Abstract
Knowledge about the phylogeny and ecology of communities along environmental gradients helps to disentangle the role of competition-driven processes and environmental filtering for community assembly. In this study, we evaluated patterns in species richness, phylogenetic structure and life-history traits of bee communities along altitudinal gradients in the Alps, Germany. We found a linear decline in species richness and abundance but increasing phylogenetic clustering in communities with increasing altitude. The proportion of social- and ground-nesting species, as well as mean body size and altitudinal range of bee communities, increased with increasing altitude, whereas the mean geographical distribution decreased. Our results suggest that community assembly at high altitudes is dominated by environmental filtering effects, whereas the relative importance of competition increases at low altitudes. We conclude that inherent phylogenetic and ecological species attributes at high altitudes pose a threat for less competitive alpine specialists with ongoing climate change.
Keywords: altitudinal gradient, phylogeny, environmental filtering, life-history traits, assembly rules, insects
1. Introduction
Understanding patterns of species richness and community structure, and their underlying drivers, along environmental gradients remains a key challenge in ecology. Knowledge of the phylogenetic clustering of species, the adaptive value of life-history traits for species and communities, and the sensitivity of communities to environmental changes can allow predictions to be made of their responses to climate change. Altitudinal gradients can be used as model systems for climatic changes to analyse the role of environmental filtering effects on animal communities [1,2]. To date, studies using altitudinal gradients have primarily focused on diversity patterns in tropical regions, whereas few studies have been conducted in temperate regions [2]. The importance of traits along altitudinal gradients has been investigated within single taxa [3–5], but thorough documentation of changes in community structure, and associated adaptive traits and phylogenetic patterns, along climatic gradients are missing. To fill this gap, studies are required that combine phylogenetic methods with the ecological analysis of species distribution patterns and functional traits to explain climate-driven differences in community structure.
Two commonly reported patterns in species richness along altitudinal gradients are: (i) a decline of species richness with altitude, and (ii) a unimodal distribution with a mid-elevation peak [6]. The reported patterns in species richness depend on the geographical region, climate zone and the taxa studied. Various hypotheses have been proposed to explain these diversity patterns [6–9]. However, the causes for the diversity patterns, and the driving factors of community assembly are poorly understood [1]. The analysis of phylogenetic structure within and between communities, as well as the analysis of trait combinations, are useful approaches to explore the processes that affect the assembly and diversity of insect communities. Several recent studies have examined phylogenetic community structures using the statistical tools provided by Webb et al. [10]. The assembly of communities is driven by niche-related processes, which are deterministic, or by neutral processes, which are stochastic [11]. Important factors for niche-related assembly rules are species characteristics and their adaptive values under environmental filtering and competition, while neutral community models predict that only dispersal limitations and not other species characteristics influence the community assembly [12,13]. Phylogenetic clustering of closely related species can be interpreted in terms of niche-related processes [10,14]: only a few lineages of closely related species with similar, conserved characteristics occur under given environmental filtering conditions (phylogenetic niche conservatism) [10,15]. Conserved characteristics are passed from common ancestors to their descendants and are common in the descendants. Phylogenetic over-dispersion (i.e. phylogenetic relatedness is lower than expected from the null-model) is the opposing pattern, and can indicate that competition between species with similar conserved traits is the dominant process in community assembly [10,14]. However, there are also many other processes proposed to influence phylogenetic community structure [12]. A subsequent analysis showing that particular life-history traits dominate in communities along altitudinal gradients would reveal the adaptive value of those traits under changing climatic conditions, and the importance of niche-related (abiotic and biotic) processes on community assembly. No patterns in the dominance of traits should be detectable if neutral processes are driving the assembly of communities, or if the traits are of no adaptive value.
We use wild bees (Hymenoptera: Apoidea) as our study system, as they are important pollinators and, so the composition of bee communities under changing climatic conditions is of high economic and ecological interest; to our knowledge, there are no studies on the phylogenetic structure of bee communities along climatic gradients. In our study, we use the net relatedness index (NRI) [10] to investigate the phylogenetic structure, and driving ecological processes within wild bee communities along an altitudinal gradient, and the mean phylogenetic distances (MPDs) between communities at similar altitudes to investigate the phylogenetic turnover at a larger spatial scale.
The ability of insects to cope with different climatic conditions has been proposed to depend on several life-history traits [1,16–19]. We analysed the importance of seven traits (sociality, parasitism, nesting behaviour, number of generations per year/voltinism, diet breadth/lecty, body size and range size). Previous studies suggest that warmer lowlands (with low or no seasonality) promote a higher degree of sociality in arthropods [19]. The number of generations per year (voltinism) depends on the generation time and on the length of the season, and is therefore expected to decline with altitude [20]. Wild bees also include some cleptoparasites species (either brood or social parasites) which either lay their eggs in brood cells which the host has already provisioned, or let their brood be raised by workers of their social hosts. The strong seasonality at high altitudes is expected to increase the relative abundance of parasitic bees owing to more closely synchronized phenology of hosts and parasites [16]. The proportion of below-ground-nesting species is predicted to increase with altitude as below-ground nests may be better protected from extreme climatic conditions than above-ground nests (e.g. stems, wood, walls, rocks, snail shells). An increasing niche breadth with altitude has been shown for some species (e.g. brown hares) in situations where less food was available [21], and in more generalized plant–pollinator networks [22]. Scarce food supply at higher altitudes may therefore lead to a higher proportion of polylectic species with a wider niche breadth. Patterns in body size along altitudinal gradients remain a controversial topic [20]. However, in the majority of studies, lower temperatures and shorter seasons were associated with larger size of animals [18,20]. According to Janzen's hypothesis [23], the temperature variability as well as overlap of seasonal and daily temperature ranges is higher in temperate elevation gradients than in tropical gradients, and thus may lead to an adaptation of species in temperate regions to larger temperature fluctuations and occurrence across larger altitudinal ranges [24]. This prediction can be extended to daily fluctuations, which are higher at high elevations than at low elevations, so that species at high-elevation communities are expected to have, on average, larger altitudinal ranges, but smaller geographical distributions, than species occurring at low altitudes [24]. We performed a species-based analysis to provide insights into the importance of the earlier-mentioned traits for the occurrence of species, and an abundance-based analysis to provide information on the importance of traits for the dominance structure in communities.
