Abstract
While plant diversity is well known to increase primary productivity, whether these bottom-up effects are enhanced by reciprocal top-down effects from the third trophic level is unknown. We studied whether pine tree species diversity, aphid-tending ants and their interaction determined plant performance and arthropod community structure. Plant diversity had a positive effect on aphids, but only in the presence of mutualistic ants, leading to a threefold greater number of both groups in the tri-specific cultures than in monocultures. Plant diversity increased ant abundance not only by increasing aphid number, but also by increasing ant recruitment per aphid. The positive effect of diversity on ants in turn cascaded down to increase plant performance; diversity increased plant growth (but not biomass), and this effect was stronger in the presence of ants. Consequently, bottom-up effects of diversity within the same genus and guild of plants, and top-down effects from the third trophic level (predatory ants), interactively increased plant performance.
Keywords: arthropod community structure, bottom-up effects, plant diversity, plant growth, top-down effects
1. Introduction
The consequences of plant species diversity on ecosystem function and on the structure of associated communities of consumers have been increasingly recognized [1–7]. There is growing evidence that a greater diversity of plant species may stabilize the multi-trophic arthropod community interacting with plants [3,4,7], increase net primary production [5,8] and even provide resistance to biological invasions [9]. In particular, a greater diversity of plant species was found to positively affect plant growth as well as the abundance and diversity of associated arthropods in grasses, legumes, forbs and other herbaceous plants [3,4,7]. In this sense, two non-exclusive hypotheses have been proposed to explain ecological consequences of host-plant species diversity on the multi-trophic communities that plants support. First, the ‘resource specialization hypothesis’ argues that increasing plant species diversity will provide a greater diversity of resources and, therefore, would attract greater diversity of herbivore species [10,11]. Alternatively, the ‘more individuals hypothesis’ postulates that a high diversity of plant species increases the productivity of plant populations and, consequently, would increase the abundance of consumers and the probability of observing higher species diversity at the community level [12].
Recent studies have focused more mechanistically on how plant–neighbour interactions in plots of different plant diversity may affect the associated communities [3,4,7,13–16]. A few studies have shown that the bottom-up effects of plant diversity cascade up to higher trophic levels, including the third trophic level [3,16]. It is particularly interesting that Haddad et al. [3] observed marked increases in the ratio of predator-to-herbivore abundance associated with increasing plant diversity. However, what remains unclear is the generality of such findings, and whether these effects may affect plant fitness, such that the bottom-up effects of plant diversity interact with the top-down effects from the third trophic level.
Ants, by acting as predators, mutualists or ecosystem engineers, have large ecological effects and can play an important role in determining the structure and function of entire communities [17–19]. An interesting case is the food-for-protection mutualistic interaction established between ants and honeydew-producing hemipteran insects such as aphids. In these interactions, ants ‘tend’ aphids, feeding upon their sugary honeydew exudates in exchange for protection from predators and parasites (reviewed by Stadler & Dixon [20]). Ant–aphid interactions have been proposed to be keystone interactions [18] because aphid-attracted ants can have marked community-wide effects. Specifically, the presence/absence of ants affects the abundance of aphids, but also population dynamics of other arthropods in the community, such as aphid predators and other untended herbivores [18,21,22], which may in turn affect plant growth and fitness [23,24]. In addition to this, because aphid-tending ants may contribute to defend plants against their enemies, plants, in the presence of ants, would benefit from reducing the allocation of resources to expensive chemical defences, leaving them available for other vital strategies [25]. As a consequence, the factors that mediate ant–aphid interactions can have broad effects themselves. Although there are several studies showing that ant–aphid interactions vary across plant genotypes [26–29], the effects of plant diversity (both intra- and inter-specific) on this mutualistic interaction remain unstudied.
The aim of this study was to test for the effects of host-plant species diversity (within the genus Pinus), mutualistic ants, and the interaction between these factors on plant performance/productivity/defences and the structure of associated above-ground arthropod communities. To test for these effects, we performed a factorial field experiment where we manipulated host-plant species diversity (three levels: mono-, di- and tricultures) and the presence of mutualistic ants (two levels: absence and presence). We measured plant growth, conducted arthropod counts, and quantified the defensive and nutritional status of pine seedlings. Pine seedlings are especially vulnerable to herbivore attack, and at this stage affect survival and competitive dynamics among species regenerating in forest gaps, which may have long-term effects on forest structure. We specifically addressed four questions: (i) what are the bottom-up effects of pine species diversity on plant growth and arthropod communities? (ii) What are the top-down effects of ants on plant growth and arthropod communities? (iii) What is the relative strength of these two effects? (iv) Do they interact? We hypothesized that high plant species diversity should lead to increased plant productivity, which in turn benefits aphids directly and ants indirectly. We further hypothesized that ants could provide a positive effect on plant growth, as their effect in facilitating aphid population could be outweighed by reduced non-aphid herbivores in ant-tended plants [18]. This study thus provides the first test for multi-trophic interactions between plant diversity and predator effects.
2. Material and methods
(a). Study area and species
We used three focal species belonging to the Pinus clade, which are broadly planted worldwide, particularly in the study area, the northwest of the Iberian Peninsula: maritime pine (Pinus pinaster Ait.), Monterrey pine (Pinus radiata D. Don.) and Scots pine (Pinus sylvestris L.). These pine species coexist in mixed forests found throughout the study area, with overlapping distributions at altitudes of 400–800 m. Six-month old seedlings were provided by a local nursery (Norfor Nursery Ltd., Pontevedra; viverofigueirido@norfor.es).
The experimental plantation was established at a small agricultural plot located in Pontevedra (Galicia, northwest Spain, 42.26° N 8.39° W). The climate in this area is temperate humid Atlantic, with annual precipitation of about 1620 mm and mean annual temperature of 15.4°C. Previous inspections of the study site confirmed the presence of ant-tended aphids (mainly Cinara spp.) and aphid-tending ants (Lasius grandis) on the pine trees surrounding the plot.
