Abstract
Tissue loss diseases or white syndromes (WS) are some of the most important coral diseases because they result in significant colony mortality and morbidity, threatening dominant Acroporidae in the Caribbean and Pacific. The causes of WS remain elusive in part because few have examined affected corals at the cellular level. We studied the cellular changes associated with WS over time in a dominant Hawaiian coral, Montipora capitata, and showed that: (i) WS has rapidly progressing (acute) phases mainly associated with ciliates or slowly progressing (chronic) phases mainly associated with helminths or chimeric parasites; (ii) these phases interchanged and waxed and waned; (iii) WS could be a systemic disease associated with chimeric parasitism or a localized disease associated with helminths or ciliates; (iv) corals responded to ciliates mainly with necrosis and to helminths or chimeric parasites with wound repair; (v) mixed infections were uncommon; and (vi) other than cyanobacteria, prokaryotes associated with cell death were not seen. Recognizing potential agents associated with disease at the cellular level and the host response to those agents offers a logical deductive rationale to further explore the role of such agents in the pathogenesis of WS in M. capitata and helps explain manifestation of gross lesions. This approach has broad applicability to the study of the pathogenesis of coral diseases in the field and under experimental settings.
Keywords: Montipora, white syndrome, tissue loss, pathology, disease, pathogenesis
1. Introduction
Coral disease has been responsible for significant declines of corals in the Atlantic [1] and is having an increasing impact in the Pacific [2]. Of the numerous diseases documented in corals [3], perhaps the most damaging and important are those that cause tissue loss (white syndrome, WS). WS is a variably defined lesion [4] involving tissue loss that affects a variety of coral species. Unlike more chronic diseases such as growth anomalies [5] or bleaching [6] where corals may either recover or take many months to die, WS results in rapid loss of tissue biomass. Thus, the effects of WS on corals are immediate and irreversible, as demonstrated demographically on small and large scales in the Pacific [7,8] and in the Atlantic [1,9], where diseases there have extirpated dominant Acropora leading to changes in rugosity and complexity of those ecosystems [10].
The causes of WS in corals are probably multiple, complex and are difficult to confirm. Most studies on WS have used field or laboratory exposure experiments, or molecular or microbiological analyses in attempts to confirm various bacteria as the cause [11–13]. Unfortunately, these approaches shed little light on how bacteria affect corals at the cellular level. Indeed, at the microscopic level, a given lesion of WS may be associated with multiple different micro-organisms such as ciliates [14] or fungi [15], with scant microscopic evidence of bacteria [16,17] associated with cell death. More recently, a newly discovered agent was found associated with WS in Montipora capitata in Hawaii consisting of multicellular structures within the gastrovascular canals (invasive gastrovascular multicellular structures, IGMS) that were genetically most closely related to Montipora flabella and are hypothesized to be chimeric parasites [18].
Disease in animals is an interaction between agent, host and environment. Knowledge of how the coral host and agents associated with WS interact or how disease progresses at the cellular level is scant [7,19]. We used a deductive approach to address the hypothesis that WS is a dynamic non-specific gross lesion with multiple potential aetiologies that change over time and a host response tailored to particular agents. WS is enzootic in M. capitata [7], a dominant coral in Kaneohe Bay, Oahu, Hawaii, and this provided an opportunity to explore the temporal dynamics of this disease using histopathology, a tool that allows both detection of potential causative agents of animal disease and the cellular response of the host to that agent. Understanding host response to potential causative agents of WS at the cellular level is important because it sheds light on mechanisms of how the host and agent interact to cause cell pathology and resultant formation of gross lesions. Our specific objective was to describe the pathogenesis of Montipora white syndrome (MWS) at the gross and cellular level.
2. Methods
(a). Tagging and gross lesions
Between February 2007 and March 2008, we labelled 48 colonies of M. capitata manifesting MWS with individually numbered plastic livestock ear tags in Kaneohe Bay, Oahu, Hawaii. Because insufficient numbers of colonies with MWS were initially available to tag at the study site, we added additional colonies over time. We surveyed the colonies every two to four weeks for a total of 19 monitoring periods. During each survey, we photographed each colony, and gross lesions were classified as multifocal, localized or diffuse based on the lesion type that predominated on the colony. Multifocal MWS manifested multiple distinct variably sized amorphous areas of tissue loss ranging from roughly 1 to 4 cm wide revealing intact bare white skeleton adjacent to normally coloured tissue (figure 1a). Localized MWS manifested as a large solitary amorphous area of tissue loss encompassing less than 25 per cent of the colony revealing intact bare white skeleton indistinctly bordered by pale tissue that gradually transitioned to normal colour with increasing distance from bare skeleton (figure 1b). Diffuse MWS involved large contiguous ill-defined areas of tissue loss encompassing more than 25 per cent of the colony revealing bare intact skeleton with no associated pallor of tissues on the edges (figure 1c).
Figure 1.