Overall, in this study, we provide a comprehensive analysis of the changes in bee communities along an environmental gradient. We analysed the community composition along an altitudinal gradient and underlying adaptive life-history traits determining community assembly in alpine grasslands. We developed and tested the following predictions:
— species richness shows a linear decrease, or a hump-shaped distribution, with increasing altitude;
— the phylogenetic community structure is influenced by altitude;
— the frequency of the occurrence of functional traits in a community is influenced by altitude; and
— bee species in communities at higher altitudes show, on average, a larger altitudinal range and a smaller geographical distribution than those at lower altitudes.
2. Material and methods
(a). Study sites
The study was carried out on grasslands in the National Park Berchtesgaden and its vicinity (47,10° N, 12,15° E). The National Park is located in the northern limestone Alps in the southeast of Germany. The region is characterized by calcareous rocks, coniferous forests and mountain pastures. Many mountain pastures were extensively managed for centuries but were abandoned within the last 150 years. The region lies in the transition zone of Atlantic and continental climate with up to 2500 mm of precipitation per year.
We selected 34 study sites (60 × 60 m) on grasslands from 600 to 2000 metres above sea level (m.a.s.l.) in the western part of the National Park Berchtesgaden. Eighteen of the selected grasslands were extensively managed and 16 grasslands were not managed. The study sites were established along the slopes of two mountains and along two gently inclining valleys and their terminal mountains. Criteria for the selection of the study sites were: (i) the grasslands represent a more or less continuous altitudinal gradient; (ii) the grasslands were extensively managed (one cut per year on meadows, extensive grazing on pastures) or unmanaged; (iii) the grasslands were not fertilized; and (iv) permission from the farmers and owners.
(b). Data collection
Wild bees and honeybees were recorded in transect walks from 8 May to 10 September 2009. Sites at lower altitudes were sampled six times and sites at altitudes greater than 1200 m.a.s.l. were sampled five times owing to the shorter snow free season. This different number of transect walks did not affect species detectability and calculated saturation (see §2c). The surveys were conducted from 09.30 to 18.00 on days when the weather was sunny or when temperature at 650 m.a.s.l. was above 17°C on cloudy days. The time for a transect within every study site was 50 min. We focused on a 2 m corridor and caught bees in 10 sub-transects of about 25 m length where time was set at 5 min each. Insects were identified to species level in the laboratory. We avoided collecting Bombus queens and instead noted the exact colour code on thorax and abdomen for later determination. Bee species identification followed Scheuchl [25] for Anthophoridae; Scheuchl [26] for Megachilidae and Melittidae; Schmid-Egger & Scheuchl [27] for Andrenidae; Mauss [28] for Bombus; Amiet et al. [29] for Lasioglossum and Halictus; and Amiet et al. [29] for Dufourea, Hylaeus and Sphecodes. The taxonomy followed Michener [30].
The species were assigned to one of two categories of five life-history traits or were considered as undefined when no information was available or when the species took up an intermediate or bimodal trait value. We considered the following trait values: social versus solitary (including communal), parasitic versus non-parasitic, below-ground (endogaic) versus above-ground (epigaic) nesting, polylectic versus oligolectic (use of more than one plant family versus only one family or genus as pollen source), univoltine versus multivoltine (including bivoltine). A sixth trait was the inter-tegular distance (ITD) which is used as an estimator for body size and body mass [31]. For social bees, we used the ITD of queens as the fitness of a colony is highly dependent on the performance of the nest-founding queens [32]. Sources for trait information were the European pollinator database resulting from the ALARM and STEP projects, Westrich [33], Gogala [34], Amiet et al. [29] and ITD measurements made by the authors according to Cane [31].
For the analysis of the geographical distribution of bee communities, we extracted occurrence data of each species in our dataset in Bavaria from the website www.aculeata.eu [35]. We noted the number of quadrants (ordnance maps) in which the species were recorded at least once during the last two centuries. We used the data only for Bavaria, as data quality varies among German federal states and therefore is difficult to compare.
To be able to correct for the effect of flower cover, we estimated the cover of all flowering plant species as a percentage of the total area (60 × 60 m) of the study site after each of the transect walks. For statistical analysis, we used the mean value of the flower cover over all transect walks per site.
(c). Statistical analysis
The statistical analyses were performed using the software R v. 2.11 for Windows [36].
To test for correlations between the predictors altitude, flower cover and the categorical predictor management, Spearman's rho statistic was used. Rho was smaller than 0.5 in all combinations, implying that there was no strong covariance of the predictors.
Species accumulation curves and species-richness estimators were calculated using the spp.est function of the R package fossil. The 10 sub-transects per survey were used as replicates to calculate species-richness estimators. To avoid phenology effects, the data from the five or six surveys in 2009 were pooled. The proportion of detected species was estimated by dividing the recorded species richness per site by the estimator Jacknife1. The values for the estimated rate of detected species ranged between 58 and 83 per cent for the Jack1 estimator. The detection rates were not correlated with the number of surveys, altitude, management or flower cover. Owing to the relatively high detection rates, we used the species richness and not the calculated estimators for our analysis. As the different number of surveys on higher (five surveys) and lower altitudes (six surveys) did not affect the detection rate (t-test: five surveys mean: 70.2%; six surveys mean: 70.7%; p = 0.801) we used data from all the surveys.
General linear models were fitted with total species richness and total abundance of bees per site as response variables. The full model (type 3 sum of squares) was fitted with altitude, the quadratic term of altitude (to test for a hump-shaped distribution), management and flower cover as predictors. For model simplification, likelihood ratio tests were performed, and non-significant terms were removed from the model [37]. Flower cover was not significant in the species-richness analysis, and the quadratic term of altitude could be removed from both models. Flower cover at the site T1 was detected as a highly influential outlier. This site was removed from the species-richness and -abundance analysis. The model residuals were sufficiently normally distributed and showed variance homogeneity. Moran's I was calculated for the model residuals of species richness. The minimum distance between a pair of sites was 0.33 km, the maximum distance was 12.26 km. We calculated Moran's I for 1 km distance classes up to 5 km and a global Moran's I using the multiplicative inverted distances as weights. No significant spatial autocorrelation of species richness could be detected at distance classes up to 5 km or in the global Moran's I.
