Abstract
Global anthropogenic changes are occurring at an unprecedented rate; one change, human-facilitated introduction of species outside their native range, has had significant ecological and economic impacts. Surprisingly, what traits facilitate range expansions post-introduction is relatively unknown. This information could help predict future expansions of introduced species as well as native species shifting their ranges as climate conditions change. Here, we asked whether specific behavioural and physiological traits were important in the ongoing expansion of house sparrows (Passer domesticus) across Kenya. We predicted that birds at the site of initial introduction (Mombasa, introduced approx. 1950) would behave and regulate corticosterone, a stress hormone, differently than birds at the range edge (Kakamega, approx. 885 km from Mombasa; colonized within the last 5 years). Specifically, we predicted greater exploratory behaviour and stronger corticosterone response to stressors in birds at the range edge, which may facilitate the identification, resolution and memory of stressors. Indeed, we found that distance from Mombasa (a proxy for population age) was a strong predictor of both exploratory behaviour and corticosterone release in response to restraint (but only while birds were breeding). These results suggest that certain behavioural and neuroendocrine traits may influence the ability of species to colonize novel habitats.
Keywords: corticosterone regulation, exploratory behaviour, house sparrow, introduced species, range expansion
1. Introduction
The introduction of species outside their native range is currently one of the largest threats to global biodiversity [1]. Of the four stages of an invasion (i.e. introduction, colonization, establishment and range expansion [2,3]), range expansion is arguably the most important because it is typically the stage in which species have the largest economic and ecological impacts. Owing to the unethical nature of experimental introductions and the rarity of natural range expansions, they remain under-studied, especially in vertebrates. However, studying invasive species during a range expansion in an ecological or evolutionary context could lend great insights not only to invasion biology, but also to the spatial structure of species interactions, mechanisms of allopatric speciation and population responses to environmental stressors, such as global climate change [4].
For populations to expand, individuals must possess particular traits to allow them to exploit the novel conditions they face in new areas; many behavioural traits (e.g. boldness, aggression, response to novelty) may be especially important in novel environments [5–15]. Different selection pressures along a range expansion could select for different levels of these traits between the site of introduction and the edge of the range. For instance, male mountain bluebirds (Sialia currucoides) are significantly more aggressive towards conspecifics in novel environments; however, as populations age, selection favours less aggressive, more philopatric males exhibiting greater parental care, which increases offspring survival [13,14]. Exploratory behaviour is also likely to be an important trait mediating range expansion, as exploration facilitates the identification of novel resources [16], as well as potential stressors (e.g. predators, challenging microclimates). However, when familiar resources are available, exploration might have a reduced benefit and be lost through genetic drift [17]; further, in these areas, exploration might increase the likelihood of exposure to toxins and predators, waste time that could be devoted to other activities [18], and increase the likelihood of being out-competed by individuals procuring known resources. Indeed, exploration tends to be stronger in invasive species and invading populations [7,19,20] compared with native species and populations.
In unfamiliar environments, where the necessity for exploration is increased, stressors are probably also less predictable and potentially more frequent. Glucocorticoids (GCs), hormones released by the hypothalamic–pituitary–adrenal axis in response to stressors, help individuals cope with and resolve stressors [21]. Thus, the release of GCs in response to stressors is also apt to be stronger at range edges. Although data indicate that introduced populations are more exploratory than native ones [7,19,20] and GCs may play a role in population viability after an introduction [22], it remains untested whether increased exploration and altered stress hormone regulation facilitate range expansions.
Here, we tested whether exploratory behaviour and corticosterone (the main avian GC) response to stressors are more pronounced at the edge of a range expansion of house sparrows (Passer domesticus) in Kenya. House sparrows are one of the world's most broadly distributed species and were introduced to Mombasa, Kenya in the 1950s [23]. Since then, house sparrows have spread northwestward across the country arriving at the Ugandan border within the last few years (figure 1). Using house sparrows collected from eight cities differing in time since colonization in Kenya, we measured exploratory behaviour in a novel environment. We also measured corticosterone response to restraint stress along this range during both the breeding and non-breeding seasons.
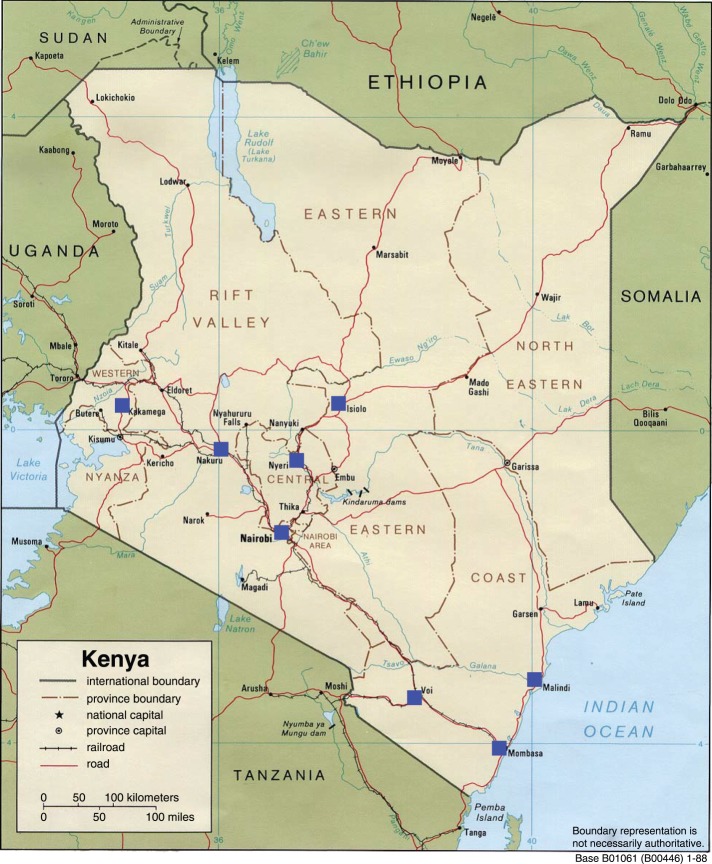
Figure 1.