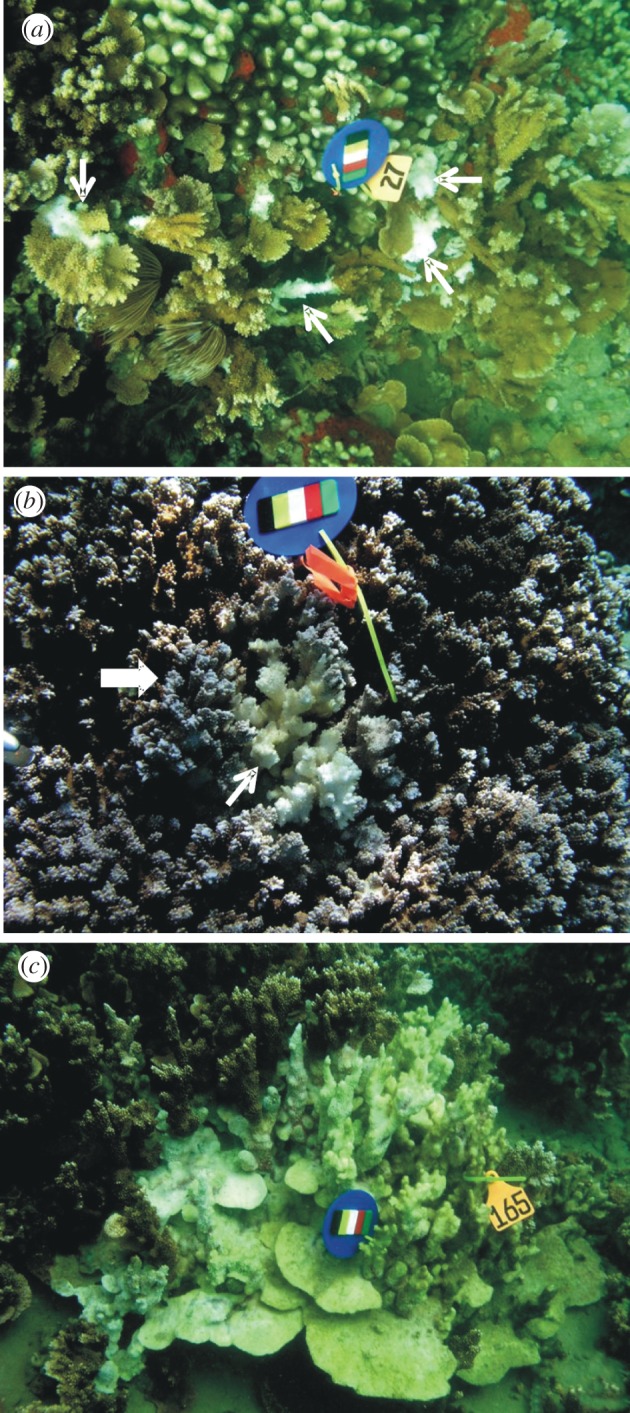
Gross lesions of WS in Montipora capitata. (a) Multifocal; note lesions indicated by white arrows. (b) Locally extensive; note central area of tissue loss revealing bare white skeleton (small white arrow) surrounded by an ill-defined band of pale tissues (block arrow). (c) Diffuse; note extensive area of tissue loss revealing intact bare white skeleton with some green colouring indicating overgrowth of algae. (a–c) Scale bars, 17 cm.
(b). Histology
If lesions of MWS were present on a tagged colony during a given survey, we sampled for histology as described [18]. Briefly, a fragment (approx. 2 × 2 cm) with a lesion and a paired apparently normal fragment from the same colony were collected in sealed plastic bags with seawater, fixed in zinc formaldehyde and processed for histopathology for subsequent microscopic examination. On histology, we classified lesions into two broad categories: (i) host response, including wound repair [20], necrosis, fragmentation or atrophy [17]; and (ii) associated agents, including helminths [21], IGMS [18], ciliates [14], molluscs [22], cyanobacteria [23], sponges [24], fungi [25] and algae [26]. Microscopic lesions were not mutually exclusive. For example, in cases where multiple agents or host responses were seen, the microscopic lesion judged most severe took precedence to facilitate statistical comparisons. To avoid bias, histology was not done until termination of the study, whereupon all slides were read blind and, upon completion, histology findings then reconciled with gross lesions.
(c). Steady state and persistence of lesions
We estimated transition probabilities for (i) type of gross lesion (multifocal, locally extensive, diffuse, no lesion) and (ii) type of organism associated with lesions at the microscopic level (IGMS, ciliates, helminth and ‘other’, representing a pool of diagnoses, each comprising n < 10, including fungi, cyanobacteria, mollusc, snail, algae and sponge). We calculated transition matrices by summing the number of transition types that occurred from one survey to another. For example, for gross lesions, four row (from) and column (to) categories existed (multifocal, locally extensive, diffuse, no lesion) for a total of 16 possible transitions. Numbers of each transition type (multifocal → no lesion, multifocal → multifocal, multifocal → diffuse, etc.) were summed and row totals used to estimate transition probabilities for each transition type (see the electronic supplementary material, table S1). We then used an eigen-analysis of transition matrices to estimate the percentage of expected (steady-state or long-term frequency distribution) observations in each category [27]. Persistence of a particular lesion type i over time was calculated for gross and microscopic lesions by 1 − (pij) days, where pij is the probability of transitioning from type i to type j between survey periods.
(d). Agents over time and mixed infections
To examine temporal trends for agents associated with lesions, we plotted percentage of lesion fragments that had particular agents over time (ciliates, helminths, IGMS, other). For gross lesions associated with agents only, we tabulated frequency distribution of single (e.g. IGMS only) versus multiple (e.g. IGMS and helminth) infections.
(e). Gross lesion type versus microscopic finding
To assess association between lesion type and microscopic finding, locally extensive and diffuse lesions were grouped as ‘acute’ (lesions that based on transition matrix had a less than 50% of remaining the same over time), whereas multifocal were grouped as ‘chronic’ (more than 50% likelihood of persisting as multifocal). We calculated the mean proportion and upper/lower 95% credible intervals of acute and chronic lesions associated with a particular microscopic finding using a multinomial model in WinBUGS and R2WinBUGS [28].
(f). Paired sample analysis
For paired within-colony-lesion/no-lesion samples only, we used logistic regression with fragment as a random effect, with primary agent (IGMS, ciliate or helminth) as a predictor and with fragment state as a response variable (1, lesion; 0, no lesion) using WinBUGS [28] and R2WinBUGS [29]. We then calculated odds ratios of being lesion or no lesion if the coral had a particular primary agent.
(g). Host response and agent
To determine the association between host response and agent, we did a multinomial analysis with host response (necrosis, fragmentation, wound repair) as the response variable and agent (IGMS, helminth, ciliate, other) as the predictor variable [30].