For the phylogenetic analysis a polytomous, ultrametric tree was compiled based on the taxonomy of bees. Branch lengths were calculated and adjusted with the 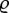 parameter set to 1 [38] using the R package APE. As an estimator for the phylogenetic relatedness of species within sites, we calculated the standardized (by the s.d. of phylogenetic distances in the null communities) NRI [10,39]. As the null model for the NRI, we calculated for each site the mean and standard deviation of the phylogenetic distances expected for the number of taxa found on the site, based on a random selection of species from the regional species pool found in our study (500 iterations per site). We also calculated the null model by weighting the sampling probability of the taxa by their abundance in the regional species pool. Patterns were almost identical to the patterns in the unweighted estimates presented in §3. A regression with altitude as predictor and the NRIs of the sites as response variables was performed to assess the influence of environmental conditions on the phylogenetic clustering of bees. Positive NRI values indicate phylogenetic clustering, whereas negative values indicate phylogenetic over-dispersion. To study the effect of environmental conditions on the phylogenetic structures at a larger spatial scale, we calculated the MPDs between sites. For all possible site combinations, we estimated the mean of the matrix of pairwise phylogenetic distances between all possible pairwise species combinations with species from the first site as rows and species from the second site as columns in the matrices. We performed an ANOVA using the average of the MPDs between site pairs at low (n = 11), medium (n = 11) and high (n = 12) altitudes as response (n = 34) with altitudinal categories as the grouping factor. We used only the MPDs between sites in the same altitudinal category (low (l): less than 1000 m.a.s.l.; intermediate (m): 1000–1500 m.a.s.l.; high (h): more than 1500 m.a.s.l.).
parameter set to 1 [38] using the R package APE. As an estimator for the phylogenetic relatedness of species within sites, we calculated the standardized (by the s.d. of phylogenetic distances in the null communities) NRI [10,39]. As the null model for the NRI, we calculated for each site the mean and standard deviation of the phylogenetic distances expected for the number of taxa found on the site, based on a random selection of species from the regional species pool found in our study (500 iterations per site). We also calculated the null model by weighting the sampling probability of the taxa by their abundance in the regional species pool. Patterns were almost identical to the patterns in the unweighted estimates presented in §3. A regression with altitude as predictor and the NRIs of the sites as response variables was performed to assess the influence of environmental conditions on the phylogenetic clustering of bees. Positive NRI values indicate phylogenetic clustering, whereas negative values indicate phylogenetic over-dispersion. To study the effect of environmental conditions on the phylogenetic structures at a larger spatial scale, we calculated the MPDs between sites. For all possible site combinations, we estimated the mean of the matrix of pairwise phylogenetic distances between all possible pairwise species combinations with species from the first site as rows and species from the second site as columns in the matrices. We performed an ANOVA using the average of the MPDs between site pairs at low (n = 11), medium (n = 11) and high (n = 12) altitudes as response (n = 34) with altitudinal categories as the grouping factor. We used only the MPDs between sites in the same altitudinal category (low (l): less than 1000 m.a.s.l.; intermediate (m): 1000–1500 m.a.s.l.; high (h): more than 1500 m.a.s.l.).
We used regression analyses to test for shifts in the dominance of traits in bee communities with altitude. Relative frequencies of trait categories per site were used as response variables. For each trait, two response variables were estimated: the proportion of species per trait category and the proportion of individuals per trait category (abundance-weighted frequencies of traits). Regressions were performed with the proportions of social, parasitic, ground-nesting, multivoltine, oligolectic species and individuals per site as response variables. For the continuous estimator of body size, ITD, we used the mean value of all species or individuals per site. Altitude was the only predictor variable.
Interpolated altitudinal ranges per species were calculated from the altitudinal difference between the highest and lowest occurrence for all species in our dataset [24]. This approach assumes that species were potentially present between their highest and lowest occurrence. Mean altitudinal ranges per site were calculated based on the altitudinal ranges of the species and were also additionally calculated as individual based mean (abundance-weighted mean). Mean geographical distributions of the species and individuals per site were calculated from the occurrence data of Bavaria (see §2b). From these data, we performed regressions with mean altitudinal ranges and mean geographical distribution per site as response variables and altitude as predictor.
3. Results
(a). Species richness
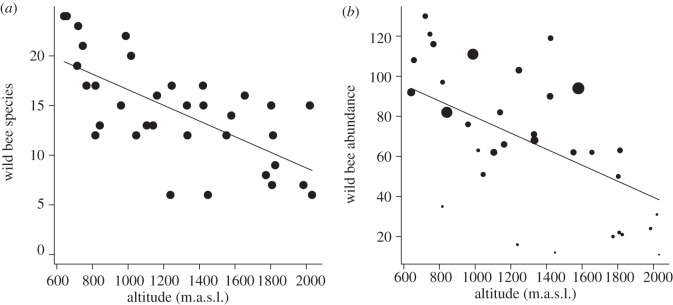
In total, we collected 2328 bees from 87 species (22 genera) with a minimum of six species and a maximum of 24 species per site. Nineteen species belonged to the genus Bombus. Seven species were found only above 1500 m.a.s.l. (Andrena lapponica, Bombus gerstaeckeri, Bombus mendax, Bombus pyrenaeus, Bombus sichelii, Dufourea alpina, Lasioglossum bavaricum), whereas 34 species were found only below 1000 m.a.s.l. Species richness of bees along an altitudinal gradient was predicted by altitude in the minimal adequate model (figure 1a). Management and flower cover showed no significant influence on the number of bee species (table 1). The abundance of bees was predicted by flower cover and altitude (table 1 and figure 1b). Both species richness and abundance showed a linear decline with increasing altitude. Abundance also linearly declined with decreasing flower cover.
Figure 1.
Altitude predicts (a) species richness of wild bees and (b) abundance of wild bees. Point size in the abundance plot is weighted by flower cover (min: 0.41%, max: 10.44%), which is also a good predictor for the abundance of wild bees. Regression lines are drawn from the minimal adequate model estimates. For statistics, see table 1.
Table 1.