(b). Experimental design
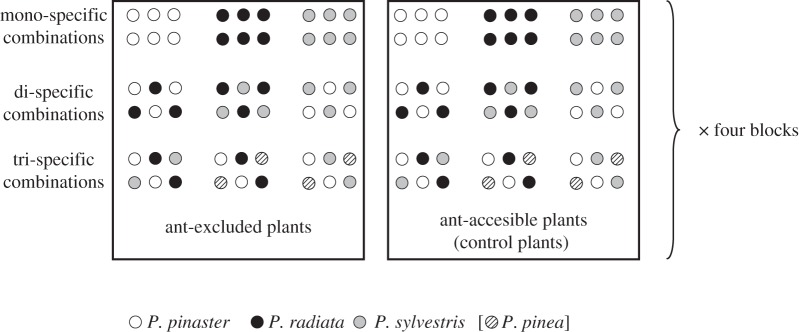
In early spring 2011, we planted six-month-old pine seedlings, manipulating the plant diversity by creating three assemblages of different pine species diversity (figure 1): (i) monocultures of the three pine species, (ii) all possible dicultures with those three species (three different combinations) and (iii) tricultures. The triculture treatment consisted of three different combinations, one with the three species studied in mono- and dicultures, and two additional combinations, including a fourth pine species (Pinus pinea, also native from the study area) not included in mono- or dicultures (figure 1). Each experimental unit (hereafter ‘combination’) consisted of six plants in two parallel rows of three plants each (figure 1). Neighbouring plants were separated by approximately 10 cm, and combinations were spaced at least 1 m apart, with the positioning of plants within the combination being randomized. The experiment followed a randomized split-plot design replicated in four blocks, with ant treatment (two levels: presence or absence) as the whole plot factor and species diversity (mono-, di- and tricultures) as the split factor, with three different combinations of each diversity treatment for a total of nine combinations per block. All blocks were separated by at least 3 m. In total, there were 432 pine seedlings, corresponding to four blocks × two ant treatments × three species diversity treatments × three combinations for each diversity treatment × six plants in each combination.
Figure 1.
Schematic of one block in the experimental design, showing the ant treatments as whole plots, the three levels of specific diversity as split-plots, and the three different mono-, di- and tri-specific combinations of the three focal pine species. Constructing three different tri-specific combinations was possible by including a fourth native pine species (P. pinea, circles with bars). Including ‘combination’ as a factor in the model allowed us to remove the possible effects of considering particular species (i.e. sampling effects), thus testing for non-additive effects of diversity. In the field, diversity combinations were randomized within the whole plots (not showed here for clarity).
On 18 April, 2 days after plantation, we measured the stem height of all the plants and carefully placed a piece of tape around the shoot (2 cm wide) of each plant. Ants were excluded from half of the plants by coating the outside surface of the tape with a sticky paste (Tanglefoot, Tanglefoot Company, MI). Control plants, with tape but without sticky paste, allowed ant access.
(c). Sampling, plant measurements and chemical analysis
We recorded the number of arthropods on each tree on August 25, when aphid populations peak in this area (X. Moreira 2010, personal observation). Arthropods were identified to species or to the taxonomic level necessary to determine their trophic level by consulting relevant literature and with the help of taxonomist Alberto Gayoso (entomologist from Xunta de Galicia). Arthropods were classified as ant-tended aphids, ants, untended (non-aphid) herbivores or aphid predators. Some aphid parasitoids were also found, but in very low numbers. Ant-tended aphids consisted either of Cinara maritimae (95%) or Cinara pini (5%). These species of aphids form small colonies on terminal shoot and branches of young and mature pine trees (X. Moreira, personal observation). Ants always consisted of aphid-tending Lasius grandis (Hymenoptera: Formicidae), all from the same ant nest. Non-aphid herbivores consisted of phloem-feeders (Pissodes castaneus, Coleoptera: Curculionidae) and sap feeders (Stictocephala bisonia, Hemiptera: Membracidae; Leucaspis pini, Hemiptera: Coccidae; and Pentatoma rufipes, Hemiptera: Pentatomidae). Aphid predators consisted of wasps (Dolichovespula media, Hymenoptera: Vespidae), ladybirds (Adalia bipunctata, Coleoptera: Coccinellidae and Coccinella septempunctata, Coleoptera: Coccinellidae), one species of assassin bug (Hemiptera: Reduviidae) and spiders (Araneae, various families).
On 26 August, plant height was measured and all pine seedlings were harvested, transported to the laboratory in ice coolers and immediately sampled for above-ground biomass determination and for further chemical analyses. One fresh 5 cm-long piece of the terminal shoot of each plant was sampled, weighed, immediately frozen and preserved at −80°C for analysis of non-volatile resin and antioxidant activity. Another subsample of terminal shoot was immediately weighed, oven-dried (45°C to constant weight) and manually ground in a mortar with liquid nitrogen for analyses of phenolic compounds, nitrogen and non-structural carbohydrates.
Concentration of non-volatile resin in the stem was estimated gravimetrically as described by Sampedro et al. [30] and Moreira et al. [31] (see the electronic supplementary material, appendix S1), and expressed as milligram of non-volatile resin × g–1 stem on a dry weight (d.w.) basis. Total phenolics in the stem were estimated by the Folin–Ciocalteu assay as described by Sampedro et al. [30] and Moreira et al. [31] (see the electronic supplementary material, appendix S1), and expressed as milligram of tannic acid equivalent × g–1 d.w. stem. These variables have been proved useful for identifying differences in resistance in previous studies [32,33]. The antioxidant capacity in aqueous extracts of stem tissue was measured by a modification of the method described by Noguera et al. [34] and Erel [35] (see the electronic supplementary material, appendix S1), and expressed as milligram of Trolox equivalent × g−1 d.w. stem. The concentrations of soluble sugars and starch in the stem were determined colorimetrically by the anthrone method [30,36] (see the electronic supplementary material, appendix S1) using glucose and potato starch, respectively, as standards and expressed as mg × g−1 d.w. Total nitrogen was determined with a CN-2000 macro elemental analyser (LECO Corporation, St Joseph, MI) at the central facilities of Universidade de Vigo, Spain (http://webs.uvigo.es/cactiweb/), and expressed in mg × g−1 d.w. of tissue. To reduce the analytical effort to reasonable levels, nutrient concentration and antioxidant capacity in the stem were analysed in a subsample of 48 selected pine trees. Specifically, we analysed only one plant per combination in the three monocultures (P. pinaster, P. radiata and P. sylvestris) and one plant of each species in the triculture including those three pine species.