3. Results
(a). Gross lesions
Forty-eight colonies were monitored from 28–389 days with a range of 2–20 monitoring periods with an average of eight fragments collected per colony. There was no evident effect of fragment collection on colonies (sites of collection healed uniformly). Time between surveys ranged from 7 to 67 days because not all colonies were relocated at each survey. By the midpoint of the study, more than 80 per cent of all colonies tagged in the study had been tagged. Over the entire study period, multifocal lesions predominated (80%) followed by localized (13%) and diffuse (7%; table 1).
Table 1.
Number of histological findings (rows) partitioned by each of three types of gross lesion (multifocal, localized and diffuse), and percentage of histology partitioned by acute (n = 156), chronic (n = 40) or both types of lesion.
| histology by gross lesion (no.) |
histology (%) |
|||||
|---|---|---|---|---|---|---|
| histology | multifocal | localized | diffuse | acute (localized and diffuse) | chronic (multifocal) | both (acute and chronic) |
| helminth | 44 | 6 | 4 | 25 | 28 | 28 |
| IGMS | 39 | 0 | 0 | 0 | 25 | 20 |
| repair | 18 | 7 | 1 | 20 | 12 | 13 |
| necrosis | 10 | 2 | 3 | 12.5 | 6 | 8 |
| no lesion | 9 | 3 | 1 | 10 | 6 | 7 |
| ciliates | 6 | 0 | 6 | 15 | 4 | 6 |
| fragmentation | 9 | 3 | 7.5 | 6 | 6 | |
| fungi | 7 | 0 | 0 | 0 | 4 | 4 |
| cyanobacteria | 6 | 0 | 0 | 0 | 4 | 3 |
| mollusc | 4 | 1 | 0 | 2.5 | 3 | 3 |
| atrophy | 1 | 3 | 0 | 7.5 | 1 | 2 |
| algae | 2 | 0 | 0 | 0 | 1 | 1 |
| sponge | 1 | 0 | 0 | 0 | 1 | 1 |
| total | 156 | 25 | 15 | |||
(b). Histology
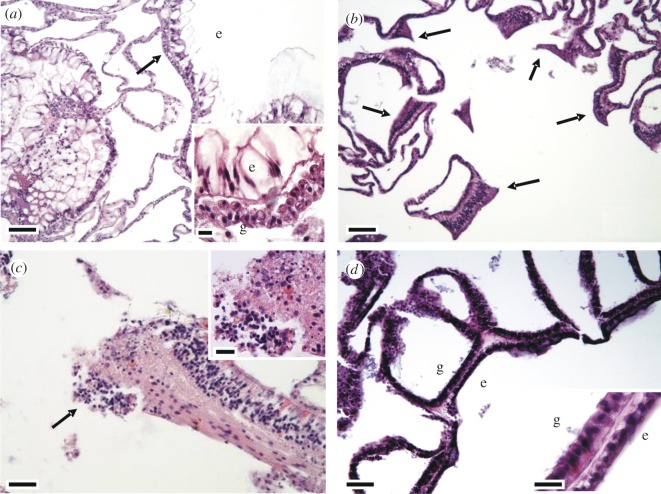
The most common host response was wound repair followed by necrosis and fragmentation (table 1). Compared with normal tissue (figure 2a), wound repair manifested as re-epithelialization of basal body wall with occasional tissue fragmentation (figure 2b), necrosis as clusters of hypereosinophilic debris with karyorrhexis or pyknosis (figure 2c), and atrophy as generalized shrinkage of epidermis and gastrodermis (figure 2d).
Figure 2.
Host response in Montipora capitata affected with WS. (a) Normal tissue; note epidermis (e) and basal body wall (arrow); epidermal cells (e) have prominent mucocytes and gastrodermis (g) is replete with zooxanthellae (inset). (b) Wound repair and fragmentation; note epidermal regeneration of basal body wall (arrows). (c) Necrosis; note fragmentation and hypereosinophilia (arrow) with pyknotic and karyorrhectic nuclei (inset). (d) Atrophy; note marked thinning of epidermis (e) and gastrodermis (g), where epidermis adopts a cuboidal appearance while gastrodermis lacks zooxanthellae (inset); compare with figure 1a (normal tissue). Scale bars: (a,b,d) 30 µm; (c) 20 µm; insets 6 µm.