ANOVA table with type III sum of squares of general linear models with species richness and abundance of bees as response variables. (Explanatory variables are flower cover, management (two levels: extensively managed, no management) and altitude.)
| response | predictor | d.f. | F | p |
|---|---|---|---|---|
| species richness | management | 1.29 | 0.77 | 0.388 |
| flower cover | 1.29 | 2.26 | 0.143 | |
| altitude | 1.29 | 11.25 | 0.002 | |
| abundance | management | 1.29 | 2.51 | 0.124 |
| flower cover | 1.29 | 10.79 | 0.003 | |
| altitude | 1.29 | 9.76 | 0.004 |
(b). Phylogeny
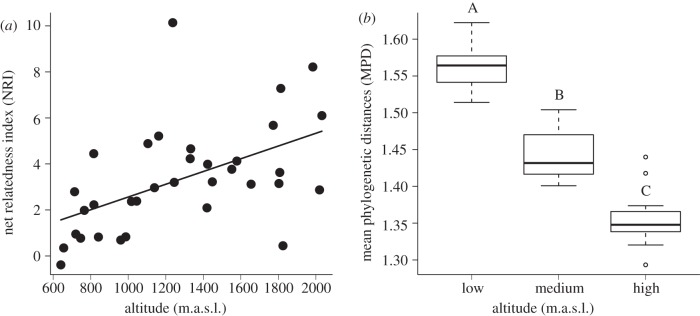
The NRI as an indicator for the phylogenetic community structure increased significantly with altitude (figure 2a; F1,32 = 10.63, p = 0.003). This implies that species in communities at higher altitudes are on average more closely related than species in communities at low altitudes. The same pattern was found in an ANOVA at a larger spatial scale where altitude predicted the site-based average of the MPDs between sites (F2,31 = 93.04, p < 0.001; figure 2b). MPDs between sites at high altitudes were smaller than at intermediate altitudes (p < 0.001), and the MPDs between sites at intermediate altitudes were significantly smaller than at low altitudes (p < 0.001). Thus, species were more closely related at high altitudes, not only within sites, but also between sites than at low altitudes. This suggests that the phylogenetic turnover at high altitudes is lower than in lowlands.
Figure 2.
Effect of altitude on (a) the phylogenetic relatedness of wild bee species within sites (NRI): F1,32 = 10.63, p = 0.003, y = −0.030 + 2.62e − 3 × altitude; and (b) the mean phylogenetic distances of wild bees between sites (MPD): F2,31 = 93.04, p < 0.001. The altitudinal categories are: low (<1000 m.a.s.l.), medium (1000–1499 m.a.s.l.), high (>1500 m.a.s.l.).
(c). Life-history traits
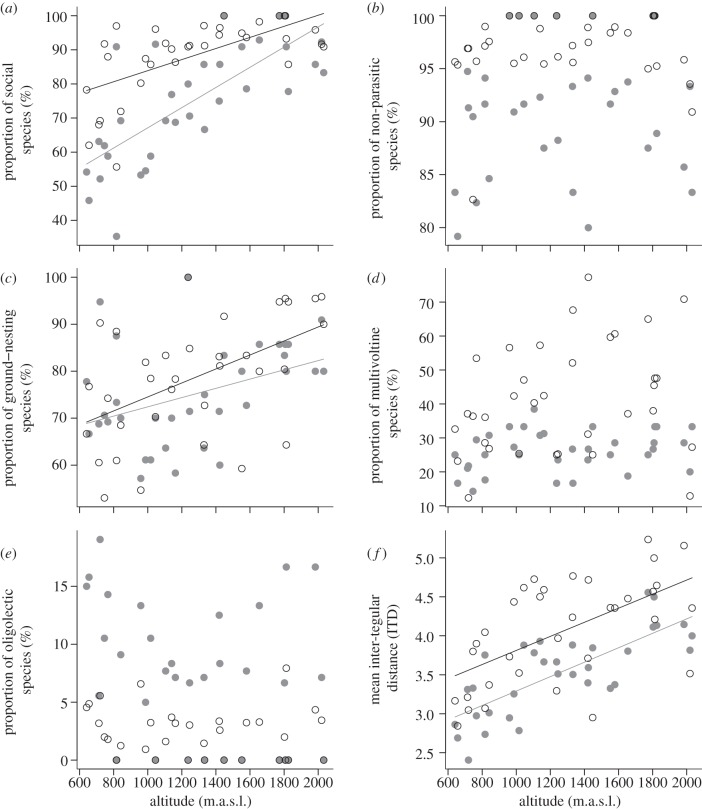
Our analysis revealed significant changes in the relative frequency of life-history traits in wild bee communities along an altitudinal gradient (table 2). The proportion of social species and individuals as well as the proportion of ground-nesting species and individuals increased significantly with altitude (figure 3a,c). The individual- and species-based means of the ITD as a proxy for body size also increased significantly with altitude (figure 3f). In our study, altitude did not affect the relative frequency of parasitism, voltinism and lecty in the communities (figure 3b,d,e and table 2). To explore how much of the pattern is owing to the dominant social bee genus Bombus, we also provide the results of the analysis after removing the 19 Bombus species (see the electronic supplementary material, appendices S1 and S2; no significant correlations with altitude except for rate of sociality and parasitism).
Table 2.
F-statistics and estimates of simple regressions on the effects of altitude (m.a.s.l.) on the proportions of social species, non-parasitic species, ground-nesting species, multivoltine species, oligolectic species and on the mean inter-tegular distance (ITD), altitudinal range and geographical distribution of wild bee communities in 34 sites. (The calculations were performed for species-based proportions/means of traits and abundance-weighted proportions/means of traits. Degrees of freedom = 1,32 in all cases.)
| F | p | estimates | |
|---|---|---|---|
| response species weighted | |||
| proportion of social species | 40.71 | <0.001 | y = 37.55 + 0.03 × altitude |
| proportion of non-parasitic species | 0.80 | 0.377 | n.s. |
| proportion of ground-nesting species | 6.06 | 0.019 | y = 62.56 + 9.84e−3 × altitude |
| proportion of multivoltine species | 2.08 | 0.159 | n.s. |
| proportion of oligolectic species | 2.05 | 0.162 | n.s. |
| mean ITD (mm) | 46.07 | <0.001 | y = 2.36 + 9.26e−4 × altitude |
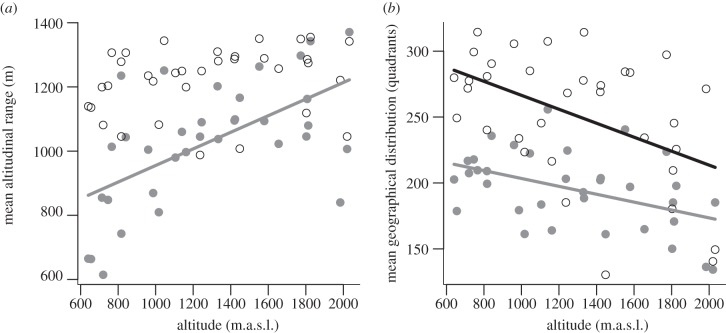
| mean altitudinal range (m) | 17.07 | <0.001 | y = 696.65 + 0.26 × altitude |
| mean geographical distribution | 8.27 | 0.01 | y = 233.33 − 0.03 × altitude |
| response abundance-weighted | |||
| proportion of social species | 21.52 | <0.001 | y = 67.64 + 0.02 × altitude |
| proportion of non-parasitic species | 0.27 | 0.609 | n.s. |
| proportion of ground-nesting species | 10.93 | 0.002 | y = 59.62 + 0.01 × altitude |
| proportion of multivoltine species | 2.47 | 0.126 | n.s. |
| proportion of oligolectic species | 0.46 | 0.501 | n.s. |
| mean ITD (mm) | 16.74 | <0.001 | y = 2.90 + 9.03e − 4 × altitude |
| mean altitudinal range (m) | 1.85 | 0.184 | n.s. |
| mean geographical distribution | 9.13 | 0.005 | y = 319.44 − 0.05 × altitude |
Figure 3.