(d). Statistical analyses
Data analysis was performed with mixed linear models for plant growth and defensive and nutritional status traits, and generalized linear mixed models for arthropod abundance, using the Mixed and Glimmix procedures, respectively (SAS 9.2 System, SAS, Cary, NC). The main effects of ants (A), diversity (D) and the A × D interaction were treated as fixed factors. The effect of the different combinations within each diversity treatment (C) and the A × C interaction were also included as fixed factors nested within the diversity treatments, in order to account for the variation between combinations and the effect of the species identity within each combination. The effects of block (B) and A × B interaction (i.e. the whole plots) were considered random factors in order to analyse the main effects of the split-plot design with the appropriate error terms [37]. To avoid confounding effects associated with size differences between pine species, final height was included as covariate in the analysis of arthropod abundance, defences and carbohydrates. Initial height was included as a covariate for the analysis of plant growth. Pearson correlations were used to evaluate the relationships among all traits separately in control and ant-excluded pine trees. Data are shown as mean ± s.e.
Diverse plots may have greater performance or arthropod abundance because of the increased probability of including species with distinct performance or communities (additive or sampling effects [38,39]). Alternatively, plant species diversity may modify plant performance and the structure of arthropod community via positive or negative interactions among neighbouring plant species (non-additive effects [38,39]). We structured our models not only to test for overall effects of diversity (and ant × diversity interactions), but also to determine whether such effects occurred through non-additive dynamics. Data were first analysed as plot means (i.e. the mean of six plants within a combination), including the combinations of each diversity treatment (nested within the diversity treatment) in the statistical model. By accounting for variation among combinations within diversity treatments, a significant diversity effect indicates that such effects are independent of the contribution coming from any single species combinations, and thus that such effects are non-additive (i.e. synergistic or antagonistic effect among species). In addition, we also analysed data for each species separately according to the same statistical models (see results in electronic supplementary material). In these tests, significant diversity effects were also indicative of non-additive dynamics, as they showed differences based upon the diversity environment within which that single species occurs. Using these two approaches, we tested for diversity effects (and ant × diversity interactions) with three focal pine species (P. pinaster, P. radiata and P. sylvestris) in mono-, di- and tricultures. Because all combinations of tricultures would by necessity be uniform in species combination, we incorporated variation in triculture species composition by adding two combinations with one additional species, P. pinea (see figure 1). Although we lack mono- and di-culture treatments of P. pinea, excluding the two triculture treatments that contained P. pinea trees from our analyses did not alter the direction or significance of any of our results (results not shown).
After determining the spatial position (x, y) of each plant, we performed an analysis of the semivariance of the residuals of the mixed models for all the studied variables to check whether spatial heterogeneity in the natural distribution of ants, aphids or soil properties could be affecting our results [40]. We observed no significant deviation from random spatial distribution (see semivariograms in the electronic supplementary material, figure S1).
3. Results
(a). Consequences of host-plant species diversity and ants on pine performance
Host-plant species diversity significantly affected pine primary growth (table 1). Specifically, final height was 12 per cent and 16 per cent greater in pine dicultures and tricultures, respectively, than in pine monocultures (figure 2a). In contrast, we found that pine above-ground biomass was not significantly affected by host-plant species diversity after four months of experiment (table 1 and figure 2b).
Table 1.
A summary of the linear mixed model for the effects of plant-specific diversity (mono-, di- and tricultures) and the presence of mutualistic ants (two levels: presence or absence) on plant performance. The effect of the particular combination of pine species (three mono-, three di- and three tricultures) nested in each diversity treatment was included in the model. Ant treatments took four months. Initial height was used as covariate. Significant p-values (p < 0.05) are indicated in bold.
| final height |
above-ground biomass |
|||||
|---|---|---|---|---|---|---|
| DFnum | DFden | F | p-value | F | p-value | |
| ant | 1 | 3 | 27.47 | 0.014 | 0.67 | 0.474 |
| diversity | 2 | 47 | 25.59 | <0.001 | 2.04 | 0.141 |
| ant × diversity | 2 | 47 | 4.23 | 0.021 | 1.09 | 0.344 |
| combination | 6 | 47 | 12.40 | <0.001 | 6.52 | <0.001 |
| ant × combination | 6 | 47 | 0.61 | 0.722 | 0.82 | 0.559 |
| initial height | 1 | 47 | 44.81 | <0.001 | 2.36 | 0.131 |
Figure 2.
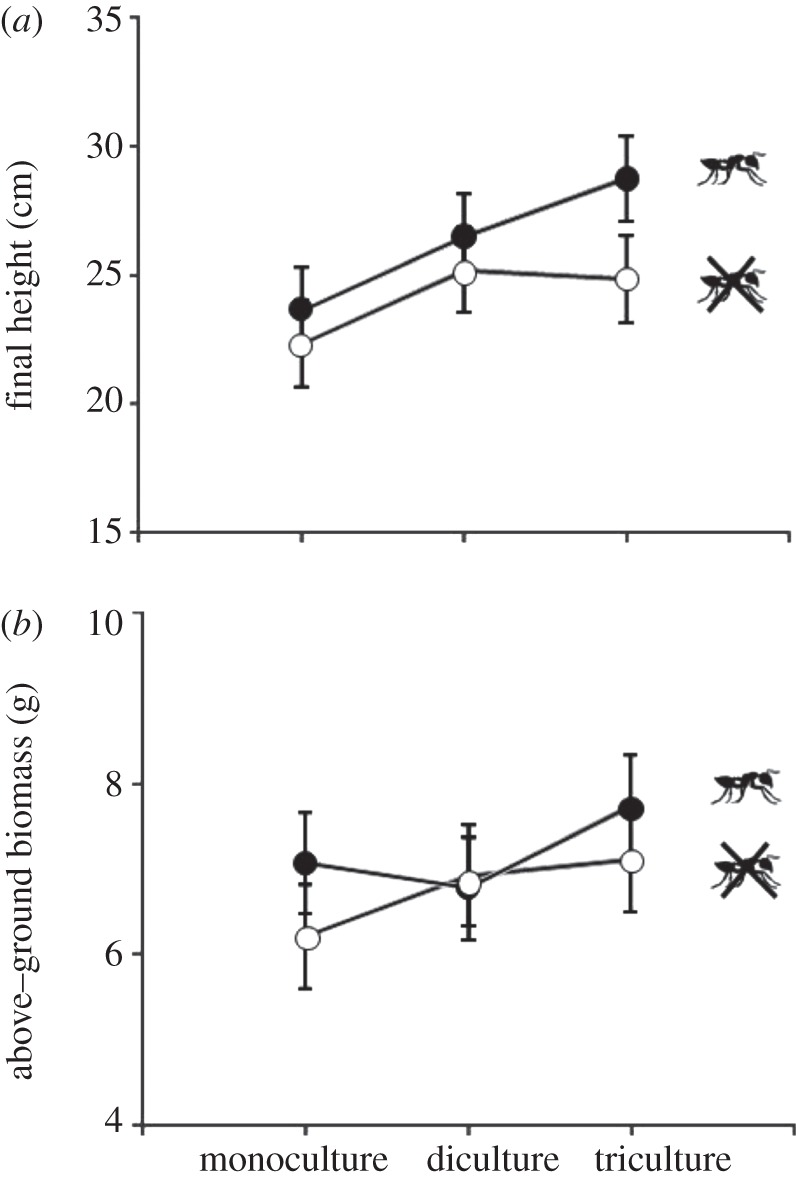
The effect of host plant species diversity (mono-, di- and tricultures) and the presence of mutualistic ants (two levels: presence or absence) on (a) final height and (b) above-ground biomass. Ant treatments were in place for four months. Initial height was used as covariate in the statistical model. Least-square means ± s.e. (n = 72).