Map of Kenya. House sparrows were introduced to Mombasa, Kenya in the 1950s and have subsequently spread across Kenya and into Eastern Africa. In this study, house sparrows were captured from eight cities (Mombasa, Malindi, Voi, Nairobi, Nakuru, Nyeri, Isiolo and Kakamega) indicated by blue squares on the map. Distance from Mombasa (km) was used as a proxy of time since colonization, as cities furthest from Mombasa are likely the most recently colonized by house sparrows. Figure was adapted from the US CIA's open database.
2. Methods
(a). Study population
Wild adult house sparrows were caught in mist nets from eight cities across Kenya during the breeding season (March–June) of 2011 (see the electronic supplementary material, table S1; figure 1). To test the effect of life-history stage on corticosterone response [24], additional house sparrows were caught from six of the eight cities in July 2010 when Kenyan birds were moulting (see the electronic supplementary material, table S1). House sparrows were introduced to Mombasa, Kenya around 1950. Although the year of house sparrow arrival to most other Kenyan cities is unknown, they probably arrived in Nairobi sometime during the late 1980s or early 1990s [25], and to cities north and west of Nairobi sometime after 2000 (National Museums of Kenya 1950–2005, unpublished data). Further, genetic analyses indicate that house sparrows expanded west from Mombasa along the Mombasa highway (i.e. from Mombasa to Voi, to Nairobi, to Nakuru, to Kakamega) with a secondary expansion north from Nairobi (i.e. from Nairobi to Nyeri, to Isiolo [26]). Therefore, we used distance from Mombasa as a proxy for time since colonization (most recently colonized cities are furthest from Mombasa). At capture, birds were banded with a numbered aluminium band and colour bands, and wing cord (mm), tarsus (mm) and mass (g) were measured before being handled as described below. Individual condition was determined using the residuals of a linear regression of mass against tarsus. All experimental procedures were approved by the University of South Florida's IACUC (W3877) and the Kenyan Ministry of Science and Technology.
(b). Exploratory behaviour
Between 8 and 18 adults were caught from each city (n = 98; see the electronic supplementary material, table S1). After capture, birds were brought into captivity and singly housed in 35.6 × 40.6 × 44.5 cm cages in ambient conditions. Birds were given ad libitum access to food (mixture of millet, red millet, sorghum and rice) and water for one week, during which time other behavioural measures were made for a separate study. Immediately following exploratory measurements, birds were released. Exploratory behaviour was measured similarly to previously published methods [27–30]. Briefly, after 1 h without access to food, birds were individually placed into a novel environment: a 2.74 × 2.13 m tent containing 10 novel items (table, cooking spoon, tent poles, broom, mop handle, antennae, stool, nest-box, rope and a bucket) and seams sufficient for perching (see the electronic supplementary material, figure S1). Birds were given 10 s to acclimate, then observed for 5 min by two individuals (and averaged for each variable). The proportion of the tent explored (measured in quarters, i.e. 25%, 50%, 75% or 100%), the number of hops (when the individual changed location, not just direction) and the number of novel perches used were recorded as measures of exploratory behaviour; additionally, the presence of stereotyped behaviours (e.g. patterns of repetitive movement) was assessed. All behavioural indices were collapsed into a single exploration score using a principal components analysis (using correlation matrix and varimax rotation). Principal component 1 (PC1) was the only factor that met the Kaiser criterion (eigenvalue greater than 1), and therefore was the only factor used in analysis; PC1 accounted for 36 per cent of the variation and varied positively with all four behavioural variables (see the electronic supplementary material, table S2).
(c). Corticosterone response
During the breeding season, 10–13 house sparrows (n = 88, electronic supplementary material, table S1) were collected from each of the eight cities; another 5–11 house sparrows (n = 58; electronic supplementary material, table S1) were collected from six of the eight cities during moult. To measure corticosterone concentrations, blood (approx. 25 μl) was taken from the brachial vein within 3 min of capture; birds were then placed in a cloth bag for 30 min to elicit a corticosterone response, and bled again. Blood was centrifuged and plasma was extracted and frozen in liquid nitrogen until corticosterone levels could be measured using a commercially available enzyme immunoassay kit (Assay Designs; average detection limit of 27 pg; validated elsewhere [31,32]). Samples were randomly distributed among eight plates; intra-plate variation was less than 10 per cent for each plate, whereas average inter-plate variation was 8 per cent. Corticosterone response (ΔCORT) was calculated by subtracting baseline values of corticosterone from elevated levels taken after 30 min of restraint. Different individuals from each site were used for this part of the study because: (i) repeated bleeding could have affected behaviour [33], and (ii) experiments were not designed to address the mechanism of action of corticosterone on exploratory behaviour.
