Abstract
Background
The molecular profile of peritumoral non-neoplastic liver parenchyma (PNLP) has recently been suggested as predictive factor of early and late recurrence of hepatocellular carcinoma (HCC). However, there is no definite cut-off point for tumor-free PNLP in terms of either histological or molecular changes. Therefore, our aim is to determine the numerical cut-off point for separating adjacent PNLP and remote PNLP in histopathologic perspective.
Methods
Peritumoral tissues from 20 resected HCC patients were sampled from 0 to 40 mm distance from the tumor border (divided into 5-mm columns). Histopathologic parameters such as necroinflammatory activity, fibrosis, bile ductular reaction, hepatic venulitis, peliosis, and steatosis were compared between each column.
Results
The morphologic changes just adjacent to the tumor were notably severe and faded with distance. The parenchyma within 10 mm of the tumor showed significantly severe inflammation, fibrosis, peliosis and hepatic venulitis compared with those from farther areas. The histopathologic changes of the parenchyma became stable beyond 20 mm.
Conclusions
Results of this study revealed that the parenchyma within 10 mm distance from the tumor, or adjacent PNLP, has histopathologic changes that are directly affected by the tumor, and the parenchyma beyond 20 mm as the remote PNLP without tumor effect.
Keywords: Nonneoplastic liver parenchyma; Carcinoma, hepatocellular; Hepatitis B, chronic
Hepatitis B virus (HBV) is the most common cause of chronic liver disease in South Korea and is one of the major risk factors of hepatocellular carcinoma (HCC) worldwide. Although various radiologic techniques for early detection and medical or surgical methods have successfully controlled the primary tumor, HCC still shows unsatisfactory long-term prognosis after surgical resection probably due to high recurrence rate.1 Most studies reported greater than 70% recurrence rate within a 5-year period. This recurrence, rather than the underlying cirrhosis, is the major cause of death.2-4 Hence, many investigations have studied risk factors for HCC recurrence after curative resection. Clinicopathologic characteristics of HCC such as micrometastasis, vascular invasion, and positive resection margin are well-known risk factors for tumor recurrence.5 Recently, peritumoral non-neoplastic liver parenchyma (PNLP) has been suggested to be an important predictive marker of HCC recurrence after surgical resection.6-8
PNLP can be defined as hepatic parenchyma without histologic evidence of tumors. However, in patients with chronic liver disease, PNLP has two different levels; the first is adjacent parenchyma that is affected by tumors and the second one is remote parenchyma that is not affected by primary tumors. Adjacent PNLP is claimed to be influenced by mass effect of tumor proper and several tumor-producing factors via paracrine or autocrine manners, indirectly depicting the trait of tumors. Meanwhile, remote PNLP may express the patient's underlying chronic liver disease and indirectly the trait of tumorigenesis, implying new occurrence of HCCs. It is therefore important to determine the exact distance of adjacent PNLP that is directly influenced by the tumor, which differs from remote PNLP, the background liver parenchyma. However, published reports lack consistency on the precise distance that defines adjacent PNLP. Some authors used the farthest non-cancerous liver parenchyma in the entire surgical specimen.9 However, there were some other studies that have limited specimens to a distance of 10 mm from the tumor,10 while others have simply made use of the peritumoral liver tissue that was available at the time of the study.11
In this study, we sought the pathologic characteristics of PNLP of HCCs in chronic HBV patients in order to suggest a reasonable cut-off point between adjacent and remote PNLP from the histologic view point.
MATERIALS AND METHODS
Patient characteristics
For this study, we selected 20 patients at Seoul National University Hospital in South Korea who had undergone hepatic resection due to HCC from July to September, 2010. These patients have clinical evidence of chronic hepatitis B, such as serologic evidence of virus, more than 6 months of abnormal liver function, or radiologic evidence of hepatic fibrosis. There were 2 female and 18 male patients with ages ranging from 48-73 years (with a median age of 58). All patients had curative surgical resection; 3 cases required liver transplantation and 17 needed partial resection. None of them underwent preoperative local treatment such as transarterial embolization, radiofrequency ablation and percutaneous ethanol injection therapy, nor preoperative systemic chemotherapy. There was one hepatitis C virus coinfection and one concomitant alcohol-related steatohepatitis with chronic hepatitis B.