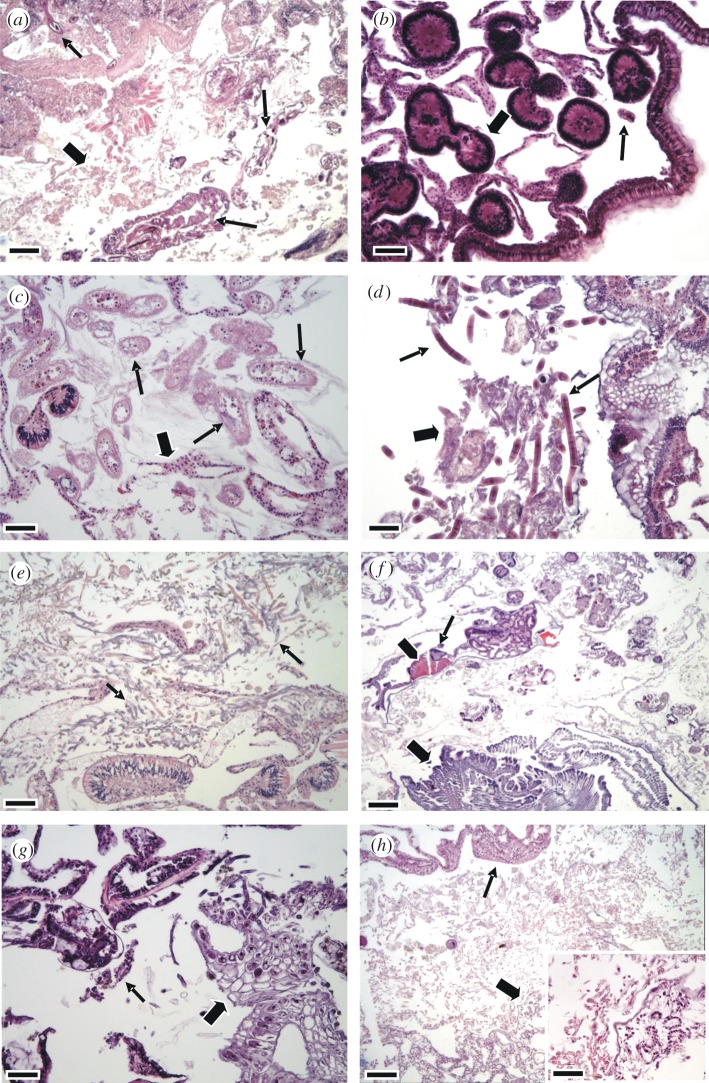
Helminths, IGMS and ciliates were most commonly associated with MWS, whereas fungi, cyanobacteria, molluscs, algae and sponges comprised a minority of associated agents (table 1). Helminth infections were characterized by non-segmented to multisegmented elongate metazoa with a coelom and gut associated with fragmentation or necrosis of coral tissues (figure 3a); IGMS consisted of multicellular structures residing in gastrovascular canals sometimes associated with necrosis or fragmentation of basal body wall (figure 3b); ciliates consisted of allantoid unicellular cilia-covered organisms effacing basal body wall and invading gastrovascular canal associated with necrosis (figure 3c); cyanobacteria consisted of elongated parallel-sided filamentous structures associated with cell death (figure 3d); fungi consisted of irregular, walled septated filamentous branching structures associated with necrosis or fragmentation (figure 3e); molluscs consisted of large metazoans with gills, hepatopancreas, striated muscle, nervous system, eyes and radula (figure 3f); algae consisted of irregular multicellular structures with distinct cell walls (figure 3g); and sponges consisted of a cellular matrix mixed with spicules, zooxanthellae and choanocytes arranged in rosettes (figure 3h).
Figure 3.
Photomicrographs of organisms associated with MWS. (a) Helminth infestation; note two types of helminth (arrows), and tissue fragmentation and necrosis (block arrow). (b) IGMS: note pleomorphic multicellular structures (block arrow) populating gastrovascular canals with associated fragmentation of basal body wall (arrow). (c) Ciliates: note numerous allantoid structures with ciliae (arrow) invading gastrovascular canals and effacing basal body wall, which is occasionally necrotic (block arrow). (d) Cyanobacteria: note parallel walled filamentous striated structures (arrows) associated with necrotic debris (block arrow). (e) Fungi: note mats of irregular-walled septate branching structures (arrows) effacing basal body wall. (f) Mollusc invading coral: note gills (block arrow), striated muscle (arrowhead) and nervous system (arrow). (g) Algae: note structure with cell walls (block arrow) associated with fragmented coral tissues (arrow). (h) Sponge: note multicellular matrix organized into multiple choanocytes arranged in rosettes with spicules (block arrow) separated from necrotic coral tissue (arrow) by mats of fungi. Inset shows detail of sponge choanocytes (lower right) apposed to mats of fungi (upper left). Scale bars: (a–h) 30 µm; inset 20 µm.
(c). Steady state and persistence of lesions
Colonies with lesions that reverted to no lesions tended to persist in that state (see the electronic supplementary material, table S1 and figure S1). Colonies with lesions had a slightly greater tendency to remain with lesions (55% of observations) than revert to no lesion between sampling periods. Localized lesions appeared most transient (probability of remaining a localized lesion from one survey to another was 17%), whereas multifocal lesions persisted in colonies the longest (probability of remaining a multifocal lesion was 51%; electronic supplementary material, table S1). For agents associated with lesions, IGMS and helminth tended to persist the longest, whereas ciliate and ‘other’ were more transient. Transitions from IGMS or helminth to ciliate were never seen (see the electronic supplementary material, table S2 and figure S1).
Steady-state distributions for gross lesions were 0.80, 0.17, 0.01 and 0.007 for no lesion, multifocal, diffuse and localized, respectively. In other words, this would be the expected frequency distribution of types of gross lesion one would expect to see over time when one started with a cohort of corals manifesting tissue loss. Steady-state distributions for microscopic lesions were 0.55, 0.36, 0.08 and 0.01 for IGMS, helminth, other and ciliate, respectively.
(d). Agents over time and mixed infections
IGMS had a peak in prevalence in February, March and July–August, whereas prevalence of helminths appeared to increase over the study period (see the electronic supplementary material, figure S2). Ciliates tended to peak during March and July (see the electronic supplementary material, figure S2). Of 138 lesion fragments with evidence of agents, mixed infections accounted for 38 (27%), with helminth and IGMS predominating in both single and mixed infections (see the electronic supplementary material, table S3).
(e). Gross lesion type versus microscopic finding
A total of 195 coral fragments were identified with localized, diffuse or multifocal lesions. Forty of those were diffuse or locally extensive lesions (acute lesions), and 155 were multifocal lesions (gross lesions). Ninety-five per cent credible intervals (range of values containing 95% of the posterior distribution of the estimate) overlapped for estimated proportions of chronic and acute lesions associated with helminths, fragmentation, ciliates, necrosis, no lesion, repair and other agents. Microscopic changes could not be predicted based on appearance of gross lesions. Twenty-five per cent of gross lesions were associated with IGMS, and there were no IGMS associated with acute lesions (see the electronic supplementary material, figure S3; table 1).
(f). Paired sample analysis
The odds (credible interval) of a fragment with a lesion having helminths was 50 (12.6, 130.6) times greater than a paired non-lesion fragment, 25 (2.6, 112.7) times greater for ciliates and four (1.9, 8.2) times greater for IGMS. In other words, helminths and ciliates tended to be restricted to lesions, whereas IGMS were more widespread in the colony.