(d). Statistical analysis
ΔCORT was ln-transformed to achieve normality. General linear models (GLMs) were used to determine whether population age (i.e. distance from Mombasa; km) predicted exploratory behaviour and ln-corrected ΔCORT responses; as corticosterone is regulated differently during breeding compared with moulting [24], corticosterone data were analysed separately by season. When GLMs indicated significant effects of distance from Mombasa on measured traits, we then used a model selection approach (using general linear mixed models, GLMMs), to determine whether distance from Mombasa was a better predictor of dependent variables than other factors known to influence behaviour or corticosterone (see below). In addition to distance from Mombasa, we used the degree of urbanization around the catching site (microhabitat), altitude (m), house sparrow density (per km2) and individual condition and sex (see the electronic supplementary material, table S1) as fixed factors in GLMMs and each individual nested within city was used as a random factor. Distance from Mombasa and altitude were determined using a GPS device (Garmin 60 CSx); microhabitat, shown to affect corticosterone regulation in other studies [34–36], was assessed as urban or non-urban by the proportion of pavement surrounding the netting site (within approx. 50 m) and the amount of vehicular and human traffic through the area; and house sparrow density was determined at the time of data collection by averaging point count estimates (two observers, 5 min fixed-radius (50 m) distributed throughout each city (8–15 per city, depending on city size [37]). Backwards model selection was conducted based on corrected Akaike information criteria (AICc) scores; each single factor was used as well as interactions between distance and microhabitat, distance and condition, distance and sex, condition and house sparrow density, and microhabitat and density; we chose these interactions because microhabitat, condition and sex may vary by distance (dependent on the dispersal mechanism) and density might influence individual condition or be influenced by microhabitat. By using AICc scores, the top five models were averaged to determine the relative importance of each variable [38]. R v. 2.14.0 was used for all statistical analyses and GraphPad Prism v. 5 was used to make the figures.
3. Results
(a). Exploratory behaviour
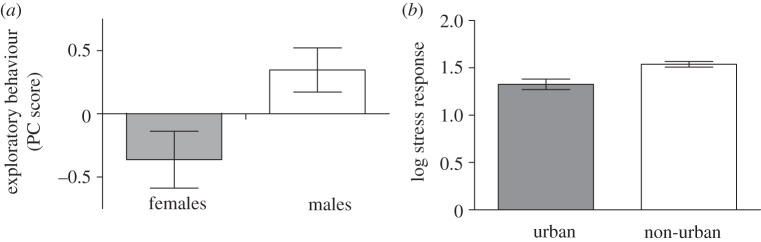
As predicted, during the breeding season, individuals at the range edge were most exploratory, whereas individuals from the site of initial introduction (Mombasa) were least exploratory (F1,97 = 7.937, p = 0.006; figure 2). Model selection indicated that distance from Mombasa was the best predictor of exploratory behaviour (estimate = 0.0010 ± 0.0004; table 1; electronic supplementary material, figure S2a). Additionally, sex was an important predictor of exploratory behaviour (estimate = −0.5034 ± 0.3330; table 1; electronic supplementary material, figure S2a): males (n = 50) were significantly more exploratory than females (n = 48; t = 2.50, p = 0.014; figure 3a).
Figure 2.
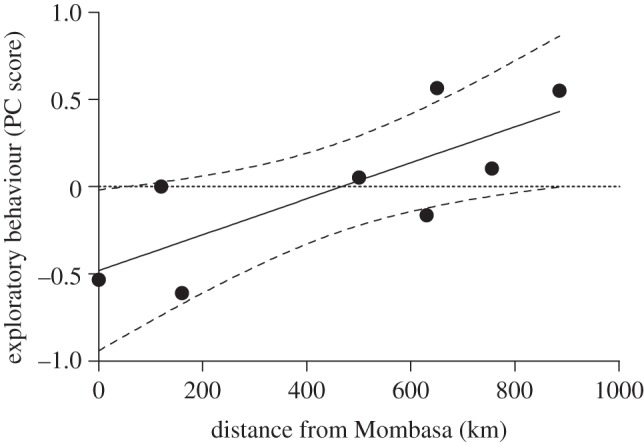
Distance from Mombasa is related to exploratory behaviour in Kenyan house sparrows (n = 98). House sparrows at the edge of the range expansion are more exploratory than birds from the site of introduction (approx. 60 years since establishment). Distance from Mombasa was the best predictor of exploration of a novel habitat (F = 7.937, p = 0.006; estimate = 0.0010 ± 0.0004). Exploratory behaviour (PC score of combined exploratory variables) is averaged by city and regression bands are 95% confidence intervals (CIs).
Table 1.