Sampling of nonneoplastic liver tissue
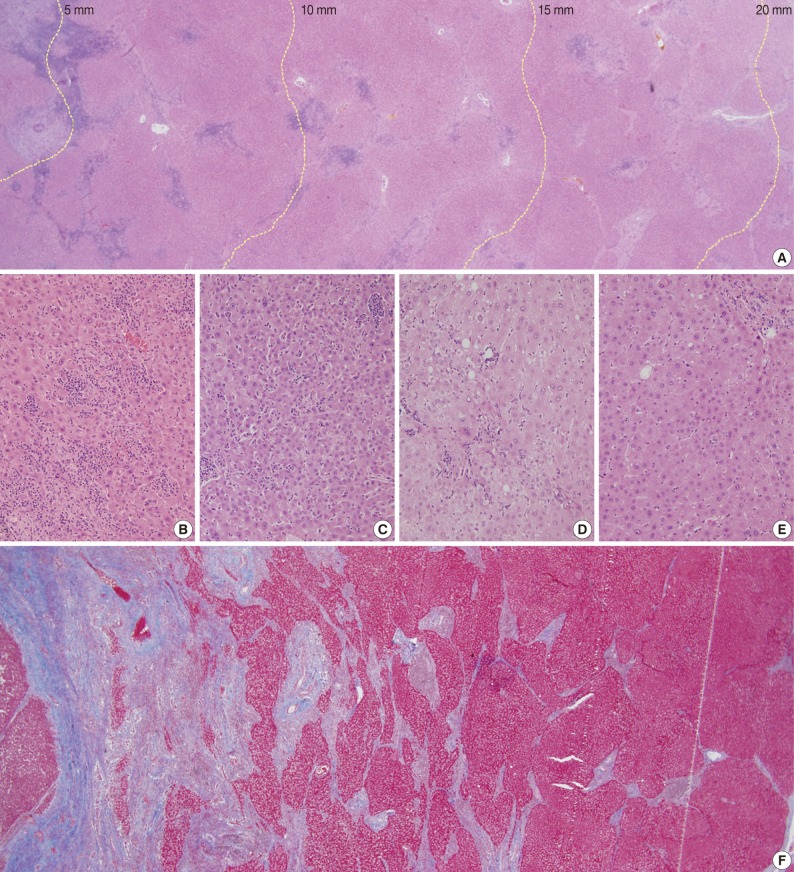
Peritumoral tissues from each fresh liver specimen were sampled from the tumor border to a distance of 40 mm from the tumor. They were fixed with 10% formalin and embedded in paraffin en bloc. Routine hematoxylin and eosin (H&E) and Masson's trichrome (M-T) stained slides were made as previously described.12,13 The adjacent PNLP was divided into eight 5 mm-sized columns, starting from the tumor border to a 40 mm distance from the tumor (Fig. 1A). The 5-mm column represents adjacent PNLP located between the tumor border and 5 mm distance from the tumor. The 10-, 15-, 20-, 25-, 30-, 35-, and 40-mm columns represent adjacent PNLP between 5 and 10 mm, 10 and 15 mm, 15 and 20 mm, 20 and 25 mm, 25 and 30 mm, 30 and 35 mm, and 35 and 40 mm from the tumor, respectively. The mean magnitude of each column was 15 mm (width)×5 mm (length), and median number of portal tracts per column was 14 (range, 3 to 17). For comparison, background liver or remote PNLP was also taken farthermost from the tumor (or at least >40 mm from the tumor) and treated similarly as the adjacent PNLP.
Fig. 1.
Necroinflammatory activity and fibrosis of adjacent peritumoral non-neoplastic liver parenchyma (PNLP) showing (A) division of PNLP with distance from tumor border from the left to right side (scanning view), (B-E) representative pictures of spotty necrosis in each 5-mm column (B, 5-mm; C, 10-mm; D, 15-mm; E, 20-mm), (F) fibrosis of PNLP and segmentation blocks for image analysis of collagen deposition (Masson's trichrome [M-T] stain, scanning view).
Grading and staging for histopathologic parameters
Parameters such as necroinflammatory activity of the hepatic parenchyma, fibrosis, bile ductular reaction, peliosis, perivenular inflammation, and steatosis were assessed by two pathologists (K.B. Lee and H.Y. Jung) after consensus meeting. A modified histologic activity index (HAI) was applied as a grading system for necroinflammatory activity.14,15 Spotty necrosis, periportal/periseptal interface hepatitis (piecemeal necrosis), and portal inflammation were scored from 0 to 4 and confluent necrosis from 0 to 6. The total modified HAI score is the sum of all of these scores (expected maximum score, 18). In addition, the number of foci of spotty necrosis per 10× objective was counted at the most severely affected area.
Fibrosis or cirrhosis was evaluated in 2 different ways. The first was scoring both H&E- and M-T-stained sections (by light microscopy) from 0 to 6 according to the Ishak fibrosis staging system.15 In addition, collagen deposition shown by M-T staining was converted into a pixel count for an objective evaluation of fibrosis/cirrhosis. Images of each column were taken using an Olympus optical microscope (BX50, Olympus, Tokyo, Japan) with 4× objective and converted into JPEG format for analysis. We divided each column as in Fig. 1F to include the majority of the samples so that we could exclude any selection bias. All images (having mean image size of 15.98 mm2) were analyzed by image J software (positive color: R, 0.8748878; G, 0.457825 65; B, 0.15829495; threshold: 0 to 290). The ratio of collagen-positive pixel count to total pixel count was expressed in percentage terms.
