI.3856-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. Reactive astrocytosis from excitotoxic injury in hippocampal organ culture parallels that seen in vivo. J Cereb Blood Flow Metab. 1997;17:26–43. doi: 10.1097/00004647-199701000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. Calcium waves precede electrophysiological changes of spreading depression in hippocampal organ cultures. J Neurosci. 1998;18:3416–3425. doi: 10.1523/JNEUROSCI.18-09-03416.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. P/Q Ca2+ channel blockade stops spreading depression and related pyramidal neuronal Ca2+ rise in hippocampal organ culture. Hippocampus. 2004;14:356–367. doi: 10.1002/hipo.10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Hulse RE, Schmitt MW, Nicholson C, Kraig RP. Optical current source density analysis in hippocampal organotypic culture shows that spreading depression occurs with uniquely reversing currents. J Neurosci. 2005;25:3952–3961. doi: 10.1523/JNEUROSCI.0491-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei BC, Susumu K, Medintz IL, Delehanty JB, Mountziaris TJ, Mattoussi H. Modular poly(ethylene glycol) ligands for biocompatible semiconductor and gold nanocrystals with extended pH and ionic stability. J Mater Chem. 2008;18:4949–4958. [Google Scholar]
- Mihrimah O. Quantum dots and other nanoparticles: what can they offer to drug discovery? Drug Discov Today. 2004;9:1065–1071. doi: 10.1016/S1359-6446(04)03291-X. [DOI] [PubMed] [Google Scholar]
- Minami SS, Sun B, Popat K, Kauppinen T, Pleiss M, Zhou Y, Ward ME, Floreanig P, Mucke L, Desai T, Gan L. Selective targeting of microglia by quantum dots. J Neuroinflammation. 2012;9:22. doi: 10.1186/1742-2094-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell HM, White DM, Kraig RP. Strategies for study of neuroprotection from cold-preconditioning. J Vis Exp. 2010;43:e2192. doi: 10.3791/2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti G. Treating lysosomal storage diseases with pharmacological chaperones: from concept to clinics. EMBO Mol Med. 2009;1:268–279. doi: 10.1002/emmm.200900036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prapainop K, Witter DP, Wentworth P. A chemical approach for cell-specific targeting of nanomaterials, small molecule-initiated misfolding of nanoparticle corona proteins. J Am Chem Soc. 2012;134:4100–4103. doi: 10.1021/ja300537u. [DOI] [PubMed] [Google Scholar]
- Pusic AD, Grinberg YY, Mitchell HM, Kraig RP. Modeling neural immune signaling of episodic and chronic migraine using spreading depression in vitro. J Vis Exp. 2011;52:e2910. doi: 10.3791/2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn B, Toga AW, Motamed S, Merlic CA. Fluoro Nissl green: a novel fluorescent counterstain for neuroanatomy. Neurosci Lett. 1995;184:169–172. doi: 10.1016/0304-3940(94)11198-r. [DOI] [PubMed] [Google Scholar]
- Rehberg M, Praetner M, Leite CF, Reichel CA, Bihari P, Mildner K, Duhr S, Zeuschner D, Krombach F. Quantum dots modulate leukocyte adhesion and transmigration depending on their surface modification. Nano Lett. 2010;10:3656–3664. doi: 10.1021/nl102100m. [DOI] [PubMed] [Google Scholar]
- Ruan Y, Yao L, Zhang B, Zhang S, Guo J. Nanoparticle-mediated delivery of neurotoxin-ii to the brain with intranasal administration: an effective strategy to improve antinociceptive activity of neurotoxin. Drug Dev Ind Pharm. 2012;38:123–128. doi: 10.3109/03639045.2011.592533. [DOI] [PubMed] [Google Scholar]
- Sapsford KE, Pons T, Medintz IL, Higashiya S, Brunel FM, Dawson PE, Mattoussi H. Kinetics of metal-affinity driven self-assembly between proteins or peptides and CdSe−ZnS quantum dots. J Phys Chem C. 2007;111:11528–11538. [Google Scholar]
- Smith BR, Cheng Z, De A, Koh AL, Sinclair R, Gambhir SS. Real-time intravital imaging of RGD-quantum dot binding to luminal endothelium in mouse tumor neovasculature. Nano Lett. 2008;8:2599–2606. doi: 10.1021/nl080141f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ. An improved staining method for rat microglial cells using the lectin from Griffonia simplicifolia (Gsa I-B4). J Histochem Cytochem. 1990;38:1683–1686. doi: 10.1177/38.11.2212623. [DOI] [PubMed] [Google Scholar]
- Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328:1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susumu K, Uyeda HT, Medintz IL, Pons T, Delehanty JB, Mattoussi H. Enhancing the stability and biological functionalities of quantum dots via compact multifunctional ligands. J Am Chem Soc. 2007;129:13987–13996. doi: 10.1021/ja0749744. [DOI] [PubMed] [Google Scholar]
- Susumu K, Oh E, Delehanty JB, Blanco-Canosa JB, Johnson BJ, Jain V, Hervey WJ, Algar WR, Boeneman K, Dawson PE, Medintz IL. Multifunctional compact zwitterionic ligands for preparing robust biocompatible semiconductor quantum dots and gold nanoparticles. J Am Chem Soc. 2011;133:9480–9496. doi: 10.1021/ja201919s. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Sakurai Y, Ichinose T, Aikawa Y, Kotani M, Itoh K. Monoclonal antibody rip specifically recognizes 2′,3′-cyclic nucleotide 3′-phosphodiesterase in oligodendrocytes. J Neurosci Res. 2006;84:525–533. doi: 10.1002/jnr.20950. [DOI] [PubMed] [Google Scholar]
- Ye L, Yong KT, Liu L, Roy I, Hu R, Zhu J, Cai H, Law WC, Liu J, Wang K, Liu J, Liu Y, Hu Y, Zhang X, Swihart MT, Prasad PN. A piplot study in non-human primates shows no adverse response to intravenous injection of quantum dots. Nat Nanotechnol. 2012;7:453–458. doi: 10.1038/nnano.2012.74. [DOI] [PubMed] [Google Scholar]