Top five models resulting from the generalized linear mixed models predicting exploratory behaviour and corticosterone release in Kenyan house sparrows across a range expansion. (Distance from Mombasa (the site of introduction; distance), sex, condition, microhabitat (urban or non-urban), altitude and house sparrow density were treated as fixed factors and individuals nested within city was treated as a random factor.)
| factors | AICc | ΔAICc | K | log-likelihood | weight |
|---|---|---|---|---|---|
| exploratory behaviour | |||||
| distance + sex | 316.28 | 2 | −151.68 | 0.52 | |
| distance + sex + distance × sex | 317.49 | 1.22 | 3 | −151.13 | 0.28 |
| distance + condition + sex + condition × sex | 319.81 | 3.53 | 4 | −151.10 | 0.09 |
| distance | 320.65 | 4.37 | 1 | −155.00 | 0.06 |
| sex | 321.39 | 5.11 | 1 | −155.37 | 0.04 |
| corticosterone release | |||||
| distance + microhabitat | 155.74 | 2 | −71.35 | 0.33 | |
| microhabitat | 156.35 | 0.62 | 1 | −72.81 | 0.24 |
| altitude + distance + microhabitat | 157.21 | 1.47 | 3 | −70.90 | 0.16 |
| distance | 157.56 | 1.82 | 1 | −73.41 | 0.13 |
| altitude + distance + microhabitat + sex | 157.68 | 1.94 | 4 | −69.93 | 0.12 |
Figure 3.
(a) Male Kenyan house sparrows (n = 50) were more exploratory than females (n = 48) (estimate = −0.5034 ± 0.3330; t = 2.50, p = 0.014) in a novel environment; means ± s.e.m. are presented. (b) House sparrows from more urban sites released significantly less corticosterone in response to 30 min restraint (estimate = 0.2803 ± 0.1309; t = 3.67, p < 0.001); means ± s.e.m. are presented.
(b). Corticosterone release
During the breeding season, individuals from the most recently colonized cities (i.e. those furthest from Mombasa) released significantly more corticosterone in response to a restraint stressor than those from the longer established cities (F1,86 = 2.131, p = 0.0359; figure 4); however, when moulting, no such relationship existed (F1,55 = 0.985, p = 0.33; figure 5). Interestingly, differences among populations were driven by corticosterone levels in response to a stressor during breeding, as baseline levels of corticosterone did not differ among cities (breeding: F1,89 = 0.974, p = 0.46; moulting: F1,47 = 1.514, p = 0.140). Model selection revealed that distance from Mombasa was one of the best predictors of ΔCORT during breeding (estimate = 0.00044 ± 0.0003; table 1; electronic supplementary material, figure S2b), however, the best model also included urbanization of the catching site (estimate = 0.2803 ± 0.1309; table 1; electronic supplementary material, figure S2b). House sparrows caught from more urban areas released significantly less corticosterone than those caught from rural areas (t = 3.67, p < 0.001; figure 3b). At the population level, no correlation was observed between exploration and corticosterone release (p = 0.815), although previous studies predict such a relationship [39].
Figure 4.
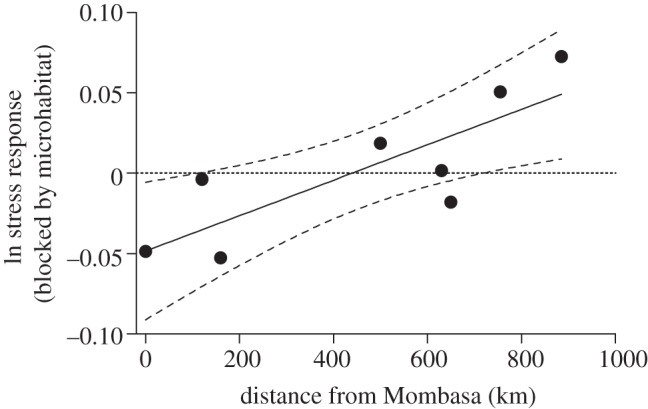
Corticosterone release is related to distance from Mombasa in Kenyan house sparrows only during the breeding season (n = 88). When breeding, house sparrows at the edge of a range expansion released more corticosterone, a stress hormone, in response to a stressor than birds from the site of introduction (F1,86 = 2.131, p = 0.0359); model selection indicated that distance from Mombasa was one of the most important predictors of variability in ΔCORT. The average residuals of ln-corrected ΔCORT and microhabitat for each population are plotted against distance from Mombasa with 95% CIs.
Figure 5.
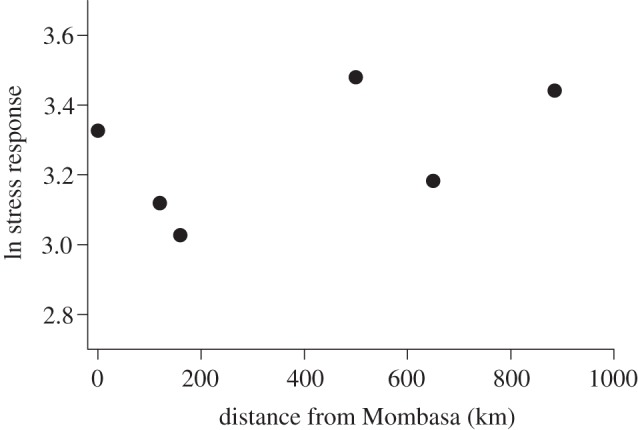
While moulting (n = 56), no significant differences in ΔCORT among Kenyan house sparrow populations were found (F1,55 = 0.985, p = 0.33). The average ln-corrected ΔCORT for each population are plotted against distance from Mombasa.
4. Discussion
Behavioural and physiological differences exist among populations of a species that has colonized a novel environment within just the last 60 years. The observed patterns are consistent with the hypothesis that these traits facilitated the range expansion. It is yet unclear whether phenotypic plasticity, genetic differentiation or both underlie the patterns described here, but below we describe how one might discriminate among these possibilities and provide possible interpretations of our present data.