Effects of altitude (m.a.s.l.) on the proportion (%) of five categorical life-history traits and on the mean ITD in wild bee communities on 34 sites. Black circles and lines represent abundance-weighted proportions/means of traits, whereas grey solid points and lines represent species-based proportions/means of traits. Lines are presented if simple regressions were significant (p < 0.05). The trait categories were: sociality (social versus solitary), parasitism (non-parasitic versus parasitic), nesting behaviour (below-ground versus above-ground-nesting), voltinism (multivoltine versus univoltine) and lecty (oligolectic versus polylectic).
Communities at high elevations consisted mainly of species with a large mean altitudinal range, whereas species in communities at low altitudes showed significantly smaller altitudinal ranges (figure 4a and table 2). No significant effect was found on the abundance-weighted analysis. The consistently large mean altitudinal ranges in this analysis imply that the communities along the complete altitudinal gradient were dominated by individuals of species with a large altitudinal range. Species with smaller ranges contributed only a few individuals to the communities (figure 4a). Both the species- and the individual-based means of the geographical distribution decreased with the altitude at which the wild bee communities were investigated (figure 4b).
Figure 4.
Effect of altitude on (a) the mean altitudinal range (m) and (b) the mean geographical distribution (number of quadrants (10 × 10 km) at which species were found in Bavaria) of wild bee communities. Black circles and lines represent abundance-weighted proportions/means of traits, whereas grey solid points and lines represent species-based proportions/means of traits. Lines are presented if simple regressions were significant (p < 0.05).
4. Discussion
(a). Species richness
Our results show that altitude strongly influences the diversity, phylogeny and ecology of wild bee communities. Species richness and abundance linearly decreased with increasing altitude and did not show a hump-shaped distribution. Higher flower cover was correlated with an increase in bee abundance and was probably the result of flowers attracting more foraging individuals, but this did not affect the species richness of wild bees. Nevertheless, the decreasing abundance with increasing altitude, after correcting for flower cover, suggests decreasing competition for floral resources with altitude. Therefore, abiotic mechanisms are most likely to be the cause of the observed decrease in diversity of bees at high altitudes. A linearly decreasing diversity with increasing altitude is consistent with temperature limitation predictions [40,41] and/or the metabolic theory of ecology [42], both of which predict a linear decline of diversity with decreasing temperatures. The same pattern is also expected from species–area effects [43], but their general validity on altitudinal gradients remains unclear [44]. Other hypotheses, such as the mid-domain effect [45], or the source-sink (mass) effects [46], predict a unimodal distribution of species richness, but do not seem to influence the diversity pattern of bees significantly in this study. However, not only species richness of communities, but also their composition, changes along the altitudinal gradient and is driven by evolutionary history and ecological processes.
(b). Phylogeny
The influence of evolutionary history and ecological processes on community assembly can be assessed by analysing the phylogenetic structures of communities. In our study, the phylogenetic relatedness within and between communities at high altitudes was on average higher than at low altitudes. The observed increase in the phylogenetic relatedness with altitude indicates that the assembly of communities at high altitudes is determined by environmental filtering processes where related species with adaptive characteristics, supposedly to low temperatures and strong seasonality, are filtered. We interpret the lower phylogenetic relatedness in lowland communities as evidence that the environmental filtering effect of abiotic factors is replaced here by an increased competition between species with similar traits. Under favourable climatic conditions, communities can even exhibit an over-dispersed phylogenetic structure and interspecific competition becomes the driving factor for the structure of communities [14]. Our finding that the phylogenetic turnover was higher between communities from low altitudes than between communities from high altitudes confirms these patterns also on larger spatial scales. Our results are in line with patterns found in tropical hummingbird communities in the Andes [47] and ant communities in temperate altitudinal gradients in the USA and in Austria [14], which were phylogenetically clustered at higher altitudes. However, for plants, an increasing phylogenetic over-dispersion with increasing altitude has been shown, whereas bacterial communities were phylogenetically clustered at all altitudes in the Colorado Rocky Mountains [48]. The contrasting results reported in these studies suggest that different taxa have different thresholds at which abiotic factors have a filtering effect [14]. However, the clustering of closely related species in communities at high altitudes, as found in our study, indicates that niche-related assembly rules and abiotic habitat constraints play a key role in community assembly [14,47]. To determine the probable causes of the observed phylogenetic structure in the communities, we examined patterns in community ecology and assessed changes in ecologically relevant species characteristics (traits) along the altitudinal gradient.
(c). Life-history traits
The species characteristics of sociality, nesting behaviour, body size, altitudinal range and geographical distribution changed in bee communities as a response to changing environmental filtering and other niche-based processes at different altitudes, and are therefore possible drivers for the observed changes in species richness, community composition and phylogenetic structures. Altitude did not significantly affect traits associated with parasitism, lecty and voltinism which implies that these characteristics are of little or no adaptive value under changing altitudinal (climatic) conditions. In the literature, a higher degree of sociality is generally expected in warmer regions with low seasonality owing to more overlapping generations, which is a prerequisite for sociality [19]. However, a higher proportion of social species in cold regions, as shown in our results, can be potentially explained by a risk spreading strategy: (i) if a generation of animals dies owing to bad weather conditions, this can be compensated for in social species through the next overlapping generation, whereas the whole brood of solitary animals might be lost; (ii) if the nest-founding queen dies, there is a good chance that at least some workers may survive and raise the brood started by the queen, or even produce new (male) brood [49]; (iii) if a gyne (mated female) in sub-social species dies, the brood can still be raised and protected by other females sharing the same nest [50]. The fact that we showed an increasing proportion of sociality with altitude in the analysis without the social genus, Bombus (see the electronic supplementary material, appendices S1 and S2), confirms the adaptive value of sociality in cold environments. However, there is a need for phylogenetically independent data from other geographical regions with additional taxa to reveal more general patterns of sociality along climatic gradients [19].