The presence of ants had significant effects on pine primary growth (table 1). After four months of growth, final height was 10 per cent greater in pines with ants than ant-excluded pines (figure 2a). Moreover, the effect of ants on pine primary growth depended on species diversity treatment (significant ant × species diversity interaction; table 1 and figure 2a). While plant diversity effects were significant for both control plants (F2,23 = 23.60, p < 0.001) and ant-excluded plants (F2,23 = 7.15, p = 0.003), the magnitude of plant diversity effects was greater for control plants (plants with ants; figure 2a). Pine above-ground biomass was not significantly affected by the presence of ants (versus absence), nor by the interaction between ant and species diversity treatments (table 1 and figure 2b).
Results for each pine species, when analysed individually, were consistent with those found at the plot level. We observed that plant species diversity significantly increased primary growth in all pine species (see the electronic supplementary material, table S1 and figure S2). Primary growth was higher in the presence than in the absence of ants for all three species, although the effect was significant only for P. sylvestris and marginally for P. radiata (see the electronic supplementary material, table S1 and figure S2). As we observed at the plot level, primary growth was greater in diverse treatments with ants (control treatment), but ant × diversity interaction was not significant (see the electronic supplementary material, table S1 and figure S2).
(b). Consequences of host-plant species diversity and ants on arthropod abundance
Four months after establishing the ant exclusion treatments, we recorded 1440 arthropods, which were classified as 561 ants (39%), 634 ant-tended aphids (44%), 215 aphid predators (15%) and 30 non-aphid herbivores (2%).
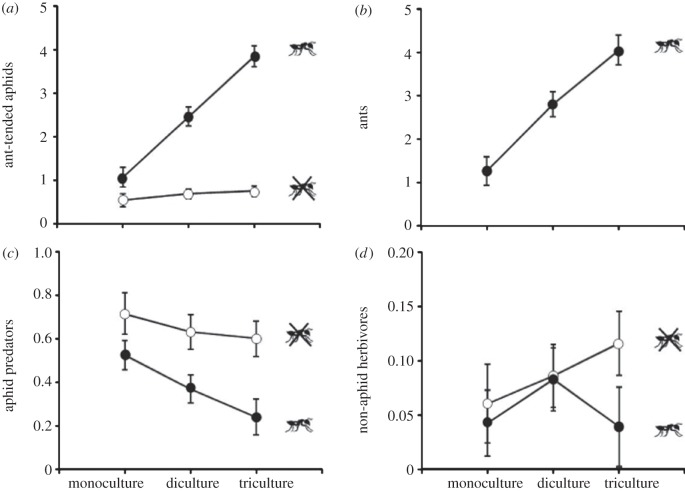
Plant species diversity significantly affected the abundance of associated arthropods (table 2). Specifically, the mean number of ant-tended aphids was approximately twofold and threefold greater in pine di- and tricultures, respectively, than in pine monocultures (figure 3a). Similarly, the mean number of ants was approximately twofold and threefold greater in pine di- and tricultures, respectively, compared with pine monocultures (figure3b). In addition to influencing ants through changes in aphid abundance, diversity also affected significantly the rate of ant recruitment to aphids (table 2). Specifically, we observed that the rate of ant recruitment (ant : aphid ratio) increases with increasing diversity (0.62 ± 0.09 in monocultures, 0.95 ± 0.08 in dicultures and 1.05 ± 0.10 in tricultures). The mean number of aphid predators and non-aphid herbivores were not significantly affected by species diversity treatment (table 2 and figure 3c,d).
Table 2.
A summary of the generalized mixed models for the effects of plant-specific diversity (mono-, di- and tricultures) and the presence of mutualistic ants (two levels: presence or absence) on the abundance of the associated arthropod community at several trophic levels. The effect of the particular combination of pine species (three mono-, three di- and three tricultures) nested in each diversity treatment was included in the model. Ant treatments took four months. Final height was used as covariate. Significant p-values (p < 0.05) are indicated in bold.
| DFnum | DFden | F | p-value | F | p-value | F | p-value | |
|---|---|---|---|---|---|---|---|---|
| ant-tended aphids | aphid predators | non-aphid herbivores | ||||||
| ant | 1 | 3 | 172.32 | 0.001 | 19.80 | 0.021 | 1.58 | 0.298 |
| diversity | 2 | 47 | 25.96 | <0.001 | 2.17 | 0.126 | 0.48 | 0.623 |
| ant × diversity | 2 | 47 | 33.29 | <0.001 | 0.81 | 0.453 | 0.88 | 0.423 |
| combination | 6 | 47 | 1.73 | 0.135 | 1.44 | 0.219 | 1.41 | 0.230 |
| ant × combination | 6 | 47 | 1.49 | 0.201 | 0.90 | 0.504 | 0.57 | 0.749 |
| final height | 1 | 47 | 1.93 | 0.171 | 2.79 | 0.101 | 0.01 | 0.919 |
| ants | ant : aphid ratio | |||||||
| diversity | 2 | 23 | 19.13 | <0.001 | 5.46 | 0.011 | ||
| combination | 6 | 23 | 2.49 | 0.053 | 1.35 | 0.278 | ||
| final height | 1 | 23 | 1.27 | 0.272 | 0.01 | 0.933 | ||
Figure 3.
The effect of host plant species diversity (mono-, di- and tricultures) and the presence of mutualistic ants (two levels: presence or absence) on the abundance (mean number per plant) of associated arthropods grouped as (a) ant-tended aphids, (b) ants, (c) aphid predators and (d) non-aphid herbivores. Ant treatments were in place for four months. Final height was used as covariate in the statistical model. Least-square means ± s.e. (n = 72).