(a). Exploratory behaviour
Although decreased exploration might be protective or unnecessary (and therefore lost owing to drift in the absence of reinforcing selection) in familiar habitats, increased exploration might be adaptive in novel environments such as those found at the edge of a range. Exploratory behaviour would allow individuals to discover and procure novel resources in unfamiliar habitats as well as identify potential stressors when such information is less readily available (e.g. from conspecifics). Within the novel environment, males were significantly more exploratory than females. Both male and female house sparrows provision and care for chicks after they hatch, but males typically locate and defend nesting sites before breeding; increased exploratory behaviour in males could enhance the acquisition of quality nesting sites, increasing fitness. Although other studies have shown that males of other species can colonize new territories first [14], it is yet unknown whether Kenyan house sparrows disperse in a sex-dependent manner.
Although exploratory behaviour was tested in an artificial environment, without any food reward, we feel our paradigm was representative of exploratory behaviour in the wild. In European starlings (Sturnus vulgaris), the amount of a novel environment (one similar to that used in this experiment) explored, but not the speed of exploration, tended to be correlated with the maximum home range size of that individual [40]. Further, although a few individuals displayed frantic movements in the tent, possibly indicative of increased stress and in search of an escape, most calmly hopped through the novel environment pecking at things on the ground or even preening (A. Liebl 2011, personal observation).
(b). Corticosterone responses to stressors
Corticosterone release also varied among populations with range-edge populations releasing the most corticosterone, although only during the breeding season. Elevated GC responses at the edge of the range may increase vigilance, aiding in the detection of stressors in novel environments, which may offset the costs of exploration, such as increased exposure to predators and parasites. Further, elevated GC responses may also facilitate the consolidation or formation of memories for novel resources and stressors alike [41]. In other words, strong, rapid elevations of corticosterone in response to stressors might allow individuals at range edges to mitigate and/or remember stressors better in environments where stressors are potentially less predictable, less familiar and/or more numerous.
Corticosterone release was also related to the degree of urbanization close to the catching site. Urbanization affects corticosterone regulation in other species as well [34–36] and a damped corticosterone response in urban areas may reflect habituation to stressors such as increased noise and human disturbance. Here, we used ΔCORT as an index of corticosterone regulation, rather than absolute levels of the hormone to control for individual variation in corticosterone regulation mechanisms (e.g. corticosterone receptors, corticosterone-binding globulins). Other studies have suggested that these factors may be controlled by baseline levels of corticosterone [42,43], which did not vary among populations at either time of year. Although this study was not designed to elucidate the hormonal mechanisms of exploratory behaviour, previous studies indicate that a relationship between corticosterone release and exploratory behaviour might exist; however, no such within-population relationship was observed. A lack of a relationship may be a result of using different birds in each population for hormonal and exploratory measurements; however, recent papers have argued this relationship may be more tenuous than previously thought [44,45].
In the Kenyan house sparrow colonization, low genetic diversity [46] and the unlikelihood of an influx of genetic variation from other areas where house sparrows occur [26] make it somewhat surprising that such extensive phenotypic distinction is observed at all among populations. In this and other examples [14,47], the rapid change of trait distributions along a range expansion suggest that phenotypic plasticity or rapid evolution allowed the differentiation among populations. However, how these patterns arise is unknown. Interestingly, ΔCORT differences among populations were significant only when individuals were breeding, a time when mothers might deposit hormones to the yolk of her developing offspring. In other taxa, maternal transfer of corticosterone to eggs has many strong physiological [48] and behavioural effects [49], including enhancement of the corticosterone response to stressors [48]. Another non-genetic parental effect could be behavioural influences: if exploratory behaviour is elevated at the range edge, offspring provisioning might be reduced if parents take longer to find food; such absence cues might be used as an indication of environmental quality, influencing offspring phenotype [50]. In rats, reduced maternal care causes an enhanced corticosterone response [51], increased vigilance [51] and improved hippocampal-dependent learning under stressful conditions owing to epigenetic alterations (methylation) of the glucocorticoid receptor promoter in the hippocampus [52]. Although it is unknown if similar mechanisms occur in birds, or what the specific developmental window might be, other studies indicate that parental behaviour during the juvenile period might also be an important time in the development of offspring behaviour and corticosterone regulation in birds [50,53,54].
5. Conclusion
Kenyan house sparrows at the edge of a range expansion were significantly more exploratory in a novel environment and released significantly more corticosterone during the breeding season compared with house sparrows at the site of initial introduction; these patterns suggest that these traits may have influenced the Kenyan colonization. Ongoing studies are investigating: (i) whether and how exploratory behaviour and corticosterone release are related to fitness among populations, and (ii) how genetic, epigenetic and maternal effects influence phenotypic diversity. We hope that our results inspire efforts to determine whether exploratory behaviour and stress-coping mechanisms affect range expansions in other species; if so, parts of species' ranges most likely to expand might be revealed and pest control efforts adjusted accordingly. Likewise, exploration and corticosterone release could prove important for species' extinction risk, as low exploratory behaviour and weak stressor responsiveness might hinder populations' adjustments to altered environments.
Acknowledgements
All experimental procedures were approved by the University of South Florida's IACUC (W3877) and the Kenyan Ministry of Science and Technology.