We defined peliosis-like lesions as blood-filled spaces, with or without sinusoidal lining, recognized with the 4× objective. The number of lesions per 4× objective field was counted for each column. The inflammatory activity around the central vein or the so-called hepatic venulitis was scored from 0 to 4 (0, absent; 1, inflammatory cells accumulation in the lumen of hepatic venule; 2, focal inflammatory cells infiltration through the vascular wall; 3, continuous infiltration of infilammatory cells around the vascular wall; and 4, fibrinoid necrosis of the vascular wall). Bile ductular proliferation was scored from 0 to 3 (0, absent; 1, mild; 2, moderate; 3, severe; or graded as <33, 33-66%, and >66% of the hepatic lobule by bile ductular proliferation, respectively). Steatosis was graded 0 to 3 based on the percentage of hepatocytes in the biopsy (0, none; 1, up to 33%; 2, 33-66%; and 3, >66%), which is adopted from the nonalcoholic steatohepatitis clinical research network scoring system.16,17
Statistical analysis
The Wilcoxon signed-rank test was used to compare pathologic characteristics of each column with those of adjacent columns, as well as remote parenchyma. Tests having p-value less than 0.05 was considered as results with significant difference.
RESULTS
Necroinflammatory activity in adjacent peritumoral hepatic parenchyma
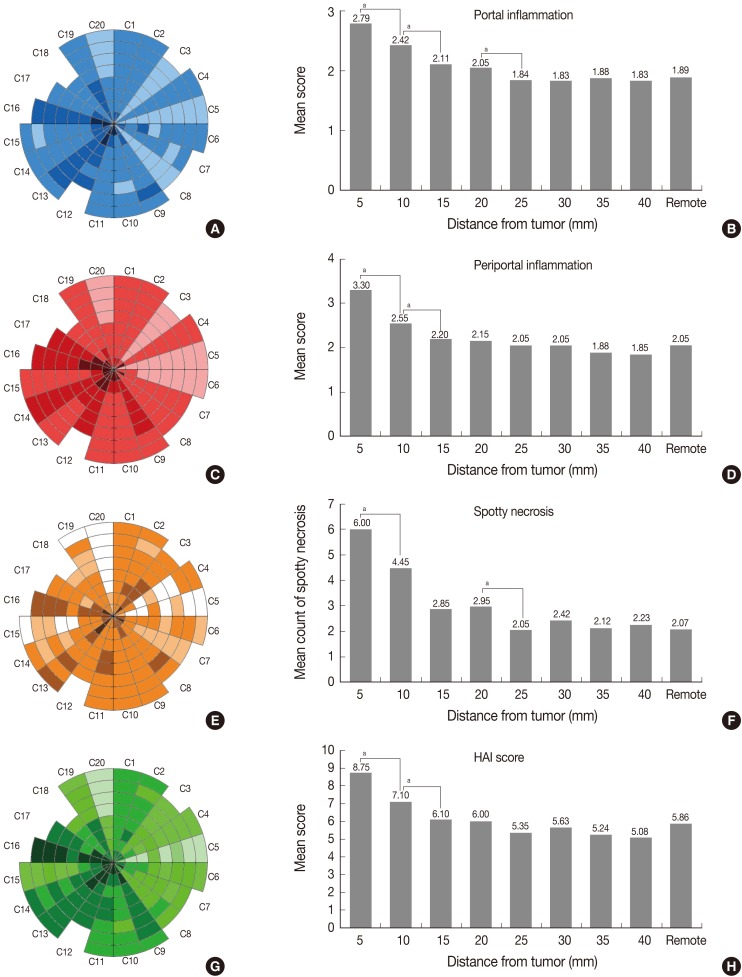
Dense inflammatory cell infiltration in the portal tracts adjacent to the tumor, which also lessened with distance, were observed as shown in Fig. 1A. Likewise, spotty necrosis was frequently observed near the tumor as in Fig. 1B-D. The lobular activities in the 5-mm (Fig. 1B) and 10-mm (Fig. 1C) columns were notably more severe than those in the 15-mm (Fig. 1D) and 20-mm (Fig. 1E) columns. The severity of portal, periportal, and lobular activity scores in adjacent PNLP were illustrated for all cases in concentric graphs by color gradation and in bar graphs by mean of scores (Fig. 2A, C, E, G). The dark-colored centers of circular graphs indicate severe necroinflammatory activity near the tumor. The plotted mean value of the necroinflammatory activity as bar graph demonstrates a pattern of decreasing inflammatory cell infiltration in adjacent PNLP (Fig. 2B, D, F, H). The inflammation adjacent to the tumor was notably severe, but decreased with distance. However, at certain distances, the inflammation became stable and no longer differed from that of remote PNLP.