Ant presence increased aphid abundance approximately fourfold compared with ant absence (table 2 and figure 3a). Interestingly, the effect of ant treatment on aphid abundance depended on species diversity treatment (significant ant × species diversity interaction; table 2 and figure 3a). By analysing ant-excluded and control plants separately, we observed that the effect of host-plant species diversity was significant in control plants (F2,23 = 18.57, p < 0.001) but not in ant-excluded plants (F2,23 = 1.12, p = 0.344). The presence of ants decreased aphid predator abundance by approximately 1.7-fold when compared with ant exclusion treatment (table 2 and figure 3c). This effect was similar in all species diversity treatments as revealed by the non-significant ant × species diversity interaction (table 2 and figure 3c). The mean number of non-aphid herbivores was not significantly affected by ant treatment, nor by the interaction between ant and species diversity treatments (table 2 and figure 3d).
In most cases, the effects of plant species diversity and ants on arthropod abundance for each pine species analysed individually mirrored the effects found at the plot level (see the electronic supplementary material, tables S2 and S3 and figure S3). Plant species diversity significantly increased the abundance of ants for all pine species and that of aphids in P. pinaster and P. radiata (see the electronic supplementary material, tables S2 and S3 and figure S3). Ant presence significantly increased the abundance of aphids in the three studied species (see the electronic supplementary material, table S2 and figure S3). As we observed at the plot level, aphid abundance was higher in high diverse treatments with ants (control treatment), but ant × diversity interaction was not significant (see the electronic supplementary material, table S2 and figure S3).
(c). Consequences of host-plant species diversity and ants on pine defensive status, nitrogen and non-structural carbohydrates in the stem
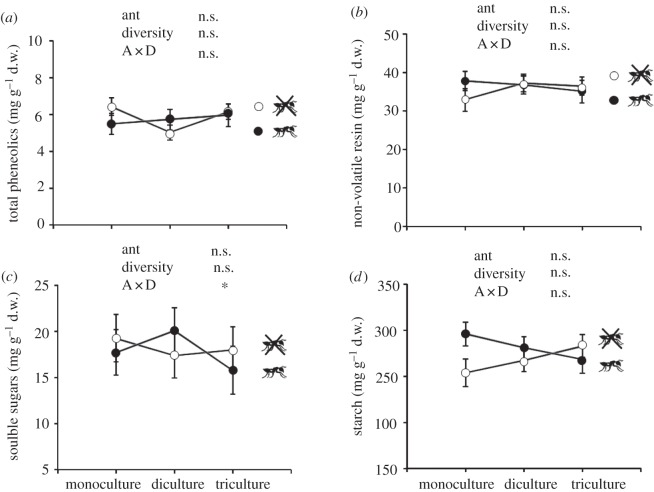
Host-plant species diversity did not significantly affect the concentration of quantitative pine chemical defences (measured as total phenolics and non-volatile resin; see the electronic supplementary material, table S4; figure 4), antioxidant capacity (see the electronic supplementary material, table S6 and figure S5), nitrogen (see the electronic supplementary material, table S6 and figure S5) and non-structural carbohydrates (measured as soluble sugars and starch; see the electronic supplementary material, table S4; figure 4) in the stem. Similarly, the presence of ants did not affect the concentration of pine chemical defences, antioxidant activity, nitrogen or non-structural carbohydrates (see the electronic supplementary material, tables S4 and S6 and figure S5; figure 4). However, the interaction between ant and species diversity treatments was significant for the concentration of soluble sugars in the stem (see the electronic supplementary material, table S4; figure 4c). By comparing ant exclusion and control (with ants) treatments across the three host-plant species diversity treatments, we observed that ants slightly increased stem soluble sugars on pine dicultures, while they decreased it on pine mono- and tricultures (figure 4c).
Figure 4.
The effect of host plant species diversity (mono-, di- and tricultures) and the presence of mutualistic ants (two levels: presence or absence) on the concentration of (a) total phenolics, (b) non-volatile resin, (c) soluble sugars and (d) starch in the stem of the pine trees. Ant treatments were in place for four months. Initial height was used as covariate in the statistical analyses. Least-square means ± s.e. (n = 72). Results of the mixed model are shown in the figure, where asterisks indicate significant differences (p < 0.05). A × D, ant × diversity interaction.
Results for each pine species analysed individually were markedly close to those observed at the plot level (see the electronic supplementary material, table S5 and figure S4).
(d). Correlation between arthropod abundance and pine performance
We observed that the abundance of mutualistic ants was positively correlated with the abundance of ant-tended aphids (r = 0.85, p < 0.001, n = 216; electronic supplementary material, table S7), and negatively correlated with the abundance of aphid predators (r = −0.50, p < 0.001, n = 216; electronic supplementary material, table S7) and non-aphid herbivores (r = −0.23, p = 0.001, n = 216; electronic supplementary material, table S7).
We also observed that the relative primary growth of pine trees was positively correlated with the abundance of ants (r = 0.55, p < 0.001, n = 216; electronic supplementary material, table S7) and with the abundance of ant-tended aphids (r = 0.55, p < 0.001, n = 432; electronic supplementary material, table S7), but only in the presence of ants (control plants).
4. Discussion
This study demonstrates that diversity within the same genus and guild of plant and mutualistic ants interactively determined arthropod community structure and ecosystem functioning. Three results are noteworthy. First, plant species diversity had strong positive effects on the abundance of aphids, but this effect occurred only in the presence of aphid-mutualist ants. Second, this bottom-up effect of diversity on aphids in turn cascaded up to the third trophic level, increasing ant abundance. Third, diversity effects on ants in turn fed back to influence plant performance. While plant diversity consistently increased pine primary growth, ants increased the magnitude of these diversity effects. Taken together, these results demonstrate the importance of a multitrophic perspective for a complete understanding of the consequences and mechanisms behind plant diversity effects.
Ecological theories, such as the resource specialization hypothesis [10,11] and the more individuals hypothesis [12], predict that plant diversity is one of the primary mechanisms explaining the structure of multitrophic communities and ecosystem processes. In particular, these ecological theories propose that greater plant diversity generates greater productivity and diversity of resources, and therefore would attract greater diversity and abundance of associated arthropods. However, our findings show that the positive effects of plant diversity on the structure of arthropod communities and plant performance may be strongly mediated by top-down control from the third trophic level. In particular, our results suggest that bottom-up effects of plant diversity (i) interact with top-down effects of higher trophic levels and modify the patterns of species interactions (i.e. plant–herbivore–predator interactions) and (ii) cascade up the food web to promote positive effects on higher trophic levels, which in turn positively influence plant growth (positive effects beget positive effects).