We thank the National Science Foundation (IOS 0920475) and the University of South Florida to L.B.M., and Society for Integrative Biology (grant in aid of research) and Sigma Xi (grant in aid of research) to A.L.L. for funding. We thank Amber Brace, Courtney Coon, Titus Imboma, Cristina Lédon-Rettig, Taegan McMahon, Nicodemus Nalianya, Jason Rohr, Brittany Sears and three anonymous reviewers for help in the field and in the development of the manuscript.
References
- 1.Sala O. E., et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774 10.1126/science.287.5459.1770 (doi:10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 2.Vermeij G. J. 1996. An agenda for invasion biology. Biol. Conserv. 78, 3–9 10.1016/0006-3207(96)00013-4 (doi:10.1016/0006-3207(96)00013-4) [DOI] [Google Scholar]
- 3.Cassey P., Blackburn T. M., Russell G. J., Jones K. E., Lockwood J. L. 2004. Influences on the transport and establishment of exotic bird species: an analysis of the parrots (Psittaciformes) of the world. Glob. Change Biol. 10, 417–426 10.1111/j.1529-8817.2003.00748.x (doi:10.1111/j.1529-8817.2003.00748.x) [DOI] [Google Scholar]
- 4.Holt R. D. 2003. On the evolutionary ecology of species’ ranges. Evol. Ecol. Res. 5, 159–178 [Google Scholar]
- 5.Cote J., Clobert J. 2007. Social personalities influence natal dispersal in a lizard. Proc. R. Soc. B 274, 383–390 10.1098/rspb.2006.3734 (doi:10.1098/rspb.2006.3734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cote J., Clobert J., Brodin T., Fogarty S., Sih A. 2010. Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Phil. Trans. R. Soc. B 365, 4065–4076 (doi:10.1098/rstb.2010.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cote J., Fogarty S., Weinersmith K., Brodin T., Sih A. 2010. Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc. R. Soc. B 277, 1571–1579 10.1098/rspb.2009.2128 (doi:10.1098/rspb.2009.2128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogarty S., Cote J., Sih A. 2011. Social personality polymorphism and the spread of invasive species: a model. Am. Nat. 177, 273–287 10.1086/658174 (doi:10.1086/658174) [DOI] [PubMed] [Google Scholar]
- 9.Holway D. A., Suarez A. V. 1999. Animal behavior: an essential component of invasion biology. Trends Ecol. Evol. 14, 328–330 10.1016/S0169-5347(99)01636-5 (doi:10.1016/S0169-5347(99)01636-5) [DOI] [PubMed] [Google Scholar]
- 10.Rehage J. S., Sih A. 2004. Dispersal behavior, boldness, and the link to invasiveness: a comparison of four Gambusia species. Biol. Invasions 6, 379–391 10.1023/B:BINV.0000034618.93140.a5 (doi:10.1023/B:BINV.0000034618.93140.a5) [DOI] [Google Scholar]
- 11.Sol D., Timmermans S., Lefebvre L. 2002. Behavioural flexibility and invasion success in birds. Anim. Behav. 63, 495–502 10.1006/anbe.2001.1953 (doi:10.1006/anbe.2001.1953) [DOI] [Google Scholar]
- 12.Wright T. F., Eberhard J. R., Hobson E. A., Avery M. L., Russello M. A. 2010. Behavioral flexibility and species invasions: the adaptive flexibility hypothesis. Ethol. Ecol. Evol. 22, 393–404 10.1080/03949370.2010.505580 (doi:10.1080/03949370.2010.505580) [DOI] [Google Scholar]
- 13.Duckworth R. A. 2008. Adaptive dispersal strategies and the dynamics of a range expansion. Am. Nat. 172, S4–S17 10.1086/588289 (doi:10.1086/588289) [DOI] [PubMed] [Google Scholar]
- 14.Duckworth R. A., Badyaev A. V. 2007. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA 104, 15 017–15 022 10.1073/pnas.0706174104 (doi:10.1073/pnas.0706174104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin L. B., Fitzgerald L. 2005. A taste for novelty in invading house sparrows, Passer domesticus. Behav. Ecol. 16, 702–707 10.1093/beheco/ari044 (doi:10.1093/beheco/ari044) [DOI] [Google Scholar]
- 16.Cole E. F., Quinn J. L. 2011. Personality and problem-solving performance explain competitive ability in the wild. Proc. R. Soc. B 279, 1168–1731 10.1098/rspb.2011.1539 (doi:10.1098/rspb.2011.1539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawson Handley L. J., Estoup A., Evans D., Thomas C., Lombaert E., Facon B., Aebi A., Roy H. 2011. Ecological genetics of invasive alien species. BioControl 56, 409–428 (doi:10.1007/s10526-011-9386-2) [Google Scholar]
- 18.Greenberg R., Mettke-Hofmann C. 2001. Ecological aspects of neophobia and neophilia in birds. In Current ornithology (eds Nolen V., Jr, Thompson C. F.), pp. 119–178 New York, NY: Kluwer Academic/Plenum Publishers [Google Scholar]
- 19.Russell J. C., McMorland A. J. C., MacKay J. W. B. 2010. Exploratory behaviour of colonizing rats in novel environments. Anim. Behav. 79, 159–164 10.1016/j.anbehav.2009.10.020 (doi:10.1016/j.anbehav.2009.10.020) [DOI] [Google Scholar]