Our results showed an increase of the mean body size with increasing altitude. Other studies on body size along altitudinal gradients at the community level have found declines, inclines or no trends [20]. A larger body size at higher altitudes can be explained by the hypothesis that a greater size provides an enhanced tolerance against starvation or desiccation [51]. Another explanation for the detected pattern can be that larger animals have a higher energy efficiency [52] which is an advantage in environments where low temperatures, bad weather periods and starvation can be a problem. Probably the main advantage for bees is that larger species can more effectively thermoregulate and fly at lower temperatures, therefore enhancing their foraging ability. We assume that body size, thermoregulation or dispersal ability are traits with adaptive value. However, we cannot disentangle in our study the adaptive value of body size from the possible phylogenetic signal of the genus Bombus, as this genus combines the traits of sociality, active thermoregulation and large body size and dominates bee communities at high altitudes in our study. Studying dominant traits along altitudinal gradients in regions where Bombus does not occur might help to further disentangle the adaptive values of sociality and body size.
We found an increasing proportion of ground-nesting species with increasing altitude. To our knowledge, there are no other studies about the effects of altitude on the nesting behaviour of insects. However, better protection against extreme climatic conditions of below-ground nests, on the one hand, and a decline of available above-ground-nesting sites (such as shrubs) at higher altitudes, on the other hand, are plausible explanations for our findings.
We found a larger mean altitudinal range in species in upland communities, but no significant patterns in the abundance-based analysis. Additionally, we show that the mean geographical distribution was smaller in upland communities than in lowland communities in both the species- and the abundance-based analysis. This is consistent with results from studies on the altitudinal ranges of vertebrates [24], which can be explained by an adaptation of Janzen's hypothesis [23], also called Rapoport's rule: species living at high altitudes have broader environmental tolerances and therefore larger altitudinal ranges owing to larger seasonal fluctuations [24]. The fact that abundance-weighted altitudinal ranges of communities were constantly high across the altitudinal gradient raises the issue of whether highly abundant, generalist species with large altitudinal and geographical ranges dominate at all altitudes. However, our results show that bee species in communities at high altitudes, even though they have on average a wider environmental range, were restricted in their distribution to mountainous areas in Bavaria, and were less abundant. This suggests that high altitudinal communities consisted of a few widespread generalists, which are dominant at low, but not at high altitudes (such as Bombus jonellus, Bombus terrestris, Lasioglossum calceatum), and of less competitive, alpine specialists with large altitudinal ranges but small geographical distributions (such as B. mendax, B. monticola, B. mucidus, B. wurfleinii, L. fratellum, and L. alpigenum). By contrast, communities at low altitudes consisted of many less abundant species with small altitudinal ranges but were dominated by abundant generalists with large altitudinal ranges and geographical distribution.
5. Conclusions
Our combined phylogenetic and ecological analyses show that competition-driven processes in community assembly are more important at low altitudes than at high altitudes where environmental filtering processes are more important in determining the assembly and phylogenetic structure of communities. The critical characteristics needed for survival under adverse and fluctuating environmental conditions seem to have evolved in a few phylogenetic lineages (e.g. Bombus and Lasioglossum), and at the expense of competitive strength in most of the bee species in alpine communities. Therefore, less competitive alpine species are restricted in their geographical distribution. With warmer climates, the importance of environmental filtering processes is reduced and competition-driven processes can be assumed to increase in their importance. This could lead to a threat for alpine specialists with high environmental tolerance but low competitive capacities.
Acknowledgements
We thank Annette Leingärtner, Alice Claßen and Michaela Bellach for valuable comments on the manuscript; Michael Vogel, Helmut Franz and the National Park Berchtesgaden for their support and the permission to work in the national park; the owners of the study sites outside the national park for their allowance; Peter Hartmann and Reiner Theunert for the verification of our bee identifications; Marcell Peters for statistical discussions. This study was performed within the framework of the joint research center FORKAST and was funded by the ‘Bavarian Climate Programme 2020’; the traits database was supported by the STEP project (EU FP7, 244090, Status and Trends of European Pollinators, www.STEP-project.net). B.H., J.K. and I.S.D. designed the study; B.H. collected data, analysed data, performed phylogenetic analysis; B.H., I.S.D. and J.K. interpreted the results; S.G.P. and S.R. provided trait data. B.H. wrote the first draft of the manuscript and all authors contributed to revisions.