Our results showed that plant species diversity increased aphid abundance, but only in the presence of ants, and these diversity effects in turn indirectly increased ant abundance. There are different potential mechanisms to explain these effects (i) The positive direct effect of diversity on plant growth may indirectly increase aphid abundance and, in turn, indirectly increase the abundance of tending ants. Because aphids depend on ants for protection, these effects are observed only in the presence of ants (bottom-up effect of diversity on plant performance plus direct effect of mutualistic ants on aphids). Some other plant properties that affect aphid performance could be potentially trading off with plant growth in more diverse assemblages (e.g. plant defences [41]); however, we did not find evidence of altered defensive or oxidative status in plants growing in more diverse species mixtures with ants. (ii) Plant diversity could be positively increasing aphid populations directly owing to greater attraction of dispersing aphids to airborne volatiles from more diverse assemblages, as has been reported elsewhere [15,42]. (iii) Finally, the effect of plant diversity could be mediated by the third trophic level, such that greater aphid abundance could be due to an increase in the protective services of ants provided to aphids in the context of more diverse host plant resources. Aphid honeydew varies by host plant species [43,44], and a mixture of honeydew types may thus be more attractive to ants than any single honeydew type due to a more complete nutritive value. Our results are consistent with this last hypothesis because the rate of ant recruitment (i.e. ant/aphid ratio) was about 1.5-fold higher in diverse plots than in monocultures. All these direct bottom-up effects could potentially be contributing to greater aphid populations on more diverse assemblages, and in fact interacting with the direct top-down effects of ants on aphid predators, non-aphid herbivores and aphid performance, and subsequently leading to the observed pattern of increased plant performance.
Consistent with past biodiversity-ecosystem function (BEF) studies [7,45–48], our results showed that plant species diversity increased plant performance (measured as primary growth), presumably through niche partitioning. Competition for limiting resources (carbon, water, light, nutrient, etc.) is lower among than within species; so plants in diverse species mixtures may occupy more niches and more efficiently uptake the limiting resources [45,49]. As this study was based upon relatively small seedlings, the marked differences observed in pine growth between poly- and mono-cultures after just four months were unlikely to be associated with light competition, but rather were likely to be due to below-ground interactions (i.e. water acquisition [50]).
In addition to the strong direct effects of plant species diversity on pine growth, our results offer clear support for the presence of mutualistic ants to have enhanced the strength of diversity effects on plant performance. In particular, we observed that plant diversity promoted greater ant abundance, and that these ants in turn increased pine primary growth. Similarly, observations from a long-term BEF experiment suggest that the positive effect of plant species diversity on plant productivity might be due entirely, or in part, to stronger top-down suppression of herbivores in diverse plots [4]. Although we did not detect effects of ants on non-aphid herbivores in our late August sampling, they were relatively rare at this time and we speculate that ant effects were probably stronger and indirectly promoted pine growth earlier in the season. Contrary to our early predictions, we found that plant species diversity and the presence of mutualistic ants had no detectable effects on above-ground biomass production, probably because it is necessary to allow more time than four months in a growing season to find significant differences in above-ground biomass production [2]. Nevertheless, primary growth may be the most important measure of plant performance in forest seedlings in terms of long-term consequences for individual plant performance and forest structure due to the need to overcome understory vegetation.
In summary, this study showed that host-plant species diversity, even within the same genus and plant guild, strongly influenced plant performance and the associated arthropod community at several trophic levels. We found greater plant growth rates and more ants and aphids in the most diverse assemblages, independently of which species compose them. However, in the absence of mutualistic ants, plant species diversity did not have marked effects on the community structure of associated arthropods, nor on plant growth. These results together suggest that plant diversity effects cascaded up to higher trophic levels, which generated, at least in part, a positive feedback on plant performance.
Acknowledgements
We thank Joaquín Moreira and Silvia Portela for their technical assistance in the experimental set-up, assessments and plant sampling. We also thank César Cendán, Luz Pato and Rocío Campañó for their help with chemical analyses, and Alberto Gayoso, Fina Lombardero, Jorge Tizado and Xavier Espadaler for their guidance with arthropod identification. Special thanks to Dr José Carlos Noguera and Andrea Tato for their help and guidance with the antioxidant capacity assays. Comments and suggestions by Jessica Pratt, Luis Abdala-Roberts, Will Petry and Tadj Schreck helped to improve the manuscript. This research was supported by funding from AEET, the Spanish Association of Terrestrial Ecology (www.aeet.org), and AGL2010-18724 COMPROPIN grant from the Spanish Ministry of Education and Science. L.S. and X.M. received financial support from DOC-INIA and post-doctoral Fulbright/Ministry of Education grant programmes, respectively. Data supporting this paper are available at Dryad Digital Repository (doi:10.5061/dryad.fk11b).