(g). Host response and agent
Ciliates were associated only with necrosis. Necrosis, fragmentation and repair responses were approximately equally distributed for IGMS and other agents, whereas helminths were mostly associated with necrosis and wound repair (see the electronic supplementary material, figure S4).
4. Discussion
MWS is not a monotypic static disease but rather has different temporal manifestations that can be deciphered from the appearance of gross lesions. Thus, MWS can be partitioned into a longer lasting chronic state exemplified by multifocal lesions and a more transient (acute) phase exemplified by locally extensive or diffuse lesions. Two pieces of evidence justify such a classification. First, multifocal lesions tended to persist longer than locally extensive or diffuse lesions; second, transition matrix probabilities suggested that multifocal lesions persisted longest, whereas locally extensive and diffuse lesions were more transient. The latter evidence also highlighted that a particular gross lesion can change over time. Ideally, it would have been desirable to have equal monitoring intervals (e.g. every two weeks), but given the logistical limitations of this study, transition probabilities over different time periods (i.e. one week, two weeks, etc.) were treated identically and data pooled across individuals.
Progression of lesions of WS has been documented in epidemiological surveys of disease in Acropora [19] and Montipora [7]; however, no attempts have been made to assign a temporal component to lesions based on gross morphology and probabilities. Incorporating temporal behaviour of gross lesions in future epidemiological surveys may be informative. For example, the predominance of acute lesions (locally extensive or diffuse) on a reef would indicate a recent or rapidly evolving process that has the potential of being more immediately damaging than more slowly progressing lesions such as multifocal tissue loss.
The lack of a consistent association between a particular type of gross lesion and microscopic morphology confirmed previous findings [15,17,31], highlighting the danger of inferring causation of coral disease based on gross appearance of lesions alone [16]. While associations between gross lesion type and microscopic lesions were not significant, the absence of certain aetiologies was notable. In particular, IGMS, algae, cyanobacteria, fungi and sponges were absent in acute lesions, suggesting that these organisms take longer to associate with gross lesions in corals. Transition probabilities (see the electronic supplementary material, table S2) and persistence over time (see the electronic supplementary material, figure S1) suggested that helminths and IGMS tended to persist in lesions the longest, whereas ciliates and other microscopic diagnoses were more transient. Parallels with other animals apply where diseases caused by helminths [32], fungi [33] and algae [34] tend to be chronic, whereas those caused by ciliates have a relatively more rapid course of action [35].
Host response also provides further support for the temporal nature of micro-organisms associated with lesions of MWS. Wound repair in corals is a chronic process taking several weeks [20,36], whereas necrosis and fragmentation of tissues are more rapid and transient [37]. For examples, ciliates were associated only with necrosis, indicating an active invasive process with little opportunity for the host to regenerate tissues. On the other hand, wound repair was more commonly associated with helminths and IGMS, indicating host regeneration concomitant with tissue destruction more suggestive of a chronic active process. Because scar tissue has yet to be documented in Acroporidae [20], an analogous example in animals would be chronic active diseases such as vascular trematodiasis in green turtles [38]. Indeed, the absence of inflammatory cells or other evident changes such as excess density of mucocytes in Montipora affected by MWS indicated this species has a very limited host response repertoire, at least as detectable by light microscopy using haematoxylin and eosin. This contrasts to other species such as Porites sp. and Acropora millepora, which seem to evince an inflammatory response [39].
Save for cyanobacteria, the absence of microscopic evidence of prokaryote or bacterial infections associated with tissue loss was compatible with microscopic morphology of this disease in corals elsewhere [15–17,31]. Indeed, eukaryotes were the predominant organisms associated with lesions, and this contrasts with many studies that have implicated bacteria as a cause of WS in Acroporidae [12,13,40]. Given the paucity of microscopic evidence linking bacteria to lesions at the cellular level in these studies, it is difficult to judge the role that bacteria played in causing disease. The laboratory techniques used here are clearly capable of detecting intracellular [15,41] bacteria and extracellular filamentous cyanobacteria [42], so it is unlikely that these would have been missed. Agar embedding of tissues prior to sectioning and use of fluorescent in situ hybridization have been proposed to enhance detectability of bacteria in corals [43], but bacteria are commonly detected in tissues of other animals without resorting to such techniques [44]. Bacteria associated with tissue loss in other organisms typically cause cell death with microcolonies of bacteria evident at the light microscopy level [37], something not seen here. The disjunct between controlled experimental studies showing bacterial aetiologies of MWS on the one hand and lack of histological evidence of bacterial invasion in lesions on the other merits further investigation.
We saw a variety of organisms associated with MWS that could plausibly be causative and could explain the transmissible behaviour of the disease in the field [7]. A majority (77%) of fragments with gross lesions where organisms were seen were infected with only a single organism; mixed infections comprised a minority of cases. This suggests that these organisms were directly involved with the presence of gross lesions. That said, two other explanations could explain these phenomena. One is that an organism not visible on light microscopy (such as a virus) killed tissues that were then invaded by eukaryotes. However, viruses reproduce and kill cells, typically leading to necrosis [45], and this host response alone in absence of associated visible agents was seen only in 8 per cent of corals. We did not see evidence of inclusions or syncitia [45] that could highlight the presence of viruses, and we did not do electron microscopy, so at this stage, the role of viruses in the pathogenesis of MWS remains speculative.
A second explanation is that corals were somehow immunocompromised, allowing for secondary invaders. Immunologically compromised hosts can at times be susceptible to invasion by single or multiple organisms. For example, humans infected with AIDS often have multiple fungal and bacterial infections [46], and sea turtles with fibropapillomatosis that are immunosuppressed have heavy infections with parasites and bacteria [47,48]. However, tools to understand coral immunology are limited [49]. Controlled empirical laboratory studies will be needed to assess the role of the eukaryotes we found on microscopy in the pathogenesis of MWS and to better understand the process of what actually initiates a lesion in a coral.