References
- 1.Hodkinson I. 2005. Terrestrial insects along elevation gradients: species and community responses to altitude. Biol. Rev. 80, 489–513 10.1017/S1464793105006767 (doi:10.1017/S1464793105006767) [DOI] [PubMed] [Google Scholar]
- 2.Beck J., Altermatt F., Hagmann R., Lang S. 2010. Seasonality in the altitude-diversity pattern of alpine moths. Basic Appl. Ecol. 11, 714–722 10.1016/j.baae.2010.08.009 (doi:10.1016/j.baae.2010.08.009) [DOI] [Google Scholar]
- 3.Dingle H., Mousseau T. A. 1994. Geographic variation in embryonic development time and stage of diapause in a grasshopper. Oecologia 97, 179–185 10.1007/BF00323147 (doi:10.1007/BF00323147) [DOI] [PubMed] [Google Scholar]
- 4.Cronin A. L. 2001. Social flexibility in a primitively social allodapine bee (Hymenoptera: Apidae): results of a translocation experiment. Oikos 94, 337–343 10.1034/j.1600-0706.2001.940214.x (doi:10.1034/j.1600-0706.2001.940214.x) [DOI] [Google Scholar]
- 5.Berner D., Körner C., Blanckenhorn W. U. 2004. Grasshopper populations across 2000 m of altitude: is there life history adaptation? Ecography 27, 733–740 10.1111/j.0906-7590.2005.04012.x (doi:10.1111/j.0906-7590.2005.04012.x) [DOI] [Google Scholar]
- 6.Rahbek C. 2005. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 8, 224–239 10.1111/j.1461-0248.2004.00701.x (doi:10.1111/j.1461-0248.2004.00701.x) [DOI] [Google Scholar]
- 7.Nogues-Bravo D., Araujo M., Romdal T., Rahbek C. 2008. Scale effects and human impact on the elevational species richness gradients. Nature 453, 216–219 10.1038/nature06812 (doi:10.1038/nature06812) [DOI] [PubMed] [Google Scholar]
- 8.Field R., et al. 2009. Spatial species-richness gradients across scales: a meta-analysis. J. Biogeogr. 36, 132–147 10.1111/j.1365-2699.2008.01963.x (doi:10.1111/j.1365-2699.2008.01963.x) [DOI] [Google Scholar]
- 9.Hoiss B., Gaviria J., Leingärtner A., Krauss J., Steffan-Dewenter I. In press. Combined effects of climate and management on plant diversity and pollination type in alpine grasslands. Divers. Distrib. (doi:10.1111/j.1472-4642.2012.00941.x) [Google Scholar]
- 10.Webb C., Ackerly D., McPeek M., Donoghue M. J. 2002. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 33, 475–505 10.1146/annurev.ecolsys.33.010802.150448 (doi:10.1146/annurev.ecolsys.33.010802.150448) [DOI] [Google Scholar]
- 11.Götzenberger L., et al. 2012. Ecological assembly rules in plant communities: approaches, patterns and prospects. Biol. Rev. 87, 111–127 10.1111/j.1469-185X.2011.00187.x (doi:10.1111/j.1469-185X.2011.00187.x) [DOI] [PubMed] [Google Scholar]
- 12.Cavender-Bares J., Kozak K. H., Fine P. V. A., Kembel S. W. 2009. The merging of community ecology and phylogenetic biology. Ecol. Lett. 12, 693–715 10.1111/j.1461-0248.2009.01314.x (doi:10.1111/j.1461-0248.2009.01314.x) [DOI] [PubMed] [Google Scholar]
- 13.Jabot F., Etienne R. S., Chave J. 2008. Reconciling neutral community models and environmental filtering: theory and an empirical test. Oikos 117, 1308–1320 10.1111/j.0030-1299.2008.16724.x (doi:10.1111/j.0030-1299.2008.16724.x) [DOI] [Google Scholar]
- 14.Machac A., Janda M., Dunn R. R., Sanders N. J. 2011. Elevational gradients in phylogenetic structure of ant communities reveal the interplay of biotic and abiotic constraints on diversity. Ecography 34, 364–371 10.1111/j.1600-0587.2010.06629.x (doi:10.1111/j.1600-0587.2010.06629.x) [DOI] [Google Scholar]
- 15.Vamosi S. M., Heard S. B., Vamosi J. C., Webb C. O. 2009. Emerging patterns in the comparative analysis of phylogenetic community structure. Mol. Ecol. 18, 572–592 10.1111/j.1365-294X.2008.04001.x (doi:10.1111/j.1365-294X.2008.04001.x) [DOI] [PubMed] [Google Scholar]
- 16.Petanidou T., Ellis W. N., Ellis-Adam A. C. 1995. Ecogeographical patterns in the incidence of brood parasitism in bees. Biol. J. Linn. Soc. 55, 261–272 10.1111/j.1095-8312.1995.tb01064x (doi:10.1111/j.1095-8312.1995.tb01064x) [DOI] [Google Scholar]
- 17.Diamond S. E., Frame A. M., Martin R. A., Buckley L. B. 2011. Species’ traits predict phenological responses to climate change in butterflies. Ecology 92, 1005–1012 10.1890/i0012-9658-92-5-1005 (doi:10.1890/i0012-9658-92-5-1005) [DOI] [PubMed] [Google Scholar]
- 18.Dillon M., Frazier M., Dudley R. 2006. Into thin air: physiology and evolution of alpine insects. Integr. Comp. Biol. 46, 49–61 10.1093/icb/icj007 (doi:10.1093/icb/icj007) [DOI] [PubMed] [Google Scholar]
- 19.Purcell J. 2011. Geographic patterns in the distribution of social systems in terrestrial arthropods. Biol. Rev. 86, 475–491 10.1111/j.1469-185X.2010.00156.x (doi:10.1111/j.1469-185X.2010.00156.x) [DOI] [PubMed] [Google Scholar]
- 20.Chown S. L., Gaston K. J. 2010. Body size variation in insects: a macroecological perspective. Biol. Rev. 85, 139–169 10.1111/j.1469-185X.2009.00097.x (doi:10.1111/j.1469-185X.2009.00097.x) [DOI] [PubMed] [Google Scholar]
- 21.Rödel H. G., Völkl W., Kilias H. 2004. Winter browsing of brown hares: evidence for diet breadth expansion. Mamm. Biol. 69, 410–419 10.1078/1616-5047-00163 (doi:10.1078/1616-5047-00163) [DOI] [Google Scholar]
- 22.Ramos-Jiliberto R., Dominguez D., Espinoza C., Lopez G., Valdovinos F., Bustamante R., Medel R. 2010. Topological change of Andean plant–pollinator networks along an altitudinal gradient. Ecol. Complexity 7, 86–90 10.1016/j.ecocom.2009.06.001 (doi:10.1016/j.ecocom.2009.06.001) [DOI] [Google Scholar]
- 23.Janzen D. H. 1967. Why mountain passes are higher in the tropics? Am. Nat. 101, 233–249 10.1086/282487 (doi:10.1086/282487) [DOI] [Google Scholar]
- 24.McCain C. M. 2009. Vertebrate range sizes indicate that mountains may be ‘higher’ in the tropics. Ecol. Lett. 12, 550–560 10.1111/j.1461-0248.2009.01308.x (doi:10.1111/j.1461-0248.2009.01308.x) [DOI] [PubMed] [Google Scholar]