References
- 1.Tilman D., Lehman C. L., Thomson K. T. 1997. Plant diversity and ecosystem productivity: theoretical considerations. Proc. Natl Acad. Sci. USA 94, 1857–1861 10.1073/pnas.94.5.1857 (doi:10.1073/pnas.94.5.1857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardinale B. J., Wright J. P., Cadotte M. W., Carroll I. T., Hector A., Srivastava D. S., Loreau M., Weis J. J. 2007. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl Acad. Sci. USA 104, 18 123–18 128 10.1073/pnas.0709069104 (doi:10.1073/pnas.0709069104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddad N. M., Crutsinger G. M., Gross K., Haarstad J., Knops J. M. H., Tilman D. 2009. Plant species loss decreases arthropod diversity and shifts trophic structure. Ecol. Lett. 12, 1029–1039 10.1111/j.1461-0248.2009.01356.x (doi:10.1111/j.1461-0248.2009.01356.x) [DOI] [PubMed] [Google Scholar]
- 4.Haddad N. M., Crutsinger G. M., Gross K., Haarstad J., Tilman D. 2011. Plant diversity and the stability of foodwebs. Ecol. Lett. 14, 42–46 10.1111/j.1461-0248.2010.01548.x (doi:10.1111/j.1461-0248.2010.01548.x) [DOI] [PubMed] [Google Scholar]
- 5.Hector A., et al. 2010. General stabilizing effects of plant diversity on grassland productivity at multiple sites through population asynchrony and overyielding. Ecology 91, 2213–2220 10.1890/09-1162.1 (doi:10.1890/09-1162.1) [DOI] [PubMed] [Google Scholar]
- 6.Dias A. T., van Ruijven J., Berendse F. 2010. Plant species richness regulates soil respiration through changes in productivity. Oecologia 163, 805–813 10.1007/s00442-010-1569-5 (doi:10.1007/s00442-010-1569-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook-Patton S. C., McArt S. H., Parachnowitsch A. L., Thaler J. S., Agrawal A. A. 2011. A direct comparison of the consequences of plant genotypic and species diversity on communities and ecosystem function. Ecology 92, 915–923 10.1890/10-0999.1 (doi:10.1890/10-0999.1) [DOI] [PubMed] [Google Scholar]
- 8.Isbell F. I., Polley H. W., Wilsey B. J. 2009. Biodiversity, productivity and the temporal stability of productivity: patterns and processes. Ecol. Lett. 12, 443–451 10.1111/j.1461-0248.2009.01299.x (doi:10.1111/j.1461-0248.2009.01299.x) [DOI] [PubMed] [Google Scholar]
- 9.Levine J. M. 2000. Species diversity and biological invasions: relating local process to community pattern. Science 288, 852–854 10.1126/science.288.5467.852 (doi:10.1126/science.288.5467.852) [DOI] [PubMed] [Google Scholar]
- 10.Keddy P. A. 1984. Plant zonation on lakeshores in Nova Scotia: a test of the resource specialization hypothesis. J. Ecol. 72, 797–808 10.2307/2259532 (doi:10.2307/2259532) [DOI] [Google Scholar]
- 11.Hurlbert A. H. 2004. Species–energy relationships and habitat complexity in bird communities. Ecol. Lett. 7, 714–720 10.1111/j.1461-0248.2004.00630.x (doi:10.1111/j.1461-0248.2004.00630.x) [DOI] [Google Scholar]
- 12.Srivastava D. S., Lawton J. H. 1998. Why more productive sites have more species: an experimental test of theory using tree hole communities. Am. Nat. 152, 510–529 10.1086/286187 (doi:10.1086/286187) [DOI] [PubMed] [Google Scholar]
- 13.Crutsinger G. M., Collins M. D., Fordyce J. A., Gompert Z., Nice C. C., Sanders N. J. 2006. Plant genotypic diversity predicts community structure and governs an ecosystem process. Science 313, 966–968 10.1126/science.1128326 (doi:10.1126/science.1128326) [DOI] [PubMed] [Google Scholar]
- 14.Genung M. A., Bailey J. K., Schweitzer J. A. 2012. Welcome to the neighbourhood: interspecific genotype by genotype interactions in Solidago influence above- and belowground biomass and associated communities. Ecol. Lett. 15, 65–73 10.1111/j.1461-0248.2011.01710.x (doi:10.1111/j.1461-0248.2011.01710.x) [DOI] [PubMed] [Google Scholar]
- 15.Ninkovic V., Al Abassi S., Ahmed E., Glinwood R., Pettersson J. 2011. Effect of within-species plant genotype mixing on habitat preference of a polyphagous insect predator. Oecologia 166, 391–400 10.1007/s00442-010-1839-2 (doi:10.1007/s00442-010-1839-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Utsumi S., Ando Y., Craig T. P., Ohgushi T. 2011. Plant genotypic diversity increases population size of a herbivorous insect. Proc. R. Soc. B 278, 3108–3115 10.1098/rspb.2011.0239 (doi:10.1098/rspb.2011.0239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heil M., Mckey D. 2003. Protective ant–plant interactions as model systems in ecological and evolutionary research. Ann. Rev. Ecol. Evol. Syst. 34, 425–453 10.1146/annurev.ecolsys.34.011802.132410 (doi:10.1146/annurev.ecolsys.34.011802.132410) [DOI] [Google Scholar]
- 18.Styrsky J. D., Eubanks M. D. 2007. Ecological consequences of interactions between ants and honeydew-producing insects. Proc. R. Soc. B 274, 151–164 10.1098/rspb.2006.3701 (doi:10.1098/rspb.2006.3701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trager M. D., Bhotika S., Hostetler J. A., Andrade G. V., Rodriguez-Cabal M. A., McKeon C. S., Osenberg C. W., Bolker B. M. 2010. Benefits for plants in ant–plant protective mutualisms: a meta-analysis. PLoS ONE 5, e14308. 10.1371/journal.pone.0014308 (doi:10.1371/journal.pone.0014308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stadler B., Dixon A. F. G. 2005. Ecology and evolution of aphid–ant interactions. Ann. Rev. Ecol. Evol. Syst. 36, 345–372 10.1146/annurev.ecolsys.36.091704.175531 (doi:10.1146/annurev.ecolsys.36.091704.175531) [DOI] [Google Scholar]
- 21.James D. G., Stevens M. M., Faulder R. J. 1999. Ant foraging reduces the abundance of beneficial and incidental arthropods in citrus canopies. Biol. Control. 14, 121–126 10.1006/bcon.1998.0678 (doi:10.1006/bcon.1998.0678) [DOI] [Google Scholar]
- 22.Eubanks M. D. 2001. Estimates of the direct and indirect effects of red imported fire ants on biological control in field crops. Biol. Control. 21, 35–43 10.1006/bcon.2001.0923 (doi:10.1006/bcon.2001.0923) [DOI] [Google Scholar]
- 23.Mooney K. A. 2006. The disruption of an ant–aphid mutualism increases the effects of birds on pine herbivores. Ecology 87, 1805–1815 10.1890/0012-9658(2006)87[1805:TDOAAM]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[1805:TDOAAM]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 24.Mooney K. A. 2007. Tritrophic effects of birds and ants on a canopy food web, tree growth, and phytochemistry. Ecology 88, 2005–2014 10.1890/06-1095.1 (doi:10.1890/06-1095.1) [DOI] [PubMed] [Google Scholar]