Thirty-four per cent of microscopic findings associated with gross lesions were not associated with any organisms and revealed either a host response (27%) or no evident microscopic abnormalities (7%). Either the organisms that caused these lesions came and went or the lesions were hosts response to traumatic events such as predation, or some other unexplained phenomenon. Work & Aeby [17] found that 50 per cent of Acropora sp. with WS (Acropora white syndrome, AWS) had wound repair, necrosis or no lesions and postulated predation by crown of thorns starfish (Acanthaster plancii) as a potential explanation for some of the wound repair. As in this study, helminths, ciliates, sponges and algae were also associated with Acropora WS; however, IGMS were absent. Given our limited understanding of whether (or how) these organism are transmitted between hosts, and what makes certain hosts susceptible, reasons for the differences seen between AWS and MWS are speculative, but could include host specificity, geographical region or seasonal factors.
Infectious diseases in animals can be systemic (affecting multiple organs) or localized (affecting a single organ). Malaria would be an example of a systemic disease [50], whereas cutaneous anthrax [51] would be a localized disease. Corals do not have organs per se, but a similar analogy applies in that infectious agents can be disseminated throughout the colony or restricted to the lesion. A good measure of this would be comparing the strength of association between presence of an organism and a lesion. When paired-lesion versus no-lesion samples were evaluated, helminths were 50 times more likely to be associated with lesions, ciliates 25 times more likely and IGMS only four times more likely. In other words, helminths and ciliates were more likely to be restricted to the lesion (localized), whereas IGMS were almost equally likely to be found in normal versus abnormal tissues (systemic). Past work with IGMS indicates them to be chimeric parasites, probably originating from the host [18], and that would explain their being a systemic disease. Significantly higher numbers of IGMS associated with lesions compared with normal tissues provide compelling evidence of their involvement with MWS, either directly by creating the lesion or indirectly by compromising host health, allowing other pathogen invasion; however, controlled empirical studies will be needed to confirm this.
This study illustrates that careful morphological examination of the development of disease at the microscopic level can shed light on potential causes of lesions in corals and the response that the coral host mounts to these agents. MWS has two behaviours: one where lesions progress and persist slowly over time, and another where lesions proceed rapidly but are more transient. The disease is associated with various eukaryotic organisms, of which IGMS, helminths and ciliates predominate. IGMS and helminths tend to persist over time and are associated with a chronic active host response (wound repair and necrosis), whereas ciliates are more transient and mainly associated with necrosis. Infections with multiple organisms are infrequent, and when they occur, they are dominated by helminths and IGMS, suggesting a potential interaction between them. This is also evident temporally as prevalences of these two organisms have similar patterns. Finally, helminths and ciliates tend to be localized infections, whereas IGMS are more systemic. Applying this methodology to WS in corals from other regions may shed more light on this disease and may reveal commonalities across geographical regions.
Acknowledgements
The authors thank Renee Breeden and Bob Rameyer for technical assistance. This study was funded in part by the US Geological Survey and the Hawaii Coral Reef Initiative. All collections were done under a State of Hawaii Department of Land and Natural Resources collecting permit issued to the Hawaii Institute of Marine Biology. Mention of products or trade names does not imply endorsement by the US Government.
References
- 1.Porter J., et al. 2002. Detection of coral reef change by the Florida Keys coral reef monitoring project. In The everglades, Florida Bay, and coral reefs of the Florida keys an ecosystem sourcebook (eds Porter J., Porter K.), pp. 749–769 Boca Raton, FL: CRC Press. [Google Scholar]
- 2.Willis B. L., Page C. A., Dinsdale E. A. 2004. Coral disease on the Great Barrier Reef. In Coral health and disease (eds Loya Y., Rosenberg E.), pp. 69–104 Heidelberg, Germany: Springer [Google Scholar]
- 3.Sutherland K. P., Porter J. W., Torres C. 2003. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Prog. Ser. 266, 273–302 10.3354/meps266273 (doi:10.3354/meps266273) [DOI] [Google Scholar]
- 4.Lindop A. M. M., Hind E. J., Bythell J. C. 2008. The unknowns in coral disease identification: an experiment to assess consensus of opinion amongst experts. In Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, Florida, 7–11 July, pp. 190–196 See www.nova.edu/ncri/11icrs/proceedings/files/m07-04.pdf [Google Scholar]
- 5.Yamashiro H., Yamamoto M., van Woesik R. 2000. Tumor formation on the coral Montipora informis. Dis. Aquat. Org. 41, 211–217 10.3354/dao041211 (doi:10.3354/dao041211) [DOI] [PubMed] [Google Scholar]