Fig. 2.
Necroinflammatory activity of adjacent peritumoral non-neoplastic liver parenchymas howing (A, B) portal inflammation, (C, D) periportal inflammation, (E, F) spotty necrosis (all based on semiquantitative four grade system), and (G, H) the modified histologic activity index (HAI) grading. The center of the circle represents the tumor border, and each concentric circle line indicates an increment of 5 mm from the tumor. C1-C20 indicate each case (C1=case 1). ap-value of Wilcoxon signed-rank test between two adjacent columns <0.05.
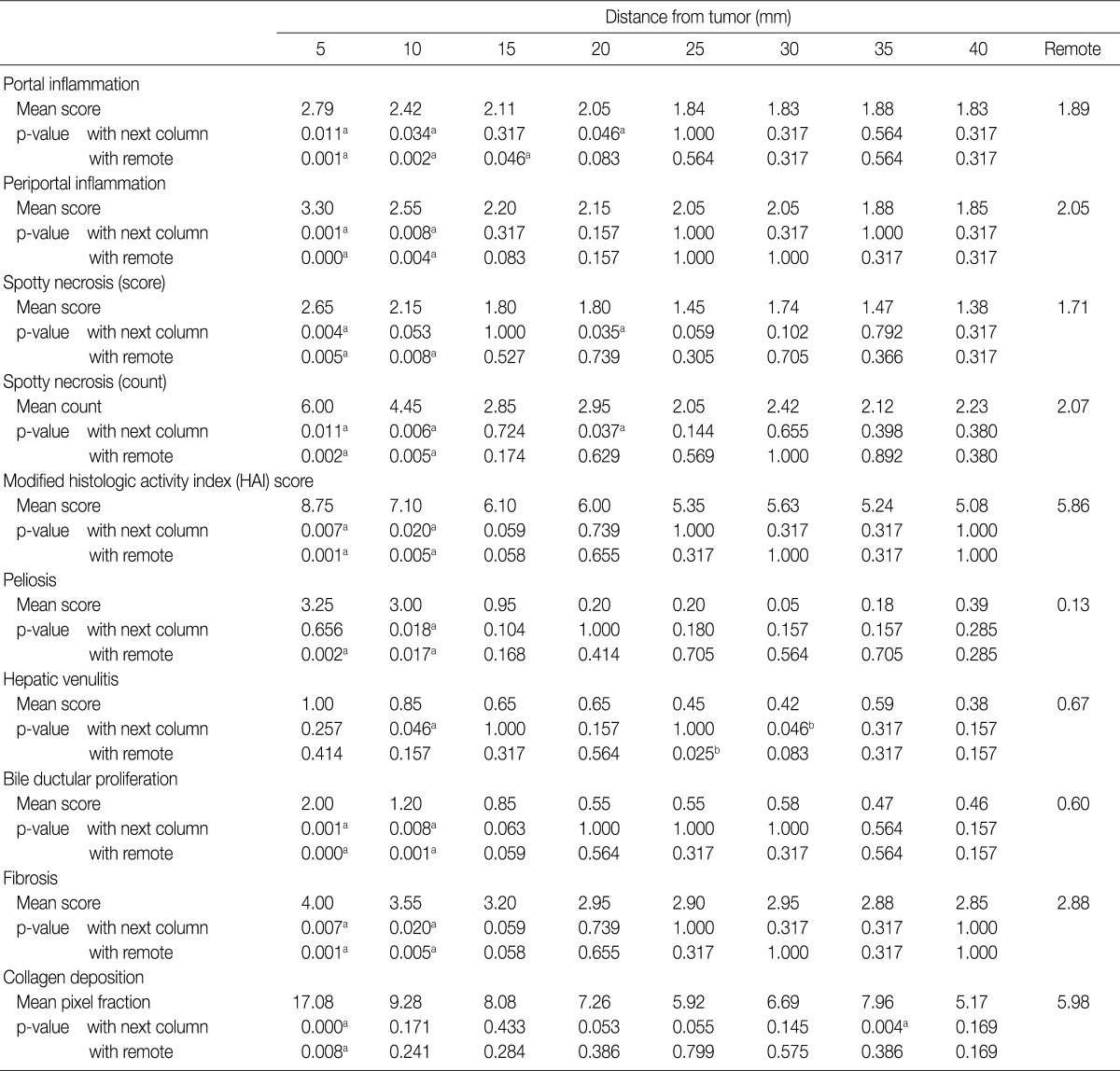
Moreover, the necroinflammatory activity in each column was compared using Wilcoxon signed-rank test (Table 1). Portal inflammation was markedly severe in the 5-mm column (mean score, 2.79), and decreased with distance until the 25-mm column (mean score, 1.84) (p=0.011 between 5-mm and 10-mm column; p=0.034 between 10-mm and 15-mm column; p=0.046 between 20-mm and 25-mm column). There was no discernible difference of portal inflammation from the 25-mm column. Therefore, in comparison to adjacent PNLP, the considerable cut-off point for portal inflammation could be 20 mm distance from the tumor.