- 20.Rehage J. S., Barnett B. K., Sih A. 2005. Behavioral responses to a novel predator and competitor of invasive mosquitofish and their non-invasive relatives (Gambusia sp.). Behav. Ecol. Sociobiol. 57, 256–266 10.1007/s00265-004-0850-1 (doi:10.1007/s00265-004-0850-1) [DOI] [Google Scholar]
- 21.Wingfield J. C., Maney D. L., Breuner C. W., Jacobs J. D., Lynn S., Ramenofsky M., Richardson R. D. 1998. Ecological bases of hormone–behavior interactions: the ‘emergency life history stage’. Am. Zool. 38, 191–206 [Google Scholar]
- 22.Martin L. B., Gilliam J., Han P., Lee K., Wikelski M. 2005. Corticosterone suppresses cutaneous immune function in temperate but not tropical house sparrows, Passer domesticus. Gen. Comp. Endocrinol. 140, 126–135 10.1016/j.ygcen.2004.10.010 (doi:10.1016/j.ygcen.2004.10.010) [DOI] [PubMed] [Google Scholar]
- 23.Anderson T. R. 2006. Biology of the ubiquitous house sparrow: from genes to populations, 547 p Oxford, UK: Oxford University Press [Google Scholar]
- 24.Romero L. M. 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 128, 1–24 10.1016/s0016-6480(02)00064-3 (doi:10.1016/s0016-6480(02)00064-3) [DOI] [PubMed] [Google Scholar]
- 25.Lewis A., Pomeroy D. 1989. A bird atlas of Kenya. Rotterdam, The Netherlands: A. A. Balkema [Google Scholar]
- 26.Schrey A., Liebl A. L., Richards C., Martin L. B. In preparation. Mechanisms of expansion: genetic admixture and human facilitated movement likely aided in the range expansion of introduced house sparrows (Passer domesticus) in Kenya.
- 27.Verbeek M. E. M., Drent P. J., Wiepkema P. R. 1994. Consistent individual differences in early exploratory behaviour of male great tits. Anim. Behav. 48, 1113–1121 10.1006/anbe.1994.1344 (doi:10.1006/anbe.1994.1344) [DOI] [Google Scholar]
- 28.Minderman J., Reid J. M., Hughes M., Denny M. J. H., Hogg S., Evans P. G. H., Whittingham M. J. 2011. Novel environment exploration and home range size in starlings Sturnus vulgaris. Behav. Ecol. 21, 1321–1329 10.1093/beheco/arq151 (doi:10.1093/beheco/arq151) [DOI] [Google Scholar]
- 29.Mutzel A., Kempenaers B., Laucht S., Dingemanse N. J., Dale J. 2011. Circulating testosterone levels do not affect exploration in house sparrows: observational and experimental tests. Anim. Behav. 81, 731–739 10.1016/j.anbehav.2011.01.001 (doi:10.1016/j.anbehav.2011.01.001) [DOI] [Google Scholar]
- 30.Dingemanse N. J., Both C., Drent P. J., van Oers K., van Noordwijk A. J. 2002. Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim. Behav. 64, 929–938 10.1006/anbe.2002.2006 (doi:10.1006/anbe.2002.2006) [DOI] [Google Scholar]
- 31.Breuner C. W., Lynn S. E., Julian G. E., Cornelius J. M., Heidinger B. J., Love O. P., Sprague R. S., Wada H., Whitman B. A. 2006. Plasma-binding globulins and acute stress response. Horm. Metab. Res. 38, 260–268 10.1055/s-2006-925347 (doi:10.1055/s-2006-925347) [DOI] [PubMed] [Google Scholar]
- 32.Kuhlman J. R., Martin L. B. 2010. Captivity affects immune redistribution to skin in a wild bird. Funct. Ecol. 24, 830–837 10.1111/j.1365-2435.2010.01710.x (doi:10.1111/j.1365-2435.2010.01710.x) [DOI] [Google Scholar]
- 33.van Oers K., Carere C. 2007. Long-term effects of repeated handling and bleeding in wild caught great tits (Parus major). J. Ornithol. 148, 185–190 10.1007/s10336-007-0200-y (doi:10.1007/s10336-007-0200-y) [DOI] [Google Scholar]
- 34.Fokidis H. B., Orchinik M., Deviche P. 2009. Corticosterone and corticosteroid binding globulin in birds: relation to urbanization in a desert city. Gen. Comp. Endocrinol. 160, 259–270 10.1016/j.ygcen.2008.12.005 (doi:10.1016/j.ygcen.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 35.Partecke J., Schwabl I., Gwinner E. 2006. Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology 87, 1945–1952 10.1890/0012-9658(2006)87[1945:satcua]2.0.co;2 (doi:10.1890/0012-9658(2006)87[1945:satcua]2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 36.Zhang S., Lei F., Liu S., Li D., Chen C., Wang P. 2011. Variation in baseline corticosterone levels of tree sparrow (Passer montanus) populations along an urban gradient in Beijing, China. J. Ornithol. 152, 801–806 10.1007/s10336-011-0663-8 (doi:10.1007/s10336-011-0663-8) [DOI] [Google Scholar]
- 37.Martin L. B., Coon C. A. C., Liebl A. L. Submitted. Surveillance for microbes and range expansion in house sparrows. [DOI] [PMC free article] [PubMed]