- 6.Brown B. E., Le Tissier M. D. A., Bythell J. C. 1995. Mechanisms of bleaching deduced from histological studies of reef corals sampled during a natural bleaching event. Mar. Biol. 122, 655–663 10.1007/BF00350687 (doi:10.1007/BF00350687) [DOI] [Google Scholar]
- 7.Aeby G. S., Ross M., Williams G. J., Lewis T. D., Work T. M. 2010. Disease dynamics of Montipora white syndrome within Kaneohe Bay, Oahu, Hawaii: distribution, seasonality, virulence, and transmissibility. Dis. Aquat. Org. 91, 1–8 10.3354/dao02247 (doi:10.3354/dao02247) [DOI] [PubMed] [Google Scholar]
- 8.Bruno J., Selig E., Casey K., Page C., Willis B., Harvell C., Sweatman H., Melendy A. 2007. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. 5, 1–8 10.1371/journal.pbio.0050001 (doi:10.1371/journal.pbio.0050001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson K. L., Porter J. W., Ritchie K. B., Polson S. W., Mueller E., Peters E. C., Santavy D. L., Smith G. W. 2002. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc. Natl Acad. Sci. USA 99, 8725–8730 10.1073/pnas.092260099 (doi:10.1073/pnas.092260099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronson R. B., Precht W. F. 2001. White-band disease and the changing face of Caribbean coral reefs. Hydrobiology 460, 25–38 10.1023/A:1013103928980 (doi:10.1023/A:1013103928980) [DOI] [Google Scholar]
- 11.Luna G. M., Bongiorni L., Gili C., Biavasco F. D., Danovaro R. 2010. Vibrio harveyi as a causative agent of the White Syndrome in tropical stony coral. Environ. Microbiol. Rep. 2, 120–127 10.1111/j.1758-2229.2009.00114.x (doi:10.1111/j.1758-2229.2009.00114.x) [DOI] [PubMed] [Google Scholar]
- 12.Sussman M., Willis B. L., Victor S., Bourne D. G. 2008. Coral pathogens identified for white syndrome (WS) epizootics in the Indo-Pacific. PLoS ONE 3, e2393. 10.1371/journal.pone.0002393 (doi:10.1371/journal.pone.0002393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutherland K. P. S., Shaban S., Joyner J. L., Porter J. W., Lipp E. K. 2011. Human pathogen shown to cause disease in the threatened eklhorn coral Acropora palmata. PLoS ONE 6, E23468. 10.1371/journal.pone.0023468 (doi:10.1371/journal.pone.0023468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourne D. G., Boyett H. V., Henderson M. E., Muirhead A., Willis B. L. 2008. Identification of a ciliate (Oligohymenophorea: Scuticociliatia) associated with brown band disease on corals of the Great Barrier Reef. Appl. Environ. Microbiol. 74, 883–888 10.1128/AEM.01124-07 (doi:10.1128/AEM.01124-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Work T. M., Rameyer R. A. 2005. Characterizing lesions in corals from American Samoa. Coral Reefs 24, 384–390 10.1007/s00338-005-0018-0 (doi:10.1007/s00338-005-0018-0) [DOI] [Google Scholar]
- 16.Ainsworth T. D., Kvennefors E. C., Blackall L., Fine M., Hoegh-Guldberg O. 2006. Disease and cell death in white syndrome of Acroporid corals on the Great Barrier Reef. Mar. Biol. 151, 19–29 10.1007/s00227-006-0449-3 (doi:10.1007/s00227-006-0449-3) [DOI] [Google Scholar]
- 17.Work T. M., Aeby G. S. 2011. Pathology of tissue loss (white syndrome) in Acropora sp. corals from the Central Pacific. J. Invert. Pathol. 107, 127–131 10.1016/j.jip.2011.03.009 (doi:10.1016/j.jip.2011.03.009) [DOI] [PubMed] [Google Scholar]
- 18.Work T. M., Forsman Z. H., Szabó Z., Lewis T. D., Aeby G. S., Toonen R. J. 2011. Inter-specific coral chimerism: genetically distinct multicellular structures associated with tissue loss in Montipora capitata. PLoS ONE 6, e2869. 10.1371/journal.pone.0022869 (doi:10.1371/journal.pone.0022869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roff G., Kvennefors E. C. E., Fine M., Ortiz J., Davy J. E., Hoegh-Guldberg O. 2011. The ecology of ‘acroporid white syndrome’, a coral disease from the southern Great Barrier Reef. PLoS ONE 6, e26829. 10.1371/journal.pone.0026829 (doi:10.1371/journal.pone.0026829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Work T. M., Aeby G. S. 2010. Wound repair in Montipora capitata. J. Invert. Pathol. 105, 116–119 10.1016/j.jip.2010.05.009 (doi:10.1016/j.jip.2010.05.009) [DOI] [PubMed] [Google Scholar]
- 21.Hyman L. H. 1940. Platyhelminthes and Rhynchocoela, the acoelomate Bilateria. New York, NY: McGraw-Hill [Google Scholar]
- 22.Ruppert A. E., Fox R. S., Barnes R. D. 2004. Invertebrate zoolog y. Belmont, CA: BrooksCole [Google Scholar]
- 23.Stanier R. J., Cohen-Bazire G. 1977. Phototropic prokaryotes: the cyanobacteria. Annu. Rev. Microbiol. 31, 225–274 10.1146/annurev.mi.31.100177.001301 (doi:10.1146/annurev.mi.31.100177.001301) [DOI] [PubMed] [Google Scholar]
- 24.Hyman L. H. 1940. The invertebrates: Protozoa through Ctenophora, p. 726 New York, NY: McGraw-Hill [Google Scholar]
- 25.Larone D. H. 1976. Medically important fungi: a guide to identification, p. 417 Washington, DC: American Society for Microbiology [Google Scholar]
- 26.McCook L. J., Jompa J., Diaz-Pulido G. 2001. Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 19, 400–417 10.1007/s003380000129 (doi:10.1007/s003380000129) [DOI] [Google Scholar]
- 27.Horn R. A., Johnson C. F. 1985. Matrix analysis, p. 561 Cambridge, UK: Cambridge University Press [Google Scholar]