Table 1.
The mean values of the pathologic parameters for each column and the p-values for the differences between columns
ap<0.05; the grade of this column is higher than the next column or the remote parenchyma; bp<0.05; the grade of this column is lower than the next column or the remote parenchyma.
Likewise, the foci of spotty necrosis were observed most frequently in the 5-mm column (mean score, 2.65; mean count, 6.0) and decreased with distance until 25-mm column (mean score, 1.45; mean count, 2.05). Both the score and the frequency of spotty necrosis did not change significantly beyond the 20 mm distance from the tumor.
On the other hand, periportal inflammation were markedly severe in the 5-mm column (mean score, 3.30) and significantly decreased in the 10-mm (mean score, 2.55; p=0.001) and 15-mm column (mean score, 2.20; p=0.008). However, it did not differ beyond 10 mm distance from the tumor.
The modified HAI, which is the sum of scores for portal, periportal inflammation and spotty necrosis, was markedly severe in the 5-mm column (mean score, 8.75) and decreased until 15-mm column (mean score, 6.10), while it did not significantly change beyond 10 mm distance from the tumor.
Peliosis and perivenular inflammation in adjacent peritumoral hepatic parenchyma
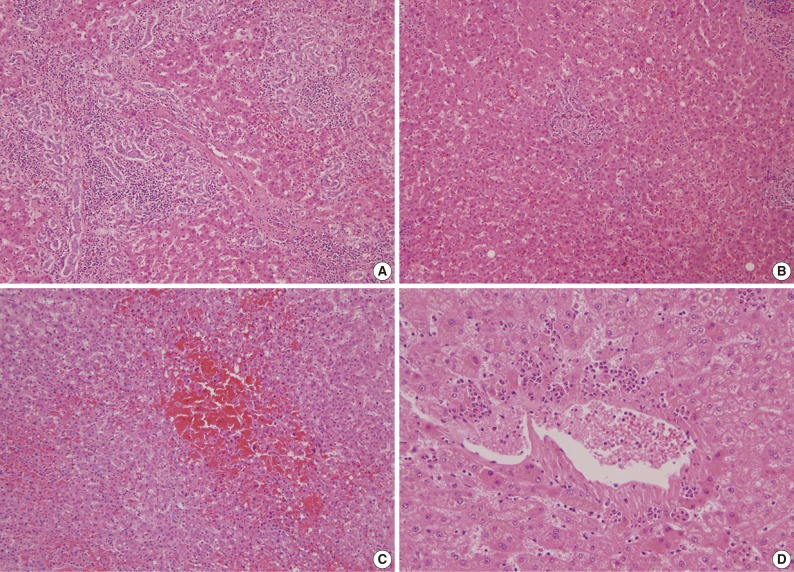
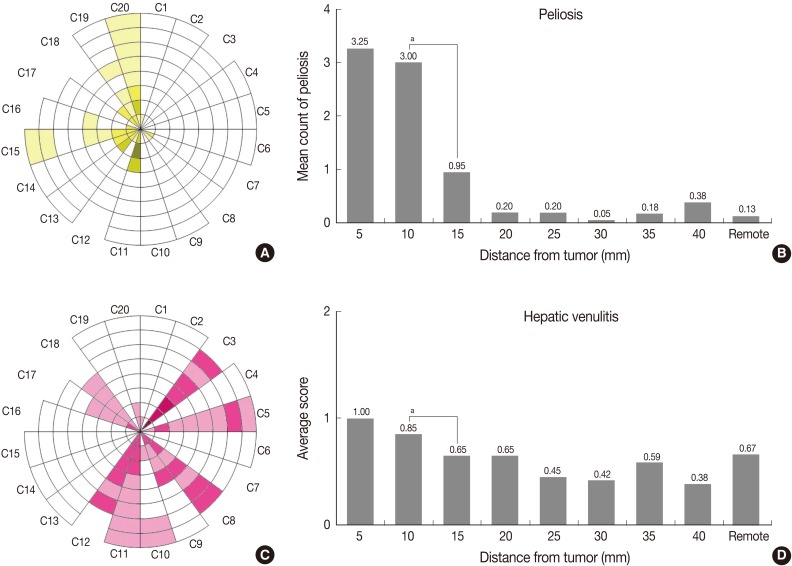
Microscopically, we found 9 cases of peliosis in adjacent PNLP. The lesions were irregularly dilated sinusoidal spaces filled with blood and fibrinoid material accompanied by damaged hepatocytes (Fig. 3C). The lesions were more frequent and extensive around the tumor (Fig. 4A, B), especially starting from the tumor border towards the 10-mm column, while nearly no peliosis was found beyond the 10-mm column, denoted by a p-value of 0.018 between 10-mm and 15-mm column (Table 1).