- 38.Bolker B. M., Brooks M. E., Clark C. J., Geange S. W., Poulsen J. R., Stevens M. H. H., White J.-S. S. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 10.1016/j.tree.2008.10.008 (doi:10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 39.Koolhaas J. M., Korte S. M., De Boer S. F., Van der Vegt B. J., Van Reenen C. G., Hopster H., De Jong I. C., Ruis M. A. W., Blokhuis H. J. 1999. Coping styles in animals: current status in behavior and stress physiology. Neurosci. Biobehav. Rev. 23, 925–935 10.1016/S0149-7634(99)00026-3 (doi:10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- 40.Minderman J., Reid J. M., Hughes M., Denny M. J. H., Hogg S., Evans P. G. H., Whittingham M. J. 2010. Novel environment exploration and home range size in starlings Sturnus vulgaris. Behav. Ecol. 21, 1321–1329 10.1093/beheco/arq151 (doi:10.1093/beheco/arq151) [DOI] [Google Scholar]
- 41.de Kloet E. 1991. Brain corticosteroid receptor balance and homeostatic control. Front. Neuroendocrinol. 12, 95–164 [DOI] [PubMed] [Google Scholar]
- 42.Müller C., Almasi B., Roulin A., Breuner C. W., Jenni-Eiermann S., Jenni L. 2009. Effects of corticosterone pellets on baseline and stress-induced corticosterone and corticosteroid-binding-globulin. Gen. Comp. Endocrinol. 160, 59–66 10.1016/j.ygcen.2008.10.018 (doi:10.1016/j.ygcen.2008.10.018) [DOI] [PubMed] [Google Scholar]
- 43.de Kloet E. R., Vreugdenhil E., Oitzl M. S., Joels M. 1998. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 19, 269–301 10.1210/er.19.3.269 (doi:10.1210/er.19.3.269) [DOI] [PubMed] [Google Scholar]
- 44.Coppens C. M., de Boer S. F., Koolhaas J. M. 2010. Coping styles and behavioural flexibility: towards underlying mechanisms. Phil. Trans. R. Soc. B 365, 4021–4028 10.1098/rstb.2010.0217 (doi:10.1098/rstb.2010.0217). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koolhaas J. M., De Boer S. F., Coppens C. M., Buwalda B. 2010. Neuroendocrinolgy of coping styles: towards understanding the biology of individual variation. Front. Neuroendocrinol. 31, 307–321 10.1016/j.yfrne.2010.04.001 (doi:10.1016/j.yfrne.2010.04.001) [DOI] [PubMed] [Google Scholar]
- 46.Schrey A., et al. 2011. Broad-scale latitudinal patterns of genetic diversity among native European and introduced house sparrow (Passer domesticus) populations. Mol. Ecol. 20, 1133–1143 10.1111/j.1365-294X.2011.05001.x (doi:10.1111/j.1365-294X.2011.05001.x) [DOI] [PubMed] [Google Scholar]
- 47.Gunnarsson T. G., Sutherland W. J., Alves J. A., Potts P. M., Gill J. A. 2012. Rapid changes in phenotype distribution during range expansion in a migratory bird. Proc. R. Soc. B 279, 411–416 10.1098/rspb.2011.0939 (doi:10.1098/rspb.2011.0939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bakker J. M., van Bel F., Heijnen C. 2001. Neonatal glucocorticoids and the developing brain: short-term treatment with life-long consequences? Trends Neurosci. 24, 649–653 10.1016/S0166-2236(00)01948-2 (doi:10.1016/S0166-2236(00)01948-2) [DOI] [PubMed] [Google Scholar]
- 49.Freire R., van Dort S., Rogers L. J. 2006. Pre- and post-hatching effects of corticosterone treatment on behavior of the domestic chick. Horm. Behav. 49, 157–165 10.1016/j.yhbeh.2005.05.015 (doi:10.1016/j.yhbeh.2005.05.015) [DOI] [PubMed] [Google Scholar]
- 50.Love O. P., Williams T. D. 2008. Plasticity in the adrenocortical response of a free-living vertebrate: the role of pre- and post-natal developmental stress. Horm. Behav. 54, 496–505 10.1016/j.yhbeh.2008.01.006 (doi:10.1016/j.yhbeh.2008.01.006) [DOI] [PubMed] [Google Scholar]
- 51.Meaney M. J. 2001. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 24, 1161–1192 10.1146/annurev.neuro.24.1.1161 (doi:10.1146/annurev.neuro.24.1.1161) [DOI] [PubMed] [Google Scholar]
- 52.Weaver I. C. G., Cervoni N., Champagne F. A., D'Alessio A. C., Sharma S., Seckl J. R., Dymov S., Szyf M., Meaney M. J. 2004. Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854 10.1038/nn1276 (doi:10.1038/nn1276) [DOI] [PubMed] [Google Scholar]
- 53.Cyr N. E., LM R. 2007. Chronic stress in free-living European starlings reduces corticosterone concentrations and reproductive success. Gen. Comp. Endocrinol. 151, 82–89 10.1016/j.ygcen.2006.12.003 (doi:10.1016/j.ygcen.2006.12.003) [DOI] [PubMed] [Google Scholar]
- 54.Banerjee S. B., Arterbery A. S., Fergus D. J., Adkins-Regan E. 2011. Deprivation of maternal care has long-lasting consequences for the hypothalamic–pituitary–adrenal axis of zebra finches. Proc. R. Soc. B 279, 759–766 10.1098/rspb.2011.1265 (doi:10.1098/rspb.2011.1265) [DOI] [PMC free article] [PubMed] [Google Scholar]