- 28.Gilks W. R., Thomas A., Spiegelhalter D. J. 1994. A language and program for complex Bayesian modelling. The Statistician 43, 169–178 10.2307/2348941 (doi:10.2307/2348941) [DOI] [Google Scholar]
- 29.Sturtz S., Ligges U., Gelman A. 2005. R2WinBUGS: a package for running WinBUGS from R. J. Stat. Soft. 12, 1–16 [Google Scholar]
- 30.Hosmer D. W., Lemeshow S. 2000. Applied logistic regression, p. 375 New York, NY: Wiley [Google Scholar]
- 31.Williams G. J., Work T. M., Aeby G. S., Knapp I. S., Davy S. K. 2010. Gross and microscopic morphology of lesions in Cnidaria from Palmyra Atoll, Central Pacific. J. Invert. Pathol. 106, 165–170 10.1016/j.jip.2010.08.002 (doi:10.1016/j.jip.2010.08.002) [DOI] [PubMed] [Google Scholar]
- 32.Damian R. T., Greene N. D., Meyer K. F., Cheever A. W., Hubbard W. J., Hawes M. E., Clark J. D. 1976. Schistosoma mansoni in baboons. III. The course and characteristics of infection with additional observations on immunity. Am. J. Trop. Med. Hyg. 25, 299–306 [PubMed] [Google Scholar]
- 33.Pare J. A. 2003. Fungal diseases of amphibians: an overview. Vet. Clin. North Am. Exot. Anim. Pract. 6, 315–326 10.1016/S1094-9194(03)00006-9 (doi:10.1016/S1094-9194(03)00006-9) [DOI] [PubMed] [Google Scholar]
- 34.Hollingsworth S. R. 2000. Canine protothecosis. Vet. Clin. North Am. Sm Anim. Pract. 30, 1091–1101 [DOI] [PubMed] [Google Scholar]
- 35.Morado J. F., Small E. B. 1995. Ciliate parasites and related diseases of Crustacea: a review. Rev. Fish. Sci. 3, 275–354 10.1080/10641269509388575 (doi:10.1080/10641269509388575) [DOI] [Google Scholar]
- 36.Lester R. T., Bak R. P. M. 1985. Effects of environment on regeneration rate of tissue lesions in the reef coral Montastrea annularis (Scleractinia). Mar. Ecol. Prog. Ser. 24, 183–185 10.3354/meps024183 (doi:10.3354/meps024183) [DOI] [Google Scholar]
- 37.Cheville N. F. 1988. Introduction to veterinary pathology. Ames, IA: Iowa State University Press [Google Scholar]
- 38.Santoro M., Morales J. A., Rodríguez-Ortíz B. 2007. Spirorchiidiosis (Digenea: Spirorchiidae) and lesions associated with parasites in Caribbean green turtles (Chelonia mydas). Vet. Rec. 161, 482–486 10.1136/vr.161.14.482 (doi:10.1136/vr.161.14.482) [DOI] [PubMed] [Google Scholar]
- 39.Palmer C. V., Mydlarz L. D., Willis B. L. 2008. Evidence of an inflammatory-like response in non-normally pigmented tissues of two scleractinian corals. Proc. R. Soc. B 275, 2687–2693 10.1098/rspb.2008.0335 (doi:10.1098/rspb.2008.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kline D. I., Vollmer S. V. 2011. White band disease (type I) of endangered Caribbean Acroporid corals is caused by pathogenic bacteria. Sci. Rep. 1, 1–5 10.1038/srep00007 (doi:10.1038/srep00007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters E. C., Oprandy J. J., Yevich P. P. 1983. Possible causal agent of ‘white band disease’ in Caribbean Acroporid corals. J. Invert. Pathol. 41, 394–396 10.1016/0022-2011(83)90260-4 (doi:10.1016/0022-2011(83)90260-4) [DOI] [Google Scholar]
- 42.Sato Y., Willis B. L., Bourne D. G. 2010. Successional changes in bacterial communities during the development of black band disease on the reef coral, Montipora hispida. ISME J. 4, 203–214 10.1038/ismej.2009.103 (doi:10.1038/ismej.2009.103) [DOI] [PubMed] [Google Scholar]
- 43.Bythell J. C., Barer M. R., Cooney R. P., Guest J. R., O'Donnell A. G., Pantos O., Le Tisser M. D. A. 2002. Histopathological methods for the investigation of microbial communities associated with disease lesions in reef corals. Lett. Appl. Microbiol. 34, 359–364 10.1046/j.1472-765X.2002.01097.x (doi:10.1046/j.1472-765X.2002.01097.x) [DOI] [PubMed] [Google Scholar]
- 44.Egidius E. 1987. Vibriosis: pathogenicity and pathology. A review. Aquaculture 67, 15–28 10.1016/0044-8486(87)90004-4 (doi:10.1016/0044-8486(87)90004-4) [DOI] [Google Scholar]
- 45.Cheville N. F. 1976. Cell pathology, p. 681 Ames, IA: Iowa State University Press [Google Scholar]
- 46.Eliezerb M., DeTeresa R. M., Mallory M. E., Hansen L. A. 2000. Changes in pathological findings at autopsy in AIDS cases for the last 15 years. AIDS 14, 69–74 10.1097/00002030-200001070-00008 (doi:10.1097/00002030-200001070-00008) [DOI] [PubMed] [Google Scholar]
- 47.Aguirre A. A., Spraker T. R., Balazs G. H., Zimmerman B. 1998. Spirorchidiasis and fibropapillomatosis in green turtles from the Hawaiian islands. J. Wildl. Dis. 34, 91–98 [DOI] [PubMed] [Google Scholar]
- 48.Work T. M., Balazs G. H., Wolcott M., Morris R. M. 2003. Bacteraemia in Hawaiian green turtles, Chelonia mydas, with fibropapillomatosis. Dis. Aquat. Org. 53, 41–46 10.3354/dao053041 (doi:10.3354/dao053041) [DOI] [PubMed] [Google Scholar]
- 49.Mydlarz L. D., Jones L. E., Harvell C. D. 2006. Innate immunity, environmental drivers, and disease ecology of marine and freshwater invertebrates. Annu. Rev. Ecol. Evol. Syst. 37, 251–288 10.1146/annurev.ecolsys.37.091305.110103 (doi:10.1146/annurev.ecolsys.37.091305.110103) [DOI] [Google Scholar]
- 50.Anstey N. M., Russell B., Yeo T. W., Price R. N. 2009. The pathophysiology of vivax malaria. Trends Parasitol. 25, 220–227 10.1016/j.pt.2009.02.003 (doi:10.1016/j.pt.2009.02.003) [DOI] [PubMed] [Google Scholar]
- 51.Roche K. J., Chang M. W., Lazarus H. 2001. Cutaneous anthrax infection. New Engl. J. Med. 345, 1611. 10.1056/NEJMicm010777 (doi:10.1056/NEJMicm010777) [DOI] [PubMed] [Google Scholar]