Fig. 3.
Bile ductular proliferation, peliosis-like lesions, and heptic venulitis in (A) 5-mm column, (B) 15-mm column, (C) 5-mm column, (D) 5-mm column.
Fig. 4.
Peliosis and hepatic venulitis. (A, B) The number of peliosis-like lesions per 4× objective field. (C, D) Hepatic venulitis. ap-value of Wilcoxon signed-rank test between two adjacent columns <0.05.
Apart from the usual occurrence of chronic viral hepatitis and neutrophic infiltration around the central veins, 10 cases of perivenular inflammation was observed in adjacent PNLP. A few cases were accompanied by fibrinoid necrosis of the vascular wall. Perivenular inflammation was markedly severe from the tumor border until 10 mm distance from the tumor (Table 1, Fig. 3D, 4C, D).
Fibrosis, bile ductular reaction and steatosis in adjacent peritumoral hepatic parenchyma
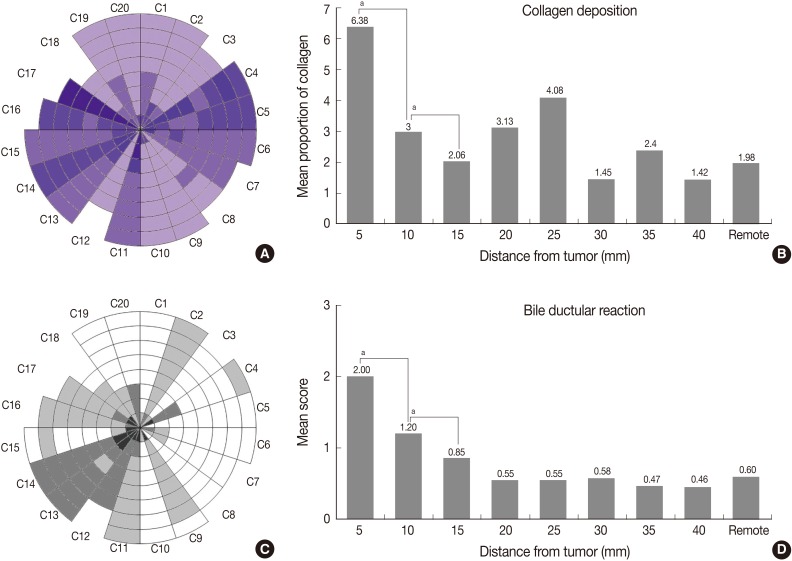
Although all cases in this study resulted from cirrhosis or chronic hepatitis B backgrounds, the degree of fibrosis in PNLP varies with distance from the tumor (Fig. 1F). The Ishak grade of fibrosis for all cases is illustrated in concentric circles (Fig. 5A). The mean value of the pixel fraction of collagen deposition for each column is presented as a bar graph (Fig. 5B).
Fig. 5.
Architectural changes including fibrosis and bile ductular proliferation. (A) Ishak fibrosis staging system. (B) Collagen deposition by image analysis. (C, D) Bile ductular proliferation. ap-value of Wilcoxon signed-rank test between two adjacent columns <0.05.
As shown in Table 1, the Ishak grade of fibrosis was markedly higher in the 5-mm and 10-mm column (p=0.007 between 5-mm and 10-mm column; p=0.020 between 10-mm and 15-mm column). To quantify the amount of fibrosis, pixel fraction of collagen deposition was evaluated. This evaluation revealed that collagen deposition was much more severe in the 5-mm column than in more distant areas. Thus, 10 mm could be the considerable cut-off point for the Ishak grade of fibrosis, and 5 mm for pixel fraction of collagen deposition.
Bile ductular proliferation around the tumor was so severe that it was difficult to find remaining hepatocytes immediately adjacent to the tumor (Fig. 3A). However, it decreased with distance (Fig. 3B). As shown in Table 1 and Fig. 5C and D, its pattern was decreasing with distance but become stable beyond the 10 mm distance from the tumor border (p=0.001 between 5-mm and 10-mm column; p=0.008 between 10-mm and 15-mm column). However, there were no differences in steatosis between the columns (data not shown).
Comparison with adjacent and remote non-neoplastic hepatic parenchyma
To confirm the gradation of histopathologic features in adjacent PNLP, we evaluated the same factors beyond 40 mm in remote PNLP, and then compared with each column of adjacent PNLP within 40 mm (Table 1). Parameters compared included portal inflammation, periportal inflammation, spotty necrosis score, spotty necrosis count, the modified HAI score, peliosis, bile ductular proliferation and the Ishak grade of fibrosis (Table 1). The comparison demonstrated that most of the pathologic changes were markedly severe in 5-mm and 10-mm columns than those of those in remote parenchyma.