- 25.Scheuchl E. 2000. Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs, Band 1: Schlüssel der Gattungen und der Arten der Familie Anthophoridae, 2nd edn Velden, Germany: Eigenverlag [Google Scholar]
- 26.Scheuchl E. 2006. Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs, Band 2: Schlüssel der Arten der Familien Megachilidae und Melittidae, 2nd edn Velden, Germany: Eigenverlag [Google Scholar]
- 27.Schmid-Egger C., Scheuchl E. 1997. Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs, Band 3: Schlüssel der Arten der Familie Andrenidae, 1st edn Velden, Germany: Eigenverlag [Google Scholar]
- 28.Mauss V. 1996. Bestimmungsschlüssel für Hummeln, 6th edn Hamburg, Germany: Deutscher Jugendbund für Naturbeobachtung [Google Scholar]
- 29.Amiet F., Müller A., Neumeyer R. 2001. Apidae 2. Colletes, Dufourea, Hylaeus, Nomia, Nomioides, Rhophitoides, Rophites, Sphecodes, Systropha. Neuchâtel, Switzerland: Schweizerische Entomologische Gesellschaft [Google Scholar]
- 30.Michener C. D. 2007. The bees of the world, 2nd edn Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 31.Cane J. H. 1987. Estimation of bee size using intertegular span (Apoidea). J. Kansas Entomol. Soc. 60, 145–147 [Google Scholar]
- 32.Bommarco R., Biesmeijer J., Meyer B., Potts S., Poyry J., Roberts S., Steffan-Dewenter I., Ockinger E. 2010. Dispersal capacity and diet breadth modify the response of wild bees to habitat loss. Proc. R. Soc. B 277, 2075–2082 10.1098/rspb.2009.2221 (doi:10.1098/rspb.2009.2221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westrich P. 1989. Die Wildbienen Baden-Württembergs: Spezieller Teil. Stuttgart, Germany: Ulmer [Google Scholar]
- 34.Gogala A. 1999. Bee fauna of Slovenia: checklist of species (Hymenoptera: Apoidea). Scopolia 42, 1–79 [Google Scholar]
- 35.Prosi-CAD 2011. Website für Freunde der aculeaten Hymenopteren See http://www.aculeata.eu/
- 36.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 37.Crawley M. J. 2007. The R book. 1. Auflage. West Sussex, UK: John Wiley & Sons [Google Scholar]
- 38.Grafen A. 1989. The phylogenetic regression. Phil. Trans. R. Soc. Lond. B 326, 119–157 10.1098/rstb.1989.0106 (doi:10.1098/rstb.1989.0106) [DOI] [PubMed] [Google Scholar]
- 39.Webb C. O., Ackerly D. D., Kembel S. W. 2008. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24, 2098–2100 10.1093/bioinformatics/btn358 (doi:10.1093/bioinformatics/btn358) [DOI] [PubMed] [Google Scholar]
- 40.Clarke A., Gaston K. 2006. Climate, energy and diversity. Proc. R. Soc. B 273, 2257–2266 10.1098/rspb.2006.3545 (doi:10.1098/rspb.2006.3545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCain C. M. 2007. Could temperature and water availability drive elevational species richness patterns? A global case study for bats. Glob. Ecol. Biogeogr. 16, 1–13 10.1111/j.1466-8238.2006.00263.x (doi:10.1111/j.1466-8238.2006.00263.x) [DOI] [Google Scholar]
- 42.Sanders N. J., Lessard J., Fitzpatrick M. C., Dunn R. R. 2007. Temperature, but not productivity or geometry, predicts elevational diversity gradients in ants across spatial grains. Glob. Ecol. Biogeogr. 16, 640–649 10.1111/j.1466-8238.2007.00316.x (doi:10.1111/j.1466-8238.2007.00316.x) [DOI] [Google Scholar]
- 43.Jones J., Li W., Maberly S. 2003. Area, altitude and aquatic plant diversity. Ecography 26, 411–420 10.1034/j.1600-0587.2003.03554.x (doi:10.1034/j.1600-0587.2003.03554.x) [DOI] [Google Scholar]
- 44.Beck J., Chey V. 2008. Explaining the elevational diversity pattern of geometrid moths from Borneo: a test of five hypotheses. J. Biogeogr. 35, 1452–1464 10.1111/j.1365-2699.2008.01886.x (doi:10.1111/j.1365-2699.2008.01886.x) [DOI] [Google Scholar]
- 45.Colwell R. K., Lees D. C. 2000. The mid-domain effect: geometric constraints on the geography of species richness. Trends Ecol. Evol. 15, 70–76 10.1016/S0169-5347(99)01767-X (doi:10.1016/S0169-5347(99)01767-X) [DOI] [PubMed] [Google Scholar]
- 46.Grytnes J., Heegaard E., Romdal T. 2008. Can the mass effect explain the mid-altitudinal peak in vascular plant species richness? Basic Appl. Ecol. 9, 373–382 10.1016/j.baae.2007.05.001 (doi:10.1016/j.baae.2007.05.001) [DOI] [Google Scholar]
- 47.Graham C. H., Parra J. L., Rahbek C., McGuire J. A. 2009. Phylogenetic structure in tropical hummingbird communities. Proc. Natl Acad. Sci. USA 106, 19 673–19 678 10.1073/pnas.0901649106 (doi:10.1073/pnas.0901649106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bryant J. A., Lamanna C., Morlon H., Kerkhoff A. J., Enquist B. J., Green J. L. 2008. Microbes on mountainsides: contrasting elevational patterns of bacterial and plant diversity. Proc. Natl Acad. Sci. USA 105, 11 505–11 511 10.1073/pnas.0801920105 (doi:10.1073/pnas.0801920105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gadagkar R. 1990. Evolution of eusociality: the advantage of assured fitness returns. Phil. Trans. R. Soc. Lond. B 329, 17–25 10.1098/rstb.1990.0146 (doi:10.1098/rstb.1990.0146) [DOI] [Google Scholar]
- 50.Jones T. C., Riechert S. E. 2008. Patterns of reproductive success associated with social structure and microclimate in a spider system. Anim. Behav. 76, 2011–2019 10.1016/j.anbehav.2008.07.033 (doi:10.1016/j.anbehav.2008.07.033) [DOI] [Google Scholar]
- 51.Chown S. L., Gaston K. J. 1999. Exploring links between physiology and ecology at macro-scales: the role of respiratory metabolism in insects. Biol. Rev. 74, 87–120 10.1111/j.1469-185X.1999.tb00182.x (doi:10.1111/j.1469-185X.1999.tb00182.x) [DOI] [Google Scholar]
- 52.Ellington C. P. 1999. The novel aerodynamics of insect flight: applications to micro-air vehicles. J. Exp. Biol. 202, 3439–3448 [DOI] [PubMed] [Google Scholar]