- 25.Herms D. A., Mattson W. J. 1992. The dilemma of plants: to grow or defend. Q. Rev. Biol. 67, 283–335 10.1086/417659 (doi:10.1086/417659) [DOI] [Google Scholar]
- 26.Mooney K. A., Agrawal A. A. 2008. Plant genotype shapes ant–aphid interactions: implications for community structure and indirect plant defense. Am. Nat. 171, E195–E205 10.1086/587758 (doi:10.1086/587758) [DOI] [PubMed] [Google Scholar]
- 27.Abdala-Roberts L., Agrawal A. A., Mooney K. A. In press. Ant–aphid interactions on Asclepias syriaca are mediated by plant genotype and caterpillar damage. Oikos. 10.1111/j.1600-0706.2012.20600.x (doi:10.1111/j.1600-0706.2012.20600.x) [DOI] [Google Scholar]
- 28.Wimp G. M., Whitham T. G. 2001. Biodiversity consequences of predation and host plant hybridization on an aphid–ant mutualism. Ecology 82, 440–452 10.2307/2679871 (doi:10.2307/2679871) [DOI] [Google Scholar]
- 29.Johnson M. T. 2008. Bottom-up effects of plant genotype on aphids, ants, and predators. Ecology 89, 145–154 10.1890/07-0395.1 (doi:10.1890/07-0395.1) [DOI] [PubMed] [Google Scholar]
- 30.Sampedro L., Moreira X., Zas R. 2011. Costs of constitutive and herbivore-induced chemical defenses in pine trees emerge only under low resources availability. J. Ecol. 99, 818–827 10.1111/j.1365-2745.2011.01814.x (doi:10.1111/j.1365-2745.2011.01814.x) [DOI] [Google Scholar]
- 31.Moreira X., Zas R., Sampedro L. 2012. Differential allocation of constitutive and induced chemical defenses in pine tree juveniles: a test of the optimal defense theory. PLoS ONE 7, e34006. 10.1371/journal.pone.0034006 (doi:10.1371/journal.pone.0034006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreira X., Sampedro L., Zas R. 2009. Defensive responses of Pinus pinaster seedlings to exogenous application of methyl-jasmonate: concentration effect and systemic response. Environ. Exp. Bot. 67, 94–100 10.1016/j.envexpbot.2009.05.015 (doi:10.1016/j.envexpbot.2009.05.015) [DOI] [Google Scholar]
- 33.Sampedro L., Moreira X., Zas R. 2011. Resistance and response of Pinus pinaster seedlings to Hylobius abietis after induction with methyl jasmonate. Plant Ecol. 212, 397–401 10.1007/s11258-010-9830-x (doi:10.1007/s11258-010-9830-x) [DOI] [Google Scholar]
- 34.Noguera J. C., Lores M., Alonso-Alvarez C., Velando A. 2011. Thrifty development: early-life diet restriction reduces oxidative damage during later growth. Funct. Ecol. 25, 1114–1153 10.1111/j.1365-2435.2011.01856.x (doi:10.1111/j.1365-2435.2011.01856.x) [DOI] [Google Scholar]
- 35.Erel O. 2004. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 37, 277–285 10.1016/j.clinbiochem.2003.11.015 (doi:10.1016/j.clinbiochem.2003.11.015) [DOI] [PubMed] [Google Scholar]
- 36.Hansen J., Møller I. 1975. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 68, 87–94 10.1016/0003-2697(75)90682-X (doi:10.1016/0003-2697(75)90682-X) [DOI] [PubMed] [Google Scholar]
- 37.Littell R. C., Milliken G. A., Stroup W. W., Wolfinger R., Schabenberger O. 2006. SAS system for mixed models, 2nd edn Cary, NC: SAS Institute [Google Scholar]
- 38.Johnson M. T., Lajeunesse M. J., Agrawal A. A. 2006. Additive and interactive effects of plant genotypic diversity on arthropod communities and plant fitness. Ecol. Lett. 9, 24–34 10.1111/j.1461-0248.2005.00833.x (doi:10.1111/j.1461-0248.2005.00833.x) [DOI] [PubMed] [Google Scholar]
- 39.Genung M. A., et al. 2010. Non-additive effects of genotypic diversity increase floral abundance and abundance of floral visitors. PLoS ONE 5, e8711. 10.1371/journal.pone.0008711 (doi:10.1371/journal.pone.0008711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zas R., Solla A., Sampedro L. 2007. Variography and kriging allow screening Pinus pinaster resistant to Armillaria ostoyae in field conditions. Forestry 80, 201–209 10.1093/forestry/cpl050 (doi:10.1093/forestry/cpl050) [DOI] [Google Scholar]
- 41.Kidd N. A. C. 1994. Resource deprival as anti-herbivore strategy in plants, with particular reference to aphids. Eur. J. Entomol. 91, 53–56 [Google Scholar]
- 42.Glinwood R., Ahmed E., Qvarfordt E., Ninkovic V., Pettersson J. 2009. Airborne interactions between undamaged plants of different cultivars affect insect herbivores and natural enemies. Arthropod Plant Interact. 3, 215–224 10.1007/s11829-009-9072-9 (doi:10.1007/s11829-009-9072-9) [DOI] [Google Scholar]
- 43.Hendrix D. L., Wei Y., Legget J. E. 1992. Homopteran honeydew is determined by both the insect and the plant species. Comp. Biochem. Physiol. 101, 23–27 10.1016/0305-0491(92)90153-I (doi:10.1016/0305-0491(92)90153-I) [DOI] [Google Scholar]
- 44.Douglas A. E. 1993. The nutritional quality of phloem sap utilized by natural aphid populations. Ecol. Entomol. 18, 31–38 10.1111/j.1365-2311.1993.tb01076.x (doi:10.1111/j.1365-2311.1993.tb01076.x) [DOI] [Google Scholar]
- 45.Loreau M., Hector A. 2001. Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76 10.1038/35083573 (doi:10.1038/35083573) [DOI] [PubMed] [Google Scholar]
- 46.Hooper D. U., et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35 10.1890/04-0922 (doi:10.1890/04-0922) [DOI] [Google Scholar]
- 47.Flombaum P., Sala O. E. 2008. Higher effect of plant species diversity on productivity in natural than artificial ecosystems. Proc. Natl Acad. Sci. USA 105, 6087–6090 10.1073/pnas.0704801105 (doi:10.1073/pnas.0704801105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tilman D., Hill J., Lehman C. 2006. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science 314, 1598–1600 10.1126/science.1133306 (doi:10.1126/science.1133306) [DOI] [PubMed] [Google Scholar]
- 49.Finke D. L., Snyder W. E. 2008. Niche partitioning increases resource exploitation by diverse communities. Science 321, 1488–1490 10.1126/science.1160854 (doi:10.1126/science.1160854) [DOI] [PubMed] [Google Scholar]
- 50.Powers M. D., Pregitzer K. S., Palik B. J. 2008. Physiological performance of three pine species provide evidence for gap partitioning. For. Ecol. Manage. 256, 2127–2135 10.1016/j.foreco.2008.08.003 (doi:10.1016/j.foreco.2008.08.003) [DOI] [Google Scholar]