These results were consistent with comparisons between adjacent columns for such parameters as periportal inflammation, the modifieid HAI score, peliosis, bile ductular proliferation and the Ishak grade of fibrosis, verifying that 10 mm distance from the tumor border could be the considerable cut-off point for those parameters. Dense collagen deposition was observed within 5 mm PNLP, as opposed to remote PNLP. This was consistent with the result from the comparison between adjacent columns, suggesting this distance to be the considerable cut-off point for the collagen deposition.
Portal inflammation within 15 mm PNLP was markedly severe than that in remote PNLP, but the comparison between adjacent columns demonstrated changes fading from 20 mm. Hepatic venulitis of adjacent PNLP was not different from the remote PNLP.
DISCUSSION
In this study, we investigated that the gradual histologic changes from the tumor edges and tried to find reasonable cut-off points for adjacent and remote peritumoral parenchyma based on various morphologic characteristics that are believed to indicate microenvironmental changes. All histologic parameters were higher proximate to the tumor, and faded with distance.
Although the individual histologic parameters revealed several distances from the tumor edges, we found the area within 10 mm and beyond 20 mm distance from the tumor to be relatively constant. PNLP within 10 mm had significantly higher inflammatory cells infiltration around portal tract and hepatic parenchyma, bile ductular reaction, collagen deposition and peliosis than 10 mm distance from the tumor. Moreover, PNLP beyond 20 mm had no distinct pathologic features compared with background liver parenchyma. Hence, the parenchyma within 10 mm from the tumor would be considered the adjacent PNLP in which morphologic changes are believed to be directly affected by the tumor, and the parenchyma beyond 20 mm as the remote PNLP without tumor effect.
These findings are very important in understanding tumor biology of HCC. The recurrence of HCC is one of the leading causes of death after curative liver resection, and the prognoses on cases of early and late recurrence are quite different.18 Late recurrence, which is defined as that occurring more than 2 years after primary resection, has better prognosis, while early recurrence that develops within 2 year after resection carries a poor prognosis. Early recurrence is strongly negatively correlated with patient's survival.19 Hence, many predictive or potential risk factors for early recurrence have been investigated, including vascular invasion, biliary tumor thrombi, intrahepatic metastasis, and positive surgical margin.18,20,21 These results imply that early recurrence is associated with local tumor factors rather than the background liver parenchyma. On the contrary, late recurrence of HCCs represents the tumorigenic trait of the remaining liver parenchyma.
Currently, immunologic or inflammatory milieu of the adjacent peritumoral tissue, which is directly affected by the tumor, have been postulated to be important in tumor progression22 and early recurrence of HCC.7,23,24 Jia et al.22 demonstrated that the colony-stimulating factor-1 receptor level of adjacent PNLP was associated with intrahepatic metastasis and early recurrence when the adjacent PNLP was defined as within 10 mm, there-by supporting our definition of adjacent PNLP as within 10 mm distance from the tumor.
The remote PNLP, which is not affected by HCC, is the remaining hepatic parenchyma in patients after resection so it is related with multiple occurrence and late recurrence of HCC. It is reported in several studies that molecular signature of non-cancerous liver tissue can predict the risk for late recurrence of HCCs and influence the clinical outcome of HCC patients. In this study, we defined the remote PNLP as beyond 20 mm distance from the tumor, which is consistent with the results of previous studies. Okamoto et al.9 showed that specific gene-expression profiles of PNLP could predict the risk for multiple occurrence of HCC by using peritumoral liver tissue farthest from the tumor.9 Hoshida et al.11 demonstrated that specific gene-expression profiles of PNLP predicted late recurrence of HCC using the peritumoral liver tissue that was available at the time of study.
The morphological changes described here were based on the criteria for viral hepatitis, and might not be sufficient to detect all types of tumor effect on adjacent PNLP. For example, peliosis, hepatic venulitis and bile ductular proliferation could be caused by the elevation of sinusoidal and bile ductal pressure, and end up with hepatocyte damage.25 We revealed positive correlation between tumor size and the farthest distance from the tumor where the peliosis could be observed (data not shown; spearman correlation coefficient=0.556; p=0.009). However, there was no such observation with hepatic venulitis and bile ductular proliferation. Furthermore, this study was limited to a small sample size, so statistical analysis for parametric validation cannot be conducted.
However, this study is the first report on comparison between adjacent PNLP and remote PNLP based on several histologic parameters via semiquantitative and quantitative scoring systems. Results of this study can be used as an objective histologic evidence that may help many hepatologists and reseachers select proper tissue specimen for research about primary hepatic tumors of patients with chronic liver disease.
In conclusion, adjacent PNLP, especially that within 10 mm of the tumor border, showed severe inflammation, architectural changes, peliosis, and hepatic venulitis. However, remote PNLP beyond 20 mm of tumor border showed no significant histologic changes.
Acknowledgments
This study was supported by grant no. 04-2011-1253 from the SNUH Research fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Poon RT. Prevention of recurrence after resection of hepatocellular carcinoma: a daunting challenge. Hepatology. 2011;54:757–759. doi: 10.1002/hep.24569. [DOI] [PubMed] [Google Scholar]
- 2.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belghiti J, Panis Y, Farges O, Benhamou JP, Fekete F. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991;214:114–117. doi: 10.1097/00000658-199108000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz JD, Schwartz M, Mandeli J, Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol. 2002;3:593–603. doi: 10.1016/s1470-2045(02)00873-2. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Wahab M, El-Husseiny TS, El Hanafy E, El Shobary M, Hamdy E. Prognostic factors affecting survival and recurrence after hepatic resection for hepatocellular carcinoma in cirrhotic liver. Langenbecks Arch Surg. 2010;395:625–632. doi: 10.1007/s00423-010-0643-0. [DOI] [PubMed] [Google Scholar]
- 6.Utsunomiya T, Shimada M, Imura S, Morine Y, Ikemoto T, Mori M. Molecular signatures of noncancerous liver tissue can predict the risk for late recurrence of hepatocellular carcinoma. J Gastroenterol. 2010;45:146–152. doi: 10.1007/s00535-009-0164-1. [DOI] [PubMed] [Google Scholar]
- 7.Xu HX, Zhu XD, Zhuang PY, et al. Expression and prognostic significance of placental growth factor in hepatocellular carcinoma and peritumoral liver tissue. Int J Cancer. 2011;128:1559–1569. doi: 10.1002/ijc.25492. [DOI] [PubMed] [Google Scholar]
- 8.Grudé P, Conti F, Mennecier D, et al. MDR1 gene expression in hepatocellular carcinoma and the peritumoral liver of patients with and without cirrhosis. Cancer Lett. 2002;186:107–113. doi: 10.1016/s0304-3835(02)00155-6. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto M, Utsunomiya T, Wakiyama S, et al. Specific gene-expression profiles of noncancerous liver tissue predict the risk for multicentric occurrence of hepatocellular carcinoma in hepatitis C virus-positive patients. Ann Surg Oncol. 2006;13:947–954. doi: 10.1245/ASO.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Ju MJ, Qiu SJ, Gao Q, et al. Combination of peritumoral mast cells and T-regulatory cells predicts prognosis of hepatocellular carcinoma. Cancer Sci. 2009;100:1267–1274. doi: 10.1111/j.1349-7006.2009.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masson P. Some histological methods: trichrome stainings and their preliminary technique. J Technol Methods. 1929;12:75–90. [Google Scholar]
- 13.Foot NC. The Masson trichrome staining methods in routine laboratory use. Biotech Histochem. 1933;8:101–110. [Google Scholar]
- 14.Nakaji M, Hayashi Y, Ninomiya T, et al. Histological grading and staging in chronic hepatitis: its practical correlation. Pathol Int. 2002;52:683–690. doi: 10.1046/j.1440-1827.2002.01410.x. [DOI] [PubMed] [Google Scholar]
- 15.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 16.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 18.Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah SA, Greig PD, Gallinger S, et al. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg. 2006;202:275–283. doi: 10.1016/j.jamcollsurg.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. [PubMed] [Google Scholar]
- 21.Li C, Wen TF, Liao ZX, et al. Recurrence of hepatocellular carcinoma after liver transplantation: recurrence characteristics and risk factors. Hepatogastroenterology. 2010;57:567–570. [PubMed] [Google Scholar]
- 22.Jia JB, Wang WQ, Sun HC, et al. High expression of macrophage colony-stimulating factor-1 receptor in peritumoral liver tissue is associated with poor outcome in hepatocellular carcinoma after curative resection. Oncologist. 2010;15:732–743. doi: 10.1634/theoncologist.2009-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H, Huang H, Shi J, et al. Prognostic value of interleukin 2 and interleukin 15 in peritumoral hepatic tissues for patients with hepatitis B-related hepatocellular carcinoma after curative resection. Gut. 2010;59:1699–1708. doi: 10.1136/gut.2010.218404. [DOI] [PubMed] [Google Scholar]
- 24.Ju MJ, Qiu SJ, Fan J, et al. Peritumoral activated hepatic stellate cells predict poor clinical outcome in hepatocellular carcinoma after curative resection. Am J Clin Pathol. 2009;131:498–510. doi: 10.1309/AJCP86PPBNGOHNNL. [DOI] [PubMed] [Google Scholar]
- 25.Turányi E, Dezsö K, Csomor J, Schaff Z, Paku S, Nagy P. Immunohistochemical classification of ductular reactions in human liver. Histopathology. 2010;57:607–614. doi: 10.1111/j.1365-2559.2010.03668.x. [DOI] [PubMed] [Google Scholar]